Abstract
Background
Zipf's law and Heaps' law are two representatives of the scaling concepts, which play a significant role in the study of complexity science. The coexistence of the Zipf's law and the Heaps' law motivates different understandings on the dependence between these two scalings, which has still hardly been clarified.
Methodology/Principal Findings
In this article, we observe an evolution process of the scalings: the Zipf's law and the Heaps' law are naturally shaped to coexist at the initial time, while the crossover comes with the emergence of their inconsistency at the larger time before reaching a stable state, where the Heaps' law still exists with the disappearance of strict Zipf's law. Such findings are illustrated with a scenario of large-scale spatial epidemic spreading, and the empirical results of pandemic disease support a universal analysis of the relation between the two laws regardless of the biological details of disease. Employing the United States domestic air transportation and demographic data to construct a metapopulation model for simulating the pandemic spread at the U.S. country level, we uncover that the broad heterogeneity of the infrastructure plays a key role in the evolution of scaling emergence.
Conclusions/Significance
The analyses of large-scale spatial epidemic spreading help understand the temporal evolution of scalings, indicating the coexistence of the Zipf's law and the Heaps' law depends on the collective dynamics of epidemic processes, and the heterogeneity of epidemic spread indicates the significance of performing targeted containment strategies at the early time of a pandemic disease.
Introduction
Scaling concepts play a significant role in the field of complexity science, where a considerable amount of efforts is devoted to understand these universal properties underlying multifarious systems[1]–[4]. Two representatives of scaling emergence are the Zipf's law and the Heaps' law. G.K. Zipf, sixty years ago, found a power law distribution for the occurrence frequencies of words within different written texts, when they were plotted in a descending order against their rank[5]. This frequency-rank relation also corresponds to a power law probability distribution of the word frequencies[32]. The Zipf's law is found to hold empirically for a great deal of complex systems, e.g., natural and artificial languages[5]–[9], city sizes[10], [11], firm sizes[12], stock market index[13], [14], gene expression[15], [16], chess opening[17], arts[18], paper citations[19], family names[20], and personal donations[21]. Many mechanisms are proposed to trace the origin of the Zipf's law[22]–[24].
Heaps' law is another important empirical principle describing the sublinear growth of the number of unique elements, when the system size keeps on enlarging[25]. Recently, particular attention is paid to the coexistence of the Zipf's law and the Heaps' law, which is reported for the corpus of web texts[26], keywords in scientific publication[27], collaborative tagging in web applications[28], [29], chemoinformatics[30], and more close to the interest in this article, global pandemic spread[31], and etc.
In [33], [34], an improved version of the classical Simon model[35] was put forward to investigate the emergence of the Zipf's law, which is deemed to be a result from the existence of the Heaps' law. However, [26], [32] concluded that the Zipf's law leads to the Heaps' law. In fact, the interdependence of these two laws has hardly been clarified. This embarrassment comes from the fact that the empirical/simulated evidence employed to show the emergence of Zipf's law mainly deals with static and finalized speicmens/results, while the Heaps' law actually describes the evolving characteristics.
In this article, we investigate the relation between these scaling laws from the perspective of coevolution between the scaling properties and the epidemic spread. We take the scenarios of large-scale spatial epidemic spreading for example, since the empirical data contain sufficient spatiotemporal information making it possible to visualize the evolution of the scalings, which allows us to analyze the inherent mechanisms of their formation. The Zipf's law and the Heaps' law of the laboratory confirmed cases are naturally shaped to coexist during the early epidemic spread at both the global and the U.S. levels, while the crossover comes with the emergence of their inconsistency as the epidemic keeps on prevailing, where the Heaps' law still exists with the disappearance of strict Zipf's law. With the U.S. domestic air transportation and demographic data, we construct a fine-grained metapopulation model to explore the relation between the two scalings, and recognize that the broad heterogeneity of the infrastructure plays a key role in their temporal evolution, regardless of the biological details of diseases.
Results
Empirical and Analytical Results
With the empirical data of the laboratory confirmed cases of the A(H1N1) provided by the World Health Organization(WHO)(see the data description in
Materials and Methods
), we first study the probability-rank distribution(PRD) of the cumulative confirmed number(CCN) of every infected country at several given dates sampled about every two weeks. 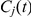 denotes the CCN in a given country
denotes the CCN in a given country 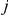 at time
at time  . Since
. Since 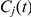 grows with time, the distributions at different dates are normalized by the global CCN,
grows with time, the distributions at different dates are normalized by the global CCN, 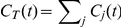 , for comparison. Fig. 1(A) shows the Zipf-plots of the PRD
, for comparison. Fig. 1(A) shows the Zipf-plots of the PRD
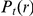 of the infected countries' confirmed cases by arranging every
of the infected countries' confirmed cases by arranging every 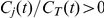 in a descending order for each specimen. The maximal rank
in a descending order for each specimen. The maximal rank 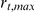 (on x-axis) for each specimen denotes the total number of infected countries at a given date, and grows as the epidemic spreading.
(on x-axis) for each specimen denotes the total number of infected countries at a given date, and grows as the epidemic spreading.
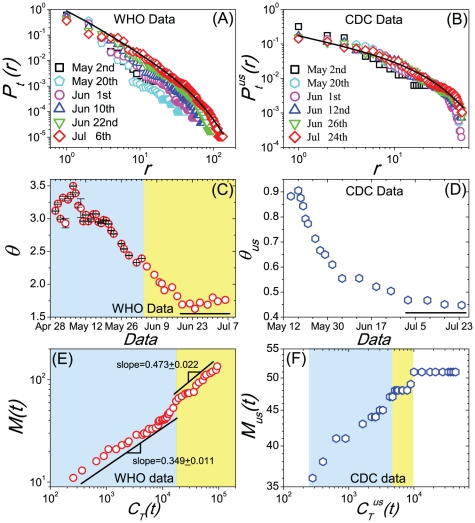
Figure 1. The empirical results of A(H1N1).
(A) The Zipf-plots of the normalized probability-rank distributions 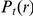 of the cumulated confirmed number of every infected country at several given date sampled about every two weeks, data provided by the WHO. (B) The Zipf-plots of
of the cumulated confirmed number of every infected country at several given date sampled about every two weeks, data provided by the WHO. (B) The Zipf-plots of 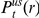 at several given data sampled about every two weeks, data provided by the CDC. (C) Temporal evolution of the estimated exponent
at several given data sampled about every two weeks, data provided by the CDC. (C) Temporal evolution of the estimated exponent 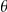 of the normalized distribution
of the normalized distribution 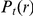 . (D) Temporal evolution of the estimated exponent
. (D) Temporal evolution of the estimated exponent 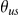 of the normalized distribution
of the normalized distribution 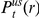 of the period after May 15th. (E) The sublinear relation between the number of infected countries
of the period after May 15th. (E) The sublinear relation between the number of infected countries 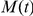 and the cumulative number of global confirmed cases
and the cumulative number of global confirmed cases 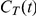 , data collected by the WHO. (F) The sublinear relation between the number of infected states
, data collected by the WHO. (F) The sublinear relation between the number of infected states 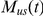 and the cumulative number of national confirmed cases
and the cumulative number of national confirmed cases 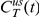 , data collected by the CDC. The shaded areas in the figures (C,E,F) corresponds to their different evolution stages, respectively.
, data collected by the CDC. The shaded areas in the figures (C,E,F) corresponds to their different evolution stages, respectively.
At the early stage(the period between April 30th and June 1st, 2009), 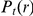 shows a power law pattern
shows a power law pattern 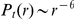 , which indicates the emergence of the Zipf's law. We estimate the power law exponent
, which indicates the emergence of the Zipf's law. We estimate the power law exponent 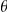 for each specimen of this stage by the maximum likelihood method[22], [37], and report its temporal evolution in the left part of Fig. 1(C). About sixty countries were affected by the A(H1N1) on June 1st, and most of them are countries with large population and/or economic power, e.g., U.S., Mexico, Canada, Japan, Australia, China. After June 1st, the disease swept much more countries in a short time, and the WHO announcement on June 11th[38] raised the pandemic level to its highest phase, phase 6(see Text S1), which implied that the global pandemic flu was occurring. At this stage(after June 1st, 2009),
for each specimen of this stage by the maximum likelihood method[22], [37], and report its temporal evolution in the left part of Fig. 1(C). About sixty countries were affected by the A(H1N1) on June 1st, and most of them are countries with large population and/or economic power, e.g., U.S., Mexico, Canada, Japan, Australia, China. After June 1st, the disease swept much more countries in a short time, and the WHO announcement on June 11th[38] raised the pandemic level to its highest phase, phase 6(see Text S1), which implied that the global pandemic flu was occurring. At this stage(after June 1st, 2009), 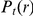 gradually displays a power law distribution with an exponential cutoff
gradually displays a power law distribution with an exponential cutoff 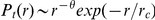 , where
, where 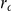 is the parameter controlling the cutoff effect(see Text S1), and the exponent
is the parameter controlling the cutoff effect(see Text S1), and the exponent 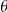 gradually reduces to around 1.7, as shown in Fig. 1(C). Surprisingly,
gradually reduces to around 1.7, as shown in Fig. 1(C). Surprisingly, 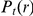 at different dates eventually reaches a stable distribution as time evolves(see those curves since June in Fig. 1(A)). Indeed, after June 19th,
at different dates eventually reaches a stable distribution as time evolves(see those curves since June in Fig. 1(A)). Indeed, after June 19th, 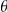 seems to reach a stable value with mild fluctuations, as shown in Fig. 1(C). The characteristics of the temporal evolution of the parameter
seems to reach a stable value with mild fluctuations, as shown in Fig. 1(C). The characteristics of the temporal evolution of the parameter 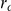 is similar to
is similar to 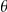 , thus we mainly present the empirical results of the exponent
, thus we mainly present the empirical results of the exponent 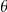 in the main text and hold the results of
in the main text and hold the results of 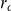 in Figure S1. In the following, we analyze the evolution of the normalized distribution
in Figure S1. In the following, we analyze the evolution of the normalized distribution 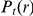 by the contact process of an epidemic transmission, regardless of the biological details of diseases.
by the contact process of an epidemic transmission, regardless of the biological details of diseases.
