Abstract
Chaperonin CCT containing t-complex polypeptide 1 is a cytosolic molecular chaperone that assists in the folding of actin, tubulin, and other proteins and is a member of the 60-kDa heat shock protein (Hsp60) family. We examined antibody titers against human CCT and other Hsp60 family members in the sera of patients with rheumatic autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematodes, Sjögren syndrome, and mixed connective tissue disease. Autoantibody titers against not only human mitochondrial Hsp60 but also CCT were significantly higher in the sera of patients with rheumatic autoimmune diseases than in healthy control sera. Although immunoglobulin G (IgG) titers against Escherichia coli GroEL were high in all the groups of sera tested, no significant differences in anti-GroEL responses were detected between patients and healthy controls. IgG titers against mycobacterial Hsp65 showed a similar pattern to titers of autoantibodies recognizing GroEL. Immunoabsorption experiments demonstrated that most of the autoantibodies recognizing CCT were cross-reactive with mitochondrial Hsp60, E coli GroEL, and mycobacterial Hsp65. Although most of the anti-Hsp60 IgG recognized CCT, anti-GroEL (or antimycobacterial Hsp65) IgG contained antibodies specific for GroEL (or mycobacterial Hsp65) in addition to antibodies cross-reactive with CCT and Hsp60. Results from immunoblot analyses, together with weak (15% to 20%) amino acid sequence identities between CCT and the other Hsp60 family members, suggested that CCT-reactive autoantibodies recognize conformational epitopes that are conserved among CCT and other Hsp60 family members.
INTRODUCTION
Heat shock proteins (Hsps) play essential roles as molecular chaperones and are conserved across a wide evolutionary range from prokaryotes to eukaryotes. Members of the Hsp60 protein family are made up of subunits that have an approximate molecular mass of 60 kDa and assist in the folding of newly synthesized and denatured proteins (Ellis and van der Vies 1991; Hartl et al 1992). The Hsp60 family (also called the chaperonin family) can be divided into 2 groups (Kubota et al 1995a). Hsp60 of mitochondria, Hsp65 of mycobacteria (the homologue of Escherichia coli is GroEL), and ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) subunit binding protein of plastid fall into group 1, whereas cytosolic chaperonin containing t-complex polypeptide 1 (CCT, also called TRiC or c-cpn) of eukaryotes and chaperonins of archea are classified into group 2. CCT is a hetero-oligomeric molecular chaperone that assists in folding of cytosolic proteins (Kubota et al 1995a; Lewis et al 1996) and is known to facilitate the folding of actin, tubulin, and certain other cytosolic proteins in the presence of adenosine triphosphate (ATP) (Tian et al 1995; Frydman and Hartl 1996; Farr et al 1997). Eight subunit species, α, β, γ, δ, ε, ζ-1 (plus ζ-2 in testis), η, and θ, constitute the mammalian CCT complex and show approximately 30% amino acid sequence identity to each other (Kubota et al 1994, 1995b). These subunits are assembled into a hexadecameric complex (Llorca et al 1999) similar to the GroEL tetradecameric complex.
The relation between mycobacterial Hsp65 and rheumatic diseases has been the subject of much discussion, and the T-cell response to Hsp65 is thought to be involved in the generation of rheumatic diseases (Holoshitz et al 1986; van Eden et al 1998; Zügel and Kaufmann 1999). In terms of B-cell response, patients with rheumatoid arthritis (RA) showed higher levels of immunoglobulin G (IgG) and IgA against Hsp65 than healthy controls in a number of studies (Tsoulfa et al 1989a, 1989b; McLean et al 1990; Winfield and Jarjour 1991a, 1991b). In addition, high antibody titers against E coli GroEL relative to those against mycobacterial Hsp65 have been reported in the sera of patients with RA (Hirata et al 1997) and healthy adults (Handley et al 1996). Autoantibodies against mitochondrial Hsp60 are thought to be raised as a result of molecular mimicry by mycobacterial Hsp65 (or E coli GroEL), because there is a high amino acid sequence identity (approximately 50% to 60%) (Gupta 1990, 1996). Although the T-cell epitopes of Hsp family proteins have been analyzed in detail (van Eden et al 1988; van der Zee et al 1998), the epitopes recognized by antimitochondrial Hsp60 autoantibodies remain obscure.
Herein, we report that serum titers of CCT-reactive antibodies are significantly higher in patients with rheumatic autoimmune diseases than in healthy controls. The anti-CCT autoantibodies cross-reacted with mitochondrial Hsp60, E coli GroEL, and mycobacterial Hsp65 despite weak (15% to 20%) amino acid sequence identity between CCT and these group 1 chaperonins. The antibodies appeared to recognize conformational epitope(s) shared by these antigens. We discuss the characteristics of the anti-CCT autoantibodies and their role in rheumatic autoimmune diseases.
