Abstract
Heat shock proteins (Hsps) act as molecular chaperones and are generally constitutively expressed in the absence of stress. Hsps are also inducible by a variety of stressors whose effects could be disastrous on the brain. It has been shown previously that Hsps are differentially expressed in glial and neuronal cells, as well as in the different structures of the brain. This differential expression has been related to specific functions distinct from their general chaperone function, such as intracellular transport. We investigated here the constitutive expression of 5 Hsps (the small Hsp, Hsp25, the constitutive Hsc70 and Hsp90β, the mainly inducible Hsp70 and Hsp90α), and of a molecular chaperone, TCP-1α during mouse nervous system development. We analyzed, by immunohistochemistry, their distribution in the central nervous system and in the ganglia of the peripheral nervous system from day 9.5 (E9.5) to day 17.5 (E17.5) of gestation. Hsps are expressed in different cell classes (neuronal, glial, and vascular). The different proteins display different but often overlapping patterns of expression in different regions of the developing nervous system, suggesting unique roles at different stages of neural maturation. Their putative function in cell remodeling during migration or differentiation and in protein transport is discussed. Moreover we consider Hsp90 function in cell signaling and the role of Hsp25 in apoptosis protection.
INTRODUCTION
Cells of living organisms synthesize a distinct set of highly conserved proteins in response to hyperthermia. These proteins are called heat shock proteins (Hsps), although their synthesis can be induced by a variety of other stressors such as anoxia, heavy metals, and viral infections (for review see Craig 1985; Parsell and Linquist 1993). Some of these proteins act as key mediators to correctly assemble and to localize the nascent polypeptides in cells under normal conditions. Stress conditions that increase Hsp synthesis generate an accumulation of denatured and abnormally folded proteins. Thus the interaction of these Hsps with abnormal proteins during stress appears to be equivalent to their interaction under normal, nonstressed conditions. This does not prevent these Hsps from having specific functions such as clathrin uncoating for Hsc70, depending on the partners and cochaperones with which they interact.
In addition some of the Hsps, such as the small Hsp (Hsp25) and Hsp90 seem to have a restricted range of targets and/or to have specific functions. This characteristic correlates with the differential expression of these proteins in different tissues of the organism. Both Hsp25 and αB-crystallin, which is very similar to Hsp25, are present at high levels in the lens where these abundant proteins were first described, in the heart (and more generally in the striated muscle), but also in certain other tissues (Bhat and Nagineni 1989; Klemenz et al 1993).
It is well documented that in the adult nervous system some Hsp genes are constitutively expressed. For example Hsp90s account for 1–2% of total cellular protein in the rabbit nervous system (Quraishi and Brown 1995) and Hsc70 for 2–3% of the total protein content of the rat spinal cord (Aquino et al 1993). This constitutive expression is preferentially localized to the neuronal cell population (Brown and Rush 1990; Izumoto and Herbert 1993; Gass et al 1994; D'Souza and Brown 1998). As for sHsps, Hsp25 (referred to as Hsp27 in the rat and the human) could only be detected by immunohistochemistry in adult rat brain, where high levels of Hsp27 characterized specific classes of neurons of the brain stem and of the spinal cord (Plumier et al 1997).
The mammalian brain is highly sensitive to different stresses such as fever, ischemia, seizure, and drugs (such as amphetamines or LSD). These stressful conditions induce Hsp70 and Hsp90α synthesis, generally in glial cells though Hsp70 is sometimes induced in neurons, depending to the type of stress applied (reviewed by Brown 1994). Hsp27 has been reported to be induced in a large number of astrocytes of ischemic or kainic acid–stressed rats (Kato et al 1994; Plumier et al 1996). Moreover, Hsp27 has been detected in neurons in pathological conditions, such as Alzheimer disease (Renhawek et al 1994) and Creutzfeldt-Jakob disease (Kato et al 1992). Because Hsps have been proposed to exhibit protective properties in tissue culture systems (Riabowol et al 1988), they may offer strategies for tissue protection in the nervous system.
Although an alteration in Hsp expression in pathologic or stressed brain compared to normal adult brain is now well documented, far less is known about the expression of these proteins during formation of the brain. The expression of heat shock genes during embryonic development has been investigated in different vertebrate and invertebrate models. Many heat shock genes display complex patterns of constitutive and inducible expression (Heikkila 1993a, 1993b; Hightower and Nover 1991; Morange 1997a, 1997b). However, although significant progress has been made on the chaperone machinery, little is known about the specific role of these proteins during embryogenesis, and, in particular, during development of the brain.
The rodent brain, and the rat brain in particular, is the most commonly used model for neurophysiological studies. As the mouse is the best known vertebrate in terms of development the present study was conducted in the mouse.
Neurons and glial cells of the central nervous system (CNS) originate from a specialized region of ectoderm, the neural plate. Following neural induction, the neuroectodermal cells lining the neural tube (neuroepithelium) proliferate, migrate, and differentiate into the numerous types of neurons and glial cells of the adult CNS. The lateral margins of the neural plate are the origins of a significant number of cells that invade the mesoderm, the so-called neural crest cells (NCC). Some of these give rise to neurons and glial cells of the peripheral nervous system (PNS) (Le Douarin and Smith 1988). The regional specification defines developmental territories within which cells are specified. At the molecular level a large number of regulatory genes are expressed in regionally restricted patterns in the brain vesicles (Lumdsen and Keynes 1989; Puelles and Rubenstein 1993). This process of regionalization provides positional information that may regulate both the production of the neuron pattern, neuronal migration, and the elongation of the axons. Neuronal cell death is an important phenomenon in the series of steps involved in development. During the development of the vertebrate nervous system 50% or more of many types of neurons normally die soon after they fail to form synaptic connections with their target cells (Oppenheim 1991).
Neuronal differentiation begins in the mouse between E8.5 as soon as the neural tube closes (Easter et al 1993) and E11 (Nornes and Das 1974). Generation of glial cells is delayed and has been reported to commence in rat at E11 for radial glia (Hockfield and McKay 1985), E16 for astrocytes, and postnatally for the oligodendrocytes, the myelin-forming cells of the CNS (Raff et al 1983).
The molecules involved in the construction of the neural tube are largely unknown, thus any molecule that displays a specific pattern of expression and parallels important morphogenetic events would be a valuable marker for the biologist. Hsps are involved in cell protection against injury and death, control of cytoskeletal structure and intracellular transport, as well as in the activation of transcription factors: they are good candidates to play an active part in development of the neural tube. Our goal in the present paper was to consider the distribution of 5 different Hsps: Hsp25, Hsp70, Hsc70, Hsp90α and β, as well as the abundance of a chaperonin, TCP-1α, specifically involved in actin and tubulin folding (Melki and Cowan 1994) in the developing mouse brain between E9.5 and E17.5.
MATERIALS AND METHODS
Animals
Embryos from outbred OF1 mice (Iffa Credo) were obtained at E9.5, E10.5, E12.5, E15.5, E16.5, and E17.5. Mice were mated with midnight taken as the time of mating and E0.5 designed as the following morning. The females were killed by cervical dislocation and the embryos dissected free from the uterus in phosphate-buffered saline (PBS). For each embryonic stage analyzed several embryos were examined to compare and establish similar patterns of normal gene expression during development.
Isolation of protein homogenates
Whole brain was isolated or the telencephalon, diencephalon, mesencephalon, and hindbrain dissected from the CNS of embryonic stage E16.5 and homogenized into Laemmli sample buffer (Laemmli 1970). Protein concentrations were determinated using the BioRad protein assay kit.
Protein separation and Western blot procedure
Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrophoretically transferred to Hybond-ECL nitrocellulose membranes.
The filters were blocked with 5% dry milk in PBST (PBS, 0.1% Tween) for 1 hour at room temperature then incubated 1 hour with the primary antibody diluted in PBST. The following antibodies were used: anti-Hsp25, anti-Hsc70, anti-Hsp70, anti-Hsp90α, and anti-Hsp90β; references and dilutions for these antibodies are listed in Table 1. After washing with PBS the filters were incubated with an appropriate horseradish peroxidase-conjugated secondary antibody (see Table 2 for references and dilutions used) for 45 minutes at room temperature. Then the signals were detected using the ECL system (Amersham).
