Abstract
Acute stress–induced injury in tissues has been revealed by both biochemical markers in plasma and microscopy. However, little is known of the mechanisms by which tissue integrity is restored. Recently, induction of early response genes such as c-fos has been reported in the heart and stomach of immobilized animals. Herein, we show that immobilization stress in mice increased plasma alanine aminotransferase activity, a marker of liver damage. c-Fos protein accumulation in liver was induced by stress after 20 minutes of immobilization and persisted for 3 hours. Immobilization also induced the release of epidermal growth factor (EGF) from submandibular salivary glands and a transient increase in EGF concentration in plasma. Although EGF administration induced a 2.5-fold increase in c-Fos mass in the liver of anesthetized mice, sialoadenectomy (which abolished the effect of immobilization on plasma EGF) did not affect the stress-induced rise in plasma alanine aminotransferase activity or liver c-Fos accumulation. Therefore, we conclude that immobilization stress induces c-Fos accumulation in liver and that this effect is not triggered by the increase in plasma EGF concentration.
INTRODUCTION
c-Fos, together with a member of the Jun family, heterodimerizes to form the activator protein 1 (AP-1) transcription factor. The Fos and Jun families belong to the early response genes, whose transcription is induced by a variety of stimuli that lead to cell proliferation, differentiation, or transformation (Angel and Karin 1991). In the liver, c-Fos is induced immediately after partial hepatectomy (Taub 1996) and also by many stressors that affect cellular integrity, such as ischemia and reperfusion (Bernelli-Zazzera et al 1992), toxic injury (Schiaffonati and Tiberio 1997), or oxidative stress (Mendelson et al 1996; Hutchinson et al 1998). c-Fos induction is also associated with cell death by apoptosis (Sanchez et al 1996) or necrosis (Xu et al 1997).
Immobilization is one of the most potent stress models in rodents: it strongly activates both components of the sympathoadrenal system (Kopin 1995). The release of catecholamines has immediate effects on both metabolism and the cardiovascular system but also produces cell injury in several tissues. Thus, immobilization has been used as a model to induce ulceration in the stomach (Buchanan and Caul 1974). Damage to the heart was demonstrated by the increase of marker enzymes in blood plasma (Arakawa et al 1997). Alterations in mitochondria ultrastructure were observed in the heart of immobilized animals (Jönsson and Johansson 1974). In both the stomach (Ueyama et al 1998) and heart (Ueyama et al 1996), immobilization induces c-Fos expression, suggesting a role in repairing mechanisms.
Immobilization increases lipid peroxidation in the liver (Kovacs et al 1996), suggesting that this tissue may also be damaged in this acute stress model. In fact, Salas et al (1980) found rough endoplasmic fragmentation and dilation, mitochondrial enlargement, and an increased number of autophagic vacuoles after 48 hours in several stress models, including restraint. Therefore, we investigated whether immobilization induces c-Fos accumulation in the liver. Given that epidermal growth factor (EGF) is one of the most potent stimuli of c-Fos expression (Angel and Karin 1991) and that catecholamines stimulate the release of EGF from submandibular glands (Byyny et al 1974; Grau et al 1994; Grau et al 1997), we examined the role of EGF in c-Fos accumulation in the liver of immobilized mice.
MATERIALS AND METHODS
Animals
Adult, Swiss, CD1 mice were obtained from Interfauna (Barcelona, Spain). All animals were male, fed ad libitum, and maintained under a constant 12-hour light, 12-hour dark cycle (lights on at 8:00 am) and controlled conditions of humidity (45–55%) and temperature (22°C ± 1°C). All experimental procedures were approved by the Committee on Animal Care of the University of Barcelona.
Sialoadenectomy
In diethyl ether–anesthetized mice, a small incision was made to expose the submandibular salivary glands, which were then ligated and excised. In control (sham-operated) animals, the glands were exposed, and a ligature was passed but not tied. The wound was stitched and disinfected. Sham-operated and sialoadenectomized animals fasted for the next 24 hours. Five weeks later, the mice had recovered completely and were used for experiments.
