Abstract
While activation of serum complement mediates antibody-initiated vascular allograft injury, increasing evidence indicates that complement also functions as a modulator of alloreactive T cells. We tested whether blockade of complement activation at the C5 convertase step affects T cell-mediated cardiac allograft rejection in mice. The anti-C5 mAb BB5.1, which prevents the formation of C5a and C5b, synergized with sub-therapeutic doses of CTLA4Ig to significantly prolong the survival of C57BL/6 heart grafts that were transplanted into naive Balb/c recipients. Anti-C5 mAb treatment limited the induction of donor-specific IFNγ-producing T cell alloimmunity without inducing Th2 or Th17 immunity in vivo and inhibited primed T cells from responding to donor antigens in secondary mixed lymphocyte responses. Additional administration of anti-C5 mAb to the donor prior to graft harvest further prolonged graft survival and concomitantly reduced both the in vivo trafficking of primed T cells into the transplanted allograft and decreased expression of T cell chemoattractant chemokines within the graft. Together these results support the novel concept that C5 blockade can inhibit T cell-mediated allograft rejection through multiple mechanisms, and suggest that C5 blockade may constitute a viable strategy to prevent and/or treat T cell-mediated allograft rejection in humans.
Keywords: complement, C5, transplant rejection, T cell, CTLA4Ig
Introduction
Despite recent progress in improving short and long term outcomes following human organ transplantation graft survival remains suboptimal (1), necessitating the development of novel immunosuppression strategies. The recognition that inflammation and innate immunity can injure allografts through both nonspecific mechanisms and the modulation of adaptive T and B alloimmunity (reviewed in 2–5) suggests that approaches targeting elements of the innate immune system could have a beneficial effect on transplant outcomes.
The complement cascade is one component of the innate immune system important for host defense against invading pathogens (6) and has an established role as a pathogenic effector mechanism in transplant rejection. Several lines of evidence support the involvement of alloantibody-initiated serum complement activation in certain forms of vascular allograft injury (7, 8). For example, C6 deficient animals are resistant to antibody-mediated rejection (9), C4d deposition is a marker of human antibody-mediated vascular rejection (7, 10), a blocking anti-mouse C5 mAb prevents heart graft rejection in sensitized mice (11–13), and preliminary reports indicate that an anti-human C5 mAb can limit graft injury in human kidney transplant recipients with donor reactive anti-HLA antibodies (14).
Over the last decade several paradigm-shifting observations have expanded our understanding of the role of complement in transplant injury. As one illustration, C3-deficient mouse kidneys are accepted by wild-type allogeneic hosts with normal serum complement activity (10). This result is in part explained by subsequent work demonstrating that kidney-derived (rather than serum) complement participates in ischemia reperfusion injury (15) and contributes to the development of renal fibrosis (16). Work from our joint group (17–23), among others (24–27), showed that alternative pathway complement components are upregulated and released during cognate interactions between alloreactive T cells and APCs. Local complement activation subsequently leads to the formation of C5a and other split products, which function as key intermediaries driving T cell activation, expansion and differentiation.
This mechanistic linkage of T cell immune responses with locally produced and activated C5a raises the intriguing possibility that targeting the C5 convertase could be used clinically to inhibit alloreactive T cells in vivo. To address this possibility we explored the clinical effects and determined underlying mechanisms of a novel immunosuppression strategy comprised of low dose CTLA4Ig in combination with an anti-C5 mAb, on T cell mediated heart graft rejection in mice. The results reveal that these reagents synergize to positively affect allograft survival and identify multiple mechanisms through which complement inhibition functions to limit T cell mediated graft injury. These preclinical observations support the future testing of analogous immunosuppression approaches in human transplant recipients.
Materials and Methods
Mice
C57BL/6 (Thy1.2,H-2b), C57BL/6 Thy1.1 (H-2b), and BALB/c (H-2d) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). 2C (H-2b) CD8 TCR transgenic mice reactive to Ld were a kind gift of Gregg Hadley, Ohio State University, Columbus OH. 2C mice were crossed with Thy1.1 to produce Thy1.1+ 2C animals. All mice were housed in the Mount Sinai School of Medicine Center for Comparative Medicine and Surgery and all procedures were performed in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International.
Antibodies and reagents
Recombinant anti-C5 mAb BB5.1 (28) and an isotype control mAb were each stably expressed in Chinese hamster ovary (CHO) cells and highly purified over a modified protein A resin (MabSelect Xtra, GE Healthcare, Piscataway, NJ). Final preparations contained ~0.5 EU endotoxin per mg protein and thus were suitable for use in the in vivo studies described herein. CTLA4 Ig was purchased from BioXCell, West Lebanon NH. MAb 1B2 (clonotypic anti-2C) was obtained as a gift from Greg Hadley. Anti-Foxp3 was obtained from eBioscience (San Diego, CA). All other antibodies for flow cytometry and ELISPOT were purchased from BD Pharmingen (San Diego, CA). Carboxy-fluorescein diacetate succinimydyl ester (CFSE) was obtained from Molecular Probes (Invitrogen, Carlsbad, CA).
Surgical procedures
Heterotopic heart transplantation and skin graft placement was performed as described elsewhere (17, 29, 30). We performed pilot studies to optimize CTLA4Ig dosing. In the B6 to Balb/c strain combination a single 1 mg intravenous (i.v.) injection on day 2 posttransplant resulted in >60 d survival while a single 0.2 mg i.v. injection on day 2 post-transplantation prolonged heart graft survival by ~2 weeks. The remaining studies were performed using this 0.2 mg dose. Because the dosage effects may be dependent upon the strain combination and because potency can vary among purchased lots, we suggest performing pilot studies to confirm optimal dosing. Based on published work (13), anti-C5 or isotype control mAb was administered as a single intraperitoneal (ip) dose (800 µg) to the donor mouse one day prior to organ harvest, and/or to recipient mice (800 µg) on d 0, 1, 2 and then twice weekly thereafter. This dose was confirmed to block serum hemolytic complement activity in vivo for up to 72 h (see Figure 1). Heart graft function was monitored daily by palpation and rejection was defined as the day on which a palpable heartbeat was no longer detectable.
