Abstract
Toxoplasma gondii Hsp70, T gondii Hsp30/bag1, and surface antigen 1 messenger RNAs were shown to be useful in analyzing stage conversion of T gondii between bradyzoites and tachyzoites. The high-level expression of T gondii Hsp70 was correlated with mortality in interferon-γ knockout mice infected with T gondii. T gondii Hsp70 inhibited the induction of nitric oxide release by peritoneal macrophages of T gondii-infected mice. These findings identify T gondii Hsp70 as a danger signal during lethal, acute T gondii infection.
INTRODUCTION
In intermediate hosts, Toxoplasma gondii exists in 2 forms, the proliferative tachyzoite, responsible for the dissemination of infection during the acute phase of disease, and the bradyzoite, which forms dormant tissue cysts. The unique ability of T gondii bradyzoites and tachyzoites to interconvert is clinically important, because it is considered to be the underlying cause of toxoplasmic inflammation, such as encephalitis, chorioretinitis, and lymphadenitis, in immunocompromised hosts.
Immunity to T gondii is strictly dependent on interferon-γ (IFN-γ) production by CD8+ T lymphocytes and natural killer and CD4+ T cells (Khan et al 1991; Gazzinelli et al 1992; Denkers et al 1993; Hunter et al 1995). It has been shown that IFN-γ seems to be a major mediator of resistance against T gondii infection (Deckert-Schluter et al 1996; Scharton-Kersten et al 1998) and also plays a role in interconversion of T gondii between bradyzoites and tachyzoites (Bohne et al 1993, 1994; Gross et al 1997). Very recently, the gene for Hsp70 of T gondii has been cloned (Weiss et al 1998; Yano et al 1998; Mun et al 1999). In addition, it has been shown that T gondii Hsp70 may regulate the stage conversion of T gondii, although the roles of T gondii Hsp70 are still controversial based on the results of previous studies (Silva et al 1998; Weiss et al 1998). This prompted us to analyze the roles of IFN-γ and T gondii Hsp70 in interconversion of T gondii between bradyzoites and tachyzoites by using IFN-γ knockout (IKO) mice. Mortality of wild type (WT) and IKO mice infected perorally or intraperitoneally with T gondii was analyzed. We used the intraperitoneal infection model in these mice, because it permits collection of a sufficient number of T gondii for determination of their stage and molecular and biological characterization of the stage.
MATERIALS AND METHODS
Mice and parasites
Six- to 8-week-old WT BALB/c (B/c) and WT C57BL/6 (B6) mice were purchased (SLC, Hamamatsu, Japan). Both IKO mice in a B/c background and a B6 background were used at the ages of 6 to 8 weeks. IKO mice were genotyped by polymerase chain reaction (PCR) (Tagawa et al 1997). The avirulent cyst-forming Fukaya strain of T gondii was prepared as described previously (He et al 1997; Kobayashi et al 1999).
Assay for protective immunity
Mice were intraperitoneally or perorally infected with 200 cysts of T gondii to assess the protective effects of IFN-γ. Mice were divided into WT B/c and WT B6 mice, IKO B/c and IKO B6 mice, and IKO B/c and IKO B6 mice treated with 250 units of recombinant murine (rm) IFN-γ. Survival of the animals was then monitored daily and cumulative mortality calculated. Twelve mice were used for each experimental group, and the experiment was repeated at least 2 times.
Assay of T gondii intracellular replication and expression of the messenger RNAs of T gondii Hsp70, T gondii Hsp30/bag1, and surface antigen 1 in vivo
WT B6, IKO B6, and IKO B6 mice treated with rmIFN-γ were intraperitoneally infected with 200 cysts of T gondii. On postinfection days 1 through 12, peritoneal exudate cells (PECs) were harvested by paracentesis and washed with Hank's balanced salt solution. After centrifugation at 1500 × g for 15 minutes, the pellets were used. The number of T gondii was determined by quantitative competitive–polymerase chain reaction (QC-PCR) of the surface antigen 1 (SAG1) gene as previously described (Luo et al 1997; Yamashita et al 1998). In brief, genomic DNA (1 μg) extracted from the brain tissue of each mouse was coamplified with a constant amount of competitor DNA by use of a set of SAG1-specific primers for 36 cycles in a final volume of 50 μL in a TSR-300 thermal sequencer (IWAKI Glass Co Ltd, Chiba, Japan). The amplified products were separated by agarose gel electrophoresis and stained with ethidium bromide. The ratios of the staining intensities of the amplified target and competitor sequences were determined by densitometry (IPLab Gel densitometer, Signal Analytical Corp, Vienna, VA, USA). By comparing the ratio obtained to a standard curve, the T gondii number in the brain was calculated. The expression of T gondii Hsp70, T gondii Hsp30/bag1 (as a marker of bradyzoites) (Bohne et al 1995), and SAG1 (as a marker of tachyzoites) (Burg et al 1988), messenger RNAs (mRNAs) were investigated by reverse transcription–polymerase chain reaction (RT-PCR) (Mun et al 1999). As internal control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used. The expression levels of the mRNAs of T gondii Hsp70, T gondii Hsp30/bag1, and SAG1 were calculated from the densities of their RT-PCR products using the following formula: mRNA expression index = mRNA of T gondii Hsp70, T gondii Hsp30/bag1, or SAG1) / (mRNA of GAPDH) / (T gondii number). Three mice were used for each experimental group, and the experiment was repeated at least 2 times.