Immobilization
Animals (under light ether anesthesia) were fixed with adhesive tape to a table in a supine position. In less than 1 minute, the effect of ether completely disappeared, and acute stress symptoms were observed. In some experiments, animals were liberated and returned to their cages after 3 hours of immobilization. At the indicated time, mice were anesthetized (sodium pentobarbital, 60 mg kg−1) to obtain samples. Blood was collected into heparinized syringes from the inferior vena cava. Blood plasma was obtained by centrifugation. A sample was deproteinized and neutralized as indicated elsewhere (Grau et al 1996) and used for glucose quantification (Trinder 1969). Another sample was processed as indicated (Grau et al 1994) for EGF quantification. Alanine aminotransferase (ALT) activity in plasma was determined by standard procedures (Boehringer Mannheim, Mannheim, Germany; assay kit ALT MPR1). In some experiments, plasma corticosterone was determined as indicated (Benavides et al 1998). Immediately after bleeding, liver and submandibular salivary glands were excised, frozen in liquid nitrogen, and stored at −80°C until further processed (in less than 1 week).
Tissue homogenization
Submandibular glands were homogenized in 10 mL of phosphate-buffered saline (PBS). After centrifugation (100 000 × g for 60 minutes at 4°C), the supernatant was stored at −40°C for EGF quantification (Grau et al 1994). A piece of liver was digested (20 minutes at 100°C) in 1 M sodium hydroxide for protein (Lowry et al 1951) and DNA (Vytasek 1982) quantification. Another sample was homogenized (8 mL of buffer per gram of tissue) in 50 mM Tris, pH 7.4 (containing 1 mM ethyleneglycoltetracetic acid, 150 mM sodium chloride, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 10 mU/mL of aprotinin, 1 μM leupeptin, 1 μM pepstatin, and 0.2 mM phenylmethyl-sulfonylfluoride) and used for protein (Lowry et al 1951) and c-Fos (see below) quantification.
Western blot analysis of c-Fos
Liver homogenates were diluted to 1.5 mg/mL of protein. Aliquots were mixed with sampling buffer and then run in 10% polyacrylamide gels in denaturing and reducing conditions (Laemmli 1970). The separated proteins were then transferred to Immobilon-P membranes (Millipore, Bedford, MA, USA) by electroblotting. After the transfer, membranes were soaked in blocking solution (5% defatted powdered milk in PBS) for 60 minutes at 37°C, rinsed (5 × 5 minutes in 100 mL of TM buffer: 0.05 Tween-20, 0.5% defatted powdered milk in PBS), and incubated overnight at 4°C with the c-Fos antibody (Upstate Biotechnology, Lake Placid, NY, USA) diluted 1/2000 in TM buffer. The membranes were rinsed as indicated above and incubated for 60 minutes at room temperature with the secondary antibody (horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G; Nordic, Tilburg, the Netherlands) diluted 1/10 000 in TM buffer, rinsed again, and developed with the ECL system (Amersham, Little Chalfont, Bucks, UK). Films were analyzed with Phoretix 1D Gel Analysis software after scanning in an EPSON GT-8500. To allow comparison of the results obtained in different films, a lane was loaded in each gel with a constant amount of a reference liver homogenate.
RESULTS
In Figure 1, we show a representative Western blot corresponding to a liver sample from control mice (A). A single band of 55 K corresponding to c-Fos was observed. The signal was linear with the amount of liver homogenate protein loaded onto the gel (B), indicating that it could be used for quantitative purposes. Thirty minutes after intraperitoneal injection of EGF, c-Fos mass in the liver had increased 2.5-fold (Fig 2). At this time, EGF concentration in plasma was higher in EGF-injected than in control mice.
Fig 1.