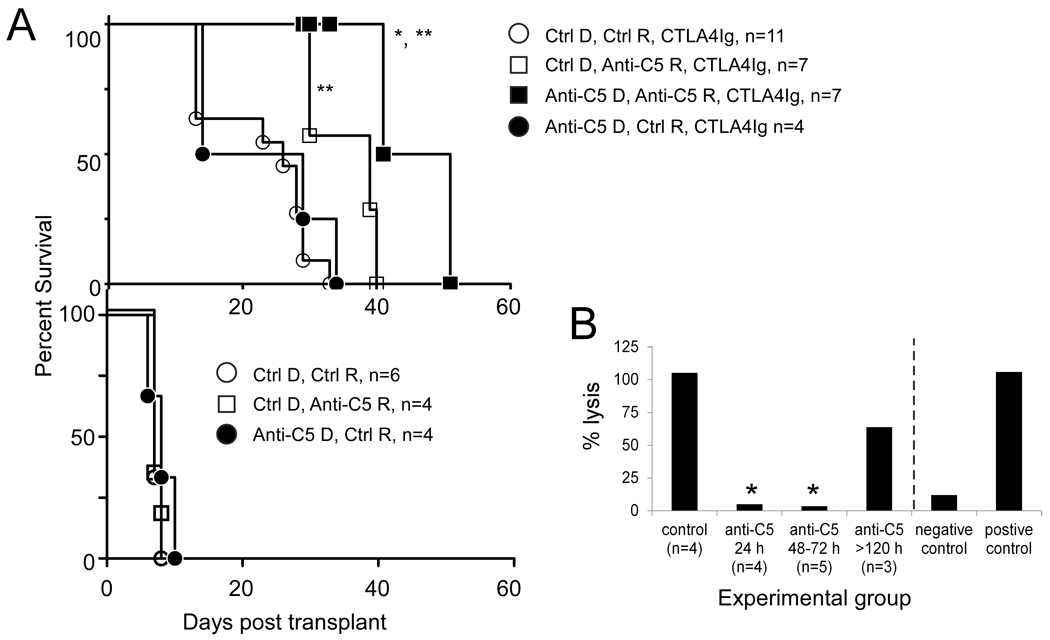
Figure 1.
Anti-C5 mAb synergizes with CTLA4Ig to prolong cardiac allograft survival. A. Graft survival curves for B6 cardiac allografts transplanted into recipient Balb/c mice. Anti-C5, anti-C5 mAb; Ctrl, isotype control mAb, D, donor treated (0.8 mg i.p. 1 day prior to harvest); R, recipient treated (800 µg on day 0, 1, 2 and twice weekly thereafter). * p<0.05 vs. open squares (Ctrl D, Anti-C5 R, CTLA4Ig), ** p<0.05 vs. all other groups in top and bottom panels. B. Anti-C5 mAb treatment inhibits serum hemolytic complement activity. Serum was obtained 2–3 weeks posttransplant in recipients treated with CTLA4Ig alone (control) or in recipients treated with CTLA4Ig plus anti-C5 mAb. The sera were obtained 24h, 48–72h or >120 h after the last administered dose of anti-C5 mAb as indicated. Mean values for hemolytic complement activity are shown for each group. *p<0.05 vs. control group. Standard deviations were <10%.
Isolation of graft- infiltrating lymphocytes
Immediately following euthanasia, the vasculature of mice was perfused with ~10mL sterile PBS+10mM EDTA (PBS/EDTA) by intracardiac injection until the kidney and liver blanched. The heart tissue was harvested, minced and washed 3 times with PBS/EDTA, followed by collagenase digestion (0.2 mg/ml collagenase type IV (Clostridium histolyticum, Sigma, St. Louis, MO) diluted in RPMI supplemented with 10% fetal bovine serum (FBS) for 2.5 h at 37° C. The digested tissue was then dissociated through a 19g needle and filtered through a 70 micron nylon mesh. Following 3 washes in RPMI/10% FBS the isolated single cell suspension enriched for tissue-derived leukocytes was processed for flow cytometric analysis.
Flow Cytometry
Cells were evaluated for surface antigen expression following incubation with fluorescently conjugated antibodies in PBS or a buffer consisting of 2% rat serum 2 mM EDTA according to standard procedures (17, 18, 29). Samples were collected using a FACS Canto II flow cytometer (BD Biosciences) and analyzed using FlowJo software (Ashland OR).
ELISPOT
ELISPOT assays were performed using single cell suspensions of spleen cells as previously described (18, 23, 31). Briefly, MultiScreen ELISPOT plates (Millipore, Bedford, MA) were coated overnight with capture antibodies specific for various cytokines including rat anti-mouse IFN-γ (clone R4-6A2), rat anti-mouse IL-4 (clone BVD4-1D11), rat anti-mouse IL-17A (clone TC11-18H10). Recipient spleen cells or magnetic-bead enriched (Miltenyi) splenic T cells (0.2 to 1 × 106 per well) were plated and incubated with donor splenocyte stimulator cells (400,000 per well) at 37°C, 5% CO2 for 24 hours. Donor splenocytes were previously treated with mitomycin C to limit proliferation and cytokine secretion (32). In some experiments, CTLA4Ig (200 µg/ml) and/or either anti-C5 mAb (100 µg/ml) or an isotype control (100 µg/ml) was included during stimulation. After a 24 h incubation, the plates were washed and incubated overnight with the appropriate biotinylated detection antibodies: rat anti-mouse IFNγ (clone XMG1.2), rat anti-mouse IL-4 (clone BVD6-24G2), rat anti-mouse IL-17A (clone TC11-8H4). After washing, an alkaline phosphatase-conjugated anti-biotin antibody (Vector Laboratories, Burlingame, CA) diluted 1:2000 in PBS supplemented with 0.1%Tween and 1% bovine serum albumin (BSA) was added for 90 min, the plates were developed by addition of 1-Step NBT/BCIP substrate (Thermo Scientific, Rockland, IL), and the resulting spots were counted on an ImmunoSpot Series 3 Analyzer (Cellular Technology Ltd., Shaker Heights, OH).
Alloantibody detection
Serum samples from recipient mice were diluted in PBS as indicated and incubated for 60 minutes at 4° C with syngeneic, donor or third-party thymocytes as target cells (33, 34). Following a wash step with PBS 1% albumin, the bound antibody was detected by incubation with fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse IgG (eBioscience, San Diego CA) and the % positive cells quantified by flow cytometry using FlowJo software.
Histological evaluation
Formalin-fixed paraffin sections of graft tissues were stained with hematoxylin and eosin (H&E) as previously described (33, 34) and evaluated by a blinded investigator.
Real time polymerase chain reaction (RT-PCR)
RNA isolation was performed using a Qiagen RNeasy Mini Kit (Qiagen,Inc., Valencia, CA) and cDNA was reverse-transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) as per the manufacturer’s instructions. The PCR Primers for CXCL1, CXCL9, and CXCL10, together with the primers for control gene MRP132 were purchased from TaqMan Gene Expression Assays (Applied Biosystems). RT- PCR was performed using the Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA).