Western blot of T gondii Hsp70
The protein expression of T gondii Hsp70 was ascertained by Western blotting as previously described using mouse anti-T gondii Hsp70 monoclonal antibody (mAb) (T gondii NCR A5, IgG1) (Yang et al 1997). In brief, PEC lysates and rT gondii Hsp70 (10 μg in 10 μL) were dissociated by boiling in a sodium dodecyl sulfate sample buffer and run under reducing conditions using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Yano et al 1998; Mun et al 1999). After electrophoresis, separated proteins were electroblotted onto nitrocellulose membrane (NitroBind MSI, Westboro, MA, USA) as previously described. Blots were blocked with 10% milk in Tris-buffer saline (pH 7.6) containing 0.1% Tween 20 (TBST), probed with anti–T gondii Hsp70 mAb in TBST for 1 h, incubated with biotinylated rabbit anti-mouse IgG antibody (Sigma Biosciences, St Louis, MO, USA) at 1:2000 for 1 hour, and incubated with horseradish peroxidase–conjugated streptavidin (Sigma) at 1:2000 for 20 minutes. Protein bands were visualized with an ECL detection system (Amersham, Arlington Height, IL, USA) according to the manufacturer's specifications. Three mice were used for each experimental group, and the experiment was repeated at least 2 times.
Peroral infectivity
It has been reported that cysts or bradyzoites of T gondii have peroral infectivity, whereas tachyzoites do not (Gross et al 1996). The peroral infectivity of T gondii in PECs of the mice was investigated by perorally injecting T gondii into WT B6 mice (indicator mice) and then measuring antibody formation against SAG1 and measuring the number of T gondii in brain tissues of indicator mice. PECs from WT B6, IKO B6, and IKO B6 mice treated intraperitoneally with rmIFN-γ who were intraperitoneally infected with cysts of T gondii were perorally injected into the indicator WT B6 mice. WT, IKO, or IKO mice treated intraperitoneally with rmIFN-γ were intraperitoneally infected with 200 cysts, and PECs were collected by paracentesis and washed with Hank's balanced salt solution from those mice on days, 2, 4, 6, 8, and 10 days after cyst injection. Mice were fed PECs using a 4-cm-long, 22-gauge feeding needle with a bulbous end (Biomedical Needles, Popper and Sons Inc, New York, NY, USA). Survivors were killed 5 weeks later, and a 1:100 dilution of serum from each mouse was tested for antibodies to SAG1 using enzyme-linked immunosorbent assay. The number of T gondii in the brain from each mouse was measured using QC-PCR as reported previously (Luo et al 1997; Yamashita et al 1998; Mun et al 1999). Three mice were used for each experimental group, and the experiment was repeated at least 2 times.
Determination of nitric oxide in vivo
WT B6, IKO B6, and IKO B6 mice treated with rmIFN-γ were intraperitoneally infected with 200 cysts of T gondii plus phosphate-buffered saline or 200 cysts of T gondii plus 100 μg of rT gondii Hsp70. At 0, 3, 6, 12, 24, and 48 hours after infection, the ascites of mice were assayed for nitrite (NO2−) by the Griess reaction (Langermans et al 1992). Three mice were used for each experimental group, and the experiment was repeated at least 2 times.
Determination of nitric oxide in vitro
At 3 hours after infection, PECs from mice intraperitoneally infected with 200 cysts of T gondii were harvested, and purified peritoneal macrophages were obtained by adhering to plastic plates. The resulting peritoneal macrophages population was sorted to a purity of >97%. For further handling, the peritoneal macrophages were cultured at a concentration of 2 × 105 cells per well in RPMI 1640 medium supplemented with 10% fetal calf serum in a 96-well microtiter plate. Both rmIFN-γ and rT gondii Hsp70 were simultaneously added with peritoneal macrophages and were present in culture medium for the entire cultivation time of 12 hours. NO2− was measured 12 hours after treatment. Three mice were used for each experimental group, and the experiment was repeated at least 2 times.
Statistics
The significance of the differences between groups was determined by Student's t-test, except for statistical analysis of the survival experiments, which was performed using the Kaplan-Meier method. P < 0.05 was considered significant.