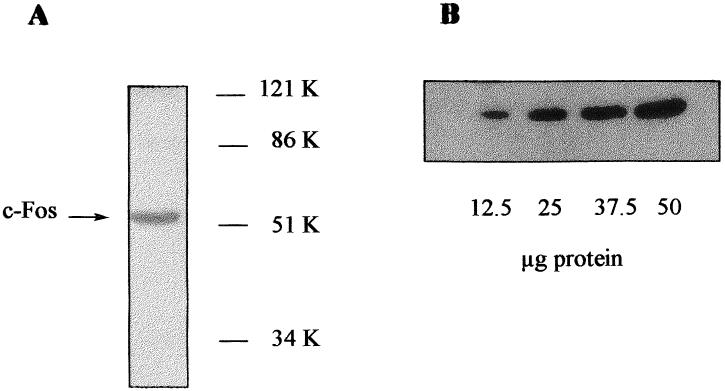
Western blot analysis of c-Fos in mice liver homogenates. (A) Liver homogenate (50 μg of protein) was loaded onto 10% polyacrylamide gel. After transfer, the membrane was incubated with the primary antibody (directed against a N-terminal fragment containing residues 75–155 of v-Fos but conserved both in human and mouse c-Fos). After washing, the membrane was incubated with a secondary antibody and then developed with the ECL system. (B) 10% Polyacrylamide gel was loaded with increasing amounts of liver homogenate protein and processed as indicated above.
Fig 2.
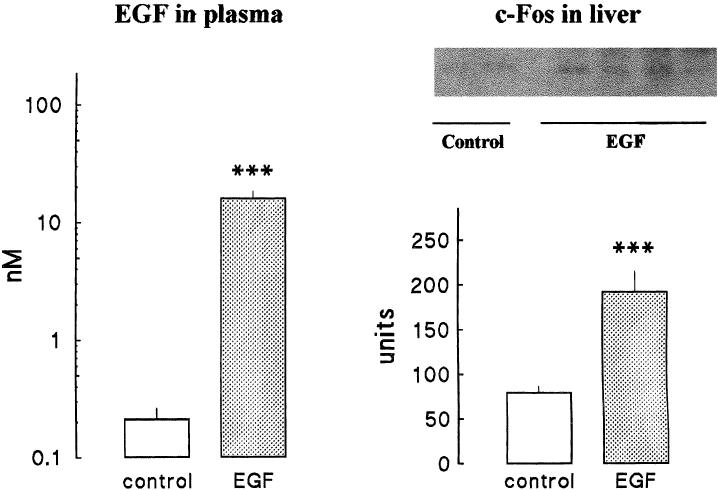
EGF administration induces c-Fos accumulation in mice liver. Mice (anesthetized with sodium pentobarbital, 60 mg kg−1) were injected with EGF (0.25 mg kg−1 intraperitoneally) (dotted bars). Control animals (white bars) received an identical volume of saline (6 mL kg−1). After 30 minutes, animals were killed and samples were obtained to determine EGF in plasma and c-Fos in liver. Left panel shows EGF in plasma and bars represent the mean ± SE of 5 animals per group. Right panel shows c-Fos protein. The upper side shows the results of 2 control and 4 EGF-injected mice (25 μg of protein per lane). The lower side of this panel shows the results of the quantification (arbitrary units) of 5 animals per group. Statistical comparisons were made by Student's t-test. *** P < 0.001
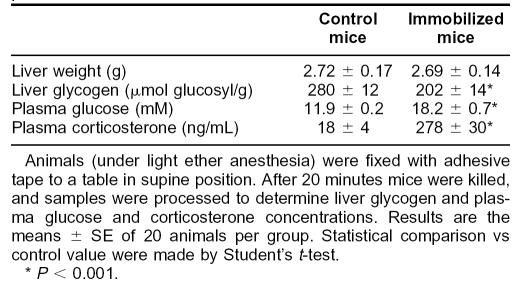
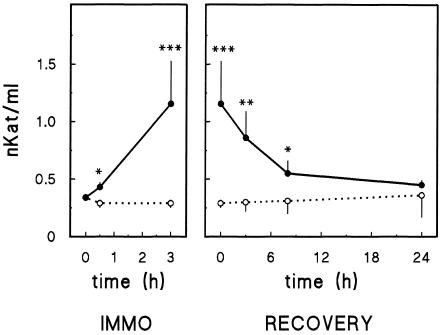
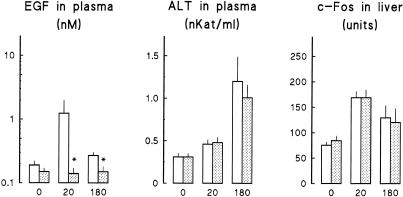
Immobilization of mice in the supine position decreased the glycogen content of the liver (Table 1). In keeping with this effect, plasma glucose concentration was increased significantly. Plasma corticosterone concentration was 15-fold higher in immobilized than in control mice. These results indicate that both the hypothalamus-hypophysis-adrenal axis and the sympathetic nervous system were activated in immobilized mice, and, therefore, animals were stressed. Plasma ALT activity had increased after 20 minutes of immobilization, and it further increased after 180 minutes (Fig 3). When the animals immobilized at 180 minutes were liberated, their plasma ALT activity progressively returned to control values 24 hours later.