Serum hemolytic activity assay
Terminal complement activity in recipient mouse sera was determined by standard methods to assess its ability to lyse chicken erythrocytes, presensitized with erythrocyte-specific Abs as previously described (12).
Statistical analysis
Graft survival was compared using Log Rank Survival statistics. Immunology assay results were compared using the Student’s t test wherein p <0.05 was considered significant.
Results
Anti-C5 synergizes with CTLA4Ig to prolong allograft survival
The effect of anti-C5 mAb, alone or in combination with a subtherapeutic dose of CTLA4Ig, on the survival of B6 hearts transplanted into fully allogeneic Balb/c recipients was explored (Fig 1A). While untreated mice or mice given an isotype control mAb rejected the allografts with a median survival time (MST) of 7 d, a single 200 µg dose of CTLA4Ig significantly prolonged the MST to 26 d (p<0.05). Graft survival in recipient mice treated with anti-C5 mAb alone on days 0, 1, 2, and then twice weekly thereafter was not different from the isotype controls (MST 8 d). Combination therapy with 200 µg CTLA4Ig plus anti-C5 mAb, according to the above schedule, significantly prolonged graft survival compared with CTLA4Ig plus an isotype control (MST 39 d, p<0.05). Serum hemolytic activity assays confirmed an absence of antibody-initiated, complement dependent RBC destruction in the sera obtained from animals within 72 h of anti-C5 mAb administration (Fig 1B).
In light of published work indicating that complement activation within donor organs contributes to post-transplant ischemic injury and graft failure (35–37), the effect of additional anti-C5 mAb administration (0.8 mg) to the donor 1 day prior to transplantation on graft survival was explored. As shown in Fig 1A, treatment of both the donor and recipient with anti-C5 mAb together with a single dose of CTLA4Ig significantly (p<0.05) extended graft survival to a MST of 46 d (p<0.05 vs. CTLA4Ig plus anti-C5 mAb administered to recipient alone). In control experiments, anti-C5 mAb-treated donor allografts were rejected with a MST of 8 d in untreated recipients.
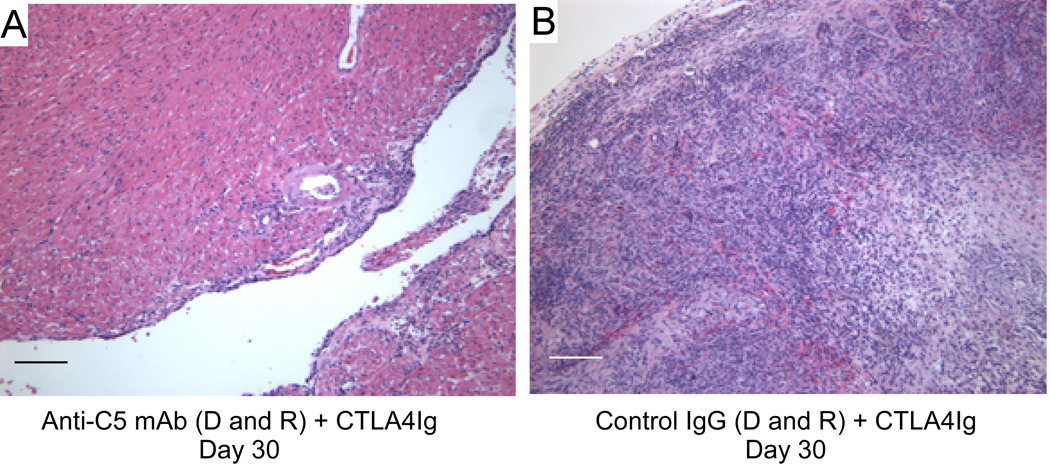
Histological examination of all grafts that ceased beating showed diffuse mononuclear cell infiltration and intra-parenchymal hemorrhage consistent with rejection (Fig 2A). In contrast, beating heart grafts obtained on day 30 from mice treated with both CTLA4Ig and anti-C5 and which received grafts that were also pre-treated with anti-C5 (Fig 2B) exhibited patchy mononuclear cell infiltrates (International Society of Heart and Lung Transplantation grade 1R) and were consistently devoid of arterial vasculitis, suggesting ongoing cellular but not antibody-mediated allograft injury. We did not observe cardiac allograft vasculopathy in any of the heart grafts examined.
Figure 2.
Anti-C5 mAb plus CTLA4Ig limits T cell mediated allograft rejection. Representative H&E stained paraffin embedded sections of heart allografts obtained on day 30 posttransplant from mice treated with CTLA4Ig plus Anti-C5 mAb (A) or CTLA4Ig alone (B). scale bar-100 microns.
Anti-C5 limits alloreactive T cell immunity in vivo
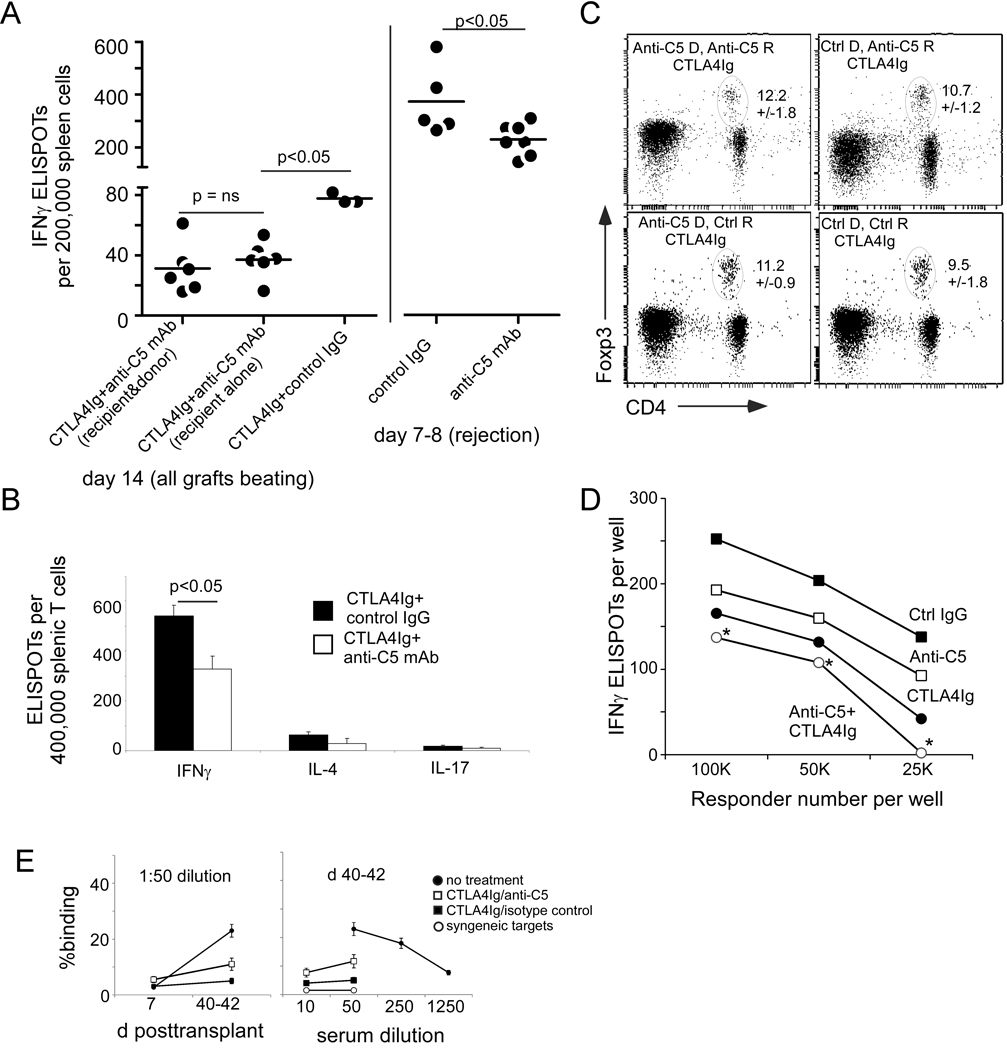
Because our previous work showed that complement activation is a key regulator of T cell immunity (17–23), we tested whether anti-C5 mAb treatment affects the strength and/or cytokine profile of alloreactive T cells following transplantation (Fig 3). Spleen cells were harvested from groups of recipient mice with beating allografted hearts 2 weeks posttransplant in the treated groups, or at rejection (on day 7–8) in the untreated groups, and assayed for reactivity to donor antigen by cytokine ELISPOT (Fig 3A). These assays revealed that treatment with CTLA4Ig alone reduced the frequency of donor-reactive IFNγ-producing cells ~5-fold compared to recipients of isotype control mAb or anti-C5 mAb alone (mean of 350 vs. 75 IFNγ producers per 200,000 spleen cells, p<0.05). The combination of CTLA4Ig plus anti-C5 mAb further reduced donor-reactive T cell immunity another 2-fold below that observed in recipients treated with CTLA4Ig alone (mean of ~40 per 200,000, p<0.05 vs. CTLA4 Ig + isotype control mAb). Administration of anti-C5 mAb to the donor prior to transplant had no additional effect (Fig 3A), despite the observed survival benefit following administration of anti-C5 to the donor (Fig 1). Monotherapy with anti-C5 mAb decreased the mean frequency of donor-reactive IFNγ-producing splenocytes at rejection by 40% on day 8 compared to the isotype control (Fig 3A, right), but this decrease was not associated with a prolongation of graft survival (Fig 1).