MATERIALS AND METHODS
Sera
Sera were donated from 25 patients with RA (22 women and 3 men; mean ± SD age, 55.6 ± 12.1 years; mean ± SD years affected, 6.7 ± 5.0), 25 patients with systemic lupus erythematodes (SLE; 23 women and 2 men; mean ± SD age, 39.0 ± 12.5 years; mean ± SD years affected, 12.6 ± 7.5), 9 patients with Sjögren syndrome (SS; all women; mean ± SD age, 49.0 ± 14.2 years; mean ± SD years affected, 4.6 ± 6.0), 15 patients with mixed connective tissue disease (MCTD; 12 women and 3 men; mean ± SD age, 44.1 ± 11.9 years; mean ± SD years affected, 9.2 ± 7.4 years), and 25 asymptomatic healthy donors with age and sex comparable with the patient groups. Diagnosis of RA (Arnett et al 1988), SLE (Tan et al 1982), SS (Vitali et al 1993), and MCTD (Kasukawa et al 1987) was based on published criteria.
Antigens
CCT was purified from the human B-cell leukemia cell line BALL-1 (Miyoshi et al 1977) by a combination of methods described previously (Frydman et al 1992; Gao et al 1992; Norcum 1996). BALL-1 cell extract was prepared by freeze thawing, applied to a Q-Sepharose FF (Amersham Pharmacia Biotech, Uppsala, Sweden) column equilibrated in buffer A (0.1 M sodium chloride, 2 mM ethylenediamine-tetraacetic acid, 5 mM 2-mercaptoethanol, 1% glycerol, 50 mM N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid–potassium hydroxide [pH 7.6]), and then eluted with a linear gradient of 0.1 M to 0.5 M sodium chloride. CCT peak fractions were identified by Western blotting (Lewis et al 1992), pooled, applied to a heparin-Sepharose CL-6B (Amersham Pharmacia) column in buffer A, and then eluted with a linear gradient of 0.1 M to 0.5 M sodium chloride. CCT fractions were pooled and applied to a Sephacryl S-400 (Amersham Pharmacia) column in 10 mM Tris-hydrochloride (pH7.6) containing 0.15 M sodium chloride. Approximately 10 mg of CCT was purified from 20 g of total soluble protein in cell lysate.
Purified preparations of human mitochondrial Hsp60, E coli GroEL, Mycobacterium bovis BCG Hsp65, and human Hsp70 were purchased from StressGen (Victoria, British Columbia, Canada).
Enzyme-linked immunosorbent assay
Antigen was diluted in 50 mM sodium carbonate buffer (pH 9.6) to a final concentration of 1 μg/mL and dispensed to 96-well multiplates (50 μL per well). After incubation at 4°C overnight, the wells were blocked with 2% human serum albumin (HSA) at 37°C for 2 hours. Human sera were diluted 1000-fold in phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBST) and 2% HSA, applied to the antigen-coated plate, and then incubated at 37°C for 90 minutes. After washing in PBST, 30 000-fold diluted horseradish peroxidase–conjugated goat anti-human IgG (gamma-specific) antibody (BioSource International, Carmellio, CA, USA) was added, and the plates were incubated at 37°C for 90 minutes. After washing with PBST, specific binding was detected using 3,3′,5,5′-tetramethylbenzidine solution (KPL Laboratories Inc, Gaithersburg, MD, USA) as a substrate. After the reaction was terminated by addition of 1 M phosphoric acid, absorbance at 450 nm was measured and used as antibody titer.
Preabsorption of sera
Solutions of Hsp60 family proteins or HSA (as a control) in PBS (5 μg of protein in 20 μL) were mixed with the same volume of diluted human serum (10% in PBST). After incubation at 37°C for 1 hour, the mixture was diluted 100-fold in PBST containing 2% HSA and analyzed for residual reactivity to specific antigens by enzyme-linked immunosorbent assay (ELISA) as above.
Immunoblot analysis
Purified antigens (0.2 μg per lane) were subjected to native (nondenaturing) polyacrylamide gel electrophoresis (PAGE) (Ornstein 1964) on 3% to 10% linear gradient polyacrylamide gels or to sodium dodecyl sulfate (SDS)–PAGE on 10% to 20% linear gradient polyacrylamide gels. Immunoblotting was carried out as described previously (Kubota et al 1999). Briefly, after electrophoresis, proteins were transferred to polyvinylidene difluoride filters, and the filters were blocked with 5% skim milk in PBS. After incubation of the filter with human sera (diluted 100-fold with PBST containing 5% skim milk), immunoreactive bands were visualized using alkaline phosphatase–labeled goat anti-human IgG (gamma chain–specific) antibody (BioSource International) and tetrazolium 5-bromo-4-chloro-3-indolylphosphate/Nitro Blue tetrazolium solution as second antibody and substrate, respectively.
Statistical analysis
Antibody titers between 2 groups were compared using an unpaired Student's t-test. Correlation of the levels of antibodies against 2 antigens was calculated by Spearman's rank correlation test.
RESULTS
Purification of CCT protein
Human CCT purified from BALL-1 cells migrated as a single band of approximately 900 kDa on native PAGE and as a cluster of several bands of 59 kDa to 65 kDa on SDS-PAGE (Fig 1). This is consistent with the electrophoretic patterns described previously (Frydman et al 1992; Gao et al 1992; Lewis et al 1992; Kubota et al 1994) and reflects the fact that CCT is a hexadecameric complex of 8 subunit species of approximately 60 kDa. No other bands were observed by Coomassie brilliant blue staining, and no contamination with mitochondrial Hsp60 was detected by Western blotting analysis using an Hsp60-specific monoclonal antibody (data not shown). Contamination with Hsp60 was estimated to be less than 0.1% from the detection limit of the experiment.