Table 1.
Effect of immobilization stress on liver glycogenolysis and plasma corticosterone
Fig 3.
Effect of immobilization on plasma ALT activity. Left: mice were immobilized (•) for the indicated period. Control animals (○) were maintained in their cages. At indicated time, animals were processed to determine plasma ALT activity. Right: animals were immobilized for 3 hours (•) and then returned to their cages. Control animals (○) were maintained in their cages all the time. At the indicated time of recovery, animals were processed to determine plasma ALT activity. Results are the mean ± SE of 15 animals per group. Statistical comparisons vs corresponding control value were made by Student's t-test. * P < 0.05; ** P < 0.01; *** P < 0.001
To evaluate the extent of the injury (measured by the plasma ALT activity), we studied the effect of hepatotoxins causing irreversible liver damage: lipopolysaccharide (10 or 100 μg kg−1) combined with galactosamine (750 mg kg−1). Eight hours after the treatment, ALT activity in plasma increased from a control value of 0.19 ± 0.02 nkat/mL to 5.49 ± 2.19 nkat/mL in mice treated with 10 μg kg−1 of lipopolysaccharides (P < 0.05) or to 118 ± 31 nkat/mL in mice treated with 100 μg kg−1 of lipopolysaccharides (P < 0.01).
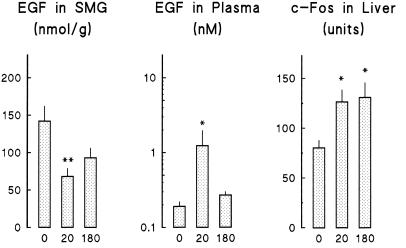
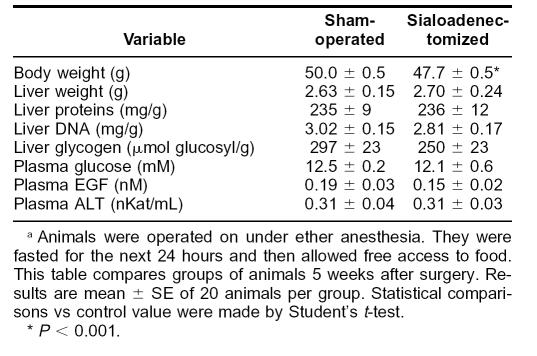
Immobilization produced a transient decrease in the EGF content of submandibular glands and an increase in the EGF concentration of plasma (Fig 4). c-Fos content in liver increased after 20 minutes of immobilization and was still higher than controls at 180 minutes. To determine the relation between these parameters, we studied the effect of immobilization in sialoadenectomized mice. Although these animals had a lower daily body weight gain and, therefore, lower whole body weight at the time of the experiment than control (sham-operated) animals (Table 2), both groups had similar liver weight and composition (protein, DNA, and glycogen content). They had also similar plasma glucose and EGF concentrations and ALT activity. Sialoadenectomy abolished the rise in EGF concentration in plasma (Fig 5) observed in control mice on immobilization. In contrast, it had no effect on the rise in ALT activity in plasma and on c-Fos accumulation in the liver.