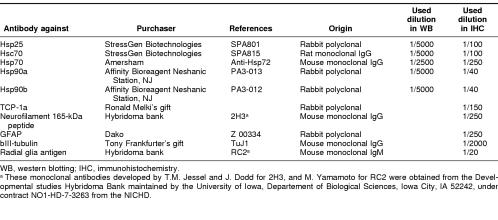
Table 1.
Primary antibody references
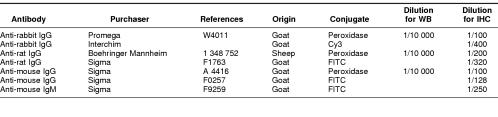
Table 2.
Secondary antibody references
Immunostaining on cryosections of embryos or isolated neural tubes
Embryos were fixed overnight by immersion in 4% paraformaldehyde in phosphate buffer (0.12 M, pH 7.2) at 4°C, or in 80% methanol at −20°C for tubulin detection. The neural tubes of embryos older than E10.5 were further dissected and isolated. Whole embryos of E9.5 stage and isolated neural tubes of E12.5, E15.5, and E17.5 were embedded in 7.5% gelatin/15% sucrose in PBS, frozen at −56°C, and the neural tubes examinated by 17-μm cryosections mounted on slides and stored at −20°C until required.
Prior to immunoreaction sections were rehydrated by incubating for 10 minutes in a 50-mM NH4Cl solution, blocked for 1 hour with 3% bovine serum albumin in PBS, then permeabilized with 0.5% Triton X-100 and 0.6% H2O2 to inhibit endogenous peroxidase activity.
Serial sections were alternatively incubated with antibodies raised against different Hsps and chaperones or against neural- or glial-specific markers detailed in Table 1 or with specific staining. Sections were incubated 1 hour at room temperature with 1 primary antibody, rinsed, then incubated 30 minutes with the appropriate horseradish-peroxidase–conjugated secondary antibody (Table 2). Peroxidase activity was identified using 0.03% diaminobenzidine tetrahydrochloride (Sigma)/0.005% H2O2 in 0.1 M Tris-HCl (pH 7.6). For double-labeling experiments the same section was incubated successively with an anti-Hsp antibody and a specific cell marker (Table 1). For neuronal characterization we used TuJ1 that recognizes the neurospecific βIII isoform of tubulin and 2H3 as a marker for the high molecular weight form of neurofilamin. For glial cell detection, RC2 is specific for radial glial cells (Marcus and Mason 1995), and we used a specific antibody against glial fibrillary acidic protein (GFAP), of the intermediate filaments of astrocytes. For vascular cell recognition, the BAND lectin from Bandeiraea simplicifolia (BAND), at 1 mg/ml as recommended (Sigma), binds the surface of developing endothelial cells. The presence of apoptotic nuclei was examined by for with Hoechst 33342 (Riedel de Haën) staining at a concentration of 5.4 μM in the final rinse. At each step, appropriate secondary antibodies conjugated to distinct fluorochromes revealed the immunoreaction (Table 2). In each series hybridization was compared with a negative control using the second antibody only. Serial sections were stained with toluidine blue.
Mapping the labeled regions of the brain at different embryonic stages was performed and identified with reference to Altman and Bayer (1995) and Shambra et al (1992).
RESULTS
The present study examines the constitutive expression of Hsps and chaperones during embryonic development using Western blotting, to have an overall picture of the protein level, and immunohistochemistry to define the pattern of Hsp and chaperone expression at stages from E9.5 to E17.5. To identify the neural, glial, or vascular nature of the cells that are labeled by antibodies targeted against Hsps, we performed double labeling. Because this study is the first to describe the expression of Hsps in the developing mouse brain, we commence with an overview of the expression of the Hsps before focusing on small Hsp and Hsp70 that exhibit a very specific pattern of expression.
Hsp level during CNS development
A Western blotting analysis of each Hsp protein during embryonic brain development in mouse embryo was carried out. Hsp25 is undectable in Western blot analysis and Hsp70 appears only at day E15.5 of development. A Western blot at E16.5 is representative of the Hsp expression in the embryonic brain (Fig 1).
Fig 1.
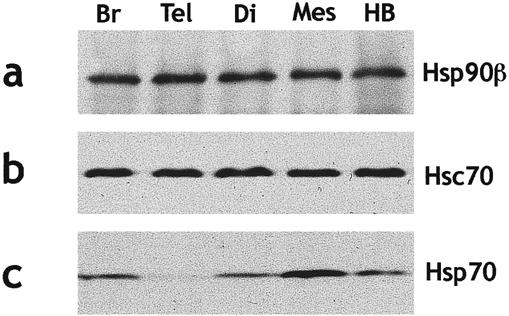
Comparative Western blot analysis of Hsps level in the brain of E16.5 mouse embryos. Samples containing 20 μg of proteins were loaded per lane for whole brain (Br), telencephalon (Tel), diencephalon (Di), mesencephalon (Mes), and hindbrain (HB). Hsp90β (a), Hsc70 (b), and Hsp70 (c) were successively analyzed. Hsp90β and Hsc70 show comparable levels in each brain region, whereas the amount of Hsp70 is greater in mes- than in di-, telencephalon, or hindbrain
Comparison of Hsp levels between various brain regions (telencephalon, diencephalon, mesencephalon, and hindbrain) shows comparable levels for Hsp90β and Hsc70 (Fig 1 a,b). The amount of Hsp70 is greater in mesencephalon than in diencephalon, hindbrain, or telencephalon (Fig 1c).
The accuracy of Western blot analysis being insufficient to predict a specific role for each Hsp, we carried out immunohistochemichal analysis.
Distribution of Hsps during CNS development
Embryonic stage E9.5
At this stage the neural tube has just closed and is undergoing an active proliferation phase. At this stage, the inducible Hsps, Hsp70 and Hsp90α, are not detectable, Hsp25 is present at a very low level (Fig 2b), whereas Hsp90β is ubiquitously expressed in all embryonic tissues (Fig 2a). Only Hsc70 is expressed at a significant level in the cells of the roof of the diencephalon and in the basal plate of the rhombencephalic medulla, at the place where the medial longitudinal tracts will form (Fig 2c).
Fig 2.
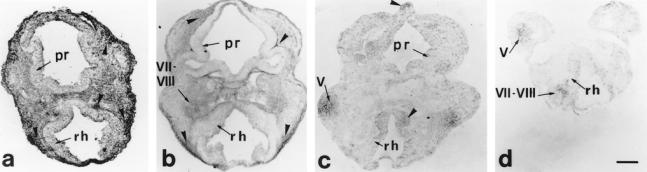
Hsp detection on cephalic coronal sections of an E9.5 mouse embryo. Sections cut the neural tube twice, at the prosencephalon (pr) and at the rhombencephalon (rh) levels. (a) Hsp90β ubiquitous is enriched along the pathway of the neural crest cells (NCC) and ganglion condensations (arrowheads); (b) Hsp25: migration paths of the NCC in the cephalic mesenchyme (arrowheads) and condensations of the VII–VIII ganglia (arrow) are enriched in Hsp25. (c) Hsc70: tectum of the forebrain, basal plate of the medulla (arrowheads), and the protrusion of the trigeminal (V) ganglion (arrow) are revealed by the antibody. (d) TCP-1α is localized on trigeminal (V) and on acoustico-facial (VII–VIII) condensations (arrows). Scale 100 μm
Rhombomeric NCC are labeled during their migration with antibodies against Hsp25, Hsc70, and Hsp90β, as are the ganglia that have just formed (ganglia V) or that are in the initial phase of their formation (ganglia VII–VIII) (Fig 2 a–c). These cell condensations are also labeled with antibodies against TCP-1α (Fig 2d).
Double-labeling experiments show a clear enrichment of Hsp25 in the first differentiating motoneurons of the truncal spinal cord that are strongly reactive to neural marker TuJ1 (not shown).
Embryonic stage E12.5
Brain development has progressed with the formation of numerous nuclei, the ontogeny of tracts, and the first signs of neurodifferentiation (cells expressing βIII-tubulin).