Figure 3.
Anti-C5 mAb synergizes with CTLA4Ig to inhibit alloreactive T cell immunity. A. Frequencies of donor-reactive IFNγ producing spleen cells from groups of allograft recipients as indicated. Each dot represents an individual animal. Bars are placed at the mean value for each group. B. Pooled splenic T cells were isolated from 3 animals per group and tested in IL-4 and IL-17 ELISPOT assays. C. Representative CD4+Foxp3+ flow cytometry plots performed on peripheral blood obtained 13–15 days posttransplant from 4 animals per group as indicated. Ovals encompass the CD4/Foxp3 double positive subsets. Numbers in the upper right of each plot are the percentage of Foxp3+ cells within the CD4 gate. Means and sd for n=4 per group are shown. There was no statistical difference among the groups. D. Serial dilutions of spleen cells obtained at the time of rejection from untreated Balb/c recipients of B6 hearts were tested in donor-reactive IFNγ ELISPOT assays in the presence or absence of Anti-C5 mAb, isotype control (100 µg/ml), and or CTLA4Ig (200 µg/ml). Each point represents the mean of triplicate wells. * p<0.05 vs. each of the other groups. The experiment was repeated with similar results. E. Serum anti-donor alloantibody. Serum samples were obtained at d 7 or 40–42 posttransplant as noted, diluted 1:10 – 1:1250 and tested against donor or syngeneic thymocytes. Note that the reactivity in control animals that rejected allografts (closed circles) is significantly greater than all other groups and titrates down with serial dilution. Reactivity in CTLA4-Ig treated animals with or without anti-C5 is not significantly different and does not titrate.
Treatment of recipients with CTLA4Ig plus anti-C5 mAb did not result in a shift in cytokine profiles toward Th2 or Th17 immunity (Fig 3B). Frequencies of donor-reactive IL-4 and IL-17 producers were low in all animals and did not increase in mice treated with anti-C5 mAb. To test whether the anti-C5 mAb treatment altered the frequency of regulatory T cells (Treg) in CTLA4Ig treated recipients we performed a separate set of transplants, isolated PBMC on d14 in all animals and quantified CD4+Foxp3+ expressing T cells by flow cytometry (Fig 3C). This analysis revealed no significant differences in peripheral blood Treg among the groups.
We, with collaborators, previously showed that in addition to regulating primary T cell activation and differentiation, immune cell-derived complement products modulate the reactivation of primed alloreactive T cells following a second encounter with donor antigen in the graft (17, 18). We therefore explored the potential for anti-C5 mAb to inhibit the reactivation of primed alloreactive T cells in this model. We isolated spleen cells from untreated recipient mice at the point of allogeneic heart graft rejection and stimulated them with donor splenocytes in serum-free culture medium containing anti-C5 mAb (or an isotype control) in the absence or presence of CTLA4Ig (Fig 3D). IFNγ ELISPOT assays revealed that CTLA4Ig and anti-C5 mAb each modestly but significantly inhibited T cell IFNγ production by primed alloreactive T cells, and that the two agents administered together exhibited additive inhibitory effects.
We tested the sera obtained from the heart transplant recipients for IgG alloantibodies reactive to donor MHC (Fig 3E). Weak anti-donor alloantibodies were detected in sera obtained at rejection in recipients treated with CTLA4Ig +/− anti-C5, and there was no difference among the groups. Control sera from mice that rejected their grafts by day 8 reacted strongly to donor antigens.