Fig 4.
Effect of immobilization on submandibular gland and plasma EGF and on liver c-Fos protein. Animals were immobilized for the indicated period (0, 20, or 180 minutes). Mice were killed, and samples were obtained to determine EGF in submandibular salivary glands and plasma and to quantify relative mass of c-Fos protein in liver homogenates. Each bar corresponds to the mean ± SE of 9–13 animals. Statistical comparisons vs zero time were made by Student's t-test. * P < 0.05; ** P < 0.01
Table 2.
Sialoadenectomy does not affect liver composition or plasma biochemistrya
Fig 5.
Sialoadenectomy does not modify the effect of immobilization on plasma ALT activity and on liver c-Fos protein. Animals were sialoadenectomized (dotted bars) or sham-operated (control animals, white bars) 5 weeks before immobilization for the indicated period (0, 20, or 180 minutes). Mice were killed, and samples were obtained to determine plasma EGF and ALT activity and to quantify relative mass of c-Fos protein in liver homogenates. Each bar corresponds to the mean ± SE of 6–11 animals. Statistical comparisons vs corresponding control (sham-operated) value were determined by Student's t-test. * P < 0.05
DISCUSSION
It was shown that acute stress induces cell damage in many tissues, including stomach (Buchanan and Caul 1974), heart (Jönsson and Johansson 1974), and liver (Salas et al 1980). Herein, we show that immobilization stress induced a rise in ALT activity in plasma. Judging by the extent of the rise in plasma ALT activity in immobilized mice compared with that in lipopolysaccharide-treated animals, the injury was indeed mild. Damage was reversible, as indicated by the recovery of ALT activity in plasma after liberation. This suggests that repair mechanisms were induced on immobilization. In agreement with this hypothesis, Kitamura et al (1997) found that immobilization induced interleukin 6 expression in the liver. Interleukin 6 expression might be involved in the expression of acute-phase proteins or in the induction of growth factors (Cohen et al 1996; Ohira et al 1996). The induction of interleukin 6 may be the consequence of the hypothalamus-hypophysis-adrenal axis activation (Zhou et al 1993).
Immobilization induces the early expression of both c-Fos and c-Jun in localized areas of the myocardium and the smooth muscle layer of coronary arteries (Ueyama et al 1996). These authors also described the induction of c-Fos expression in gastric epithelial cells and the smooth muscle layer of both small blood vessels and the stomach wall (Ueyama et al 1998). Our main finding is that immobilization stress also induces c-Fos accumulation in liver. Both the rise in plasma ALT activity and the induction of c-Fos in liver can be attributed to immobilization and not to the ether exposure, since a brief exposure to ether without immobilization had no effect on either parameter (data not shown).
c-Fos expression is regulated by a variety of stimuli, including growth factors (Angel and Karin 1991). In liver cells, EGF strongly induces c-Fos expression. Thus, it was shown that both in hepatocytes and hepatocarcinoma 7777 cells EGF increased c-Fos messenger RNA by 15- and 5-fold, respectively (Nadori et al 1997). The effect on c-Fos messenger RNA was the highest among the members of the Fos and Jun families. Herein, we show that, in the whole animal, EGF increases the amount of c-Fos protein 2.5-fold.
In mice, submandibular salivary glands accumulate a large amount of EGF (Fisher 1990). Exogenous administration of catecholamines induces the release of EGF from submandibular glands to saliva and blood plasma (Byyny et al 1974; Tuomela 1990; Grau et al 1994). Herein, we show that immobilization stress induces both a decrease in EGF content in submandibular glands and an increase in EGF concentration in plasma. We conclude that submandibular glands are the origin of EGF in plasma, since in sialoadenectomized mice immobilization did not increase plasma EGF concentration. The liver is a major target of EGF action. A role of submandibular gland EGF in the control of liver regeneration after partial hepatectomy was recognized (Michalopoulos and Defrances 1997). Therefore, it was important to determine whether submandibular gland EGF was involved in the induction of c-Fos accumulation in the liver of immobilized mice.