HSP25 is expressed throughout the brain, with a higher level of expression in the tracts limiting the different prosomeres (the zona limitans interthalamica), in the peripheral tracts and in longitudinal tracts of the ventral regions of the tegmentum, pons, and medulla (Fig 3b). In addition, as we shall see later, Hsp25 is very selectively expressed in isolated or grouped neurons.
Fig 3.
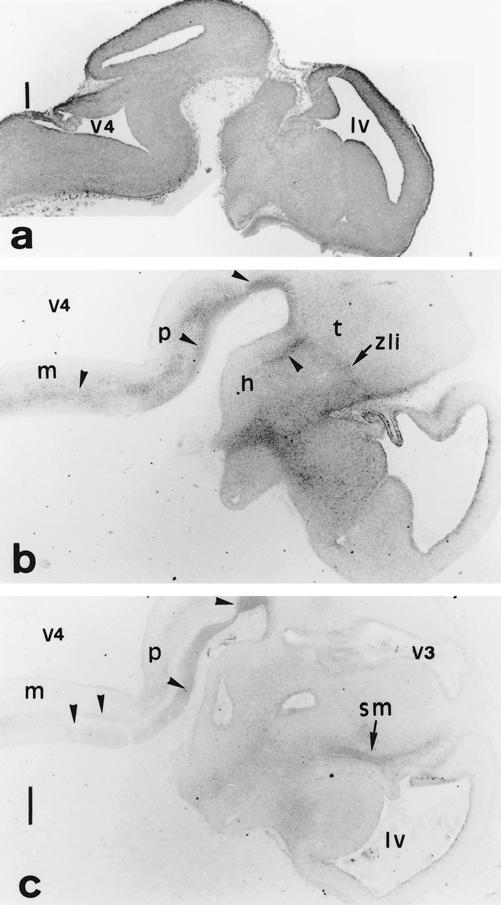
(a) Hsp90β, (b) Hsp25, and (c) Hsc70 distribution on E12.5 neural tube parasagittal sections. (b,c) Focused at the forebrain and the ventral hindbrain levels. (a) Hsp90β displays an ubiquitous distribution in the marginal zone. (b,c) The longitudinal tracts along the pons (p) and the medulla (m) (arrowheads), the zona limitans interthalamica (zli), a bundle that limits prosomeres in the diencephalon and the peripheral descending tracts, as the stria medullaris (sm) are recognized by anti-Hsp25 and anti-Hsc70. h, Hypothalamus; lv, lateral ventricle of telencephalon; t, thalamus; v3, v4, third and fourth ventricles. Scale 250 μm
The localization of Hsc70 is similar to the localization of Hsp25 (Fig 3c). The longitudinal and descending fasciculi, as stria medullaris, are strongly TuJ1 immunoreactive and are also enriched in Hsc70. In the spinal cord, the marginal layer is enriched in Hsc70, but it is poorly expressed in the mantle layer. The ventricular layer and spinal cord ganglia are devoid of HSC70.
By contrast, Hsp90β is ubiquitously expressed in the CNS, with a higher level of expression in the derivatives of the subventricular layer of the neural tube (Fig 3a).
The TCP-1α chain of the cytoplasmic chaperonin is less abundant in the E12.5 embryonic brain than the 3 previously described Hsps. However, its expression is clearly detectable in the cortex, striatum, and at a weaker level in the thalamus, pons, medulla, and spinal cord (data not shown).
At E12.5, the 2 inducible forms of Hsp, Hsp70 and Hsp90α, are not detectable under normal conditions.
Embryonic stage E15.5
This stage is characterized by the formation of a stratified primary cortex in the telencephalon, the neopallium, and the first steps in the formation of the cerebellum. In addition, olfactory bulbs and choroid plexi fully differentiate at this stage of development.
Despite its ubiquitous synthesis, Hsp25 exhibits a specific pattern of increased expression in the epithelium of the olfactory bulbs, in the rhinencephalon, the septum and the striatum of the basal ganglia, and the neocortex in the anterior brain (Fig 4b). Hsp25 is also heavily expressed in the meninges (essentially the dura mater) as well as in isolated neurons or groups of neurons (see later, Fig 7).
Fig 4.
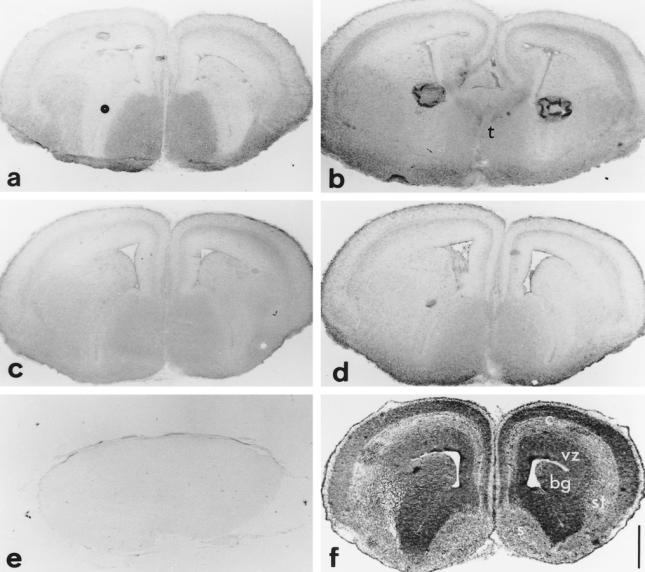
Hsc70 (a), Hsp25 (b), Hsp90α (c) and Hsp90β (d) distribution on E15.5 telencephalic coronal sections compared to toluidine blue staining (f) and to a negative control section where the first antibody was omitted (e). Hsc70, Hsp25, and the Hsp90s are differentially expressed in some layers of the developing stratification of the cortex (c), in septum (s), basal ganglia (bg), striatum (st), but not in the ventricular zone (vz). The more caudal section (b) shows that Hsp25 is also expressed in thalamic (t) structures. Note in (b) that the strong staining in the middle of each hemisphere is an artifact due to a bubble of nonadherant material. Toluidine blue intensely (f) stains the differentiating cortex (c) and the ventricular zone (vz), regions where the cell nuclei population is very dense. Scale 500 μm.
Fig 7.
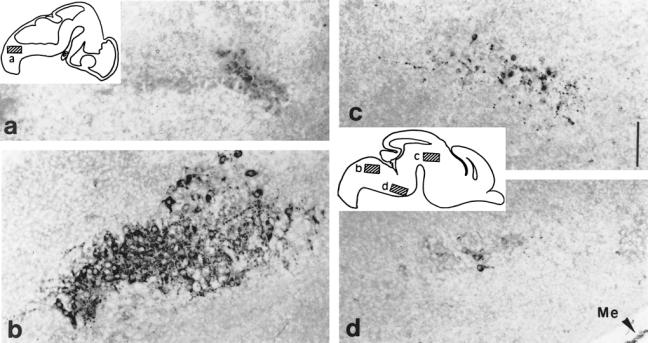
Hsp25 expression in brain nuclei: E12.5 and E15.5 parasagittal sections. Diagrams of these sections localize the level of highpower pictures: E12.5 (a), E15.5 (b–d) (a) Nascent hypoglossal neurons start to express Hsp25. (b) Cytoplasmic presence of Hsp25 in the somata and processes of the large motoneurons of the hypoglossal nucleus at E15.5. (c) Neurons of an indeterminate nucleus in the differentiating field of the tegmentum. (d) Some neurons of the ventral area of the pontic region express Hsp25. Notice that among the meningae (Me), the dura mater is strongly labeled with anti-Hsp25. Scale 250 μm.
As with Hsp25, Hsc70 is present in the neocortex, the basal ganglia, the preoptic areas of the anterior brain and the posterior commissure (Fig 4a). A comparison between immunolabeling and staining with toluidine blue (Fig 4f) of a coronal section of the telencephalon confirms the observation at E12.5: Hsc70 is more abundant in those regions that are poor in cell nuclei (Fig 4a). In addition, Hsc70 shows a very characteristic localization in the tegmental epithelium of the isthmus, where it is present in very dense cytoplasmic particles attached to the nuclear external membrane (see later). The localization of the molecular chaperonin TCP-1α is similar to that of Hsc70 but with a lower intensity.