RESULTS
Influence of IFN-γ defect on survival of mice infected with T gondii
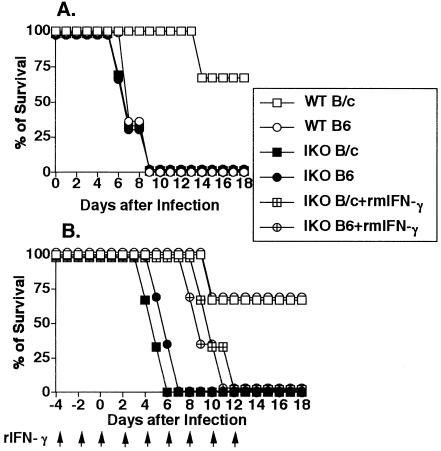
We investigated whether IFN-γ defect could influence survival period in the T gondii–infected mice. Sixty-seven percent of WT B/c mice infected perorally with 200 cysts of T gondii survived more than 18 days, whereas all WT B6 mice succumbed within 9 days after infection. There was a significant difference (P < 0.0001) of susceptibility between WT B/c and WT B6 mice (Fig 1A). Not only IKO B/c but also IKO B6 mice succumbed within 9 to 13 days after peroral infection with 200 cysts (Fig 1A).
Fig 1.
IFN-γ-dependent resistance to lethal T gondii challenge. Mice were perorally (A) or intraperitoneally (B) challenged with 200 cysts of T gondii, and survival was monitored. Twelve mice were used for each experimental group, and the experiment was repeated at least 2 times. Arrows indicate the date of rmIFN-γ treatment of rmIFN-γ-treated IKO mice
Sixty-seven percent of WT B6 mice and resistant WT B/c mice survived more than 18 days when infected intraperitoneally with 200 cysts of T gondii. However, all IKO B/c and IKO B6 mice died 6 to 7 days after the intraperitoneal infection. The treatment of IKO B/c and IKO B6 mice with rmIFN-γ prolonged their survival to 12 and 11 days after infection, respectively. IKO mice treated with rmIFN-γ displayed a modest but nonetheless significant prolongation of survival (P < 0.0001) relative to untreated IKO mice (Fig 1B).
Kinetics of expression of T gondii Hsp70, T gondii Hsp30/bag1, and SAG1 mRNAs and of the number of T gondii in PECs of WT mice
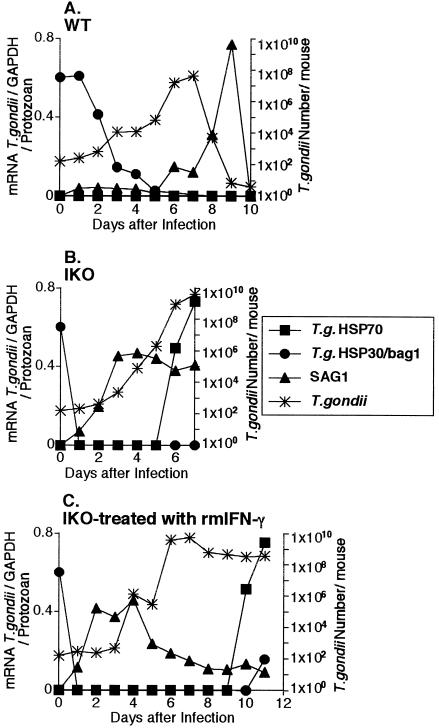
To examine the stage conversion of T gondii in vivo, the kinetics of expression of T gondii Hsp70, T gondii Hsp30/bag1, and SAG1 mRNAs were investigated (Fig 2A). T gondii Hsp70 mRNA transcripts were not detected by RT-PCR in PECs of WT B6 mice even after intraperitoneal infection with 200 cysts of T gondii. On the other hand, the level of T gondii Hsp30/bag1 expression per protozoan continued at a high level until 2 days after infection. The levels of SAG1 expression per protozoan in WT B6 mice increased gradually from 6 to 9 days and disappeared rapidly to background level by 10 days after infection. The disappearance of SAG1 mRNA was correlated with disappearance of T gondii itself from the peritoneal cavity. The number of T gondii in the peritoneal cavity of WT B6 mice reached a peak 7 days after intraperitoneally infection, and thereafter the protozoa decreased and finally disappeared from the peritoneal cavity 10 days after intraperitoneal infection. These results show that bradyzoites in the peritoneal cavity of WT B6 mice quickly began to convert into the tachyzoite stage 2 days after infection.
Fig 2.
Time course of the expression of T gondii Hsp70, T gondii Hsp30/bag1 and SAG1 mRNAs per protozoan and the number of T gondii after infection intraperitoneally with 200 cysts of T gondii. The expression of T gondii Hsp70, T gondii Hsp30/bagl (as a market of bradyzoites) and SAG1 (as a market of tachyzoites) mRNAs per protozoan and the number of T gondii were investigated in PECs of WT (A), IKO (B) and IKO treated with rmIFN-γ (C). The results are expressed as the ratio between the optical density (OD) value of the RT-PCR product of each molecule and the OD value of the RT-PCR product of GAPDH, relative to the number of T gondii estimated by QC-PCR, according to the following formula: {(OD of molecule/OD of GAPDH)/T gondii number × 10 000. There mice were used for each experimental group and the experiment was repeated at least 2 times
Kinetics of expression of T gondii Hsp70, T gondii Hsp30/bag1, and SAG1 mRNAs and of the number of T gondii in PECs in IKO mice
To examine whether IFN-γ defect could influence stage conversion in vivo, the kinetics of expression of T gondii Hsp70, T gondii Hsp30/bag1, and SAG1 mRNAs were investigated by using the T gondii-infected IKO mice (Fig 2B). The levels of T gondii Hsp30/bag1 mRNA expression in peritoneal T gondii of IKO B6 mice were drastically decreased (<0.02 per protozoan) 1 day after infection. Thereafter, T gondii Hsp30/bag1 mRNA transcripts were not detected in PECs of IKO B6 mice. The levels of SAG1 expression per protozoan in IKO B6 mice increased gradually from 1 day after infection until death (7 days after infection). T gondii in PECs of IKO B6 mice infected intraperitoneally expressed T gondii Hsp70 mRNA 2 days before death. These findings about T gondii Hsp70 mRNA expression suggested that T gondii Hsp70 is a danger signal in T gondii–infected mice. The number of T gondii in PECs in IKO B6 mice increased starting 2 days after infection and reached a peak 7 days after infection.