Although sialoadenectomized mice had no submandibular glands, resting plasma EGF concentration was not decreased. This has been reported by many authors (Byyny et al 1974; Perheentupa et al 1984; Tuomela 1990; Grau et al 1994) and is attributed to the fact that EGF is also synthesized in other cell types, some of which can overexpress the gene as a consequence of sialoadenectomy (Dagogo 1992). Surgical removal of submandibular glands abolishes the increase in plasma EGF concentration on adrenergic stimulation (Byyny et al 1974; Grau et al 1994) or, as shown above, on physiological stress. This explains why both groups have similar plasma EGF concentration when not exposed to stress.
The results obtained in sialoadenectomized mice suggest that EGF released from submandibular glands is not essential for the induction of c-Fos accumulation in the liver of stressed mice. Our results, however, do not rule out the possibility that EGF synthesized in other cells or other members of its family, such as transforming growth factor-α (Fausto 1991) or heparin-binding EGF-like growth factor (Ito et al 1996), may be responsible for or may contribute to c-Fos accumulation. It is also conceivable that stimuli other than growth factors may be responsible for c-Fos induction. It is known that immobilization causes oxidative stress in liver (Kovacs et al 1996) and that oxidative stress induces c-Fos expression in hepatocytes (Sanchez et al 1996). Many other stress conditions induce c-Fos expression in both whole mice (Matsuda et al 1996; Fu et al 1997; Muller et al 1997; Kaufer et al 1998; Nikulina et al 1998) and culture cell systems (van Wijk et al 1993; Kawanishi and Fujioka 1994; Wilhelm et al 1997; Muller and Gebel 1998).
Further research is required to elucidate the mechanisms involved in the induction of c-Fos accumulation in the liver of stressed mice. Nevertheless, our results indicate that the liver experiences stress-induced injury like other organs (stomach or heart). As in these tissues, this results in the induction of c-Fos expression, which leads to c-Fos protein accumulation and an increase in AP-1 activity.
Acknowledgments
This study was supported by grants PB94-0863 and PB97-0936 from Ministerio de Educación y Ciencia, Spain. Our gratitude goes to Robin Rycroft for editorial help.
REFERENCES
- Angel P, Karin M. The role of Jun, Fos and AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Kodama H, Matsuoka N, Yamaguchi I. Stress increases plasma enzyme activities in rats: differential effects of adrenergic and cholinergic blockades. J Pharmacol Exp Ther. 1997;280:1296–1303. [PubMed] [Google Scholar]
- Bernelli-Zazzera A, Cairo G, Schiaffonati L, Tacchini L. Stress proteins and reperfusion stress in the liver. Ann N Y Acad Sci. 1992;663:120–124. doi: 10.1111/j.1749-6632.1992.tb38655.x. [DOI] [PubMed] [Google Scholar]
- Buchanan DC, Caul WF. Gastric ulceration in rats induced by self-imposed immobilization or physical restraint. Physiol Behav. 1974;13:583–588. doi: 10.1016/0031-9384(74)90291-1. [DOI] [PubMed] [Google Scholar]
- Byyny RL, Orth DN, Cohen S, Doyne ES. Epidermal growth factor: effects of androgen and adrenergic agents. Endocrinology. 1974;95:776–782. doi: 10.1210/endo-95-3-776. [DOI] [PubMed] [Google Scholar]
- Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- Dagogo JS. Sialoadenectomy increases the concentration of immunoreactive epidermal growth factor in the mouse thyroid gland. Thyroid. 1992;2:137–140. doi: 10.1089/thy.1992.2.137. [DOI] [PubMed] [Google Scholar]
- Fausto N. Growth factors in liver development, regeneration and carcinogenesis. Prog Growth Factor Res. 1991;3:219–234. doi: 10.1016/0955-2235(91)90008-r. [DOI] [PubMed] [Google Scholar]