Anti-C5 limits early T cell trafficking into the allograft
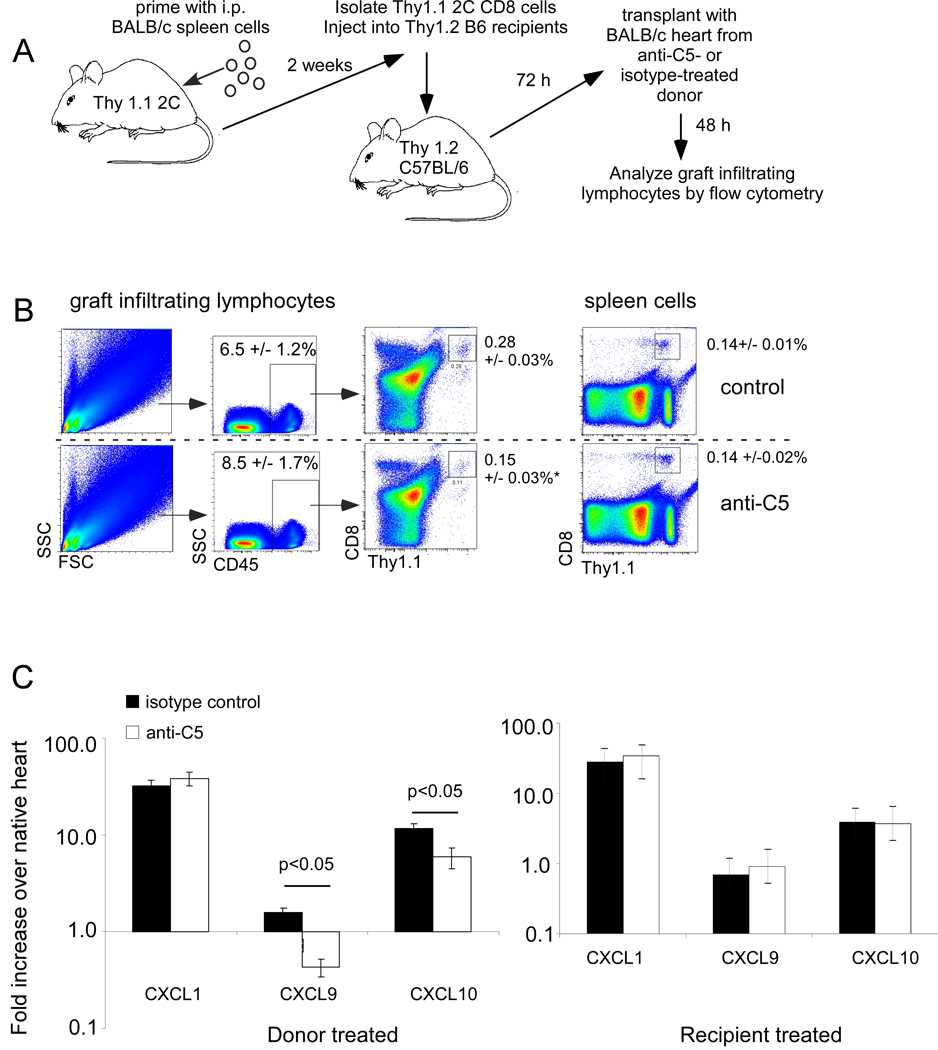
The beneficial effects on graft survival of anti-C5 mAb administration to the donor mouse (Fig 1) prompted a hypothesis in which graft-derived complement activation induced by ischemia reperfusion injury facilitates T cell entry into the graft. To test this hypothesis, Thy1.1+ 2C (CD8 TCR transgenic Ld-reactive) mice were primed with an intraperitoneal injection of BALB/c spleen cells. Two weeks later, splenic Thy1.1+ 2C T cells were harvested from the primed mice and adoptively transferred (4 × 106 cells/recipient) into congenic Thy1.2 recipients (Fig 4A). These adoptive hosts then received Balb/c heart grafts obtained from donors that had been pretreated with anti-C5 or an isotype control. Forty-eight hours after grafting, graft-infiltrating lymphocytes were isolated from the transplanted hearts and the number of Thy1.1+ 2C CD8 T cells within each graft quantified by flow cytometry (Fig 4B).
Figure 4.
Donor treatment with anti-C5 mAb limits trafficking of primed T cells into the allograft. A. Schematic representation of experimental design (see text for details). B. Representative flow cytometry plots for graft infiltrating lymphocytes (left) or spleen cells (right) identifying adoptively transferred Thy1.1+ CD8+ 2C cells 48 h after transplantation. Percentages shown are the means and standard deviations of 5 animals per group studied in 2 separate experiments. Anti-C5 mAb significantly inhibited graft infiltration of 2C cells, *p<0.01. C. Anti-C5 mAb treatment of donor but not recipient inhibits transplant-induced upregulation of T cell chemokine mRNA. Left. Balb/c donor mice were treated with Anti-C5 mAb or isotype control 24 h prior to transplantation. Right, recipient mice were treated with anti-C5 mAb or isotype control on the day of transplantation but donor mice were not treated. Total RNA was produced from B6 hearts 24 h after transplantation into Balb/c recipients, cDNA was prepared and tested by real time PCR for expression of CXCL1, CXCL9 and CXCL10 as noted. Each bar represents the mean expression normalized to MRP132 of triplicate assays. The experiments were repeated with similar results. *p<0.05.
Strikingly, there were ~2 -fold fewer Thy1.1+ 2C CD8 T cells within the graft-infiltrating lymphocyte population obtained from mice transplanted with hearts from anti-C5 treated donors (0.15 ±0.03%) compared with those from animals transplanted with hearts from control mAb-treated donors (0.28 ±0.03% p<0.01). Similar numbers of T cells were detected in the spleens of animals in each group (Fig 4B). Together the data support the conclusion that anti-C5 mAb inhibits the ability of primed T cells to enter the allograft.
To further assess the potential underlying mechanism of these effects, hearts from Balb/c mice treated either with anti-C5 or isotype control mAb were transplanted into B6 recipients. After 24 h, RNA was isolated from each graft and assayed for the expression of the transcripts for T cell chemoattractant chemokines CXCL9 (MIG) and CXCL10 (IP-10), and, as a control, for the neutrophil chemoattractant chemokine CXCL1 (GROα, KC) using real-time PCR (Fig 4C). Compared with the isotype control, anti-C5 mAb treatment of the donors alone not only blocked the transplantation-induced increase in CXCL9 expression, but lowered it below the baseline observed in the native heart. Treatment of donors alone with anti-C5 partially blocked the transplant induced upregulation of CXCL10 but had no effect on expression of the neutrophil chemoattractant CXCL1. In contrast, treatment of the recipient alone with anti-C5 antibody had no effect on the transplant-induced levels of expression of CXCL9, CXCL10 or CXCL1 relative to that observed among recipient mice treated with the isotype control.