Kinetics of expression of T gondii Hsp70, T gondii Hsp30/bag1, and SAG1 mRNAs and of the number of T gondii in PECs in IKO mice treated with rmIFN-γ
To study the effects of the treatment of IFN-γ in the stage conversion in vivo, the kinetics of expression of T gondii Hsp70, T gondii Hsp30/bag1, and SAG1 mRNAs were investigated by using T gondii-infected IKO mice treated with rmIFN-γ (Fig 2C). Treatment with rmIFN-γ prolonged the survival of IKO mice infected with 200 cysts. T gondii Hsp70 was expressed 2 days before death in PECs in IKO B6 mice treated with rmIFN-γ. The expression of T gondii Hsp30/bag1 mRNA in peritoneal T gondii of IKO mice treated with rmIFN-γ continued until 12 hours and then disappeared until 10 days after infection. The expression of T gondii Hsp30/bag1 mRNA began to increase slightly 11 days after infection in IKO mice treated with rmIFN-γ. Additionally, the level of SAG1 expression per protozoan in IKO B6 mice treated with rmIFN-γ reached a peak at 2 to 4 days and then decreased by 6 days after infection. The levels of SAG1 expression per protozoan in IKO B6 mice treated with rmIFN-γ continued to be low from 6 days until death. These results indicate that IFN-γ down-regulated the expression of the SAG1 gene in IKO mice. The number of T gondii in the peritoneal cavities of IKO B6 mice treated with rmIFN-γ began to increase 2 days after infection and reached a plateau 6 days after infection. Thereafter, the plateau levels of protozoa number were maintained until death (11 days after infection).
Representative RT-PCR data used to assess the mRNA expression of T gondii Hsp70, T gondii Hsp30/bag1, and SAG1 and QC-PCR data used for measuring the number of T gondii in PECs of WT, IKO, and IKO mice treated with rmIFN-γ and infected intraperitoneally with cysts of T gondii are shown in Figure 3A. T gondii Hsp70 protein expression was assessed by Western blotting with T gondii Hsp70–specific mAb (T gondii NCR A5, IgG1) (Fig 3B). The lysates of PECs from 6 days after infection in IKO B/c mice and 7 days after infection in IKO B6 mice were positive, whereas the lysates of PECs of both IKO B/c and IKO B6 mice 1 day after infection were negative.
Fig 3.
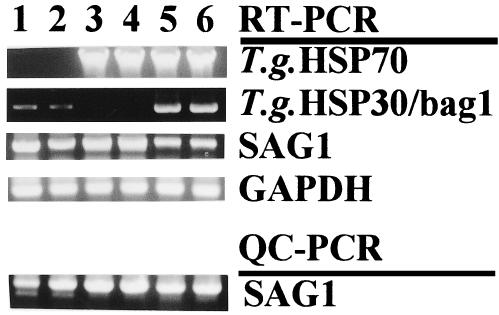
The expression of T gondii Hsp70, T gondii Hsp30/bag1 and SAG1 and the number of T gondii after infection i.p. with 200 cysts of T gondii. (A) Representative data of RT-PCR to assess the expression of T gondii Hsp70, T gondii Hsp30/bag1 and SAG1, and QC-PCR for measuring the number of T gondii in PECs of the mice infected with cysts of T gondii i.p. Lane 1, day 7 of WT B/c; lane 2, day 7 of WT B6; lane 3, day 6 of IKO B/c; lane 4, day 7 of IKO B6; lane 5, day 12 of IKO B/c treated with rmIFN-γ lane 6, day 11 of IKO B6 treated with rmIFN-γ. The reaction product was visualized by electrophoresis using 10 μl of the reaction mixture at 100 V in a
Fig 3.