Straightforwardly, according to the mass action principle in the mathematical epidemiology[39], [40](see Text S1), which is widely applied in studying the epidemic spreading process on a network[41]–[56], we consider the SIR epidemic scheme here,
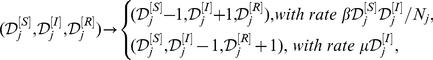 |
(1) |
where 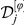 denotes the number of individuals in compartment
denotes the number of individuals in compartment 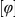 (susceptible(S), infectious(I) or permanently recovered(R)) in a given country
(susceptible(S), infectious(I) or permanently recovered(R)) in a given country 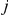 ,
, 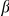 denotes the disease transmission rate, and infectious individuals recover with a probability
denotes the disease transmission rate, and infectious individuals recover with a probability 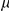 . The population in a given country
. The population in a given country 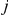 at time
at time  is
is 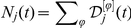 , where
, where 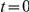 means the time when initially confirmed cases in the entire system are reported. At the early stage of a pandemic outbreak, the new introductions of infectious individuals dominate the onset of outbreak in unaffected countries. However, after the disease already lands in these countries, the ongoing indigenous transmission gradually exceeds the influence of the new introductions, and becomes the mainstream of disseminators[57], [58]. According to Eq.(1), in a given infected country
means the time when initially confirmed cases in the entire system are reported. At the early stage of a pandemic outbreak, the new introductions of infectious individuals dominate the onset of outbreak in unaffected countries. However, after the disease already lands in these countries, the ongoing indigenous transmission gradually exceeds the influence of the new introductions, and becomes the mainstream of disseminators[57], [58]. According to Eq.(1), in a given infected country 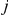 , there are
, there are
| (2) |
new infected individuals on average at 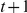 days, and the average number of illness at
days, and the average number of illness at 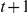 days is
days is
| (3) |
Defining 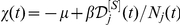 and
and 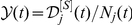 , we have
, we have
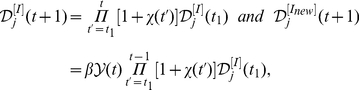 |
(4) |
where 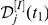 denotes the number of initially confirmed or introduced cases in country
denotes the number of initially confirmed or introduced cases in country 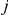 , and is always a small positive integer. The CCN of country
, and is always a small positive integer. The CCN of country 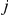 at
at 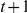 days is
days is 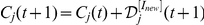 . When
. When  is large enough, we have
is large enough, we have
| (5) |
Before the disease dies out in country 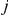 ,
, 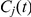 keeps increasing from the onset of outbreak[59]. When
keeps increasing from the onset of outbreak[59]. When  is large enough, it is obviously
is large enough, it is obviously 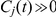 ,
, 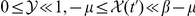 , thus
, thus 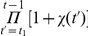 is definitely larger than
is definitely larger than 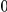 and can hardly be infinity.
and can hardly be infinity. 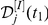 is a small positive integer, thus
is a small positive integer, thus 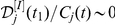 when
when  is large enough. We therefore have
is large enough. We therefore have 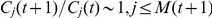 for large
for large  , where
, where 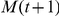 is the total number of infected countries after
is the total number of infected countries after 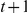 days of spreading. Thus the normalized probability
days of spreading. Thus the normalized probability 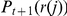 at
at 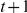 day is:
day is:
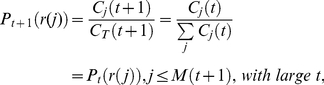 |
(6) |
where 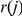 is the rank of the CCN of country
is the rank of the CCN of country 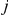 in the descending order of the CCN list of all infected countries. Eq.(6) indicates that each probability
in the descending order of the CCN list of all infected countries. Eq.(6) indicates that each probability 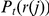 is invariant for large
is invariant for large  , thus the normalized distribution
, thus the normalized distribution 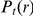 becomes stable when
becomes stable when  is large enough. The intrinsic reasons for the emergence of these scaling properties are discussed in Modeling and Simulation Results.
is large enough. The intrinsic reasons for the emergence of these scaling properties are discussed in Modeling and Simulation Results.
Since the normalized PRD
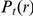 displays the Zipf's law pattern
displays the Zipf's law pattern 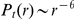 at the early stage of the epidemic, the CCN of the country ranked
at the early stage of the epidemic, the CCN of the country ranked  is
is 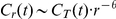 at this stage. Considering the CCN of the countries with ranks between
at this stage. Considering the CCN of the countries with ranks between  and
and 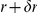 , where
, where 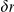 is any infinitesimal value, we have
is any infinitesimal value, we have 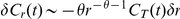 . Supposing
. Supposing 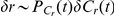 with
with 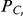 denoting the probability density function, we have
denoting the probability density function, we have
| (7) |
Thus
| (8) |
where 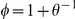 ,
, 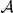 is a constant. According to the normalization condition
is a constant. According to the normalization condition 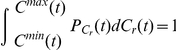 , where
, where 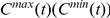 is the CCN of the country with the maximal(minimal) value at a give time
is the CCN of the country with the maximal(minimal) value at a give time  , we have
, we have 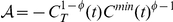 because
because 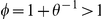 and
and 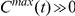 . Then
. Then
| (9) |
At a given date,  can be regarded as the number of countries with the amount of cumulated confirmed cases which is no less than
can be regarded as the number of countries with the amount of cumulated confirmed cases which is no less than 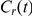 , then
, then
| (10) |
Recalling 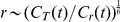 , we have
, we have
| (11) |
where 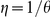 . At the early stage corresponding to the period between April 30th and June 1st,
. At the early stage corresponding to the period between April 30th and June 1st, 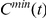 is one according to the WHO data. Therefore, we have
is one according to the WHO data. Therefore, we have
| (12) |
which indicates that the Heap's law[25], [26], [31], [32] can be observed in this case. The empirical evidence for the emergence of the Heap's law at this stage is shown in the middle part of Fig. 1(E). The Heaps' exponent 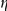 is obtained by the least square method[31], [32], and the relevance between
is obtained by the least square method[31], [32], and the relevance between 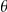 and
and 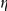 is reported in Table 1.
is reported in Table 1.
Table 1. The empirical results of the parameters 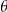 and
and 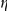 , and their relevance at the early time(the period between April 30th and June 1st, 2009), using 2009 Pandemic A(H1N1) data collected by the WHO.
, and their relevance at the early time(the period between April 30th and June 1st, 2009), using 2009 Pandemic A(H1N1) data collected by the WHO.
| Date |
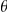
|
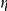
|
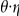
|
| April 30th | 3.12 | 0.349 | 1.046 |
| May 1st | 3.23 | 0.349 | 1.127 |
| May 2th | 3.00 | 0.349 | 1.047 |
| May 3th | 3.32 | 0.349 | 1.159 |
| May 4th | 2.93 | 0.349 | 1.022 |
| May 5th | 3.29 | 0.349 | 1.148 |
| May 6th | 3.35 | 0.349 | 1.169 |
| May 7th | 3.5 | 0.349 | 1.222 |
| May 8th | 3.39 | 0.349 | 1.183 |
| May 9th | 3.2 | 0.349 | 1.117 |
| May 10th | 3.16 | 0.349 | 1.103 |
| May 11th | 2.96 | 0.349 | 1.033 |
| May 12th | 3.06 | 0.349 | 1.068 |
| May 13th | 2.96 | 0.349 | 1.033 |
| May 14th | 3.00 | 0.349 | 1.047 |
| May 15th | 3.07 | 0.349 | 1.071 |
| May 16th | 3.07 | 0.349 | 1.071 |
| May 17th | 2.95 | 0.349 | 1.030 |
| May 18th | 2.93 | 0.349 | 1.023 |
| May 19th | 2.98 | 0.349 | 1.040 |
| May 20th | 2.97 | 0.349 | 1.037 |
| May 21th | 2.92 | 0.349 | 1.019 |
| May 22th | 2.82 | 0.349 | 0.984 |
| May 23th | 2.77 | 0.349 | 0.967 |
| May 26th | 2.62 | 0.349 | 0.914 |
| May 27th | 2.54 | 0.349 | 0.886 |
| May 29th | 2.44 | 0.349 | 0.852 |
| June 1st | 2.33 | 0.349 | 0.813 |
At the latter stage(the period after June 1st, 2009), the exponential tail of the distribution 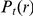 leads to a deviation from the strict Zipf's law. However, with a steeper exponent
leads to a deviation from the strict Zipf's law. However, with a steeper exponent 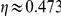 , the Heaps' law still exists, as shown in the right part of Fig. 1(E). Though the two scaling laws are naturally shaped to coexist during the early epidemic spreading, their inconsistency gradually emerges as the epidemic keeps on prevailing. Indeed, in the Discussion of [32], without empirical or analytical evidence, Lü et al have intuitively suspected that there may exist some unknown mechanisms only producing the Heaps' law, and it is possible that a system displaying the Heaps' law does not obey the strict Zipf's law. Here we not only verify this suspicion with the empirical results, but also explore the substaintial mechanisms of the evolution process in Modeling and Simulation Results, where we uncover the important role of the broad heterogeneity of the infrastructure in the temporal evolution of scaling emergence.