Hsp70, which was undetectable before this stage of development, exhibits a very specific pattern of expression that will be described further in section IV.
As previously observed at earlier stages of development, Hsp90β is ubiquitously expressed but as, however, a reduced level in the ventricular layer of the neuroepithelium (Fig 4d). From the anterior to the posterior part of the brain, Hsp90β is most abundant in the preoptic areas, hypothalamus, pons, and medulla.
Hsp90α exhibits a distribution that is similar to the distribution of Hsp90β, but its level of expression is lower compared to that of Hsp90β (Fig 4 c,d). In contrast, Hsp90α, and not Hsp90β, is expressed at a high level in the choroidal plexi in the lateral and fourth ventricles. Only the epithelial layer bordering the cavity of the ventricle, responsible for the secretion of the cephalo-rachidian liquid, is labeled.
Embryonic stage E17.5
All of the Hsps investigated are expressed at this stage of development. This ubiquitous expression does not exclude specific patterns of synthesis in the cortex and cerebellum or very high levels of expression in specific structures, as exemplified by Hsp90α and Hsc70.
As shown, Hsp90α is abundant in 2 cell layers localized in the bed nucleus of the stria terminalis (Fig 5b), between the septum, the anterior commissure, and the preoptic area (Altman and Bayer 1986, 1995). The bed nucleus of the stria terminalis, which is a part of the hypothalamus, is derived from neurons of the medial horn of the neuroepithelium of the lateral ventricle (Altman and Bayer 1986). Hsp90α is also heavily expressed in the medial part of the hypothalamus: 2 layers of neurons with large soma are present on both sides of the third ventricle: they might correspond to the dorsomedial nuclei that form only later in the rat and that establish connections with the bed nucleus of the stria terminalis (Altman and Bayer 1986) (Fig 5a). No labeling was detected on hypophysis sections. A third group of neurons is localized in the inferior tectal neuroepithelium of the isthmus at the mes-metencephalon border, forming the lumen of the Sylvius aqueduct (Fig 5c). Hsp90α, absent in trigeminal ganglia earlier, is strongly expressed in gathered neurons (Fig 8d).
Fig 5.
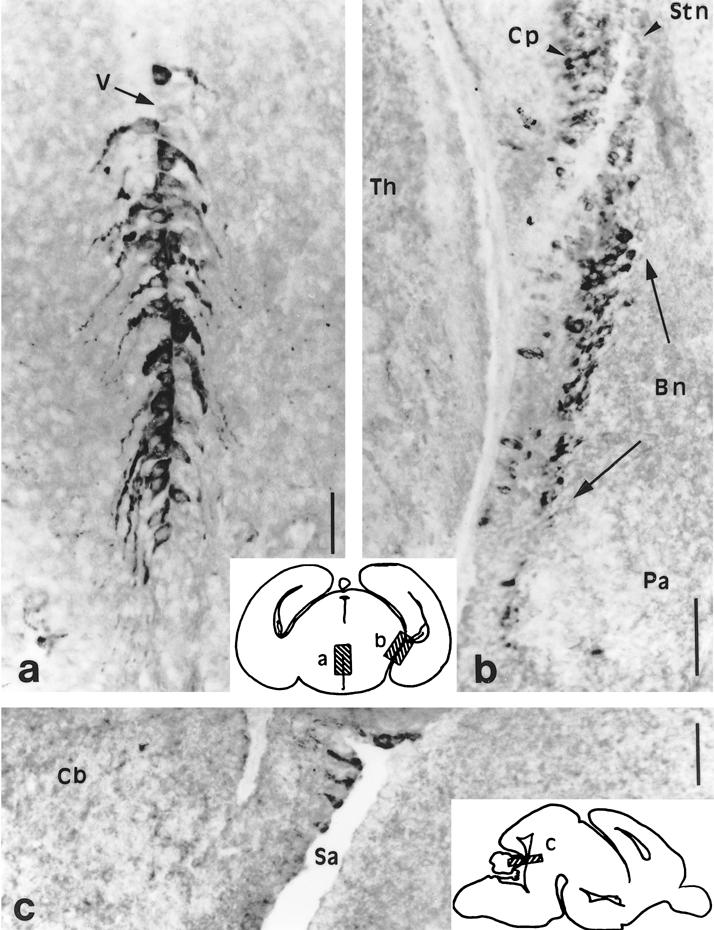
Hsp90α exhibits a highly specific distribution on the E17.5 brain hypothalamic coronal sections (a,b) and mes-metencephalic parasagittal section (c). Schematic drawings of these sections localize the high power pictures (a–c). (a) Hypothalamic neurons of the putative dorsomedial nuclei on both sides of the obturated third ventricle (V). (b) Neurons of the bed nucleus of the stria terminalis (Bn) are strongly labeled as are the choroid plexus (Cp) of the lateral ventricle to a lesser extent. pa, Pallidum; stn, strionuclear neuroepithelium; Th, thalamus. (c) Few neurons of the neuroepithelium along the border of the Sylvius aqueduct (Sa) of the isthmus (mes-metencephalic limit) (cb) cerebellum. Scales 100 μm
Fig 8.
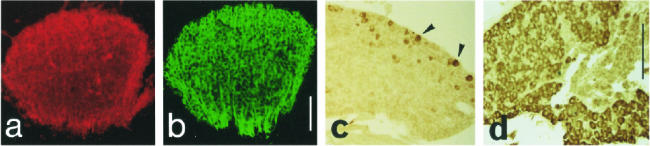
Hsp25 (a,c) and Hsp90α (d) during PNS ganglia differentiation. (a) E12.5 coronal section: Hsp25 is highly present in all differentiating neurons of the trigeminal ganglion. (b) The same neurons are labeled with TuJ1. (c) E15.5 parasagittal section: only a subpopulation of the neurons of the trigeminal ganglion is labeled with anti-Hsp25 (arrowheads). (d) E17.5 coronal section: most neurons express Hsp90α. Scales 150 μm
As already demonstrated at E15.5, an intense perinuclear labeling of the neuroepithelial tectal cells is obtained with antibodies against Hsc70. Within 2 days, this stained area has expanded to the inferior colliculus in the mesencephalon and to the cerebellar neuroepithelium in the metencephalon. As revealed by staining with Hoechst, these intensively stained cells are not undergoing apoptosis (data not shown).
Expression of Hsp25 in the brain and specific neurons
Hsp25 is strongly expressed in the earlier stages of the brain vasculature development, as recognized by BAND lectin staining (Hockfield and McKay 1985). At E12.5 the first endothelial cells, originating from the NCC, invade the periphery of the neural tube and migrate inside where they proliferate and develop into brain vessels. These cells are heavily labeled by anti-Hsp25 (data not shown).
At E12.5 the neurons generated at the ventricular surface of the neural tube start to migrate along the radial glial cells in the marginal zone where they will organize the adult cortical plate (Rakic 1972). Hsp25 expression is enhanced in the ventricular layer (Fig 6b) and has the same localization as the RC2 marker that is specific to these radial glial cells (Fig 6a). Hsp25 expression is also higher in the first differentiating neurons of the peripheral layer (Fig 6d) that are recognized by the TuJ1 antibody (Fig 6c).
Fig 6.
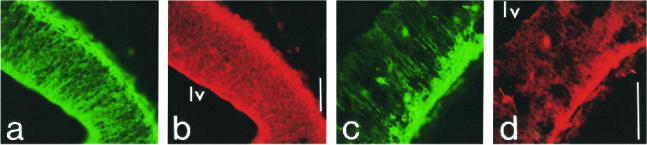
Hsp25 double labeling on E12.5 cortical parasagittal sections with, respectively, RC2 and TuJ1. (a) RC2 recognizes the early differentiating radial glial cells in the ventricular zone, and (b) Hsp25 displays a partially coincident localization with RC2. (c) TuJ1 strongly labels the early differentiating cortical neurons of the cortical plate, and (d) Hsp25 labels similarly. lv, Lateral ventricle. Scales 100 μm
At E12.5, the hypoglossal nucleus consisting of the large motoneurons that will innervate the tongue muscle initiates its formation in the central part of the medulla under the fourth ventricle (Altman and Bayer 1980; Phelps et al 1990). Hsp25 is expressed in this nucleus as early as E12.5 (Fig 7a), but its expression is maximal at E15.5, immediately preceding the formation of the first myofibrils of the tongue and the first contractions of this muscle. Hsp25 is expressed in most motoneurons, in both soma and dendrites (Fig 7b). No signs of cell death are observed.