Continued.1% agarose gel containing ethidium bromide (1 μg/ml). The gels were then examined on a UV light box and photographed. GAPDH was the positive transcription control. (B) Western blotting with T gondii Hsp70-specific mAb (T gondii NCR A5) of lysates of PECs infected with T gondii. Lane 1, day 1 of IKO B/c; lane 2, day 1 of IKO B6; lane 3, day 6 of IKO B/c; lane 4, day 7 of IKO B6; lane 5, rT gondii Hsp70. Molecular weights of natural T gondii Hsp70 and rT gondii Hsp70 were 72 kDa and 74 kDa, respectively. Three mice were used for each experimental group and the experiment was repeated at least 2 times
Peroral infectivity of T gondii from PECs of infected mice
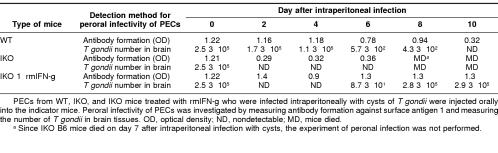
To define the bradyzoite stage of T gondii in PECs, peroral infectivity was examined by measuring antibody formation of the indicator mice perorally infected with PECs. In addition, to assess the number of T gondii in the brain quantitatively, QC-PCR was used (Table 1). We detected peroral infectivity of PECs from WT B6 mice until 8 days after infection, indicating that T gondii remained as bradyzoites in the PECs of these WT mice until 8 days after infection. On the other hand, no peroral infectivity was observed in PECs of IKO B6 mice infected with T gondii from 2 days after infection. These data show that T gondii injected intraperitoneally into IKO mice lost peroral infectivity from 2 days after infection. Peroral infectivity of PECs of IKO B6 mice treated with rmIFN-γ remained 10 days after infection when assayed by antibody formation. However, T gondii in the brain was not detected 2 to 6 days after infection in IKO B6 mice treated with rmIFN-γ. T gondii appeared in the brain starting 6 days after infection in IKO B6 mice treated with rmIFN-γ. In the groups of IKO mice treated with rmIFN-γ, antibody formation was elicited in the mice perorally infected with PECs containing very low numbers of residual live bradyzoites, although such low numbers of residual live bradyzoites in PECs could hardly be detected by QC-PCR. These results indicated that the subinoculation method is useful for detection of low numbers of bradyzoites in large amount of samples, although the method is not appropriate for a quantitative assay of bradyzoites.
Table 1.
Peroral infectivity of peritoneal exudate cells (PECs) from wild type (WT) B6 mice, interferon-{γ} knockout (IKO) B6 mice, and interferon-{γ} knockout B6 mice treated with recombinant murine (rm) interferon-{γ} (IKO + rmIFN-{γ}) who were infected with Toxoplasma gondii
Influence of IFN-γ defect and T gondii Hsp70 on nitric oxide release of peritoneal macrophages of mice infected with T gondii in vivo
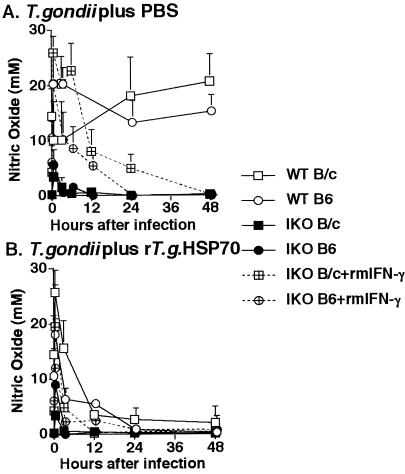
Because our data suggested that expression of T gondii Hsp70 mRNA might be a danger signal (Fig 2), we analyzed the inhibitory effect of T gondii Hsp70 on nitric oxide (NO) release of peritoneal macrophages. Activation of peritoneal macrophages was monitored after intraperitoneal infection with 200 cysts of T gondii by measurement of NO2− in the supernatant of ascites. In WT mice, peritoneal macrophages produced high levels of NO 30 minutes after intraperitoneal infection with T gondii, and the level of NO release was maintained thereafter. NO release by peritoneal macrophages in IKO and IKO mice treated with rmIFN-γ decreased gradually after T gondii infection, although the level of NO release of peritoneal macrophages in IKO mice treated with rmIFN-γ was higher than that in IKO mice (Fig 4A).
Fig 4.
Influence of IFN-γ defect and T gondii Hsp70 on NO release. Age/sex-matched WT B/C, IKO B/C and IKO B/C treated with rmIFN-γ were i.p. infected with 200 cysts of T gondii plus PBS (A) or 200 cysts of T gondii plus 100 μg of rT gondii Hsp70 (B). At 0, 3, 6, 12, 24 and 48 h postinfection, the ascites of the mice was assayed for NO2− by the Griess reaction. There mice were used for each experimental group and the experiment was repeated at least 2 times
The effects of T gondii Hsp70 on NO release of peritoneal macrophages were examined in mice injected intraperitoneally with 100 μg of rT gondii Hsp70. NO release decreased in WT, IKO, and IKO mice treated with rmIFN-γ when rT gondii Hsp70 was intraperitoneally injected (Fig 4B).