, the Heaps' law still exists, as shown in the right part of Fig. 1(E). Though the two scaling laws are naturally shaped to coexist during the early epidemic spreading, their inconsistency gradually emerges as the epidemic keeps on prevailing. Indeed, in the Discussion of [32], without empirical or analytical evidence, Lü et al have intuitively suspected that there may exist some unknown mechanisms only producing the Heaps' law, and it is possible that a system displaying the Heaps' law does not obey the strict Zipf's law. Here we not only verify this suspicion with the empirical results, but also explore the substaintial mechanisms of the evolution process in Modeling and Simulation Results, where we uncover the important role of the broad heterogeneity of the infrastructure in the temporal evolution of scaling emergence.
We also empirically study the evolution of scaling emergence of the epidemic spreading at the countrywide level. Since the United States is one of the several earliest and most seriously prevailed countries of the A(H1N1)[60], we mainly focus on the A(H1N1) spreading in the United States. With the empirical data of the laboratory confirmed cases of the A(H1N1) provided by the Centers for Disease Control and Prevention(CDC)(see the data description in
Materials and Methods
), in Fig. 1(B) we report the PRD of the CCN of infected states, 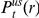 , at several given dates sampled about every two weeks. Our findings suggest a crossover in the temporal evolution of
, at several given dates sampled about every two weeks. Our findings suggest a crossover in the temporal evolution of 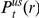 . At the early stage(the period before May 15th),
. At the early stage(the period before May 15th), 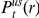 shows a power law pattern
shows a power law pattern 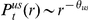 with a much smaller exponent
with a much smaller exponent 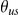 than that of the WHO results. Washington D.C. and 46 states(excluding Alaska, Mississippi, West Virginia, Wyoming) were affected by A(H1N1) on May 15th. After May 15th,
than that of the WHO results. Washington D.C. and 46 states(excluding Alaska, Mississippi, West Virginia, Wyoming) were affected by A(H1N1) on May 15th. After May 15th, 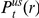 gradually becomes a power law distribution with an exponential cutoff,
gradually becomes a power law distribution with an exponential cutoff, 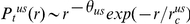 , which leads to a deviation from the strict Zipf's law. In this case, the exponent
, which leads to a deviation from the strict Zipf's law. In this case, the exponent 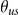 gradually reduces and reaches a stable value 0.45(see Fig. 1(D)), which conforms to the fact that
gradually reduces and reaches a stable value 0.45(see Fig. 1(D)), which conforms to the fact that 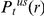 of different dates eventually reaches a stable distribution as time evolves. The temporal evolution of the exponent
of different dates eventually reaches a stable distribution as time evolves. The temporal evolution of the exponent 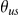 of all data are shown in Figure S2.
of all data are shown in Figure S2. 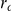 keeps the value around 14 after June 12th, 2009.
keeps the value around 14 after June 12th, 2009.
The relation between 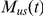 and
and 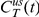 is shown in Fig. 1(F). Though at first glance this figure provides us an impression of the sublinear growth of the number of infected states
is shown in Fig. 1(F). Though at first glance this figure provides us an impression of the sublinear growth of the number of infected states 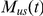 when the cumulative number of national total patients
when the cumulative number of national total patients 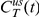 increases, we could not use the least square method here to estimate the Heaps' exponent
increases, we could not use the least square method here to estimate the Heaps' exponent 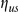 for several reasons: (i) the amount of data at each stage is quite small; (ii) there are several periods that
for several reasons: (i) the amount of data at each stage is quite small; (ii) there are several periods that 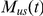 keeps unchanged(May 6th
keeps unchanged(May 6th  May 7th,
May 7th, 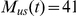 ; May 12th
; May 12th  May 13th,
May 13th, 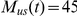 ; May 18th
; May 18th  May 27th,
May 27th, 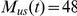 ); (iii) the magnitude of
); (iii) the magnitude of 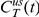 is much larger than that of
is much larger than that of 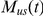 ; (iv) after June 1st, 2009, Washington D.C. and all 50 states of the United States were affected by the A(H1N1). Define
; (iv) after June 1st, 2009, Washington D.C. and all 50 states of the United States were affected by the A(H1N1). Define 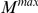 the maximal number of the geographical regions the epidemic spreads to. In the U.S. scenario,
the maximal number of the geographical regions the epidemic spreads to. In the U.S. scenario, 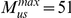 . When
. When 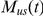 reaches
reaches 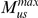 on June 1st,
on June 1st, 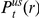 evolves and becomes stable after June 26th(see Fig. 1(B,D)). In the Modeling and Simulation Results, we explore the relation between these two scalings with a fine grained metapopulation model characterizing the spread of the A(H1N1) at the U.S. level in detail.
evolves and becomes stable after June 26th(see Fig. 1(B,D)). In the Modeling and Simulation Results, we explore the relation between these two scalings with a fine grained metapopulation model characterizing the spread of the A(H1N1) at the U.S. level in detail.
Note that these scaling properties are not exceptive for the A(H1N1) transmission. More supported exemplifications are reported in Figure S3, e.g. the cases of SARS, Avian Influenza(H5N1). It is worth remarking that the normalized distribution 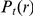 almost keeps the power law pattern during the whole spreading process of the global SARS. This phenomenon might result from the intense containment strategies, e.g. patient isolation, enforced quarantine, school closing, travel restriction, implemented by individuals or governments confronting mortal plague.
almost keeps the power law pattern during the whole spreading process of the global SARS. This phenomenon might result from the intense containment strategies, e.g. patient isolation, enforced quarantine, school closing, travel restriction, implemented by individuals or governments confronting mortal plague.
Modeling and Simulation Results
The above analyses, however, do not tell the whole story, because the intrinsic reasons for the emergence of these scaling properties have not been explained. Some additional clues from the perspective of Shannon entropy[61] of a system might unlock the puzzle.
Nowadays, population explosion in the urban areas, massive interconnectivity among different geographical regions, and huge volume of human mobility are the factors accelerating the spread of infectious disease[62], [74]. At a large geographical scale, one main class of models is the metapopulation model dividing the entire system into several interconnected subpopulations[58], [63]–[74], [87], [88]. Within each subpopulation, the infectious dynamics is described by the compartment schemes, while the spread from one subpopulation to another is due to the transportation and mobility infrastructures, e.g., air transportation. Individuals in each subpopulation exist in various discrete health compartments(status), i.e. susceptible, latent, infectious, recovered, and etc., with compartmental transitions by the contagion process or spontaneous transition, and might travel to other subpopulations by vehicles, e.g., airplane, in a short time. The metapopulation model can not only be employed to describe the global pandemic spread when we regard each subpopulation as a given country, but also be used to simulate the disease transmission within a country when each subpopulation is regarded as a given geographical region in the country. Here we mainly consider the spread of pandemic influenza at the U.S. country level for threefold reasons: (i) the computational cost of simulating global pandemic spread is too tremendous to implement on a single PC or Server[58], [70], [72], [81], [87]; (ii) the IATA or OAG flight schedule data, which is widely used to obtain the global air transportation network, do not provide the attendance and flight-connecting information(see data description in Materials and Methods ); (iii) the United States is one of the several earliest and most seriously prevailed countries[60].
We construct a metapopulation model at the U.S. level with the U.S. domestic air transportation and demographic statistical data[75]–[78](detailed data description is provided in
Materials and Methods
, and a full specification of the simulation model is reported in Text S1). Define a subpopulation as a Metropolitan/Micropolitan Statistical Areas(MSAs/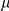 SAs)[75] connected by a transportation network, in this article, the U.S. domestic airline network(USDAN). The USDAN is a weighted graph comprising
SAs)[75] connected by a transportation network, in this article, the U.S. domestic airline network(USDAN). The USDAN is a weighted graph comprising 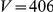 vertices(airports) and
vertices(airports) and 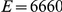 weighted and directed edges denoting flight courses. The weight of each edge is the daily amount of passengers on that flight course. The infrastructure of the USDAN presents high levels of heterogeneity in connectivity patterns, traffic capacities and population(see Fig. 2). The disease dynamics in a single subpopulation is modeled with the Susceptible-Latent-Infectious-Recovered(SLIR) compartmental scheme, where the abbreviation L denotes the latent compartment which experiences
weighted and directed edges denoting flight courses. The weight of each edge is the daily amount of passengers on that flight course. The infrastructure of the USDAN presents high levels of heterogeneity in connectivity patterns, traffic capacities and population(see Fig. 2). The disease dynamics in a single subpopulation is modeled with the Susceptible-Latent-Infectious-Recovered(SLIR) compartmental scheme, where the abbreviation L denotes the latent compartment which experiences 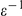 days on average for an infected person(The SIR epidemic dynamics discussed at Empirical and Analytical Results is an reasonable approximation, which actually simplifies the epidemic evolution to a Markov chain to help us study the issue, and the value of the reproductive number
days on average for an infected person(The SIR epidemic dynamics discussed at Empirical and Analytical Results is an reasonable approximation, which actually simplifies the epidemic evolution to a Markov chain to help us study the issue, and the value of the reproductive number 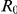 does not depend on
does not depend on  , we therefore ignore the compartment L there).
