Abstract
Methyl 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate (CDODA-Me) and the corresponding 2-trifluoromethyl analog (CF3DODA-Me) are derived synthetically from the triterpenoid glycyrrhetinic acid, a major component of licorice. CDODA-Me and CF3DODA-Me inhibited growth of highly invasive ARO, DRO, K-18 and HTh-74 thyroid cancer cells and this was due, in part, to decreased expression of specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 that are overexpressed in these cells. CDODA-Me and CF3DODA-Me also decreased expression of Sp-dependent genes, such as survivin and vascular endothelial growth factor, and induced apoptosis. In addition, pituitary tumor-transforming gene-1 (PTTG-1) protein and mRNA levels were also decreased in thyroid cancer cells treated with CDODA-Me or CF3DODA-Me and this was accompanied by decreased expression of PTTG-1-dependent c-Myc and fibroblast growth factor 2 genes. RNA interference studies against Sp1, Sp3 and Sp4 proteins showed that in thyroid cancer cells, PTTG-1 was an Sp-dependent gene. This study demonstrates for the first time that drugs, such as CDODA-Me and CF3DODA-Me, that decrease Sp protein expression also downregulate PTTG-1 in thyroid cancer cells and therefore have potential for clinical treatment of thyroid cancer and other endocrine neoplasias where PTTG-1 is a major pro-oncogenic factor.
Keywords: PTTG-1, Sp proteins, thyroid cancer, anticancer agents
INTRODUCTION
Pituitary tumor-transforming gene-1 (PTTG-1) is a unique protein which contains various domains including transactivating and DNA binding sequences in the C- and N-terminal regions, respectively [1–3]. PTTG-1 regulates expression of multiple genes including c-Myc and fibroblast growth factor-2 (FGF-2) [4, 5], and recent ChIP-on-ChIP studies showed that PTTG-1 antibodies immunoprecipitated at least 400 gene promoters [6]. Many of these genes were associated with signal transduction pathways, cell cycle and metabolic control, and there is also evidence suggesting that PTTG-1 acts cooperatively with other transcription factors including specificity protein 1 (Sp1). PTTG-1 plays a critical role in mitosis by acting as a securin protein that regulates sister chromatid separation [3]. PTTG−/− null mice exhibit multiple tissue-specific and time-dependent characteristics including sub-fertility, pituitary, splenic and testicular hypoplasia, and decreased islet and β-cell mass leading to impairment of glucose homeostasis [7, 8]. Loss of PTTG-1 protected against pituitary tumor development in retinoblastoma protein hemizygote knockout mice [9], whereas overexpression of PTTG-1 in the pituitary enhanced pituitary carcinogenesis [10].
PTTG-1 is overexpressed in tumors derived from multiple tissues including the pituitary, thyroid, liver, testis, breast, ovary, colon and esophagus and is a prognostic factor for some cancers [3, 11–21]. For example, PTTG-1 expression was correlated with aggressiveness of breast tumors [19] and higher levels of PTTG-1 were observed in esophageal tumors with increased lymph node metastasis and higher tumor grades [20]. Similar results were observed for colon tumors [21]. The precise role of PTTG-1 may be dependent on tumor type; however, there is evidence that PTTG-1 upregulates genes such as FGF-2 and vascular endothelial growth factor (VEGF) that play important roles in tumor growth, angiogenesis and metastasis [3]. The oncogenic activity of PTTG-1 suggests that this gene is a potential therapeutic target, and studies with antisense oligonucleotides and small inhibitory RNAs demonstrate the efficacy of PTTG-1 knockdown in both in vitro and in vivo models [22–24].