Hsp25 is also abundant in other nuclei in the ventral area of the pontic region (Fig 7d) and in a nucleus in the differentiating field of the tegmentum, in close proximity to the cephalic flexure (Fig 7c). In the latter nucleus, Hsp25 is still abundantly expressed at E17.5. In contrast to the hypoglossal nucleus, not all of the cells of the tegmentum nucleus are labeled. In the positive cells, Hsp25 is very abundant in axons and dendrites. Hsp25 is also abundant at E15.5 in isolated neurons of the tectum and medulla.
Hsp25 is expressed in the peripheral nervous system, in the trigemellate (V) ganglia, as early as the condensation stage (E9.5), and during their formation at E12.5, when the entire neurons (TuJ1 immunoreactive) are strongly labeled (Fig 8 a,b). Later in development, Hsp25 expression is limited to small groups of cells in these structures (Fig 8c) and has disappeared at E 17.5.
Hsp70 localization in the developing brain
Hsp70 exhibits a specific pattern of expression from E15.5
Hsp70 is not detectable in the CNS before E15.5. At this stage, in the telencephalon, Hsp70 is expressed in the external plexiform layer at the periphery of the olfactory bulb, ventrally in the septum, and dorsally in 2 layers of the neocortex. In the diencephalon, preoptic area, and both ventral (mammillothalamic) and dorsal (stria medullaris) fibers are labeled. In the mesencephalon and metencephalon, Hsp70 is present in limited areas of the inferior colliculi, in the central vermix of the cerebellum, in a well-delineated part of the neuroepithelium in the isthmus, and in the ventral tangential nucleus. In the medulla, antibodies against Hsp70 stain the ventral pyramidal tracts, the medial vestibular nuclei, and the prepositus and cuneate nuclei of the subventricular zone (Fig 9).
Fig 9.

Hsp70 localization on a E15.5 brain parasagittal section. In the forebrain derivates Hsp70 is present in the external layer of the olfactory bulb (ob), the cortex (c) (to a lesser extent in hippocampus [h]), in the septum, the preoptic area (po), and the fibers of the diencephalic tracts. On this section the labeled stria medullaris corresponds with the area of the posterior commissure (pc) and the labeling for the mammilothalamic tract (mt) corresponds with the caudal area of the mammilar region. In the midbrain Hsp70 is present in the inferior colliculus (ic) and in the ventral tegmental nucleus (vt). In the caudalmost region of the brain the immunolabeling is distributed in the central vermix of the cerebellum (cb), in the prepositus (pp), and in the cuneate (cu) nuclei of the dorsal medulla (m). p, Pons. Scale 500 μm
Cortical localization is highly specific compared to the other Hsps
At E15.5, sagittal (Figs 9, 10a) and coronal sections (Fig 4) demonstrate the striated organization of the cortex in which each Hsp exhibits a clearly distinct profile of expression. From the pial surface to the ventricular lumen the labeling reflects the layered organization of the cortex. Hsp70 is characterized by a very restricted distribution compared to the other Hsps. It is strictly present in the marginal zone, and in 2 layers of the intermediate zone (Fig 10a). Interestingly, GFAP colocalizes perfectly with Hsp70 in parasagittal sections of the brain at E15.5 (Fig 10 a,b) despite the fact that a limited number of differentiated astrocytes can be detected at this stage of development and these only in the medulla. Hsp90s, Hsp25, and TuJ1 are strongly expressed, and Hsc70 to a lesser extent in the cytoplasm of the postmigratory neurons of the marginal zone (not shown).
Fig 10.
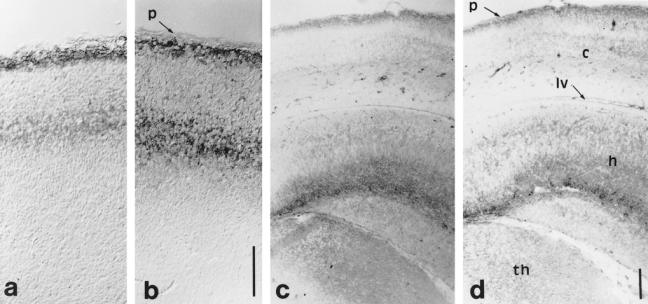
Hsp70 (a,c) and GFAP (b,d) localization on E15.5 (a,b) and E17.5 (c,d), respectively, parasagittal and coronal sections of the cortex. (a,b) Hsp70 (a) is differentially and specifically expressed throughout 2 of the 6 layers of the newly organized stratification of the neocortex as GFAP (b). (c,d) Hsp70 (c) is heavily expressed under the pial surface and in the hippocampus (h) as is GFAP (d). c, neocortex; h, hippocampus; lv, lateral ventricle; p, pial surface; th, thalamus. Scales 100 μm
At E17.5, the specific pattern of Hsp70 staining reflects the GFAP distribution (Fig 10 c,d), whereas Hsc70 and Hsp25 are more highly expressed in the subventricular zone reflecting βIII-tubulin staining, and Hsp90s are equally distributed over the cortical layers (not shown).
The neurons that will form the hippocampus and the dentate gyrus appear at E11. In the hippocampus, the formation of the neurons stops at E16, and at E17.5 the hippocampus stains with all of the antibodies. The distribution of Hsp70 resembles that of GFAP (Fig 10 c,d), whereas Hsp25 and Hsc70 distribution resembles βIII-tubulin.
DISCUSSION
Our results demonstrate that members of Hsp families are expressed in a spatially and temporally specific pattern during embryonic development of mouse brain. Similar observations have been made during postnatal development of rat brain (D'Souza and Brown 1998), but unlike these authors who found Hsp gene expression only in neuronal cells, we found expression in both neuronal and glial cells. The presence of certain Hsps seems limited to the cells exiting the cell cycle and entering differentiation. We propose that the Hsps play a role in this process. Within each family, the different members (Hsc70 and Hsp70; Hsp90α and Hsp90β) display distinct but often overlapping patterns of expression in different regions of the building brain, which suggests that they may perform unique roles at different stages of neuronal maturation.
The role of Hsp90β, Hsc70, and Hsp25 in cell migration and differentiation
Hsp25, Hsc70, and Hsp90β are expressed early during NCC migration, follow the differentiation of the first tracts, and are present in many differentiating fields, where neurons out of the proliferating zone start to undergo their morphogenesis.
Hsp role in cytoskeletal reorganization
Cells of developing tissues undergo extensive architectural remodeling during either migration, adhesion, or differentiation. This remodeling leads to extensive reorganization of the cytoskeletal system (microtubules, microfilaments, and intermediate filaments). It has been shown that many Hsps may bind to cytoskeletal elements (Liang and MacRae 1997). Coprecipitation experiments have demonstrated that Hsp90 (Koyasu et al 1986) and Hsc70 are actin and microtubule binding proteins (Whatley et al 1986). Liang and MacRae (1997), reviewing tubulin-Hsp70 interactions, suggest that Hsp70 could regulate tubulin polymerization in two ways: (1) directly by acting as an antagonist of MAPs (microtubule-associated proteins), thereby inhibiting the tubulin polymerization necessary for cell division and differentiation, or (2) through association with tau, a microtubule-associated protein.
Hsp25 is known to be associated with stress fibers and plays a role in stabilization of this actin-rich structure (Gopalakrishnan and Takemoto 1992; Lavoie et al 1993). A reduction in the level of αB-crystallin (a related member of the small Hsp family) in glioma cell cultures and of Hsp27 in human MCF-7 tumoral breast cells is correlated with altered cell morphology, disorganized microfilament network, and reduction of cell adhesiveness (Iwaki et al 1994; Mairesse et al 1996). Migrating NCC (E9.5), meninges, and neurons, either isolated or gathered in nuclei, show striking levels of Hsp25. We show that Hsp25 colocalizes with specific markers of 3 different lineages: with lectin of Bandeiraea that labels endothelial cells invading the neural tube, with the RC2 marker the in radial glial lineage, and with βIII-tubulin in differentiating neurons. Taken together these diverse localizations may reflect either multiple functions for Hsp25 or, on the other hand, the same function accomplished at a specific step of differentiation of various cell types. The association of Hsp25 with F-actin in a phosphorylation-dependent manner (Lavoie et al 1995) and its ability to affect cell motility and shape offer some suggestion as to the reason Hsp25 is selectively expressed in migrating neurons.