Fig 1.
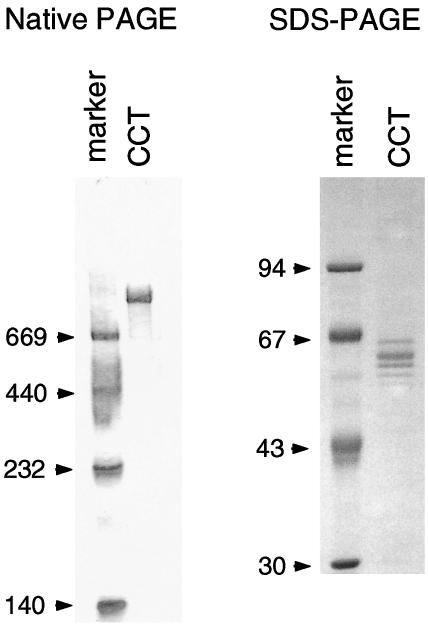
Characterization of purified CCT by native PAGE and SDS-PAGE. Native PAGE and SDS-PAGE were carried out on 3–10% polyacrylamide linear gradient gels and on 8% polyacrylamide gels, respectively. By SDS-PAGE, CCT migrates as several bands of about 60 kDa, representing the 8 different subunits. Molecular weight markers were thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa), and lactate dehydrogenase (140 kDa) for native PAGE and phosphorylase b (94 kDa), albumin (67 kDa), ovalbumin (43 kDa), and carbonic anhydrase b (30 kDa) for SDS-PAGE
IgG titers against CCT and mitochondrial Hsp60 were significantly higher in the sera of patients with autoimmune disease than in those of healthy individuals
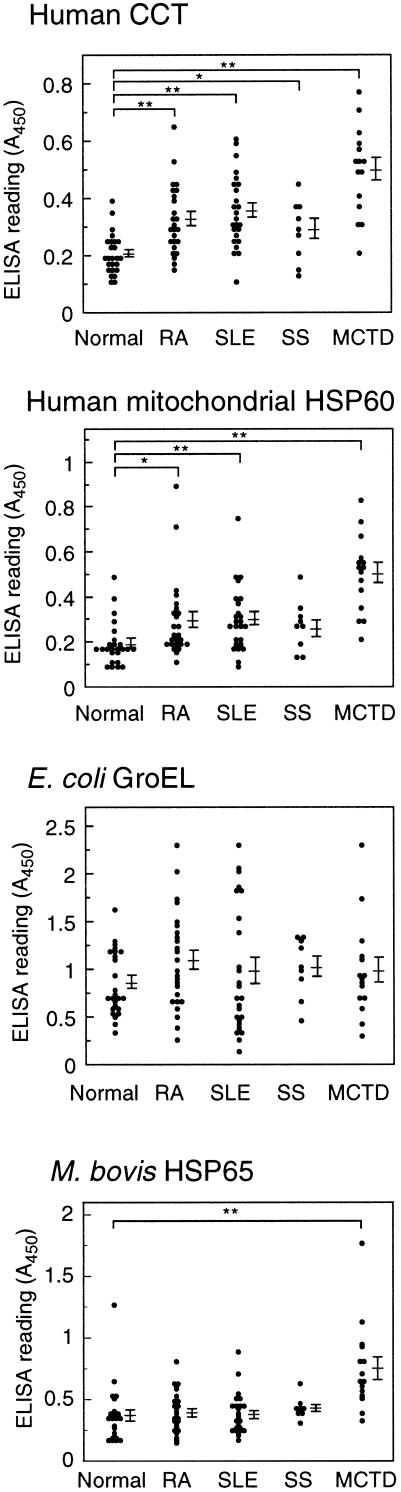
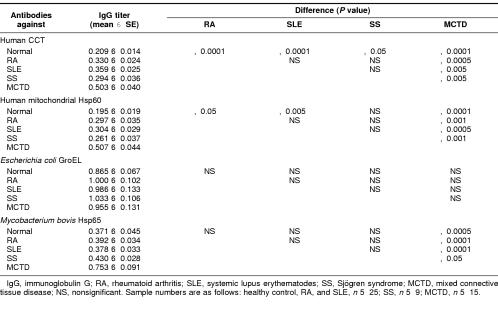
To study the role of antibodies against Hsp60 family proteins in autoimmune diseases, we examined sera of patients with rheumatic disease for antibodies against human cytosolic chaperonin CCT, human mitochondrial Hsp60, E coli GroEL, and mycobacterial Hsp65. Sera from rheumatic patients (RA, SLE, SS, and MCTD) and healthy controls were diluted 1000-fold and analyzed by ELISA. The relation between antibody titer and ELISA reading (A450) was shown to be linear under the condition of these experiments. IgG titers against CCT were significantly higher in the sera of patients with RA, SLE, SS, and MCTD than in sera from healthy controls (Fig 2 and Table 1). Similarly, IgG titers against mitochondrial Hsp60 were significantly higher in the sera of patients with RA, SLE, and MCTD than in healthy control sera. Although IgG titers against GroEL were high in both patient and healthy control sera, no significant differences were observed among the patient groups or between patients and healthy controls. Only in patients with MCTD were IgG titers against M bovis Hsp60 significantly higher than in healthy controls. When sera were used at 250-fold dilution, similar results were obtained, except that absorbance in ELISA for sera with high antibody titer to GroEL were saturated (absorbance values were more than 2.0). These results indicate that the sera of patients with rheumatic disease contain high titers of autoantibodies reactive with CCT and/or mitochondrial Hsp60.