- Fisher DA. Hormone epidermal growth factor interactions in development. Horm Res. 1990;33:69–75. doi: 10.1159/000181487. [DOI] [PubMed] [Google Scholar]
- Fu K, Sarras MPJ, De Lisle RC, Andrews GK. Expression of oxidative stress-responsive genes and cytokine genes during caerulein-induced acute pancreatitis. Am J Physiol. 1997;273:G696–G705. doi: 10.1152/ajpgi.1997.273.3.G696. [DOI] [PubMed] [Google Scholar]
- Grau M, Rodríguez C, Soley M, Ramírez I. Relationship between epidermal growth factor in mouse submandibular glands, plasma, and bile: effects of catecholamines and fasting. Endocrinology. 1994;135:1854–1862. doi: 10.1210/endo.135.5.7956907. [DOI] [PubMed] [Google Scholar]
- Grau M, Soley M, Ramírez I. Interaction between adrenaline and epidermal growth factor in the control of liver glycogenolysis in mouse. Endocrinology. 1997;138:2601–2609. doi: 10.1210/endo.138.6.5183. [DOI] [PubMed] [Google Scholar]
- Grau M, Tebar F, Ramírez I, Soley M. Epidermal growth factor administration decreases liver glycogen and causes mild hyperglycemia in mice. Biochem J. 1996;315:289–293. doi: 10.1042/bj3150289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson RW, Ing NH, Burghardt RC. Induction of c-fos, and cytochrome c oxidase subunits I and II by gossypol acetic acid in rat liver cells. Cell Biol Toxicol. 1998;14:391–399. doi: 10.1023/a:1007543510337. [DOI] [PubMed] [Google Scholar]
- Ito N, Higashiyama S, Kawata S, et al. Regulation of heparin-binding EGF-like growth factor expression by phorbol ester in a human hepatoma-derived cell line. Biochim Biophys Acta Mol Cell Res. 1996;1310:163–167. doi: 10.1016/0167-4889(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Jönsson L, Johansson G. Cardiac muscle cell damage induced by restraint stress. Virchows Arch B Cell Pathol. 1974;17:1–12. doi: 10.1007/BF02912832. [DOI] [PubMed] [Google Scholar]
- Kaufer D, Friedman A, Seidman S, Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature. 1998;393:373–377. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- Kawanishi H, Fujioka H. Heat shock cellular stress on aged gut-associated lymphocytes: mRNA expression of inducible heat shock protein gene and protooncogenes. Dev Comp Immunol. 1994;18:165–177. doi: 10.1016/0145-305x(94)90244-5. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Konno A, Morimatsu M, Jung BD, Kimura K, Saito M. Immobilization stress increases hepatic IL-6 expression in mice. Biochem Biophys Res Commun. 1997;238:707–711. doi: 10.1006/bbrc.1997.7368. [DOI] [PubMed] [Google Scholar]
- Kopin IJ. Definition of stress and sympathetic neuronal responses. Ann N Y Acad Sci. 1995;771:19–30. doi: 10.1111/j.1749-6632.1995.tb44667.x. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Juranek I, Stankovicova T, Svec P. Lipid peroxidation during acute stress. Pharmazie. 1996;51:51–53. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Fan AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Matsuda S, Peng H, Yoshimura H, Wen TC, Fukuda T, Sakanaka M. Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci Res. 1996;26:157–170. [PubMed] [Google Scholar]
- Mendelson KG, Contois LR, Tevosian SG, Davis RJ, Paulson KE. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc Natl Acad Sci U S A. 1996;93:12908–12913. doi: 10.1073/pnas.93.23.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, Defrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Muller T, Gebel S. The cellular stress response induced by aqueous extracts of cigarette smoke is critically dependent on the intracellular glutathione concentration. Carcinogenesis. 1998;19:797–801. doi: 10.1093/carcin/19.5.797. [DOI] [PubMed] [Google Scholar]
- Muller T, Haussmann HJ, Schepers G. Evidence for peroxynitrite as an oxidative stress-inducing compound of aqueous cigarette smoke fractions. Carcinogenesis. 1997;18:295–301. doi: 10.1093/carcin/18.2.295. [DOI] [PubMed] [Google Scholar]