Discussion
Inhibition of serum C5 activation through antibody blockade inhibits membrane attack complex formation thereby preventing red blood cell lysis in patients with paroxysmal nocturnal hemoglobinuria. Through a similar mechanism, anti-C5 mAb prevents antibody-mediated cardiac and renal allograft injury in sensitized mice (11–13), and preliminary clinical data suggests that anti-C5 blockade with the humanized antibody eculizumab limits vascular injury and prevents antibody-mediated rejection in presensitized human kidney transplant recipients demonstrating high levels of donor-specific alloantibodies (38, 39). In the current set of studies we show that anti-C5 mAb functions through additional mechanisms when explored in the appropriate experimental paradigm. When co-administered with CTLA4Ig, anti-C5 mAb 1) prolonged murine cardiac allograft survival in unsensitized recipients (Fig 1–2), 2) diminished the frequency of donor-reactive, IFNγ-producing effector T cells induced by the transplant in the absence of evidence of allograft tolerance, 3) did not alter T cell cytokine profiles as significant changes in IL-4 or IL-17 producing cells were not observed in the treated mice, and 4) did not induce significant alterations in the frequency of peripheral Tregs (Fig 3). Our findings additionally indicate that anti-C5 mAb can inhibit the reactivation of primed alloreactive T cells following secondary encounter with antigen (Fig 3), expanding upon our previous finding that endothelial cell-derived C5a regulates reactivation of primed alloreactive CD8 T cells in vitro and within allografts (17).
The molecular mechanisms underlying the anti-C5 effect can be partially inferred from our previous work (17–23). Using in vivo transplant models supported by in vitro analyses, we previously showed that immune cell-produced C5a promotes T cell proliferation, limits T cell apoptosis and drives T cell differentiation toward IFNγ-producing type 1 immunity. Mechanistic studies revealed that immune cells 1) upregulate complement component production, release and activation following cognate T cell/APC interactions, 2) that this upregulation is dependent upon CD28/CD80 and CD40/CD154 interactions, and 3) that the locally produced C5a binds to its receptors expressed on the T cell and the APC, activating both partners via an AKT-dependent signaling cascade (17–23). These findings, coupled with the previous reports that anti-C5 mAb blocks the proteolytic activation of C5 (28), strongly suggest that at least one mechanism by which anti-C5 mAb inhibits T cells is through blocking C5a formation and its downstream effects on T cells and APCs. Consistent with a preeminent role for C5a as a modulator of alloreactive T cells are the findings by others that C5aR deficiency or blockade prolongs murine kidney allograft survival and inhibits T cell cytokine production (40)
An additional new finding from the current work is the direct documentation that anti-C5 mAb administered to the donor prior to graft harvest has clinically important effects on subsequent graft survival (Fig 1). This result is likely in part related to the observed decrease in the trafficking of primed T cells into the allograft, accompanied by an abrogation of the transplant-induced upregulation of T cell chemoattractant chemokines CXCL9 in the allograft (Fig 4). Work by others indicates that these chemokines are indeed upregulated through a complement-dependent mechanism following ischemia and reperfusion (41–45). Notably, the anti-C5 mAb did not block upregulation of CXCL1, a neutrophil chemoattractant, by the graft, suggesting that complement activation following IR injury has a relative specificity for mononuclear cell chemoattractants.
Together, the mechanisms uncovered in these latter experiments are consistent with the concept that blockade of C5 activity in the donor organ limits IR injury, prevents complement induced upregulation of T cell chemokines and as a result, inhibits early T cell trafficking into the graft. These results are clinically relevant as the occurrence of inflammatory events early post-transplant can have a profound effect on late graft function, both in mouse models and in humans.
The T cell inhibitory effect of anti-C5 mAb as a single agent was limited despite its capacity for potent inhibition of serum C5 cleavage in vivo (Fig 1) and its negative effect on donor CXCL9 expression (Fig 4). While we are unable to ascertain whether the anti-C5 mAb fully inhibits local cleavage of immune cell derived C5, our results are consistent with the interpretation that T cell immunity is modulated by, but not strictly dependent upon, C5 cleavage.
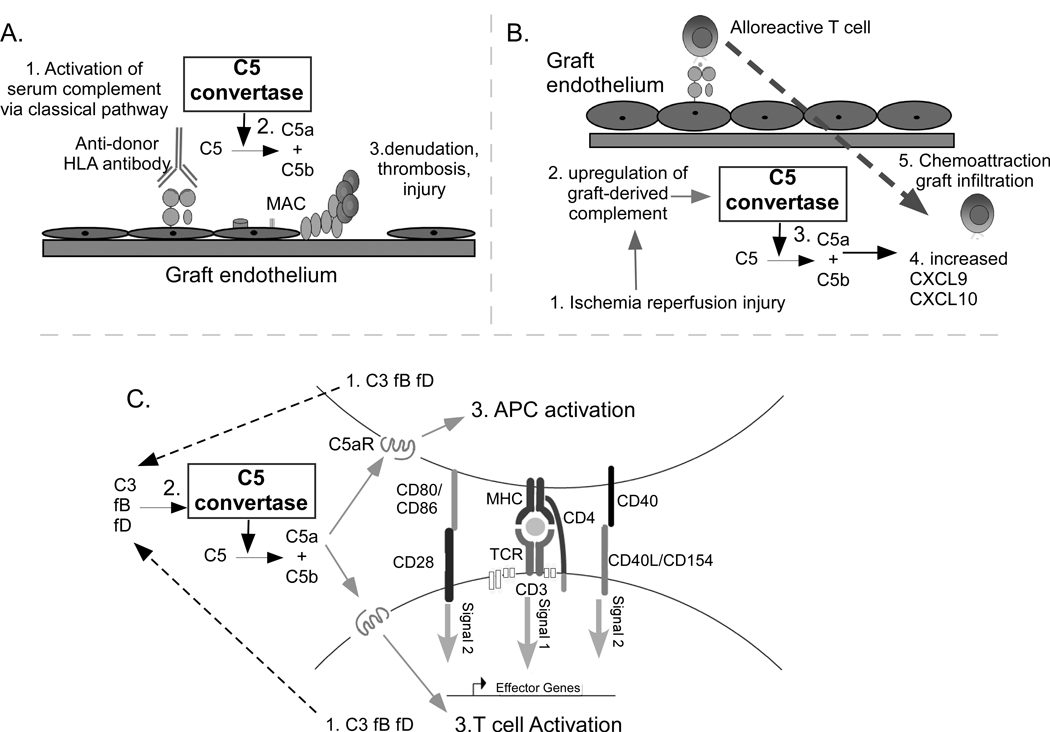
Because donor-reactive antibodies were virtually absent from the sera of animals treated with CTLA4Ig with or without anti-C5 mAb (Fig 2) we did not test graft tissue for C4d deposition as an indirect measure of antibody-initiated, complement-dependent allograft injury. Although it is unlikely, we cannot formally eliminate the possibility that blockade of low level, antibody-initiated serum complement activation contributed to the enhanced survival in the recipients treated with anti-C5 mAb in our studies. A working model summarizing putative mechanisms through which complement contributes to allograft injury and indicating how anti-C5 mAb may be functioning to prolong graft survival is shown in Figure 5.