Fig 2.
Comparison of IgG titers against human CCT, human mitochondrial Hsp60, E coli GroEL, and M bovis BCG Hsp65 in patients with RA, SLE, SS, and MCTD and healthy individuals. Each antigen was coated on a microtiter plate and incubated with human sera (diluted 1000-fold). Antibody titers determined by ELISA are expressed as absorbance at 450 nm. Mean values and standard errors are shown at the right. Statistically significant differences between groups is indicated by double (P < 0.01) or single (P < 0.05) asterisks
Table 1.
Comparison of IgG titers against Hsp60 family proteins in sera from patients with autoimmune disease and healthy controls
Antibody titers against CCT and mitochondrial Hsp60 correlated strongly in human sera
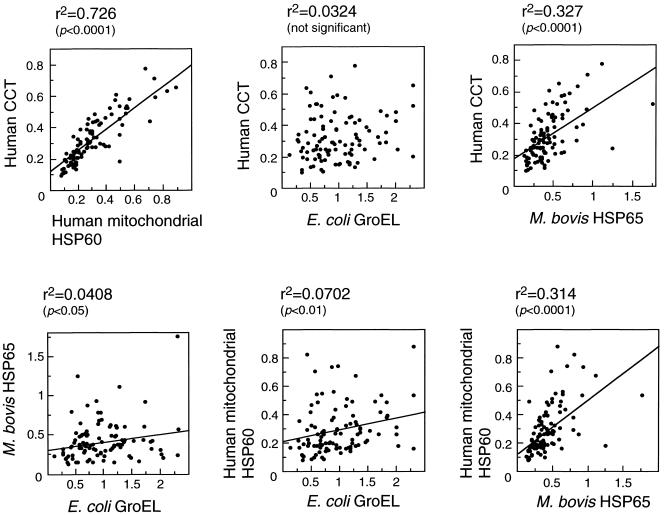
To identify possible correlations among IgG titers against the 4 Hsp60 family antigens, the data shown in Figure 2 were analyzed using scatter diagrams, without distinction between patients and healthy individuals (Fig 3). IgG titers against CCT correlated strongly with titers against mitochondrial Hsp60 (r2 = 0.726). IgG titers against Mycobacterium bovis Hsp65 showed modest correlation with anti-CCT (r2 = 0.33) and antimitochondrial Hsp60 (r2 = 0.31) titers, whereas IgG titers against E coli GroEL showed little or no correlation with titers against the other antigens. These results suggest that the observed antibodies reactive with CCT and mitochondrial Hsp60 may be raised by a similar mechanism. In all groups of sera examined, antibody titers to any antigens showed no significant correlation with period affected by diseases, history of treatment, or age of donors.
Fig 3.
Correlation between antibody titers against human CCT, mitochondrial Hsp60, E coli GroEL, and M bovis Hsp65 in human sera. Each diagram shows all of the sera presented in Figure 2 and Table 1 (without distinction among normal, RA, SLE, SS, and MCTD samples; 99 serum samples in total). The values indicated are the same as in Figure 2
Most of the IgG recognizing Hsp60 but only a portion of the IgG recognizing GroEL (or Hsp65) was cross-reactive with CCT
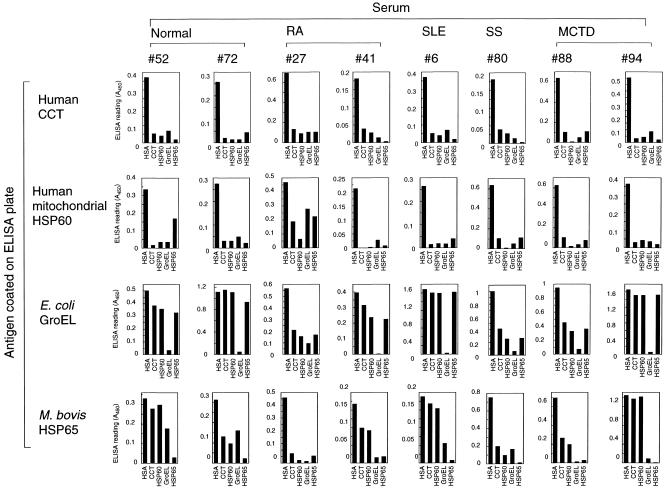
To examine cross-reactivity among antibodies against the Hsp60 family antigens, 22 serum samples with high antibody titers were preabsorbed with each antigen (or HSA as a control) before ELISA analysis. Representative results are shown in Figure 4. Levels of IgG against CCT and mitochondrial Hsp60 were almost completely depleted by each other and were effectively reduced by preabsorption with E coli GroEL or M bovis Hsp65 (except for anti-Hsp60 in RA sample no. 27). In contrast, anti-GroEL antibodies were barely depleted (normal sample no. 72, SLE sample no. 6, and MCTD sample no. 94) or only partially depleted (normal sample no. 52, RA sample no. 27, SS sample no. 80, and MCTD sample no. 88) by preabsorption with the other Hsp60 family proteins, except in RA sample no. 27. Anti-Hsp65 antibodies were almost completely (RA sample no. 27 and SS sample no. 80) or partially (normal sample no. 72, RA sample no. 41, and MCTD sample no. 88) absorbed by CCT or mitochondrial Hsp60. None of the antibodies against Hsp60 family proteins were depleted by preabsorption with the human molecular chaperone Hsp70 (data not shown). These results indicate that the antibodies reacting with CCT and Hsp60 recognize epitope(s) that are common to the 2 antigens and also present in the bacterial proteins GroEL and Hsp65. In contrast, antibodies recognizing GroEL or Hsp65 appear to recognize epitopes specific to GroEL and/or Hsp65 in addition to those shared with CCT and Hsp60.