It has also been shown to interact with peripherin, vimentin, and GFAP, 3 intermediate filament proteins (Djabali et al 1997; Perng et al 1999). Moreover, the work of Perng et al (1999) shows evidence that Hsp27 and αB-crystallin might be responsible for the maintenance of the different networks of intermediate filaments.
After axotomy in rat, the distribution of Hsp27 along axons in vivo and in the growth cone in vitro is compatible with a possible role in the growing tip of the axon (Costigan et al 1998). Depending on its phosphorylation level, Hsp25 may prevent or permit actin filament polymerization, stabilizing the axon structure (Rousseau et al 1997).
Hsps in axonal transport
Cell differentiation, especially dendrite and axonal process formation, leads to an activation of protein transport. Transport of proteins to cellular membranes involves clathrin-coated vesicles shuttling back and forth (Goldstein et al 1979). The constitutive form of Hsp70, Hsc70, functions as an ATP-dependent uncoating enzyme that releases clathrin from coated vesicles. Two components of axonal transport have been described with different rates of transport. Rapidly transported axonal proteins are primilarly associated with a particular fraction that consists of membranous material or vesicles that are routed through the Golgi apparatus and targeted to the synaptic membrane. Evidence suggests that Hsc70 is bound to clathrin during this fast axonal transport (Black et al 1991) and that it could be important in transporting correctly folded proteins to plasma membrane during cell differentiation. In slow axonal transport, soluble proteins are associated with elements of the cytoskeleton, ie, microtubule, microfilament, and neurofilament proteins. Hsp70 may serve as a cross-linker molecule between transported cellular molecules and the actin microfilament via the binding of Hsp70 directly with the cellular targeting sequence (Tsang 1993).
Thus an increase in the demand for axonal transport during neuronal development may account for the increase in the Hsp synthesis.
Hsp25 and neuronal survival
The most spectacular labeling of Hsp25 in brain is found in the labeled neurons of selected nuclei. They display staining of the soma and of the dendritic trees, in agreement with Plumier's observations (Plumier et al 1997). In the hypoglossal nucleus at E12.5, nascent neurons are weakly Hsp25 immunoreactive. At E15.5, the stage corresponding to the onset of muscular contraction, when the XIIth cranial nerve innervates the tongue muscles, the huge hypoglossal motoneurons are very heavily labeled. The coincidence of these 2 events calls to mind reciprocal interactions between the motoneurons and their target. Such a correlation is required in the neurotrophic theory of Oppenheim (1991). A hallmark of nervous system development is the extensive histogenic cell death that occurs relatively late in maturation and results in the loss of 20–80% of all neurons. The main supposition in Oppenheim's theory is that the survival of developing vertebrate neurons depends on specific neurotrophic factors secreted by the target cells that neurons innervate. The neurons that fail to obtain adequate amounts of neurotrophin die soon after they form synaptic connections with their target cells. This dependence is present also in postnatal life and reduces as growth proceeds (Snider and Thanedar 1989).
One might see in the high level of Hsp25 a way for the cells to protect themselves against apoptosis. Recent studies have shown that overexpression of Hsp25 in murine fibrosarcoma cells suppresses apoptotic death in response to FAS/APO1 ligand stimulation and to staurosporine, camptothecin or actinomycin D (Mehlen et al 1996; Samali and Cotter 1996). Costigan et al (1998) reported that PC12 cells (sympathic-like neurons derived from the NCC) transfected with an antisense Hsp27 construction undergo an apoptotic cell death. Thus, Hsp27 may play an antiapoptotic role in neurons, promoting survival in injured neurons after peripheral nerve axotomy in a Bcl-2 independent pathway (Costigan et al 1998). The protection mechanism remains unclear. In neurons developing in vitro, Hsp25 expression correlates with neuronal differentiation and survival in some systems (Mehlen et al 1999) but not in others (Davidson and Morange 2000).
A comparison between the function of Hsp70 and Hsc70
Our observations suggest that Hsc70 is localized to neurons and Hsp70 to the astrocytic compartment. Observations of Brown (1994) after heat shock on adult mammalian brain demonstrate the induction of Hsp70 in glial cells, although other trauma may induce Hsp expression in selected neuronal populations (Blake et al 1990). The authors suggest that the transport of Hsp70 may rapidly deliver the protein from induced glial cells to the adjacent neuronal cell body and processes. Our results with Hsp70 show that this protein is already present in glial cells and ready to be transported. In addition, the observations of Brown and ours suggest that Hsp70 cannot be functionally replaced by the Hsc70 that is already present in many structures in high amounts.
The role of Hsp90α in CNS development
During brain development, Hsp90β and Hsp90α are widely expressed after E15.5. The coincident localization of Hsp90s with the neuronal marker TuJ1 supports the hypothesis that these proteins may be required for cell-specific function in neurons. Hsp90 is a major molecular chaperone that protects denatured proteins against irreversible aggregation and degradation (Nathan et al 1997). But in addition, Hsp90s have been assigned chaperone functions in the mechanisms of cellular signaling through its interaction with protein kinases and transcription factors. A disruption of the Hsp90 pathway has strong repercussions for development in Drosophila (Rutherford and Linquist 1998).
Growth and/or neurotrophic factors play important biological roles in the development and maturation of the nervous system. They act as instructive signals for lineage commitment in PNS and in CNS, promote graded stages of cell differentiation, axonal guidance, survival of subsets of neurons, and maintenance of neuronal phenotype. A wide variety of neurotrophic factors have been described, any of which may potentially require Hsp90 to function correctly.
Potential target proteins for Hsp90 also include a very diverse group of transcription factors, such as steroid receptors (Picard et al. 1990), basic helix-loop-helix (bHLH) factors such as MyoD (Shaknovitch et al 1992) or single-minded (McGuire et al 1996), heat shock factor 1 (Ali et al 1998), and a mutated form of the tumor suppressor p53. Recent studies indicate that bHLH genes play an essential function in morphogenesis of the nervous system. In neural development the transition from the initial growth phase of dividing precursor cells to the subsequent differentiation phase of postmitotic cells is controlled antagonistically by multiple bHLH genes. Cascades of neuronal bHLH genes promote determination and differentiation, whereas antineuronal bHLH genes repress them under the control of Notch and maintain cells at the precursor stage (Kageyama and Nakanishi 1997).
Besides the ubiquitous distribution of the 2 Hsp90s, we established that Hsp90α is very strongly and specifically expressed at E17.5 in a few hypothalamic nuclei and in the ventricular epithelium of the mes-metencephalic border. It could be suggested that the specific labeling observed in the hypothalamic nuclei is related to Hsp90α steroid-receptor-binding activity and that this activity is completely assigned to the Hsp90α form as opposed to Hsp90β. Estrogen-receptor mRNA is detected as early as E11.5 in mouse brain vesicles (Orimo et al 1995) and in selective zones of ferret hypothalamus at midgestation (Tobet et al 1993). Glucocorticoid receptor is detected in rat at E14 in hippocampal formation and at E16 in hypothalamic paraventricular nucleus (Yi et al 1994). The hypothalamus is in mammals an important regulation center for physiological and endocrine functions. Many nuclei that synthesize peptidic releasing hormones are regulated in adults by the level of steroid hormones in the blood. Olàzabal et al (1992) demonstrated that estradiol induces Hsp90 and progestin receptor in adult rat ventromedial hypothalamus.
The distinct localization observed for the 2 Hsp90s reveals the possibility that Hsp90α and β perform different functions. A similar possibility was evoked by examining rat testis (Lee 1990) and zebrafish muscle early development (Sass et al 1996).