Influence of T gondii Hsp70 on NO release of peritoneal macrophages of T gondii–infected mice in vitro
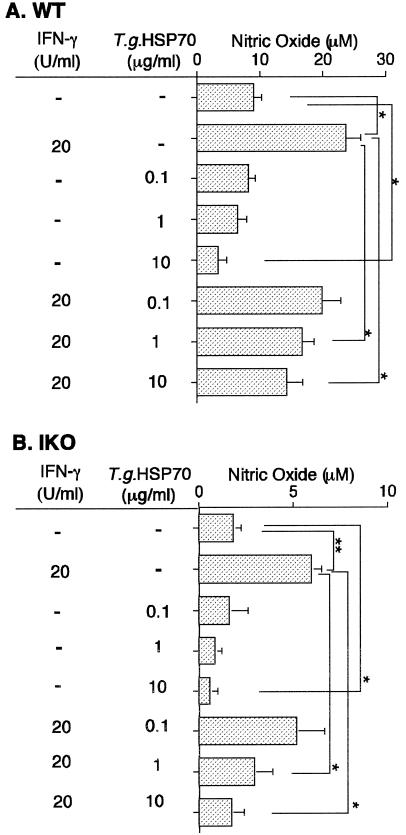
To further determine inhibitory effects of rT gondii Hsp70 on NO release of peritoneal macrophages in vitro, peritoneal macrophages from T gondii-infected mice were treated with rT gondii Hsp70, rmIFN-γ, or rT gondii Hsp70 plus rmIFN-γ, and NO release of peritoneal macrophages was monitored 12 hours after treatment. In both WT (Fig 5A) and IKO (Fig 5B) mice, peritoneal macrophages treated with rmIFN-γ released significant amounts of NO2− after incubation for 12 hours (P < 0.05). In contrast, NO release from peritoneal macrophages decreased by treatment of 10 μg/mL of rT gondii Hsp70. The stimulatory effect of rmIFN-γ was inhibited by the addition of more than 1 μg/mL of rT gondii Hsp70.
Fig 5.
Influence of T gondii HSP70 on NO release. Age/sex-matched WT B6 (A) and IKO B6 (B) were i.p. infected with 200 cysts of T gondii. At 3 h P.I., PECs from mice were harvested and highly purified peritoneal macrophages were obtained by adhering to plastic plates. For further handling, the peritoneal macrophages were cultured at a concentration of 2 × 105 cells per well in a 96-well microtiter plates. Recombinant mIFN-γ and rT gondii HSP70 were added simultaneously with peritoneal macrophages and were present in culture medium for the entire cultivation time of 12 h. NO2− was measured 12 h post treatment by the Griess reaction. The levels of NO release of non-stimulated peritoneal macrophages from non-infected WT and IKO B6 mice were 2.2 and 0.8, respectively. *,P<0.05; **, P <0.005. Three mice were used for each experimental group and the experiment was repeated at least 2 times
DISCUSSION
Hsp70 family members are known to bind peptides, and the binding motifs have been described previously (Fourie et al 1994). They may play a role in antigen processing and presentation (Vanbuskirk et al 1989; DeNagel and Pierce 1992; Manara et al 1993). We demonstrated a potential role of HSC71 in antigen processing and presentation of T gondii–infected melanoma cells to CD4+ CTL (Yang et al 1997).
In this study, we demonstrated that expression of T gondii Hsp70 was correlated with mortality in IKO mice (Figs 2 and 3) and that the T gondii Hsp70 molecule functions as a danger signal during lethal, acute T gondii infection. T gondii Hsp70 is toxic and has antagonistic functions against host protective immunity (Mun et al 1999). After T gondii infection, T gondii Hsp70 was not expressed in WT B6 mice who survived (Fig 2A). However, when WT mice were lethally infected with 1000 cysts of T gondii, T gondii Hsp70 mRNA was expressed in PECs 1 to 2 days before death (data not shown). Silva et al (1998) examined T gondii Hsp70 expression by using Leishmania Hsp70 polyclonal antibody cross-reactive with T gondii Hsp70 and showed high levels of expression of Hsp70 during a short period of conversion from bradyzoites to tachyzoites. Weiss et al (1998) analyzed T gondii Hsp70 expression with an anti-human Hsp70 mAb (C92F3A–5) cross-reactive with T gondii Hsp70 and showed that bradyzoite induction is associated with increased T gondii Hsp70 levels. Because Hsp70 is well known to be one of the most conserved proteins in all organisms and a major immunogen in infections, T gondii Hsp70 induces antibodies cross-reactive with self Hsp70 antibodies (unpublished data, Chen and Yano, in preparation). In the present study, we used T gondii Hsp70–specific mAb (T gondii NCR A5), which is non–cross-reactive with mouse Hsp70 in Western blotting analysis (Fig 3B). We also used T gondii Hsp70–specific primers, which do not cross-amplify mouse Hsp70 in RT-PCR.