Fig 4.
Cross-reactivity among antibodies against human CCT, mitochondrial Hsp60, E coli GroEL, and M bovis Hsp65. Diluted human sera were preabsorbed with the indicated antigens before ELISA analysis. The sera used in this assay were those that showed high antibody titers in the analysis shown in Figure 2. HSA was used as a control
Autoantibodies reactive with CCT and Hsp60 recognize epitopes sensitive to SDS treatment
Immunoblot analysis was used to confirm the reactivities of the autoantibodies and to further characterize epitope recognition involved. The antigens were resolved by either native PAGE or SDS-PAGE and blotted onto membranes. Sera of selected patients and healthy individuals, including the sera used in the immunoabsorption experiments shown in Figure 4, were analyzed with these membranes (Fig 5). Immunoblotting after native PAGE showed specific recognition of CCT, mitochondrial Hsp60, and E coli GroEL, with immunoreactive bands at approximately 600 kDa to 900 kDa, except for normal serum samples no. 63 and 67 (which were negative for antibodies to all Hsp60 family antigens by ELISA) and SLE sample no. 22 (CCT only). Although no immunoreactivity with CCT or mitochondrial Hsp60 was detected by immunoblotting after SDS-PAGE using the same sera that were used for native PAGE (data not shown), antibody recognition of E coli GroEL was detected by blotting after SDS-PAGE. These results indicate that some of the epitopes on GroEL are recognized by antibodies in a sequence-specific manner, whereas the epitopes on CCT and mitochondrial Hsp60 that are recognized by the sera of patients with rheumatic disease are sensitive to SDS treatment.
Fig 5.
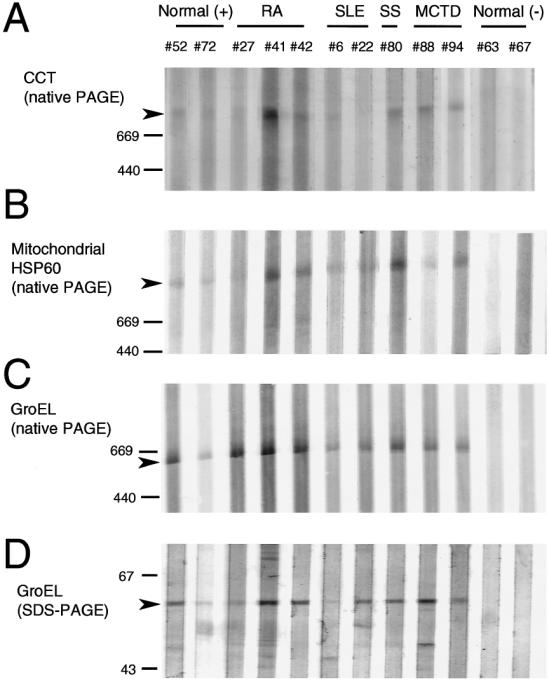
Immunoblot analysis of human CCT, mitochondrial Hsp60, and E coli GroEL using human sera. CCT, Hsp60, or GroEL were resolved on native PAGE or SDS-PAGE gels, blotted onto membranes, and incubated with human sera (diluted 100-fold) followed by alkaline phosphatase–conjugated anti-human IgG. Healthy control serum samples no. 52 and 72 were positive (+) for antibodies against all the Hsp60 family proteins by ELISA, whereas no. 63 and 67 were negative (−). The patients' sera selected for this assay showed high antibody titers against all the antigen tested by ELISA. Arrowheads indicate the position of each antigen. Mobility of molecular weight markers are indicated at the left
DISCUSSION
Our results indicate that IgG autoantibody titers against CCT and mitochondrial Hsp60 are significantly higher in the sera of patients with rheumatic autoimmune disease than in healthy control sera (Fig 2 and Table 1). Most of the autoantibodies against CCT also recognized mitochondrial Hsp60 (Fig 4). Consistent with recent reports (Handley et al 1996; Hirata et al 1997), IgG titers against GroEL were much higher than those against Hsp65 (except for anti-Hsp65 in MCTD sera), Hsp60, or CCT. However, no significant differences in anti-GroEL titers were observed between patients and healthy controls (Fig 2 and Table 1). Anti-Hsp65 antibodies (except in MCTD sera) gave results similar to anti-GroEL antibodies.