As we have shown previously, Hsp25 is strongly expressed in the differentiating heart and muscle, at the earliest stages (Loones et al 1997). The differentiation of muscle cells and neurons is under the control of bHLH transcription factors, and it can be strongly suggested that Hsps may regulate the activity of these factors during the differentiation of both cell types. Interestingly, Hsp90 is known to be involved in the folding of bHLH factors into their active configuration. As Lyons et al (1995) suggest, an interesting parallel can be drawn between muscle cells and neurons. Both cell types are electrically excitable and become postmitotic before differentiation.
Acknowledgments
We are particularly grateful to Prof Steve Easter for helpful discussions, to Drs Tony Frankfurter and Ronald Melki for their generous gift of TuJ1 and TCP-1α antibodies, to Dr Marion Wassef for her kind loan of the microtome, and to Sean Davidson for his critical correction of the manuscript. We thank Prof David Walsh for sharing unpublished results. This work was supported by a grant from the Association pour la Recherche sur le Cancer (grant 9840), by ACC from the French Ministère de la Recherche Scientifique, by a grant from the DRET (95073) and by the Ecole Normale Supérieure.
REFERENCES
- Ali A, Bharadwaj S, O'Carroll R, Ovsenek N. HSP90 interacts with and regulates the activity of heat shock factor 1 in Xenopus oocytes. Mol Cell Biol. 1998;18:4949–4960. doi: 10.1128/mcb.18.9.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat. I. Thymidine-radiographic study of the time of origin of neurons of the lower medulla. J Comp Neurol. 1980;194:1–35. doi: 10.1002/cne.901940102. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. The development of the rat hypothalamus. Adv Anat Embryol Cell Biol. 1986;100:1–178. [PubMed] [Google Scholar]
- Altman J, Bayer SA 1995 Atlas of Prenatal Rat Brain Development. CRC Press, Boca Ràton, FL. [Google Scholar]
- Aquino DA, Klipfel AA, Brosnan CF, Norton WT. The 70-kDa heat shock cognate protein (HSC70) is a major constituent of the central nervous system and is up-regulated only at the mRNA level in acute experimental autoimmune encephalomyelitis. J Neurochem. 1993;61:1340–1348. doi: 10.1111/j.1471-4159.1993.tb13627.x. [DOI] [PubMed] [Google Scholar]
- Bhat SP, Nagineni CN. αB subunit of lens-specific protein α-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989;158:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Black MM, Chestnut MH, Pleasure IT, Keen JH. Stable clathrin: uncoating protein (hsc70) complexes in intact neurons and their axonal transport. J Neurosci. 1991;11:1163–1172. doi: 10.1523/JNEUROSCI.11-05-01163.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake MJ, Gershon D, Fargnoli J, Holbrook NJ. Discordant expression of heat shock protein mRNAs in tissues of heat-stressed rats. J Biol Chem. 1990;265:15275–15279. [PubMed] [Google Scholar]
- Brown IR 1994 Induction of heat shock genes in the mammalian brain by hyperthermia and tissue injury. In: Heat Shock Proteins in the Nervous System, ed Mayer RJ, Brown IR. Academic Press, London, 31–53. [DOI] [PubMed] [Google Scholar]
- Brown IR, Rush SJ. Expression of heat shock genes (Hsp70) in the mammalian brain: distinguishing constitutively expressed and hyperthermia-inducible mRNA species. J Neurosci Res. 1990;25:14–19. doi: 10.1002/jnr.490250103. [DOI] [PubMed] [Google Scholar]
- Costigan M, Mannion RJ, Kendall G, Lewis SE, Campagna JA, Coggeshall RE, Meredith-Middleton J, Tate S, Woolf CJ. Heat shock protein 27: developmental regulation and expression after peripheral nerve injury. J Neurosci. 1998;18:5891–5900. doi: 10.1523/JNEUROSCI.18-15-05891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. The heat shock response. Crit Rev Biochem. 1985;18:239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Morange M. Hsp25 and the p38 MAPK pathway are involved in differentiation of cardiomyocytes. Dev Biol. 2000;218:146–160. doi: 10.1006/dbio.1999.9596. [DOI] [PubMed] [Google Scholar]
- Djabali K, de Néchaud B, Landon F, Portier MM. αB-crystallin interacts with intermediate filaments in response to stress. J Cell Sci. 1997;110:2759–2769. doi: 10.1242/jcs.110.21.2759. [DOI] [PubMed] [Google Scholar]
- D'Souza SM, Brown IR. Constitutive expression of heat shock proteins Hsp90, Hsp70 and Hsp60 in neural and non-neural tissues of the rat during postnatal development. Cell Stress Chaperones. 1998;3:188–199. doi: 10.1379/1466-1268(1998)003<0188:ceohsp>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easter SS, Ross LS, Frankfurter A. Initial tract formation in the mouse brain. J Neurosci. 1993;13:285–299. doi: 10.1523/JNEUROSCI.13-01-00285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass P, Schröder H, Prior P, Kiessling M. Constitutive expression of heat shock protein 90 (HSP90) in neurons of the rat brain. Neurosci Lett. 1994;182:188–192. doi: 10.1016/0304-3940(94)90794-3. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Anderson RGW, Brown MS. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979;279:679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S, Takemoto L. Binding of actin to lens alpha crystallins. Curr Eye Res. 1992;11:929–933. doi: 10.3109/02713689209033490. [DOI] [PubMed] [Google Scholar]
- Heikkila JJ. Heat shock gene expression and development. I. An overview of fungal, plant, and poikilothermic animal developmental systems. Dev Genet. 1993a;14:1–5. doi: 10.1002/dvg.1020140102. [DOI] [PubMed] [Google Scholar]
- Heikkila JJ. Heat shock gene expression and development. II. An overview of mammalian and avian developmental systems. Dev Genet. 1993b;14:87–91. doi: 10.1002/dvg.1020140202. [DOI] [PubMed] [Google Scholar]
- Hightower L, Nover L 1991 Heat shock and development. In: Results and Problems in Cell Differentiation. ed Hennig W, Nover L, Scheer U. Springer Verlag, Berlin. [PubMed] [Google Scholar]
- Hockfield S, McKay RDG. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki T, Iwaki A, Tateishi J, Goldman JE. Sense and antisense modification of glial αB-crystallin production results in alterations of stress fiber formation and thermoresistance. J Cell Biol. 1994;125:1385–1393. doi: 10.1083/jcb.125.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumoto S, Herbert J. Widespread constitutive expression of HSP90 messenger RNA in rat brain. J Neurosci Res. 1993;35:20–28. doi: 10.1002/jnr.490350104. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Nakanishi S. Helix-loop-helix factors in growth and differentiation of the vertebrate nervous system. Curr Opin Genet Dev. 1997;7:659–665. doi: 10.1016/s0959-437x(97)80014-7. [DOI] [PubMed] [Google Scholar]
- Kato H, Liu Y, Kogure K, Kato K. Induction of 27-kDa heat shock protein following cerebral ischemia in a rat model of ischemic tolerance. Brain Res. 1994;634:235–244. doi: 10.1016/0006-8993(94)91926-7. [DOI] [PubMed] [Google Scholar]
- Kato SA, Hirano A, Umahara T, Kato M, Herz F, Ohama E. Comparative immunohistochemical study on the expression of αB-crystallin, ubiquitin and stress-response protein 27 in ballooned neurons in various disorders. Neuropathol Appl Neurobiol. 1992;18:335–340. doi: 10.1111/j.1365-2990.1992.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Klemenz R, Andres A-C, Fröhli E, Schäfer R, Aoyama A. Expression of the murine small heat shock proteins Hsp25 and αB-crystallin in the absence of stress. J Cell Biol. 1993;120:639–645. doi: 10.1083/jcb.120.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S, Nishida E, Kadowaki T, Matsuzaki F, Iida K, Harada F, Kasuga M, Sakai H, Yahara I. Two mammalian heat shock proteins, HSP90 and HSP100, are actin-binding proteins. Proc Natl Acad Sci U S A. 1986;83:8054–8058. doi: 10.1073/pnas.83.21.8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–687. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N, Smith J. Development of the peripheral nervous system from the neural crest. Annu Rev Cell Biol. 1988;4:375–404. doi: 10.1146/annurev.cb.04.110188.002111. [DOI] [PubMed] [Google Scholar]