Figure 5.
Working model illustrating how complement and the C5 convertase could participate in the pathogenesis of allograft injury. A. Antibody binding to donor HLA molecules on donor endothelium activates serum complement through the classical pathway (1) resulting in formation of the C5 convertase which cleaves C5 (2), initiating membrane attack complex (MAC) formation, and ultimately injuring the endothelium (3). B. Ischemia reperfusion injury to the donor organ (1) upregulates production (2) and local activation of graft derived complement, resulting in formation of the C5 convertase (3). Downstream activation products including C5a induce production of mononuclear cell chemoattractants CXCL9 and CXCL10 (4) which together with C5a enhance T cell infiltration into the graft (5). C. Cognate alloreactive T cell/APC interactions induce immune cell production and release of alternative pathway complement components from both partners (1) including C3, factor B (fB), and factor D (fD), ultimately resulting in formation of a C5 convertase (2). The resultant C5a, along with C3a (not shown), bind in an autocrine fashion to their respective receptors expressed on both partners (3) resulting in enhanced APC activation (e.g. upregulation of CD86 and cytokine release) and driving T cell proliferation and expansion. Anti-C5 mAb, which inhibits the C5 convertase, could abrogate allograft injury through blocking these multiple, non-redundant pathways.
In summary, our data indicate that anti-C5 mAb functions as an adjuvant immunosuppressant to inhibit T cell activation and trafficking and to prolong murine cardiac allograft survival when used in conjunction with sub-therapeutic doses of CTLA4Ig. Because our work indicates that the combined use of CTLA4Ig and anti-C5 mAb is both safe (no untoward infections noted) and efficacious in mice, and because published studies support safety and preliminary efficacy of the analogous human reagents (46) future trials that include CTLA4Ig with anti-C5 mAb (potentially calcineurin-free) to prevent allograft rejection are warranted.
Acknowledgements
Funding sources: The work was supported by an NIH R01AI043578 and R01AI071185 awarded to PSH. HR is a recipient of a fellowship award from the National Kidney Foundation. WK is a recipient of a fellowship award from the American Society of Transplantation.
Footnotes
Disclosures
Commercial organizations. The manuscript was prepared in part with Alexion Pharmaceuticals, Cheshire CT. Alexion provided BB5.1 mAb but did not provide any other funding.
Conflicts of interest. S Faas, P Tamburini and R Rother are employees of Alexion and own stock in the company.
References
- 1.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. American journal of transplantation. 2011;11(3):450–462. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 2.Bromberg JS, Heeger PS, Li XC. Evolving paradigms that determine the fate of an allograft. American journal of transplantation. 2010;10(5):1143–1148. doi: 10.1111/j.1600-6143.2010.03033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valujskikh A, Baldwin WM, 3rd, Fairchild RL. Recent progress and new perspectives in studying T cell responses to allografts. American journal of transplantation. 2010;10(5):1117–1125. doi: 10.1111/j.1600-6143.2010.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein DR. Inflammation and transplantation tolerance. Seminars in immunopathology. 2011 doi: 10.1007/s00281-011-0251-2. [DOI] [PubMed] [Google Scholar]
- 5.Schenk AD, Rosenblum JM, Fairchild RL. Chemokine-directed strategies to attenuate allograft rejection. Clin Lab Med. 2008;28(3):441–454. doi: 10.1016/j.cll.2008.07.004. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin WM, Ota H, Rodriguez ER. Complement in transplant rejection: diagnostic and mechanistic considerations. Springer Semin Immunopathol. 2003;25(2):181–197. doi: 10.1007/s00281-003-0133-3. [DOI] [PubMed] [Google Scholar]
- 8.Wehner J, Morrell CN, Reynolds T, Rodriguez ER, Baldwin WM., 3rd Antibody and complement in transplant vasculopathy. Circulation research. 2007;100(2):191–203. doi: 10.1161/01.RES.0000255032.33661.88. [DOI] [PubMed] [Google Scholar]
- 9.Qian Z, Wasowska BA, Behrens E, Cangello DL, Brody JR, Kadkol SS, et al. C6 produced by macrophages contributes to cardiac allograft rejection. The American journal of pathology. 1999;155(4):1293–1302. doi: 10.1016/S0002-9440(10)65231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, et al. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10(10):2208–2214. doi: 10.1681/ASN.V10102208. [DOI] [PubMed] [Google Scholar]
- 11.Rother RP, Arp J, Jiang J, Ge W, Faas SJ, Liu W, et al. C5 blockade with conventional immunosuppression induces long-term graft survival in presensitized recipients. Am J Transplant. 2008;8(6):1129–1142. doi: 10.1111/j.1600-6143.2008.02222.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Arp J, Liu W, Faas SJ, Jiang J, Gies DR, et al. Inhibition of terminal complement components in presensitized transplant recipients prevents antibody-mediated rejection leading to long-term graft survival and accommodation. J Immunol. 2007;179(7):4451–4463. doi: 10.4049/jimmunol.179.7.4451. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Jiang J, Liu W, Kubelik D, Chen G, Gies D, et al. Prevention of acute vascular rejection by a functionally blocking anti-C5 monoclonal antibody combined with cyclosporine. Transplantation. 2005;79(9):1121–1127. doi: 10.1097/01.tp.0000161218.58276.9a. [DOI] [PubMed] [Google Scholar]
- 14.Stegall M, Tayyab D, Lynn C, Justin B, Patrick D, JM G. Terminal Complement Inhibition Decreases Early Acute Humoral Rejection in Sensitized Renal Transplant Recipients. Am J Transplant. 2010;10:s39. doi: 10.1111/j.1600-6143.2011.03757.x. s4. [DOI] [PubMed] [Google Scholar]
- 15.Sheerin NS, Risley P, Abe K, Tang Z, Wong W, Lin T, et al. Synthesis of complement protein C3 in the kidney is an important mediator of local tissue injury. FASEB J. 2008;22(4):1065–1072. doi: 10.1096/fj.07-8719com. [DOI] [PubMed] [Google Scholar]