The epitopes recognized by these autoantibodies have yet to be determined, although the T-cell epitope of mycobacterial Hsp65 has been well characterized (van Eden et al 1988; van der Zee et al 1998). To our knowledge, there have been no reports of B-cell epitopes on CCT, mitochondrial Hsp60, or E coli GroEL in autoimmune diseases, although B-cell epitopes on mycobacterial Hsp65 (amino acid no. 97–109, 179–187, and 504–512) were defined in case of atherosclerosis (Metzler et al 1997). It has been suggested that B-cell epitopes on mycobacterial Hsp65 may be conformational and composed of discontinuous regions of the molecule (Karopoulos et al 1995). In the present study, serum preabsorption experiments (Fig 4) indicated that a portion of the IgG reactive to E coli GroEL was cross-reactive with CCT and mitochondrial Hsp60, whereas the remainder was specific for GroEL. Similarly, a fraction of the IgG against GroEL cross-reacted with M bovis Hsp65. Most of the GroEL-specific antibodies seemed to recognize sequence-specific epitopes, since they recognized the antigen even after SDS-PAGE (Fig 5). In contrast, the fraction cross-reactive with CCT appeared to recognize SDS-sensitive epitope(s) of chaperonin proteins, because this recognition was observed after native PAGE but not after SDS-PAGE. Since the autoantibodies recognizing CCT were cross-reactive not only with Hsp60 but also with GroEL and Hsp65, the epitope(s) recognized by these antibodies are probably located on conserved amino acid sequences or 3-dimensional structures. The highly conserved sequences among the Hsp60 family proteins are very short and restricted to the adenosine triphosphatase (ATPase) domains (Kubota et al 1995a), which are unlikely to be exposed to the outside of the molecules (Braig et al 1994; Kim et al 1994) and thus may not be accessible to antibodies. It, therefore, appears that the CCT-reactive autoantibodies may recognize conformational epitopes conserved among the chaperonin family proteins.
IgG titers against E coli (gram-negative) GroEL were generally higher than titers against M bovis (gram-positive) Hsp65 in the present study. Infection with bacteria, especially gram-negative bacteria, may cause an immune response to Hsp60 family proteins and result in the production of antibodies against epitopes structurally related to CCT. However, production of autoantibodies cross-reactive with CCT or mitochondrial Hsp60 may be suppressed in healthy individuals by immunological tolerance. In patients with autoimmune diseases, such tolerance might be impaired or lost.
The pathogenic significance of such autoantibodies in the sera of patients with rheumatic disease with connective tissue disorders is often discussed, although the roles of anti-Hsp autoantibodies in rheumatic diseases have yet to be established (Tsoulfa et al 1989a; Zügel and Kaufmann 1999). Among the Hsp60 family proteins, only mitochondrial Hsp60 has been considered as an autoantigen to date (Winfield and Jarjour 1991b; Karopoulos et al 1995). Cell-surface Hsp60 expression has been suggested at the site of a rheumatoid inflammatory condition known as pannus (Boog et al 1992) and on γδ T-cells (Jarjour et al 1990) and heat-stressed endothelial cells (Schett et al 1995). In addition, it was recently reported that human Hsp60 and anti-Hsp60 antibodies are detectable even in sera of healthy individuals (Pockley et al 1999). The present study indicates that significantly higher titers of autoantibodies cross-reactive with both CCT and Hsp60 are present in the sera of patients with rheumatic autoimmune disease than in healthy individuals. We now propose that CCT, which is located mainly in the cytosol (Frydman et al 1992; Lewis et al 1992), is another Hsp60 family protein that acts as a target for such autoantibodies. CCT is one of the essential cytosolic proteins expressed in all cell types and abundantly expressed in proliferating cells (Kubota et al 1999; Yokota et al 1999). However, cell-surface expression of CCT has not been reported. Destruction of joints or other tissues during the progression of rheumatic diseases may cause leakage of cytosolic proteins, including CCT, into the blood stream. Autoantibodies against CCT (and also against Hsp60) could form immune complexes with these antigens and result in abnormal complement activation. Such immune complexes may increase the severity of rheumatic disease states in a manner similar to the possible contribution to lupus nephritis of SLE made by autoantibodies to DNA and nuclear antigens (Reichlin 1995; Stephanou et al 1998). Since there appeared to be generally greater differences between patient and control sera in CCT-reactive antibodies than in anti-Hsp60 antibodies, CCT may prove to be a useful diagnostic antigen with which to detect the autoantibodies prevalent in patients with rheumatic disease.
Acknowledgments
We thank Drs Tsunetaka Ohta, Mitsukiyo Fujii, and Yasunori Okuda for their contributions to the purification of CCT.