, we therefore ignore the compartment L there).
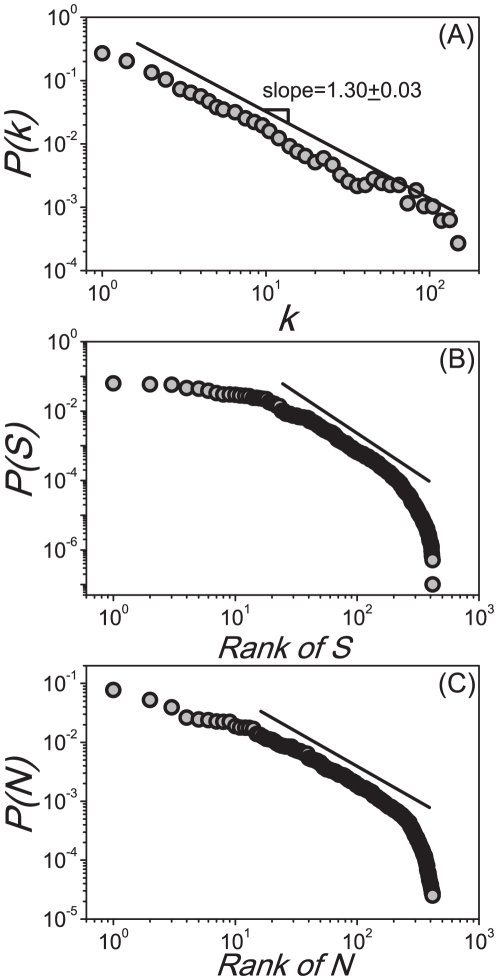
Figure 2. The heterogeneity of the USDAN's infrastructure.
(A) The degree distribution 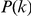 follows a power law pattern on almost two decades with an exponent 1.30
follows a power law pattern on almost two decades with an exponent 1.30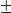 0.03. (B) shows that the probability-rank distribution of the traffic outflux
0.03. (B) shows that the probability-rank distribution of the traffic outflux 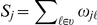 , where
, where  denotes the set of neighbors belonging to the vertex
denotes the set of neighbors belonging to the vertex 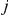 and the weight
and the weight 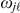 of a connection between two vertices
of a connection between two vertices 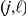 is the number of passengers traveling a given route per day, is skewed and heterogeneously distributed. (C) shows that the probability-rank distribution of populations is skewed and heterogeneously distributed.
is the number of passengers traveling a given route per day, is skewed and heterogeneously distributed. (C) shows that the probability-rank distribution of populations is skewed and heterogeneously distributed.
The key parameters determining the spreading rate of infections are the reproductive number 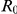 and the generation time
and the generation time 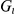 .
. 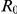 is defined as the average amount of individuals an ill person infects during his or her infectious period
is defined as the average amount of individuals an ill person infects during his or her infectious period 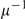 in a large fully susceptible population, and
in a large fully susceptible population, and 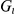 refers to the sum of the latent period
refers to the sum of the latent period 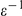 and the infectious period
and the infectious period 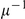 . In our metapopulation model,
. In our metapopulation model, 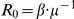 . The initial conditions of the disease are defined as the onset of the outbreak in San Diego-Carlsbad-San Marcos, CA MSA on April 17th, 2009, as reported by the CDC[79]. Assuming a short latent period value
. The initial conditions of the disease are defined as the onset of the outbreak in San Diego-Carlsbad-San Marcos, CA MSA on April 17th, 2009, as reported by the CDC[79]. Assuming a short latent period value 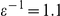 days as indicated by the early estimates of the pandemic A(H1N1)[80], which is compatible with other recent studies[81], [82], we primarily consider a baseline case with parameters:
days as indicated by the early estimates of the pandemic A(H1N1)[80], which is compatible with other recent studies[81], [82], we primarily consider a baseline case with parameters: 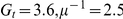 days and
days and 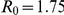 , which are higher than those obtained in the early findings of the pandemic A(H1N1)[80], but they are the median results in other subsequent analyses[81], [83]. Fixing the latency period to
, which are higher than those obtained in the early findings of the pandemic A(H1N1)[80], but they are the median results in other subsequent analyses[81], [83]. Fixing the latency period to 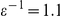 days, we also employ a more aggravated baseline scenario with parameters:
days, we also employ a more aggravated baseline scenario with parameters: 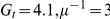 days and
days and 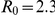 , which are close to the upper bound results in[81], [83]–[85].
, which are close to the upper bound results in[81], [83]–[85].
In succession, we characterize the disease spreading pattern by information entropy, which is customarily applied in information theory. To quantify the heterogeneity of the epidemic spread at the U.S. level, we examine the prevalence at each time  ,
, 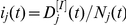 , for all subpopulations, and introduce the normalized vector
, for all subpopulations, and introduce the normalized vector 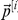 with components
with components 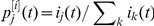 . Then we measure the level of heterogeneity of the disease prevalence by quantifying the disorder encoded in
. Then we measure the level of heterogeneity of the disease prevalence by quantifying the disorder encoded in 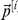 with the normalized entropy function
with the normalized entropy function
| (13) |
which provides an estimation of the geographical heterogeneity of the disease spread at time  . If the disease is uniformly influencing all subpopulations(e.g., all prevalences are equivalent), the entropy reaches its maximum value
. If the disease is uniformly influencing all subpopulations(e.g., all prevalences are equivalent), the entropy reaches its maximum value 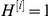 . On the other hand, starting from
. On the other hand, starting from 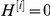 , which is the most localized and heterogeneous situation that just one subpopulation is initially affected by the disease,
, which is the most localized and heterogeneous situation that just one subpopulation is initially affected by the disease, 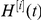 increases as more subpopulations are influenced, thus decreasing the level of heterogeneity.
increases as more subpopulations are influenced, thus decreasing the level of heterogeneity.
In order to better uncover the origin of the emergence of the scaling properties, we compare the baseline results with those obtained on a null model UNI. The UNI model is a homogeneous Erdös-Rényi random network with the same number of vertices as that of the USDAN, and the generating regulation is described as follows: for each pair of vertices 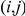 , an edge is independently generated with the uniform probability
, an edge is independently generated with the uniform probability 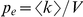 , where
, where 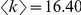 is the average out-degree of the USDAN. Moreover, the weights of the edges and the populations are uniformly equal to their average values in the USDAN, respectively. Therefore, the UNI model is completely absent from the heterogeneity of the airline topology, flux and population data.
is the average out-degree of the USDAN. Moreover, the weights of the edges and the populations are uniformly equal to their average values in the USDAN, respectively. Therefore, the UNI model is completely absent from the heterogeneity of the airline topology, flux and population data.
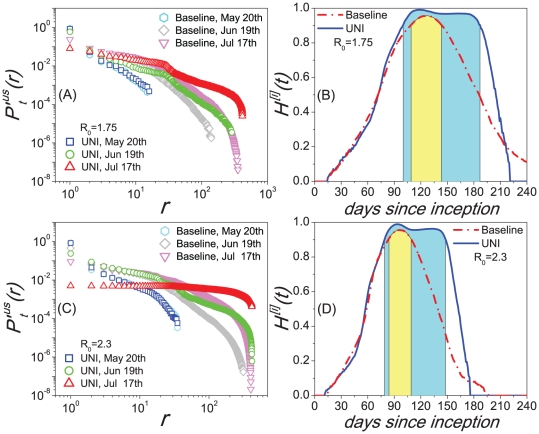
Different evolving behaviors between the UNI scenarios and the baselines(real airline cases) provide a remarkable evidence for the direct dependence between the scaling toproperties and the heterogeneous infrastructure. Fig. 3(A,C) show the comparison of the PRD between the baseline results and the UNI outputs at several given dates sampled about every 30 days, where each specimen is the median result over all runs that led to an outbreak at the U.S. level in 100 random Monte Carlo realizations. In Fig. 3(A), we consider the situation of 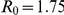 , and do observe that the evolution of PRD of the baseline case experiences two stages: a power law at the initial time and an exponentially cutoff power law at a larger time. However, the UNI scenario shows a distinct pattern: as time evolves, the middle part of the PRD grows more quickly, and displays a peak which obviously deviates scaling properties. Fig. 3(C) reports the situation of
, and do observe that the evolution of PRD of the baseline case experiences two stages: a power law at the initial time and an exponentially cutoff power law at a larger time. However, the UNI scenario shows a distinct pattern: as time evolves, the middle part of the PRD grows more quickly, and displays a peak which obviously deviates scaling properties. Fig. 3(C) reports the situation of 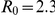 . In this aggravated instance, the PRD of the UNI scenario actually becomes rather homogeneous when
. In this aggravated instance, the PRD of the UNI scenario actually becomes rather homogeneous when  is large enough(see the curve of July 17th of the UNI scenario in Fig. 3(C)). Fig. 3(B,D) present the comparison of the information entropy profiles between the baseline results and the UNI outputs when
is large enough(see the curve of July 17th of the UNI scenario in Fig. 3(C)). Fig. 3(B,D) present the comparison of the information entropy profiles between the baseline results and the UNI outputs when 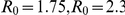 , respectively. The completely homogeneous network UNI shows a homogeneous evolution(
, respectively. The completely homogeneous network UNI shows a homogeneous evolution(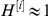 ) of the epidemic spread in a long period(see the light cyan areas in Fig. 3(B,D)), with sharp fallings at both the beginning and the end of the outbreak. However, we observe distinct results in the baselines, where
) of the epidemic spread in a long period(see the light cyan areas in Fig. 3(B,D)), with sharp fallings at both the beginning and the end of the outbreak. However, we observe distinct results in the baselines, where 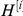 is significantly smaller than 1 for most of the time, and the long tails indicate a long lasting heterogeneity of the epidemic prevalence. These analyses signal that the broad heterogeneity of infrastructure plays an essential role in the emergence of scalings.
is significantly smaller than 1 for most of the time, and the long tails indicate a long lasting heterogeneity of the epidemic prevalence. These analyses signal that the broad heterogeneity of infrastructure plays an essential role in the emergence of scalings.