One of the roles of T gondii Hsp70 as a danger signal is inhibiting NO synthesis in T gondii-infected mice. In the group of IKO mice treated with rmIFN-γ, T gondii Hsp70 expression was delayed. In addition, IKO mice treated with rmIFN-γ survived longer compared with nontreated IKO mice, and the mice died 1 day after T gondii Hsp70 expression (Fig 2C). We demonstrated that decreased NO release in T gondii-infected IKO mice was correlated with increased T gondii replication and decreased cyst formation. Bohne et al (1994) reported that the inhibitory effect of IFN-γ on replication of T gondii was accompanied by the induction of bradyzoite-specific antigens in IFN-γ–stimulated mouse peritoneal macrophage cell cultures. Gross et al (1997) reported that IFN-γ–mediated NO formation prevents overgrowth of tachyzoites, induces stage differentiation from tachyzoites to bradyzoites, and stabilizes the cyst stage by the inhibition of the mitochondrial respiratory chain in an in vitro system. These findings suggested that T gondii Hsp70 may play a role as a danger signal by inhibiting the induction of NO in T gondii infection. In this study, the effect of IFN-γ on the stage conversion of T gondii inoculated into mice was investigated in the peritoneal cavity. Because there is no evidence of IFN-γ receptor expression in T gondii, IFN-γ down-regulated indirectly the expression of T gondii Hsp70. The fine analysis of IFN-γ–mediated down-regulation of T gondii Hsp70 remains to be answered.
Recently, Chen et al (1999) reported that human Hsp60 served as a danger signal to the innate immune system and chronic TH1-dependent tissue inflammation. They demonstrated that human Hsp60 directly induced nitrite production and cytokine synthesis in macrophages. Further molecular analysis in the differential regulation of nitrite synthesis by human Hsp60 and T gondii Hsp70 needs to be performed.
Genetic control of susceptibility and resistance of mice infected with the avirulent strain Fukaya has been analyzed previously (Luo et al 1997; Mun et al 1999), and WT B6 mice were shown to be susceptible to peroral infection with cysts of the Fukaya strain. However, WT B6 strain was resistant to intraperitoneal infection with a high dose of Fukaya cysts. On the other hand, B/c mice in which the IFN-γ gene had been destroyed lost their resistant against intraperitoneal infection of the Fukaya strain. This result clearly indicates that IFN-γ is an essential component of intraperitoneal immunity to T gondii infection in WT B/c mice. As noted above, WT B6 mice were susceptible to peroral infection with Fukaya cysts, although high levels of IFN-γ production have been shown in WT B6 mice (Luo et al 1996; He et al 1997). Furthermore, injection of a high dose of rmIFN-γ did not provide the protective immunity to WT B6 mice (Luo et al 1996). This suggests that IFN-γ alone is essential but not sufficient for protective immunity against peroral infection of T gondii in WT B6 mice and further that factors other than IFN-γ or T gondii Hsp70 may affect the outcome of the T gondii infection in mice regardless of IFN-γ.
Acknowledgments
This work was supported in part by grant-in-aid 11670239 and 10670225 from the Ministries of Education, Science, and Culture, National Health of Japan, and Japan Science Promotion Society.
REFERENCES
- Bohne W, Gross U, Ferguson DJ, Heesemann J. Cloning and characterization of a bradyzoite-specifically expressed gene (Hsp30/bag1) of Toxoplasma gondii related to genes encoding small heat-shock proteins of plants. Mol Microbiol. 1995;16:1221–1230. doi: 10.1111/j.1365-2958.1995.tb02344.x. [DOI] [PubMed] [Google Scholar]
- Bohne W, Heesemann J, Gross U. Induction of bradyzoite-specific Toxoplasma gondii antigens in gamma interferon-treated mouse macrophages. Infect Immun. 1993;61:1141–1145. doi: 10.1128/iai.61.3.1141-1145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg JL, Perelman D, Kasper LH, Ware PL, Boothroyd JC. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988;141:3584–3591. [PubMed] [Google Scholar]
- Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–3219. [PubMed] [Google Scholar]
- Deckert-Schluter M, Rang A, Weiner D, Huang S, Wiestler OD, Hof H, Schluter D. Interferon-gamma receptor-deficiency renders mice highly susceptible to toxoplasmosis by decreased macrophage activation. Lab Invest. 1996;75:827–841. [PubMed] [Google Scholar]
- DeNagel DC, Pierce SK. A case for chaperones in antigen processing. Immunol Today. 1992;13:86–89. doi: 10.1016/0167-5699(92)90147-Y. [DOI] [PubMed] [Google Scholar]
- Denkers EY, Gazzinelli RT, Martin D, Sher A. Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J Exp Med. 1993;178:1465–1472. doi: 10.1084/jem.178.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie AM, Sambrook JF, Gething MJH. Common and divergent peptide binding specificities of Hsp70 molecular chaperones. J Biol Chem. 1994;269:30470–30478. [PubMed] [Google Scholar]
- Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- Gross U, Bohne W, Soete M, Dubremetz JF. Developmental differentiation between tachyzoites and bradyzoites of Toxoplasma gondii. Parasitol Today. 1996;12:30–33. doi: 10.1016/0169-4758(96)80642-9. [DOI] [PubMed] [Google Scholar]
- Gross U, Kempf MC, Seeber F, Luder CG, Lugert R, Bohne W. Reactivation of chronic toxoplasmosis: is there a link to strain-specific differences in the parasite? Behring Inst Mitt. 1997;99:97–106. [PubMed] [Google Scholar]
- He N, Aosai F, Mun HS, Sekiya S, Yano A. Cytokine production assayed by RT-PCR in pregnant mice infected by Toxoplasma gondii as a model of congenital toxoplasmosis. Jpn J Trop Med Hyg. 1997;25:59–67. [Google Scholar]
- Hunter CA, Candolfi E, Subauste C, Van Cleave V, Remington JS. Studies on the role of interleukin-12 in acute murine toxoplasmosis. Immunology. 1995;84:16–20. [PMC free article] [PubMed] [Google Scholar]
- Khan IA, Ely KH, Kasper LH. A purified parasite antigen (p30) mediates CD8+ T cell immunity against fatal Toxoplasma gondii infection in mice. J Immunol. 1991;147:3501–3506. [PubMed] [Google Scholar]
- Kobayashi M, Aosai F, Hata H, Mun HS, Tagawa Y, Iwakura Y, Yano A. Toxoplasma gondii: difference of invasion into tissue of digestive organs between susceptible and resistant strain and influence of IFN-γ in mice inoculated with the cysts perorally. J Parasitol. 1999;85:973–975. [PubMed] [Google Scholar]
- Langermans JA, Van der Hulst ME, Nibbering PH, Hiemstra PS, Fransen L, Van Furth R. IFN-gamma-induced l-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor alpha. J Immunol. 1992;148:568–574. [PubMed] [Google Scholar]
- Luo W, Aosai F, Ueda M, Yamashita K, Shimizu K, Sekiya S, Yano A. Kinetics in parasite abundance in susceptible and resistant mice infected with an avirulent strain of Toxoplasma gondii by using quantitative competitive PCR. J Parasitol. 1997;83:1070–1074. [PubMed] [Google Scholar]
- Luo W, Yano A. Kinetics of changes in abundance of transcripts encoding cytokines, Fas, and Fas ligand in susceptible and resistant mice infected with Toxoplasma gondii. Trop Med. 1996;38:27–37. [Google Scholar]
- Manara GC, Sansoni P, Badiali-De GL, et al. New insights suggesting a possible role of a heat shock protein 70-kDa family-related protein in antigen processing/presentation phenomenon in humans. Blood. 1993;82:2865–2871. [PubMed] [Google Scholar]
- Mun HS, Aosai F, Yano A. Role of Toxoplasma gondii Hsp70 and Toxoplasma gondii Hsp30/bag1 in antibody formation and prophylactic immunity in mice experimentally infected with Toxoplasma gondii. Microbiol Immunol. 1999;43:471–479. doi: 10.1111/j.1348-0421.1999.tb02430.x. [DOI] [PubMed] [Google Scholar]
- Scharton-Kersten T, Nakajima H, Yap G, Sher A, Leonard WJ. Infection of mice lacking the common cytokine receptor gamma-chain (gamma(c)) reveals an unexpected role for CD4+ T lymphocytes in early IFN-gamma-dependent resistance to Toxoplasma gondii. J Immunol. 1998;160:2565–2569. [PubMed] [Google Scholar]
- Silva NM, Gazzinelli RT, Silva DA, Ferro EA, Kasper LH, Mineo JR. Expression of Toxoplasma gondii-specific heat shock protein 70 during in vivo conversion of bradyzoites to tachyzoites. Infect Immun. 1998;66:3959–3963. doi: 10.1128/iai.66.8.3959-3963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa Y, Sekikawa K, Iwakura Y. Suppression of Concanavalin A-induced 1 hepatitis in IFN-γ−/− mice, but not in TNF-α−/− mice. J Immunol. 1997;159:1418–1428. [PubMed] [Google Scholar]
- Vanbuskirk A, Grump BL, Margoliah E, Pierce S. A peptide binding protein having a role in antigen presentation is a member of the Hsp70 heat shock family. J Exp Med. 1989;170:1799–1809. doi: 10.1084/jem.170.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LM, Ma YF, Takvorian PM, Tanowitz HB, Wittner M. Bradyzoite development in Toxoplasma gondii and the Hsp70 stress response. Infect Immun. 1998;66:3295–3302. doi: 10.1128/iai.66.7.3295-3302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Yui K, Ueda M, Yano A. Cytotoxic T-lymphocyte-mediated lysis of Toxoplasma gondii-infected target cells does not lead to death of intracellular parasites. Infect Immun. 1998;66:4651–4655. doi: 10.1128/iai.66.10.4651-4655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TH, Aosai F, Norose K, Mun HS, Yano A. Heat shock cognate protein 71-associated peptides function as an epitope for Toxoplasma gondii-specific CD4+ CTL. Microbiol Immunol. 1997;41:553–561. doi: 10.1111/j.1348-0421.1997.tb01891.x. [DOI] [PubMed] [Google Scholar]
- Yano A, Mun HS, Yang TH, and et al. 1998 Roles of IFN-γ in effector mechanisms and pathogenicity of Hsps in mice and human infected with Toxoplasma gondii. In: ICOPA IX, ed Tada I, Kojima S, Tsuji M. International Proceedings Division, Chiba, Japan, 457–466. [Google Scholar]