REFERENCES
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Boog CJ, de Graeff-Meeder ER, Lucassen MA, van der Zee R, Voorhorst-Ogink MM, van Kooten PJ, Geuze HJ, van Eden W. Two monoclonal antibodies generated against human hsp60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J Exp Med. 1992;175:1805–1810. doi: 10.1084/jem.175.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig K, Otwinowski Z, Hegde R, Boisvert DC, Joachimiak A, Horwich AL, Sigler PB. The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature. 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, vander Vies SM. Molecular chaperones. Ann Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Farr GW, Scharl EC, Schumacher RJ, Sondek S, Horwich AL. Chaperonin-mediated folding in the eukaryotic cytosol proceeds through rounds of release of native and nonnative forms. Cell. 1997;89:927–937. doi: 10.1016/s0092-8674(00)80278-0. [DOI] [PubMed] [Google Scholar]
- Frydman J, Hartl FU. Principles of chaperone-assisted protein folding: difference between in vitro and in vivo mechanisms. Science. 1996;272:1497–1502. doi: 10.1126/science.272.5267.1497. [DOI] [PubMed] [Google Scholar]
- Frydman J, Nimmesgern E, Erdjument-Bromage H, Wall JS, Tempst P, Hartl F-U. Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. EMBO J. 1992;11:4767–4778. doi: 10.1002/j.1460-2075.1992.tb05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Thomas JO, Chow RL, Lee G-H, Cowan NJ. A cytoplasmic chaperonin that catalyze β-actin folding. Cell. 1992;69:1043–1050. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- Gupta RS. Sequence and structural homology between a mouse t-complex protein TCP-1 and the ‘chaperonin’ family of bacterial (GroEL, 60–65 kDa heat shock antigen) and eukaryotic proteins. Biochem Int. 1990;20:833–841. [PubMed] [Google Scholar]
- Gupta RS 1996 Evolutionary relationships of chaperonins. In: The Chaperonins, ed Ellis RJ. Academic Press, San Diego, CA, 27–64. [Google Scholar]
- Handley HH, Yu J, Yu DTY, Singh B, Gupta RS, Vaughan JH. Autoantibodies to human heat shock protein (hsp)60 may be induced by Escherichia coli groEL. Clin Exp Immunol. 1996;103:429–435. doi: 10.1111/j.1365-2249.1996.tb08298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Martin J, Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Ann Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453. [DOI] [PubMed] [Google Scholar]
- Hirata D, Hirai I, Iwamoto M, et al. Preferential binding with Escherichia coli hsp60 of antibodies prevalent in sera from patients with rheumatoid arthritis. Clin Immunol Immunopathol. 1997;82:141–148. doi: 10.1006/clin.1996.4280. [DOI] [PubMed] [Google Scholar]
- Holoshitz J, Klajman A, Drucker I, Lapidot Z, Yaretzky A, Frenkel A, van Eden W, Cohen IR. T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet. 1986;2:305–9. doi: 10.1016/s0140-6736(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Jarjour W, Mizzen LA, Welch WJ, Denning S, Shaw M, Mimura T, Haynes BF, Winfield JB. Constitutive expression of a groEL-related protein on the surface of human gamma/delta cells. J Exp Med. 1990;172:1857–1860. doi: 10.1084/jem.172.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karopoulos C, Rowley MJ, Handley CJ, Strugnell RA. Antibody reactivity to mycobacterial 65 kDa heat shock protein: relevance to autoimmunity. J Autoimmun. 1995;8:235–248. doi: 10.1006/jaut.1995.0018. [DOI] [PubMed] [Google Scholar]
- Kasukawa R, Tojo T, and Miyawaki S 1987 Preliminary diagnostic criteria for classification of mixed connective tissue disease. In: Mixed Connective Tissue Disease and Anti-Nuclear Antibodies, ed Kasukawa R, Sharp GC. Elsevier, Amsterdam, the Netherlands, 41–47. [Google Scholar]
- Kim S, Willison KR, Horwich AL. Cytosolic chaperonin subunits have a conserved ATPase domain but diverged polypeptide-binding domains. Trends Biochem Sci. 1994;19:543–548. doi: 10.1016/0968-0004(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Kubota H, Hynes G, Carne A, Ashworth A, Willison K. Identification of six Tcp-1-related genes encoding divergent subunits of the TCP-1-containing chaperonin. Curr Biol. 1994;4:89–99. doi: 10.1016/s0960-9822(94)00024-2. [DOI] [PubMed] [Google Scholar]
- Kubota H, Hynes G, Willison K. The chaperonin containing t-complex polypeptide 1 (TCP-1): multisubunit machinery assisting in protein folding and assembly in the eukaryotic cytosol. Eur J Biochem. 1995a;230:3–16. doi: 10.1111/j.1432-1033.1995.tb20527.x. [DOI] [PubMed] [Google Scholar]
- Kubota H, Hynes G, Willison K. The eighth Cct gene, Cctq, encoding the theta subunit of the cytosolic chaperonin containing TCP-1. Gene. 1995b;154:231–236. doi: 10.1016/0378-1119(94)00880-2. [DOI] [PubMed] [Google Scholar]