Figure 3. Comparisons of the scaling properties between the UNI scenarios and the baseline cases.
(A,C) present the comparison of the PRD
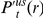 of the CCN of every infected MSA/
of the CCN of every infected MSA/
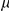 SA between the baselines and the UNI scenarios at several given date sampled about every 30 days when
SA between the baselines and the UNI scenarios at several given date sampled about every 30 days when 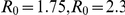 , respectively. (B,D) present the comparison of the information entropy profiles between the baselines and the UNI results when
, respectively. (B,D) present the comparison of the information entropy profiles between the baselines and the UNI results when 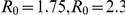 , respectively. Each data in these figures are the median results over all runs that led to an outbreak at the U.S. level in 100 random Monte Carlo realizations.
, respectively. Each data in these figures are the median results over all runs that led to an outbreak at the U.S. level in 100 random Monte Carlo realizations.
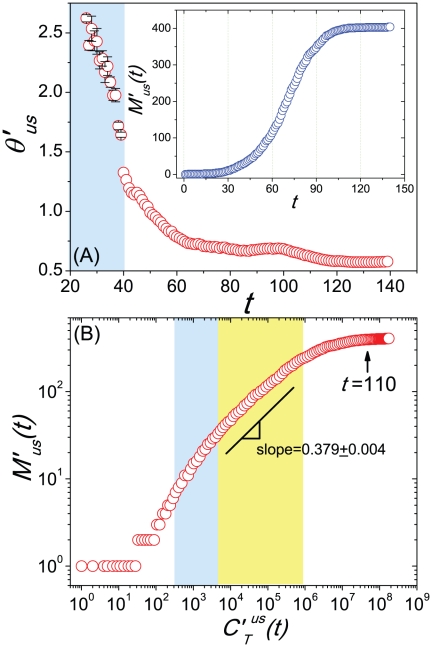
We further explore the properties of the two scalings and their relation with the baseline case of 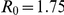 in detail. Since each independent simulation generates a stochastic realization of the spreading process, we analyze the statistical properties with 100 random Monte Carlo realizations, measure the normalized PRD of the CCN of infected MSAs/
in detail. Since each independent simulation generates a stochastic realization of the spreading process, we analyze the statistical properties with 100 random Monte Carlo realizations, measure the normalized PRD of the CCN of infected MSAs/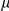 SAs for each realization that led to an outbreak at the U.S. level, and report the median result of the PRD
SAs for each realization that led to an outbreak at the U.S. level, and report the median result of the PRD
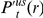 of each day. From
of each day. From 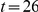 to
to 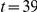 ,
, 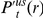 clearly shows a power law pattern
clearly shows a power law pattern 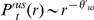 , which implies the emergence of the Zipf's law(when
, which implies the emergence of the Zipf's law(when 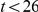 , just several regions are affected by the disease). The exponent
, just several regions are affected by the disease). The exponent 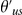 at each date is estimated by the maximum likelihood method[22], [37], and the temporal evolution of
at each date is estimated by the maximum likelihood method[22], [37], and the temporal evolution of 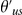 is reported in the left part of Fig. 4(A). When
is reported in the left part of Fig. 4(A). When 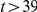 ,
, 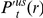 gradually becomes an exponentially cutoff power law distribution
gradually becomes an exponentially cutoff power law distribution 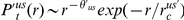 , and the exponent
, and the exponent 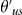 gradually reduces and reaches a stable value of 0.574 with neglectable fluctuations when
gradually reduces and reaches a stable value of 0.574 with neglectable fluctuations when 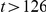 (see Fig. 4(A)). Here we do not show the error bar since the fitting error on the exponent is far less(
(see Fig. 4(A)). Here we do not show the error bar since the fitting error on the exponent is far less(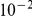 ) than the value of
) than the value of 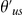 by the average of 100 random realizations. The inset of Fig. 4(A) shows the increase of the number of infected regions
by the average of 100 random realizations. The inset of Fig. 4(A) shows the increase of the number of infected regions 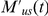 as time evolves. When
as time evolves. When 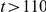 , more than 400 subpopulations reports the existence of confirmed cases, thus
, more than 400 subpopulations reports the existence of confirmed cases, thus 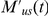 tends to reach its saturation.
tends to reach its saturation.
Figure 4. The statistical results of the scaling properties of our metapopulation model.
(A) Temporal evolution of the estimated exponent 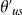 of the normalized distribution
of the normalized distribution 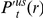 . The inset shows the growing of the number of infected subpopulations
. The inset shows the growing of the number of infected subpopulations 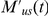 with time
with time  . (B) The relation between the number of infected subpopulations
. (B) The relation between the number of infected subpopulations 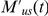 and the national cumulative confirmed cases
and the national cumulative confirmed cases 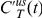 . The shaped areas in the figures corresponds to their different evolution stages, respectively. Each data in these figures are the median results over all runs that led to an outbreak at the U.S. level in 100 random Monte Carlo realizations.
. The shaped areas in the figures corresponds to their different evolution stages, respectively. Each data in these figures are the median results over all runs that led to an outbreak at the U.S. level in 100 random Monte Carlo realizations.
Fig. 4(B) shows the relation between 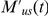 and
and 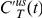 (the national cumulative number of patients). Since
(the national cumulative number of patients). Since 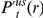 displays a power law of
displays a power law of 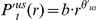 at the early stage of the period between
at the early stage of the period between 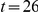 and
and 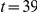 , it is reasonable to deduce the existence of the Heaps' law
, it is reasonable to deduce the existence of the Heaps' law
| (14) |
according to the analyses in Empirical and Analytical Results. In order to verify this assumption, we estimate the exponent 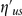 using Eq.(14), and report the relevance between
using Eq.(14), and report the relevance between 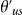 and
and 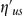 in Table 2(the amount of data in this period is not sufficient to get a accurate estimation of the exponent
in Table 2(the amount of data in this period is not sufficient to get a accurate estimation of the exponent 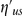 with the least square method). When
with the least square method). When 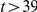 , though
, though 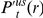 gradually deviates the strict Zipf's law, the Heaps' law of the relation between
gradually deviates the strict Zipf's law, the Heaps' law of the relation between 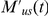 and
and 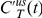 still exists till
still exists till 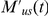 tends to reach its saturation(see the middle part in Fig. 4(B)).
tends to reach its saturation(see the middle part in Fig. 4(B)).
Table 2. The value of the parameters 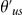 and
and 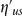 for the simulation results at the early time of the period between
for the simulation results at the early time of the period between 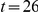 and
and 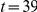 .
.
| t |
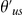
|
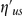
|
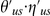
|
| 26 | 2.623 | 0.427 | 1.120 |
| 27 | 2.395 | 0.459 | 1.099 |
| 28 | 2.535 | 0.449 | 1.138 |
| 29 | 2.433 | 0.457 | 1.112 |
| 30 | 2.429 | 0.456 | 1.108 |
| 31 | 2.269 | 0.455 | 1.032 |
| 32 | 2.285 | 0.460 | 1.051 |
| 33 | 2.170 | 0.482 | 1.046 |
| 34 | 2.220 | 0.477 | 1.059 |
| 35 | 2.086 | 0.492 | 1.026 |
| 36 | 1.976 | 0.503 | 0.994 |
| 37 | 1.977 | 0.504 | 0.996 |
| 38 | 1.717 | 0.540 | 0.927 |
| 39 | 1.644 | 0.538 | 0.884 |
Discussion
Zipf's law and Heaps' law are two representatives of the scaling concepts in the study of complexity science. Recently, increasing evidence of the coexistence of the Zipf's law and the Heaps' law motivates different understandings on the dependence between these two scalings, which is still hardly been clarified. This embarrassment derives from the contradiction that the empirical or simulated materials employed to show the emergence of Zipf's law are often finalized and static specimens, while the Heaps' law actually describes the evolving characteristics.
In this article, we have identified the relation between the Zipf's law and the Heaps' law from the perspective of coevolution between the scalings and large-scale spatial epidemic spreading. We illustrate the temporal evolution of the scalings: the Zipf's law and the Heaps' law are naturally shaped to coexist at the early stage of the epidemic at both the global and the U.S. levels, while the crossover comes with the emergence of their inconsistency at a larger time before reaching a stable state, where the Heaps' law still exists with the disappearance of strict Zipf's law.