- Lee S-J. Expression of HSP 86 in male germ cells. Mol Cell Biol. 1990;10:3239–3242. doi: 10.1128/mcb.10.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, MacRae TH. Molecular chaperones and the cytoskeleton. J Cell Sci. 1997;110:1431–1440. doi: 10.1242/jcs.110.13.1431. [DOI] [PubMed] [Google Scholar]
- Loones M-T, Rallu M, Mezger V, Morange M. HSP gene expression and HSF2 in mouse development. Cell Mol Life Sci. 1997;53:179–190. doi: 10.1007/PL00000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumdsen A, Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989;337:424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Lyons GE, Micales BK, Schwarz J, Martin JF, Olson EN. Expression of mef2 genes in the mouse central nervous system suggests a role in neuronal maturation. J Neurosci. 1995;15:5727–5738. doi: 10.1523/JNEUROSCI.15-08-05727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairesse N, Horman S, Mosselmans R, Galand P. Antisense inhibition of the 27 kDa heat shock protein production affects growth rate and cytoskeletal organization in MCF-7 cells. Cell Biol Int. 1996;20:205–212. doi: 10.1006/cbir.1996.0025. [DOI] [PubMed] [Google Scholar]
- Marcus RC, Mason CA. The first retinal axon growth in the mouse optic chiasm: axon patterning and the cellular environment. J Neurosci. 1995;15:6389–6402. doi: 10.1523/JNEUROSCI.15-10-06389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J, Coumailleau P, Whitelaw ML, Gustafsson J-A, Poellinger L. The basic-loop-helix/PAS Factor Sim is associated with Hsp90. Implications for regulation by interaction with partner factors. J Biol Chem. 1996;270:31353–31357. doi: 10.1074/jbc.270.52.31353. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Coronas V, Lubjic-Thibal V, Ducasse C, Granger L, Jourdan F, Arrigo A-P. Small stress protein Hsp27 accumulation during dopamine-mediated differentiation of rat olfactory neurons counteracts apoptosis. Cell Death Differ. 1999;6:227–233. doi: 10.1038/sj.cdd.4400483. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Schulze-Osthoff K, Arrigo A-P. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks FAS/APO-1- and staurosporine-induced cell death. J Biol Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- Melki R, Cowan NJ. Facilitated folding of actins and tubulins occurs via a nucleotide-dependent interaction between cytoplasmic chaperonin and distinctive folding intermediates. Mol Cell Biol. 1994;14:2895–2904. doi: 10.1128/mcb.14.5.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morange M. Developmental control of heat shock and chaperone gene expression. Introduction. Cell Mol Life Sci. 1997a;53:78–79. doi: 10.1007/PL00000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morange M. Developmental control of heat shock and chaperone gene expression. Conclusion. Cell Mol Life Sci. 1997b;53:212–213. doi: 10.1007/PL00000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DF, Harju-Vos M, Lindquist S. In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad SciU S A. 1997;94:12949–12956. doi: 10.1073/pnas.94.24.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nornes HO, Das GD. Temporal pattern of neurogenesis in spinal cord of rat. I. An autoradiographic study-time and sites of origin and migration and settling patterns of neuroblasts. Brain Res. 1974;73:121–138. doi: 10.1016/0006-8993(74)91011-7. [DOI] [PubMed] [Google Scholar]
- Olàzabal UE, Pfaff DW, Mobbs CV. Estrogenic regulation of heat shock protein 90 kDa in the rat ventromedial hypothalamus and uterus. Mol Cell Endocrinol. 1992;84:175–183. doi: 10.1016/0303-7207(92)90028-5. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Orimo A, Inoue S, Ikeda K, Noji S, Muramatsu M. Molecular cloning, structure, and expression of mouse estrogen-responsive finger protein Efp. Co-localization with estrogen receptor mRNA in target organs. J Biol Chem. 1995;270:24406–24413. doi: 10.1074/jbc.270.41.24406. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Linquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Perng MD, Cairns L, van den Ijssel P, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and αB-crystallin. J Cell Sci. 1999;112:2099–2112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Brennan LA, Vaughn JE. Generation patterns of immunocytochemically identified cholinergic neurons in rat brainstem. Dev Brain Res. 1990;56:63–74. doi: 10.1016/0165-3806(90)90165-u. [DOI] [PubMed] [Google Scholar]
- Picard D, Kursheed B, Garabedian MJ, Fortin MG, Linquist S, Yamamoto KR. Reduced levels of Hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Plumier J-CL, Amstrong JN, Landry J, Babity JM, Robertson HA, Currie RW. Expression of the 27,000 mol. wt heat shock protein following kainic acid-induced status epilepticus in the rat. Neuroscience. 1996;75:849–856. doi: 10.1016/0306-4522(96)00317-x. [DOI] [PubMed] [Google Scholar]
- Plumier J-CL, Hopkins DA, Robertson HA, Currie RW. Constitutive expression of the 27-kDa heat shock protein (Hsp27) in sensory and motor neurons of the rat nervous system. J Comp Neurol. 1997;384:409–428. [PubMed] [Google Scholar]
- Puelles L, Rubenstein LR. Expression pattern of homeobox and other putative regulatory genes in embryonic mouse forebrain suggests a neuromeric organization. Trends Neurosci. 1993;16:472–479. doi: 10.1016/0166-2236(93)90080-6. [DOI] [PubMed] [Google Scholar]
- Quraishi H, Brown IR. Expression of heat shock protein 90 (Hsp90) in neural and non neural tissues of the control and hyperthermic rabbit. Exp Cell Res. 1995;219:358–363. doi: 10.1006/excr.1995.1239. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on the culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Renhawek K, Bosman GJ, de Jong WW. Expression of small heat-shock protein Hsp27 in reactive gliosis in Alzheimer disease and other types of dementia. Acta Neuropathol. 1994;87:511–519. doi: 10.1007/BF00294178. [DOI] [PubMed] [Google Scholar]
- Riabowol KT, Mizzen LA, Welch WJ. Heat shock is lethal to fibroblasts microinjected with antibodies against Hsp70. Science. 1988;242:433–436. doi: 10.1126/science.3175665. [DOI] [PubMed] [Google Scholar]
- Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Linquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- Sass JB, Weinberg ES, Krone PH. Specific localization of zebrafish Hsp90α mRNA to myoD-expressing cells suggests a role for Hsp90α during normal muscle development. Mech Dev. 1996;54:195–204. doi: 10.1016/0925-4773(95)00476-9. [DOI] [PubMed] [Google Scholar]
- Shaknovitch R, Shue G, Kohtz S. Conformational activation of a basic helix-loop-helix protein (MyoD1) by the C-terminal region of murine HSP90 (HSP84) Mol Cell Biol. 1992;12:5059–5068. doi: 10.1128/mcb.12.11.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambra UB, Lauder JM, and Silver J 1992 Atlas of the Prenatal Mouse Brain. Academic Press, San Diego. [DOI] [PubMed] [Google Scholar]
- Snider WD, Thanedar S. Target dependence of hypoglossal motor neurons during development and in maturity. J Comp Neurol. 1989;279:489–498. doi: 10.1002/cne.902790312. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Basham ME, Baum MJ. Estrogen receptor immunoreactive neurons in the fetal ferret forebrain. Dev Brain Res. 1993;72:167–180. doi: 10.1016/0165-3806(93)90182-a. [DOI] [PubMed] [Google Scholar]
- Tsang TC. New model for 70 kDa heat-shock proteins, potential mechanisms of function. FEBS Lett. 1993;323:1–3. doi: 10.1016/0014-5793(93)81435-3. [DOI] [PubMed] [Google Scholar]
- Whatley SA, Leung T, Hall C, Lim L. The brain 68-kilodalton microtubule-associated protein is a cognate form of the 70-kilodalton mammalian heat-shock protein and is present as a specific isoform in synaptosomal membranes. J Neurochem. 1986;47:1576–1583. doi: 10.1111/j.1471-4159.1986.tb00797.x. [DOI] [PubMed] [Google Scholar]
- Yi SJ, Masters JN, Baram TZ. Glucocorticoid receptor mRNA ontogeny in the fetal and postnatal rat forebrain. Mol Cell Neurosci. 1994;5:385–393. doi: 10.1006/mcne.1994.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]