- 16.Tang Z, Lu B, Hatch E, Sacks SH, Sheerin NS. C3a mediates epithelial-to-mesenchymal transition in proteinuric nephropathy. J Am Soc Nephrol. 2009;20(3):593–603. doi: 10.1681/ASN.2008040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raedler H, Yang M, Lalli PN, Medof ME, Heeger PS. Primed CD8(+) T-cell responses to allogeneic endothelial cells are controlled by local complement activation. Am J Transplant. 2009;9(8):1784–1795. doi: 10.1111/j.1600-6143.2009.02723.x. [DOI] [PubMed] [Google Scholar]
- 18.Pavlov V, Raedler H, Yuan S, Leisman S, Kwan WH, Lalli PN, et al. Donor deficiency of decay-accelerating factor accelerates murine T cell-mediated cardiac allograft rejection. J Immunol. 2008;181(7):4580–4589. doi: 10.4049/jimmunol.181.7.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112(5):1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Lin F, Strainic MG, An F, Miller RH, Altuntas CZ, et al. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol. 2008;180(9):5882–5889. doi: 10.4049/jimmunol.180.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28(3):425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalli PN, Strainic MG, Lin F, Medof ME, Heeger PS. Decay accelerating factor can control T cell differentiation into IFN-gamma-producing effector cells via regulating local C5a-induced IL-12 production. J Immunol. 2007;179(9):5793–5802. doi: 10.4049/jimmunol.179.9.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201(10):1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng Q, Li K, Sacks SH, Zhou W. The role of anaphylatoxins C3a and C5a in regulating innate and adaptive immune responses. Inflamm Allergy Drug Targets. 2009;8(3):236–246. doi: 10.2174/187152809788681038. [DOI] [PubMed] [Google Scholar]
- 25.Li K, Anderson KJ, Peng Q, Noble A, Lu B, Kelly AP, et al. Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood. 2008;112(13):5084–5094. doi: 10.1182/blood-2008-05-156646. [DOI] [PubMed] [Google Scholar]
- 26.Peng Q, Li K, Anderson K, Farrar CA, Lu B, Smith RA, et al. Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a-C3aR interaction. Blood. 2008;111(4):2452–2461. doi: 10.1182/blood-2007-06-095018. [DOI] [PubMed] [Google Scholar]
- 27.Peng Q, Li K, Patel H, Sacks SH, Zhou W. Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J Immunol. 2006;176(6):3330–3341. doi: 10.4049/jimmunol.176.6.3330. [DOI] [PubMed] [Google Scholar]
- 28.Frei Y, Lambris JD, Stockinger B. Generation of a monoclonal antibody to mouse C5 application in an ELISA assay for detection of anti-C5 antibodies. Mol Cell Probes. 1987;1(2):141–149. doi: 10.1016/0890-8508(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 29.Valujskikh A, Lantz O, Celli S, Matzinger P, Heeger PS. Cross-primed CD8(+) T cells mediate graft rejection via a distinct effector pathway. Nat Immunol. 2002;3(9):844–851. doi: 10.1038/ni831. [DOI] [PubMed] [Google Scholar]
- 30.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2(6):501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 31.Raedler H, Yang M, Lalli PN, Medof ME, Heeger PS. Primed CD8 T-Cell Responses to Allogeneic Endothelial Cells Are Controlled by Local Complement Activation. Am J Transplant. 2009 doi: 10.1111/j.1600-6143.2009.02723.x. [DOI] [PubMed] [Google Scholar]
- 32.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162(1):352–358. [PubMed] [Google Scholar]
- 33.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172(9):5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Demir Y, Valujskikh A, Heeger PS. Antigen location contributes to the pathological features of a transplanted heart graft. The American journal of pathology. 2004;164(4):1407–1415. doi: 10.1016/S0002-9440(10)63227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asgari E, Zhou W, Sacks S. Complement in organ transplantation. Curr Opin Organ Transplant. 2010;15(4):486–491. doi: 10.1097/MOT.0b013e32833b9cb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel H, Smith RA, Sacks SH, Zhou W. Therapeutic strategy with a membrane-localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. J Am Soc Nephrol. 2006;17(4):1102–1111. doi: 10.1681/ASN.2005101116. [DOI] [PubMed] [Google Scholar]
- 37.Farrar CA, Zhou W, Lin T, Sacks SH. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. Faseb J. 2006;20(2):217–226. doi: 10.1096/fj.05-4747com. [DOI] [PubMed] [Google Scholar]
- 38.Gloor J, Stegall MD. Sensitized renal transplant recipients: current protocols and future directions. Nat Rev Nephrol. 2010;6(5):297–306. doi: 10.1038/nrneph.2010.34. [DOI] [PubMed] [Google Scholar]
- 39.Lonze BE, Singer AL, Montgomery RA. Eculizumab and renal transplantation in a patient with CAPS. The New England journal of medicine. 2010;362(18):1744–1745. doi: 10.1056/NEJMc0910965. [DOI] [PubMed] [Google Scholar]
- 40.Li Q, Peng Q, Xing G, Li K, Wang N, Farrar CA, et al. Deficiency of C5aR prolongs renal allograft survival. J Am Soc Nephrol. 2010;21(8):1344–1353. doi: 10.1681/ASN.2009090977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banda NK, Thurman JM, Kraus D, Wood A, Carroll MC, Arend WP, et al. Alternative complement pathway activation is essential for inflammation and joint destruction in the passive transfer model of collagen-induced arthritis. J Immunol. 2006;177(3):1904–1912. doi: 10.4049/jimmunol.177.3.1904. [DOI] [PubMed] [Google Scholar]
- 42.Thurman JM, Lenderink AM, Royer PA, Coleman KE, Zhou J, Lambris JD, et al. C3a is required for the production of CXC chemokines by tubular epithelial cells after renal ishemia/reperfusion. J Immunol. 2007;178(3):1819–1828. doi: 10.4049/jimmunol.178.3.1819. [DOI] [PubMed] [Google Scholar]
- 43.Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol. 2003;170(3):1517–1523. doi: 10.4049/jimmunol.170.3.1517. [DOI] [PubMed] [Google Scholar]
- 44.De Vries B, Matthijsen RA, Wolfs TG, Van Bijnen AA, Heeringa P, Buurman WA. Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: inhibition of late apoptosis and inflammation. Transplantation. 2003;75(3):375–382. doi: 10.1097/01.TP.0000044455.05584.2A. [DOI] [PubMed] [Google Scholar]
- 45.Ferraresso M, Macor P, Valente M, Della Barbera M, D'Amelio F, Borghi O, et al. Posttransplant ischemia-reperfusion injury in transplanted heart is prevented by a minibody to the fifth component of complement. Transplantation. 2008;86(10):1445–1451. doi: 10.1097/TP.0b013e31818a68e2. [DOI] [PubMed] [Google Scholar]
- 46.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353(8):770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]