- Nadori F, Lardeux B, Rahmani M, Bringuier A, Durand-Schneider AM, Bernuau D. Presence of distinct AP-1 dimers in normal and transformed rat hepatocytes under basal conditions and after epidermal growth factor stimulation. Hepatology. 1997;26:1477–1483. doi: 10.1002/hep.510260614. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Marchand JE, Kream RM, Miczek KA. Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res. 1998;810:200–210. doi: 10.1016/s0006-8993(98)00925-1. [DOI] [PubMed] [Google Scholar]
- Ohira H, Miyata M, Kuroda M, et al. Interleukin-6 induces proliferation of rat hepatocytes in vivo. J Hepatol. 1996;25:941–947. doi: 10.1016/s0168-8278(96)80300-x. [DOI] [PubMed] [Google Scholar]
- Perheentupa J, Lakshmanan J, Hoath SB, Fisher DA. Hormonal modulation of mouse plasma concentration of epidermal growth factor. Acta Endocrinol. 1984;107:571–576. doi: 10.1530/acta.0.1070571. [DOI] [PubMed] [Google Scholar]
- Salas M, Tuchweber B, Kourounakis P. Liver ultrastructure during acute stress. Pathol Res Pract. 1980;167:217–233. doi: 10.1016/S0344-0338(80)80052-5. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Alvarez AM, Benito M, Fabregat I. Apoptosis induced by transforming growth factor-beta in fetal hepatocyte primary cultures: involvement of reactive oxygen intermediates. J Biol Chem. 1996;271:7416–7422. doi: 10.1074/jbc.271.13.7416. [DOI] [PubMed] [Google Scholar]
- Schiaffonati L, Tiberio L. Gene expression in liver after toxic injury: analysis of heat shock response and oxidative stress-inducible genes. Liver. 1997;17:183–191. doi: 10.1111/j.1600-0676.1997.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Taub R. Liver regeneration 4: transcriptional control of liver regeneration. FASEB J. 1996;10:413–427. [PubMed] [Google Scholar]
- Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–27. [Google Scholar]
- Tuomela T. Epidermal growth factor concentrations in submandibular salivary gland, plasma, bile, kidneys and urine of male mice: dynamics after phenylephrine injection. Life Sci. 1990;46:1197–1206. doi: 10.1016/0024-3205(90)90494-c. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Saika M, Koreeda C, Senba E. Water immersion-restraint stress induces expression of immediate-early genes in gastrointestinal tract of rats. Am J Physiol Gastrointest Liver Physiol. 1998;275:G287–G295. doi: 10.1152/ajpgi.1998.275.2.G287. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Umemoto S, Senba E. Immobilization stress induces c-fos and c-jun immediate early genes expression in the heart. Life Sci. 1996;59:339–347. doi: 10.1016/0024-3205(96)00303-7. [DOI] [PubMed] [Google Scholar]
- van Wijk R, Welters M, Souren JE, Ovelgonne H, Wiegant FA. Serum-stimulated cell cycle progression and stress protein synthesis in C3H10T1/2 fibroblasts treated with sodium arsenite. J Cell Physiol. 1993;155:265–272. doi: 10.1002/jcp.1041550207. [DOI] [PubMed] [Google Scholar]
- Vytasek R. A sensitive fluorometric assay for the determination of DNA. Anal Biochem. 1982;120:243–248. doi: 10.1016/0003-2697(82)90342-6. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Bender K, Knebel A, Angel P. The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol Cell Biol. 1997;17:4792–4800. doi: 10.1128/mcb.17.8.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Bradham C, Brenner DA, Czaja MJ. Hydrogen peroxide-induced liver cell necrosis is dependent on AP-1 activation. Am J Physiol. 1997;273:G795–G803. doi: 10.1152/ajpgi.1997.273.4.G795. [DOI] [PubMed] [Google Scholar]
- Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133:2523–2530. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]