With the U.S. domestic air transportation and demographic data, we construct a metapopulation model at the U.S. level. The simulation results predict main empirical findings. Employing information entropy characterizing the epidemic spreading pattern, we recognize that the broad heterogeneity of the infrastructure plays an essential role in the evolution of scaling emergence. These findings are quite different from the previous conclusions in the literature. For example, studying a phenomenologically self-adaptive complete network, Han et al. claimed that scaling properties are dependent on the intensity of containment strategies implemented to restrict the interregional travel[31]. In [36], Picoli Junior et al. considered a simple stochastic model based on the multiplicative process[23], and suggested that seasonality and weather conditions, i.e., temperature and relative humidity, also dominates the temporal evolution of scalings because they affect the dynamics of influenza transmission. In this work, without the help of any specific additional factor, we directly show that the evolution of scaling emergence is mainly determined by the contact process underlying disease transmission on an infrastructure with huge volume and heterogeneous structure of population flows among different geographic regions. (The effects of the travel-related containment strategies implemented in real world can be neglected, since the number of scheduled domestic and international passengers of the U.S. air transportation only declined in 2009 by 5.3% from 2008[86]. In fact, the travel restrictions would not be able to significantly slow down the epidemic spread unless more than 90% of the flight volume is reduced[58], [66], [69], [70], [88].)
In summary, our study suggests that the analysis of large-scale spatial epidemic spread as a promising new perspective to understand the temporal evolution of the scalings. The unprecedented amount of information encoded in the empirical data of pandemic spreading provides us a rich environment to unveil the intrinsic mechanisms of scaling emergence. The heterogeneity of epidemic spread uncovered by the metapopulation model indicates the significance of performing targeted containment strategies, e.g. vaccination of prior groups, targeted antiviral prophylaxis, at the early time of a pandemic disease.
Materials and Methods
Data Description
In this article, in order to construct the U.S. domestic air transportation network, we mainly utilize the “Air Carrier Traffic and Capacity Data by On-Flight Market report(December 2009)” provided by the Bureau of Transportation Statistics(BTS) database[76]. This report contains 12 months' data covering more than 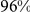 of the entire U.S. domestic air traffic in 2009, and provides the monthly number of passengers, freight and/or mail transported between any two airports located within the U.S. boundaries and territories, regardless of the number of stops between them. This BTS report provides a more accurate solution for studying aviation flows between any two U.S. airports than other data sources(the attendance and the flight-connecting information in the OAG flight schedule data are commonly unknown, while the datasets adopted in [63], [64], [66], [69] primarily consider the international passengers). In order to study the epidemic spread in the Continental United States where we have a good probability to select citizens living and moving in the mainland, we get rid of the airports as well as the corresponding flight courses located in Hawaii, and all offshore U.S. territories and possessions from the BTS report.
of the entire U.S. domestic air traffic in 2009, and provides the monthly number of passengers, freight and/or mail transported between any two airports located within the U.S. boundaries and territories, regardless of the number of stops between them. This BTS report provides a more accurate solution for studying aviation flows between any two U.S. airports than other data sources(the attendance and the flight-connecting information in the OAG flight schedule data are commonly unknown, while the datasets adopted in [63], [64], [66], [69] primarily consider the international passengers). In order to study the epidemic spread in the Continental United States where we have a good probability to select citizens living and moving in the mainland, we get rid of the airports as well as the corresponding flight courses located in Hawaii, and all offshore U.S. territories and possessions from the BTS report.
In order to obtain the U.S. demographic data, we resort to the “OMB Bulletin N0. 10–02: Update of Statistical Area Definitions and Guidance on Their Uses”
[75] provided by the United States Office of Management and Budget(OMB), and the “Annual Estimates of the Population of Metropolitan and Micropolitan Statistical Areas: April 1, 2000 to July 1, 2009”
[77] provided by the United States Census Bureau(CB). OMB defines a Metropolitan Statistical Area(MSA)(Micropolitan Statistical Area, 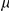 SA) as one or more adjacent counties or county equivalents that have at least one urban core area of at least 50,000 population(10,000 population but less than 50,000), plus adjacent territory that has a high degree of social and economic integration with the core. For other regions with at least 5,000 population but less than 10,000, we use the American FactFinder[78] provided by the CB to get the demographic information. We do not consider sparsely populated areas with population less than 5,000, because they are commonly remote islands, e.g. Block Island in Rhode Island, Sand Point in Alaska.
SA) as one or more adjacent counties or county equivalents that have at least one urban core area of at least 50,000 population(10,000 population but less than 50,000), plus adjacent territory that has a high degree of social and economic integration with the core. For other regions with at least 5,000 population but less than 10,000, we use the American FactFinder[78] provided by the CB to get the demographic information. We do not consider sparsely populated areas with population less than 5,000, because they are commonly remote islands, e.g. Block Island in Rhode Island, Sand Point in Alaska.
Before constructing the metapopulation model, we take into account the fact that there might be more than one airport in some huge metropolitan areas. For instance, New York-Northern New Jersey-Long Island(NY-NJ-PA MSA) has up to six airports(their IATA codes: JFK, LGA, ISP, EWR, HPN, FRG), Los Angeles-Long Beach-Santa Ana(CA MSA) has four airports(their IATA codes: LAX, LGB, SNA, BUR), and Chicago-Joliet-Naperville(IL-IN-WI MSA) has two airports(their IATA codes: MDW, ORD). Assuming a homogeneous mixing inside each subpopulation, we need to assemble each group of airports serving the same MSA/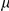 SA, because the mixing within each given census areas is quite high and cannot be characterized by fine-grained version of subpopulations for every single airport. We searched for groups of airports located close to each other and belonged to the same metropolitan areas, and then manually aggregated the airports of the same group in a single “super-hub”.
SA, because the mixing within each given census areas is quite high and cannot be characterized by fine-grained version of subpopulations for every single airport. We searched for groups of airports located close to each other and belonged to the same metropolitan areas, and then manually aggregated the airports of the same group in a single “super-hub”.
The full list of updates of the pandemic A(H1N1) human cases of different countries is available on the website of Global Alert and Response(GAR) of World Health Organization(WHO)(WHO website. http://www.who.int/csr/disease/swineflu/updates/en/index.html. Accessed 2011 May 24). It is worth remarking that WHO was no longer updating the number of the cumulated confirmed cases for each country after July 6th, 2009, but changed to report the number of confirmed cases on the WHO Region level(the Member States of the World Health Organization(WHO) are grouped into six regions, including WHO African Region(46 countries), WHO European Region(53 countries), WHO Eastern Mediterranean Region(21 countries), WHO Region of the Americas(35 countries), WHO South-East Asia Region (11 countries), WHO Western Pacific Region(27 countries). (WHO website. http://www.who.int/about/regions/en/index.html. Accessed 2011 May 24).
The cumulative number of the laboratory confirmed human cases of A(H1N1) flu infection of each U.S. state is available at the website of 2009 A(H1N1) Flu of the Centers for Disease Control and Prevention(CDC)(CDC website. http://cdc.gov/h1n1flu/updates/. Accessed 2011 May 24), where the detailed data were started from April 23, 2009, to July 24, 2009. After July 24, the CDC discontinued the reporting of individual confirmed cases of A(H1N1), and began to report the total number of hospitalizations and deaths weekly.
The data of the human cases of global SARS and global Avian influenza(H5N1) are available at the website of the Disease covered by GAR of WHO(WHO website. http://www.who.int/csr/disease/en/. Accessed 2011 May 24).
Supporting Information
(PDF)
The temporal evolution of the estimated parameter
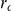 , data provided by the WHO.
, data provided by the WHO.
(EPS)
The temporal evolution of the estimated exponent
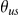 for all data provided by the CDC.
for all data provided by the CDC.
(EPS)
The empirical results of the SARS and avian influenza(H5N1). (A) shows the normalized probability-rank distribution of the cumulated confirmed number of every infected country around the world at several given date sampled about every four weeks, data provided by the WHO(WHO website. http://www.who.int/csr/sars/country/en/index.html. Accessed 2011 May 24.). (B) shows the normalized probability-rank distribution of the cumulated confirmed number of every infected country around the world at several given date sampled about every half a year, data provided by the WHO(WHO website. http://www.who.int/csr/disease/avian_influenza/country/en/. Accessed 2011 May 24.).
(EPS)
Acknowledgments
We were grateful to the insightful comments of editor, Alejandro Raul Hernandez Montoya, and the two anonymous referees, and gratefully acknowledge helpful discussions with Changsong Zhou, Xiao-Pu Han, Zhi-Hai Rong, Zhen Wang, and Yang Yang. We also thank the Bureau of Transportation Statistics (BTS), for providing us the U.S. domestic air traffic database.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: We acknowledge support from the National Key Basic Research and Development Program (No. 2010CB731403), the Natural Science Foundation of China (Grant No. 60874089), Shanghai Rising-Star Program (No. 09QH1400200) and the NECT program (No. NCET-09-0317). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stanley HE. Scaling, universality, and renormalization: Three pillars of modern critical phenomena. Rev Mod Phys. 1999;71:S358–S366. [Google Scholar]
- 2.Stanley HE, Amaral LAN, Gopikrishnan P, Ivanov PC, Keitt TH, et al. Scale invariance and universality: organizing principles in complex systems. Physica A. 2000;281:60–68. [Google Scholar]
- 3.Cardy J. Scaling and Renormalization in Statistical Physics(Cambridge University Press, New York) 1996.
- 4.Brown JH, West GB. Scaling in Biology(Oxford University Press, USA) 2000.