- Kubota H, Yokota S, Yanagi H, Yura T. Structures and coregulated expression of the genes encoding mouse cytosolic chaperonin CCT subunits. Eur J Biochem. 1999;262:492–500. doi: 10.1046/j.1432-1327.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Tian G, Vainberg IE, Cowan NJ. Chaperonin-mediated folding of actin and tubulin. J Cell Biol. 1996;132:1–4. doi: 10.1083/jcb.132.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis VA, Hynes GM, Zheng D, Saibil H, Willison K. T-complex polypeptide-1 is a subunit of a heteromeric particle in the eukaryotic cytosol. Nature. 1992;358:249–252. doi: 10.1038/358249a0. [DOI] [PubMed] [Google Scholar]
- Llorca O, Smyth MG, Carrascosa JL, Willison KR, Radermacher M, Stenbacher S, Valpuesta JM. 3D reconstruction of the ATP-bound form of CCT reveals the asymmetric folding conformation of a type II chaperonin. Nat Struct Biol. 1999;6:639–642. doi: 10.1038/10689. [DOI] [PubMed] [Google Scholar]
- McLean IL, Archer JR, Cawley MID, Pegley FS, Kidd BL, Thompson PW. Specific antibody response to the mycobacterial 65 kDa stress protein in ankylosing spondylitis and rheumatoid arthritis. Br J Rheumatol. 1990;29:426–9. doi: 10.1093/rheumatology/29.6.426. [DOI] [PubMed] [Google Scholar]
- Metzler B, Schett G, Kleindienst R, et al. Epitope specificity of anti-heat shock protein 65/60 serum antibodies in atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:536–541. doi: 10.1161/01.atv.17.3.536. [DOI] [PubMed] [Google Scholar]
- Miyoshi I, Hiraki S, Tsubota T, et al. Human B cell, T cell and null cell leukaemic cell lines derived from acute lymphoblastic leukaemias. Nature. 1977;267:843–844. doi: 10.1038/267843a0. [DOI] [PubMed] [Google Scholar]
- Norcum MT. Novel isolation method and structural stability of a eukaryotic chaperonin: the TCP-1 ring complex from rabbit reticulocytes. Prot Sci. 1996;5:1366–1375. doi: 10.1002/pro.5560050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein L. Disc electrophoresis. Ann N Y Acad Sci. 1964;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4:29–35. doi: 10.1054/csac.1998.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichlin M. Cell injury mediated by autoantibodies to intracellular antigens. Clin Immunol Immunopathol. 1995;76:215–219. doi: 10.1006/clin.1995.1118. [DOI] [PubMed] [Google Scholar]
- Schett G, Xu Q, Amberger A, Van der Zee R, Recheis H, Willeit J, Wick G. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest. 1995;96:2569–2577. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanou A, Latchman DS, Isenberg DA. The regulation of heat shock proteins and their role in systemic lupus erythematosus. Semin Arthritis Rheumatol. 1998;28:155–162. doi: 10.1016/s0049-0172(98)80032-2. [DOI] [PubMed] [Google Scholar]
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tian G, Vainberg IE, Tap WD, Lewis SA, Cowan NJ. Specificity in chaperonin-mediated protein folding. Nature. 1995;375:250–253. doi: 10.1038/375250a0. [DOI] [PubMed] [Google Scholar]
- Tsoulfa G, Rook GA, Van-Embden JD, Young DB, Mehlert A, Isenberg DA, Hay FC, Lydyard PM. Raised serum IgG and IgA antibodies to mycobacterial antigens in rheumatoid arthritis. Ann Rheum Dis. 1989a;48:118–23. doi: 10.1136/ard.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoulfa G, Rook GAW, Bahr GM, et al. Elevated IgG antibody levels to the mycobacterial 65-kDa heat shock protein are characteristic of patients with rheumatoid arthritis. Scand J Immunol. 1989b;30:519–27. doi: 10.1111/j.1365-3083.1989.tb02459.x. [DOI] [PubMed] [Google Scholar]
- van der Zee R, Anderton SM, Prakken ABJ, Paul AGAL, van Eden W. T cell responses to conserved bacterial heat-shock-protein epitopes induce resistance in experimental autoimmunity. Semin Immunol. 1998;10:35–41. doi: 10.1006/smim.1997.0103. [DOI] [PubMed] [Google Scholar]
- van Eden W, Thole JE, van der Zee R, Noordzij A, van Embden JD, Hensen EJ, Cohen IR. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988;331:171–3. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- van Eden W, van der Zee R, Paul AGAL, Prakken BJ, Wendling U, Anderton SM, Wauben MH. Do heat shock proteins control the balance of T-cell regulation in inflammatory diseases? Immunol Today. 1998;19:303–307. doi: 10.1016/s0167-5699(98)01283-3. [DOI] [PubMed] [Google Scholar]
- Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjögren's syndrome: results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993;36:340–347. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- Winfield J, Jarjour W. Do stress proteins play a role in arthritis and autoimmunity? Immunol Rev. 1991a;121:193–220. doi: 10.1111/j.1600-065x.1991.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Winfield JB, Jarjour WN. Stress proteins, autoimmunity, and autoimmune disease. Curr Top Microbiol Immunol. 1991b;167:161–189. doi: 10.1007/978-3-642-75875-1_10. [DOI] [PubMed] [Google Scholar]
- Yokota S, Yanagi H, Yura T, Kubota H. Cytosolic chaperonin is upregulated during cell growth: preferential expression and binding to tubulin at G1/S transition through early S phase. J Biol Chem. 1999;274:37 070–37 078. doi: 10.1074/jbc.274.52.37070. [DOI] [PubMed] [Google Scholar]
- Zügel U, Kaufmann SHE. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]