- 5.Zipf GK. Human Behaviour and the Principle of Least Effort: An Introduction to Human Ecology(Addison-Wesley, Massachusetts) 1949.
- 6.Ferrer-i-Cancho R, Elvevåg B. Random Texts Do Not Exhibit the Real Zipf's Law-Like Rank Distribution. PLoS ONE. 2010;5:e9411. doi: 10.1371/journal.pone.0009411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieberman E, Michel JB, Jackson J, Tang T, Nowak MA. Quantifying the evolutionary dynamics of language. Nature. 2007;449:713–716. doi: 10.1038/nature06137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanter I, Kessler DA. Markov Processes: Linguistics and Zipf's Law. Phys Rev Lett. 1995;74:4559–4562. doi: 10.1103/PhysRevLett.74.4559. [DOI] [PubMed] [Google Scholar]
- 9.Maillart T, Sornette D, Spaeth S, von Krogh G. Empirical Tests of Zipf's Law Mechanism in Open Source Linux Distribution. Phys Rev Lett. 2008;101:218701. doi: 10.1103/PhysRevLett.101.218701. [DOI] [PubMed] [Google Scholar]
- 10.Decker EH, Kerkhoff AJ, Moses ME. Global Patterns of City Size Distributions and Their Fundamental Drivers. PLoS ONE. 2007;2:e934. doi: 10.1371/journal.pone.0000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batty M. Rank clocks. Nature. 2006;444:592–596. doi: 10.1038/nature05302. [DOI] [PubMed] [Google Scholar]
- 12.Axtell RL. Zipf Distribution of U.S. Firm sizes. Science. 2001;293:1818–1820. doi: 10.1126/science.1062081. [DOI] [PubMed] [Google Scholar]
- 13.Coronel-Brizio HF, Hernández-Montoya AR. On Fitting the Pareto-Levy distribution to financial data: Selecting a suitable fit's cut off parameter. Physica A. 2005;354:437–449. [Google Scholar]
- 14.Coronel-Brizio HF, Hernández-Montoya AR. Asymptotic behavior of the Daily Increment Distribution of the IPC, the Mexican Stock Market Index. Revista Mexicana de Física. 2005;51:27–31. [Google Scholar]
- 15.Ogasawara O, Okubo K. On Theoretical Models of Gene Expression Evolution with Random Genetic Drift and Natural Selection. PLoS ONE. 2009;4:e7943. doi: 10.1371/journal.pone.0007943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furusawa C, Kaneko K. Zipf's Law in Gene Expression. Phys Rev Lett. 2003;90:088102. doi: 10.1103/PhysRevLett.90.088102. [DOI] [PubMed] [Google Scholar]
- 17.Blasius B, Tönjes R. Zipf's Law in the Popularity Distribution of Chess Openings. Phys Rev Lett. 2009;103:218701. doi: 10.1103/PhysRevLett.103.218701. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Mekler G, Martínez RA, del Río MB, Mansilla R, Miramontes P, et al. Universality of Rank-Ordering Distributions in the Arts and Sciences. PloS ONE. 2009;4:e4791. doi: 10.1371/journal.pone.0004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redner S. How popular is your paper? An empirical study of the citation distribution. Eur Phys J B. 1998;4:131–134. [Google Scholar]
- 20.Baek SK, Kiet HAT, Kim BJ. Family name distributions: Master equation approach. Phys Rev E. 2007;76:046113. doi: 10.1103/PhysRevE.76.046113. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Wang C, Wang Y. Deformed Zipf's law in personal donation. Europhys Lett. 2009;88:38001. [Google Scholar]
- 22.Newman MEJ. Power laws, Pareto distributions and Zipf's law. Contemporary Physics. 2005;46:323–351. [Google Scholar]
- 23.Sornette D. Multiplicative processes and power laws. Phys Rev E. 1997;57:4811–4813. [Google Scholar]
- 24.Saichev A, Malevergne Y, Sornette D. Theory of Zipf's Law and Beyond, Lecture Notes in Economics and Mathematical Systems(Springer) 2009.
- 25.Heaps HS. Information Retrieval: Computational and Theoretical Aspects(Academic Press, Orlando) 1978.
- 26.Serrano MÁ, Flammini A, Menczer F. Modeling Statistical Properties of Written Text. PLoS ONE. 2009;4:e5372. doi: 10.1371/journal.pone.0005372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang ZK, Lü L, Liu JG, Zhou T. Empirical analysis on a keyword-based semantic system. Eur Phys J B. 2008;66:557–561. [Google Scholar]
- 28.Cattuto C, Barrat A, Baldassarri A, Schehr G, Loreto V. Collective dynamics of social annotation. Proc Natl Acad Sci. 2009;106:10511–10515. doi: 10.1073/pnas.0901136106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cattuto C, Loreto V, Pietronero L. Semiotic dynamics and collaborative tagging. Proc Natl Acad Sci. 2007;104:1461–1464. doi: 10.1073/pnas.0610487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benz RW, Swamidass SJ, Baldi P. Discovery of power-law in chemical space. J Chem Inf Model. 2008;48:1138–1151. doi: 10.1021/ci700353m. [DOI] [PubMed] [Google Scholar]
- 31.Han XP, Wang BH, Zhou CS, Zhou T, Zhu JF. eprint arXiv; 2009. Scaling in the Global Spreading Patterns of Pandemic Influenza A and the Role of Control: Empirical Statistics and Modeling.0912.1390 [Google Scholar]
- 32.Lü L, Zhang ZK, Zhou T. Zipf's Law Leads to Heaps' Law: Analyzing Their Relation in Finite-Size Systems. PLoS ONE. 2010;5:e14139. doi: 10.1371/journal.pone.0014139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montemurro MA, Zanette DH. New perspectives on Zipf's law in linguistics: from single texts to large corpora. Glottometrics. 2002;4:86–98. [Google Scholar]
- 34.Zanette DH, Montemurro MA. Dynamics of Text Generation with Realistic Zipf's Distribution. J Quant Linguistics. 2005;12:29–40. [Google Scholar]
- 35.Simon HA. On a class of skew distribution functions. Biometrika. 1955;42:425–440. [Google Scholar]
- 36.Picoli Junior Sd, Teixeira JJV, Ribeiro HV, Malacarne LC, Santos RPBd, et al. Spreading Patterns of the Influenza A (H1N1) Pandemic. PLoS ONE. 2011;6:e17823. doi: 10.1371/journal.pone.0017823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clauset A, Shalizi CR, Newman MEJ. Power-law distributions in empirical data. SIAM Review. 2009;51:661–703. [Google Scholar]
- 38.“World now at the start of 2009 influenza pandemic”, Statement to the press by WHO Director-General Dr. Margaret Chan(June 11, 2009), World Health Organization. Available: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/. Accessed 2011 May 24. [Google Scholar]
- 39.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control(Oxford Unvi. Press, Oxford) 1991.
- 40.Hamer WH. The Milroy Lectures On Epidemic disease in England – The evidence of variability and of presistency of type. The Lancet. 1906;167:733–739. [Google Scholar]
- 41.Pastor-Satorras R, Vespignani A. Epidemic Spreading in Scale-Free Networks. Phys Rev Lett. 2001;86:3200–3203. doi: 10.1103/PhysRevLett.86.3200. [DOI] [PubMed] [Google Scholar]
- 42.Eguíluz VM, Klemm K. Epidemic Threshold in Structured Scale-Free Networks. Phys Rev Lett. 2002;89:108701. doi: 10.1103/PhysRevLett.89.108701. [DOI] [PubMed] [Google Scholar]
- 43.Barthélemy M, Barrat A, Pastor-Satorras R, Vespignani A. Velocity and Hierarchical Spread of Epidemic Outbreaks in Scale-Free Networks. Phys Rev Lett. 2004;92:178701. doi: 10.1103/PhysRevLett.92.178701. [DOI] [PubMed] [Google Scholar]
- 44.Gross T, D'Lima CJD, Blasius B. Epidemic Dynamics on an Adaptive Network. Phys Rev Lett. 2006;96:208701. doi: 10.1103/PhysRevLett.96.208701. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Wang XF. Controlling the spreading in small-world evolving networks: stability, oscillation, and topology. IEEE T AUTOMAT CONTR. 2006;51:534–540. [Google Scholar]
- 46.Zhou T, Liu JG, Bai WJ, Chen GR, Wang BH. Behaviors of susceptible-infected epidemics on scale-free networks with identical infectivity. Phys Rev E. 2006;74:056109. doi: 10.1103/PhysRevE.74.056109. [DOI] [PubMed] [Google Scholar]
- 47.Han XP. Disease spreading with epidemic alert on small-world networks. Phys Lett A. 2007;365:1–5. [Google Scholar]
- 48.Yang R, Zhou T, Xie YB, Lai YC, Wang BH. Optimal contact process on complex networks. Phys Rev E. 2008;78:066109. doi: 10.1103/PhysRevE.78.066109. [DOI] [PubMed] [Google Scholar]
- 49.Parshani R, Carmi S, Havlin S. Epidemic Threshold for the Susceptible-Infectious-Susceptible Model on Random Networks. Phys Rev Lett. 2010;104:258701. doi: 10.1103/PhysRevLett.104.258701. [DOI] [PubMed] [Google Scholar]
- 50.Castellano C, Pastor-Satorras R. Thresholds for Epidemic Spreading in Networks. Phys Rev Lett. 2010;105:218701. doi: 10.1103/PhysRevLett.105.218701. [DOI] [PubMed] [Google Scholar]
- 51.Li X, Cao L, Cao GF. Epidemic prevalence on random mobile dynamical networks: Individual heterogeneity and correlation. Eur Phys J B. 2010;75:319–326. [Google Scholar]
- 52.Pulliam JR, Dushoff JG, Levin SA, Dobson AP. Epidemic Enhancement in Partially Immune Populations. PLoS ONE. 2007;2:e165. doi: 10.1371/journal.pone.0000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scoglio C, Schumm W, Schumm P, Easton T, Roy Chowdhury S, et al. Efficient Mitigation Strategies for Epidemics in Rural Regions. PLoS ONE. 2010;5:e11569. doi: 10.1371/journal.pone.0011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matrajt L, Longini IM., Jr Optimizing Vaccine Allocation at Different Points in Time during an Epidemic. PLoS ONE. 2010;5:e13767. doi: 10.1371/journal.pone.0013767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwami S, Suzuki T, Takeuchi Y. Paradox of Vaccination: Is Vaccination Really Effective against Avian Flu Epidemics? PLoS ONE. 2009;4:e4915. doi: 10.1371/journal.pone.0004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bettencourt LMA, Ribeiro RM. Real Time Bayesian Estimation of the Epidemic Potential of Emerging Infectious Diseases. PLoS ONE. 2008;3:e2185. doi: 10.1371/journal.pone.0002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Longini IM, Jr, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, et al. Containing Pandemic Influenza at the Source. Science. 2005;309:1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 58.Bajardi P, Poletto C, Ramasco JJ, Tizzoni M, Colizza V, et al. Human Mobility Networks, Travel Restrictions, and the Global Spread of 2009 H1N1 Pandemic. PLoS ONE. 2011;6:e16591. doi: 10.1371/journal.pone.0016591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fraser C, Riley S, Anderson RM, Ferguson NM. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci USA. 2004;101:6146–6151. doi: 10.1073/pnas.0307506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Situation updates–Pandemic (H1N1) 2009, World Health Organization. Available: http://www.who.int/csr/disease/swineflu/updates/en/index.html. Accessed 2011 May 24. [Google Scholar]
- 61.Shannon CE, Weaver W. The Mathematical Theory of Communication(The University of Illinois Press, Urbana) 1964.
- 62.Barabási AL. Bursts: The Hidden Pattern Behind Everything We Do(Dutton Books, USA) 2010.
- 63.Rvachev LA, Longini IM., Jr A mathematical model for the global spread of influenza. Math Biosci. 1985;75:3–22. [Google Scholar]
- 64.Hufnagel L, Brockmann D, Geisel T. Forecast and control of epidemics in a globalized world. Proc Natl Acad Sci USA. 2004;101:15124–15129. doi: 10.1073/pnas.0308344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colizza V, Barrat A, Barthèlemy M, Vespignani A. The role of the airline transportation network in the prediction and predictability of global epidemic. Proc Natl Acad Sci USA. 2006;103:2015–2020. doi: 10.1073/pnas.0510525103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cooper BS, Pitman RJ, Edmunds WJ, Gay NJ. Delaying the International Spread of Pandemic Influenza. PLoS Med. 2006;3:e212. doi: 10.1371/journal.pmed.0030212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ovaskainen O, Cornell SJ. Asymptotically exact analysis of stochastic metapopulation dynamics with explicit spatial structure. Theor Popul Biol. 2006;69:13–33. doi: 10.1016/j.tpb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 68.Colizza V, Pastor-Satorras R, Vespignani A. Reaction-diffusion processes and metapopulation models in heterogeneous networks. Nat Phys. 2007;3:276. [Google Scholar]
- 69.Epstein JM, Goedecke DM, Yu F, Morris RJ, Wagener DK, et al. Controlling Pandemic Flu: The Value of International Air Travel Restrictions. PLoS ONE. 2007;2:e401. doi: 10.1371/journal.pone.0000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colizza V, Barrat A, Barthelemy M, Valleron AJ, Vespignani A. Modeling the worldwide spread of pandemic influenza: Baseline case and containment interventions. PLoS Med. 2007;4:e13. doi: 10.1371/journal.pmed.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cornell SJ, Ovaskainen O. Exact asymptotic analysis for metapopulation dynamics on correlated dynamic landscapes. Theor Popul Biol. 2008;74:209–225. doi: 10.1016/j.tpb.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Balcan D, Colizza V, Gonçalves B, Hu H, Ramasco JJ, et al. Multiscale mobility networks and the spatial spreading of infectious diseases. Proc Natl Acad Sci USA. 2009;106:21484–21489. doi: 10.1073/pnas.0906910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vergu E, Busson H, Ezanno P. Impact of the Infection Period Distribution on the Epidemic Spread in a Metapopulation Model. PLoS ONE. 2010;5:e9371. doi: 10.1371/journal.pone.0009371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balcan D, Vespignani A. Nat Phys; 2011. Phase transitions in contagion processes mediated by recurrent mobility patterns. doi: 10.1038/nphys1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.United States Office of Management and Budget (OMB), OMB Bulletin No. 10-02: Update of Statistical Area Definitions and Guidance on Their Uses(December 1, 2009). Available: http://www.whitehouse.gov/sites/default/files/omb/assets/bulletins/b10-02.pdf. Accessed 2011 May 24. [Google Scholar]
- 76.Bureau of Transportation Statistics (BTS), United States, Air Carrier Traffic and Capacity Data by On-Flight Market report(December 2009). Available: http://www.bts.gov/. Accessed 2011 May 24. [Google Scholar]
- 77.United States Census Bureau (CB), Annual Estimates of the Population of Metropolitan and Micropolitan Statistical Areas: April 1, 2000 to July 1, 2009. Available: http://www.census.gov/popest/metro/. Accessed 2011 May 24. [Google Scholar]
- 78.United States Census Bureau (CB), American Factfinder. Available: http://factfinder.census.gov/home/saff/main.html?_lang=en. Accessed 2011 May 24. [Google Scholar]
- 79.Centers for Disease Control and Prevention (CDC), United States, Swine Influenza A (H1N1) Infection in Two Children – Southern California, March-April. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5815a5.htm. Accessed 2011 May 24. [PubMed] [Google Scholar]
- 80.Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Kerkhove MDV, et al. Pandemic Potential of a Strain of Influenza A (H1N1): Early Findings. Science. 2009;324:1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balcan D, Hu H, Gonçalves B, Bajardi P, Poletto C, et al. Seasonal transmission potential and activity peaks of the new influenza A(H1N1): a Monte Carlo likelihood analysis based on human mobility. BMC Med. 2009;7:45. doi: 10.1186/1741-7015-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, et al. Incubation periods of acute respiratory viral infections: a systematic review. . Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Y, Sugimoto JD, Halloran ME, Basta NE, Chao DL, et al. The Transmissibility and Control of Pandemic Influenza A (H1N1) Virus. Science. 2009;326:729–733. doi: 10.1126/science.1177373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boëlle PY, Bernillon P, Desenclos JC. A preliminary estimation of the reproduction ratio for new influenza A(H1N1) from the outbreak in Mexico, March-April 2009. Euro Surveill. 2009;14:19205. doi: 10.2807/ese.14.19.19205-en. [DOI] [PubMed] [Google Scholar]
- 85.Nishiura H, Castillo-Chavez C, Safan M, Chowell G. Transmission potential of the new influenza A(H1N1) virus and its agespecificity in Japan. Euro Surveill. 2009;14:19227. doi: 10.2807/ese.14.22.19227-en. [DOI] [PubMed] [Google Scholar]
- 86.Bureau of Transportation Statistics (BTS), United States . “Summary 2009 Traffic Data for U.S and Foreign Airlines: Total Passengers Down 5.3 Percent from 2008”. 2010 Available: http://www.bts.gov/. Accessed 2011 May 24. [Google Scholar]
- 87.den Broeck WV, Gioannini C, Gonçalves B, Quaggiotto M, Colizza V, et al. The GLEaMviz computational tool, a publicly available software to explore realistic epidemic spreading scenarios at the global scale. BMC Infect Dis. 2011;11:37. doi: 10.1186/1471-2334-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colizza V, Vespignani A. Epidemic modeling in metapopulation systems with heterogeneous coupling pattern: Theory and simulations. J Theor Biol. 2008;251:450. doi: 10.1016/j.jtbi.2007.11.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
The temporal evolution of the estimated parameter
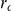 , data provided by the WHO.
, data provided by the WHO.
(EPS)
The temporal evolution of the estimated exponent
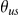 for all data provided by the CDC.
for all data provided by the CDC.
(EPS)
The empirical results of the SARS and avian influenza(H5N1). (A) shows the normalized probability-rank distribution of the cumulated confirmed number of every infected country around the world at several given date sampled about every four weeks, data provided by the WHO(WHO website. http://www.who.int/csr/sars/country/en/index.html. Accessed 2011 May 24.). (B) shows the normalized probability-rank distribution of the cumulated confirmed number of every infected country around the world at several given date sampled about every half a year, data provided by the WHO(WHO website. http://www.who.int/csr/disease/avian_influenza/country/en/. Accessed 2011 May 24.).
(EPS)