Sp proteins are also overexpressed in cancer cells and tumors [23–31], and these transcription factors regulate expression of several genes that are important for cell proliferation (cyclin D1), survival (survivin, bcl-2) and angiogenesis (VEGF, VEGFR1 and VEGFR2) [32–41]. Studies in this laboratory have identified anticancer drugs that act, in part, through repressing Sp proteins and these include betulinic acid, tolfenamic acid, and the novel synthetic triterpenoids, methyl 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate (CDODA-Me) and the corresponding 2-trifluoromethyl derivative (CF3DODA-Me) [40, 42]. Kakar and coworkers previously reported that GC-rich sites in the PTTG-1 promoter that bound Sp1 protein were responsible for approximately 70% of the promoter activity in prostate cancer cells as determined by mutations of the GC-rich sites [43]. In this study, we report that CDODA-Me and CF3DODA-Me decreased expression of Sp1, Sp3 and Sp4 in several thyroid cancer cell lines and this was accompanied by decreased expression of PTTG-1 at the mRNA, protein and promoter levels. CDODA-Me and CF3DODA-Me also decreased expression of PTTG-1-dependent genes and RNA interference confirmed that Sp proteins regulated PTTG-1 expression. This demonstrates a novel pathway for drug-induced downregulation of PTTG-1 through repression of Sp proteins.
MATERIALS AND METHODS
Cell lines
Human anaplastic thyroid cancer (ATC) cell lines ARO, DRO, K-18 (KAT-18) and HTh-74 were kindly provided by members of the Department of Endocrine Neoplasia and Hormonal Disorders, M.D. Anderson Cancer Center, Houston, TX and are available from the American Type Culture Collection (ATCC, Manassas, VA). The widely used ARO and DRO cells may be derived from melanoma cells and the tumor type designation of these cells is being investigated [44]. Fetal bovine serum was obtained from JRH Biosciences (Lenexa, KS). ATC cell lines cells were maintained in RPMI 1640 (Sigma Chemical, St. Louis, MO) supplemented with 0.22% sodium bicarbonate, 0.011% sodium pyruvate, 0.45% glucose, 0.24% HEPES, 10% FBS, and 10 ml/L of 100× antibiotic antimycotic solution (Sigma). Cells were maintained at 37°C in the presence of 5% CO2.
Antibodies and Reagents
Antibodies for Sp1, Sp3, Sp4 and VEGF were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). PTTG-1 was obtained from Invitrogen (Carlsbad, CA). Cleaved PARP, c-Myc and survivin were from Cell Signaling Technology, Inc. (Danvers, MA). Monoclonal β-actin antibody was purchased from Sigma-Aldrich. Reporter lysis buffer and luciferase reagent for luciferase studies were purchased from Promega (Madison, WI). β-Galactosidase (β-Gal) reagent was obtained from Tropix (Bedford, MA). Lipofectamine reagents were supplied by Invitrogen. Western Lightning chemiluminescence reagents were from Perkin-Elmer Life Sciences (Boston, MA).
Cell Proliferation Assay
ATC cells (4 × 104 per well) were plated in 12-well plates and allowed to attach for 24 hr. The medium was then changed to DMEM/Ham's F-12 media containing 2.5% charcoal-stripped FBS, and either vehicle (DMSO) or the indicated compounds were added for 24 and 48 hr. Cells were trypsinized and counted after 24 and 48 hr using a Coulter Z1 cell counter (Beckman Coulter, Fullerton, CA). Each experiment was done in triplicate, and results are expressed as means ± S.E. for each set of three experiments.
Transfections
The PTTG-1-luc construct containing the −1373 to +3 region of the PTTG-1 promoter was provided by Dr. Kakar (University of Louisville, Louisville, KY). ATC cells (1 × 105) were seeded in 12-well plates in DMEM/Ham's F-12 media supplemented with 2.5% charcoal-stripped FBS and grown overnight. Transient transfections were performed using Lipofectamine 2000 reagent (Invitrogen) according to the protocol provided by the manufacturer. Transfection studies were performed using 0.4 μg of PTTG-1-Luc and 0.04 μg of β-galactosidase. Six hours after transfection, the transfection mix was replaced with complete media containing either vehicle (DMSO) or the indicated compound for 20 to 22 hr. Cells were then lysed with 100 μl of 1× reporter lysis buffer, and 30 μl of cell extract was used for luciferase and β-galactosidase assays. A multifunctional microplate reader (FLUOstar OPTIMA) was used to quantify luciferase and β-galactosidase activities, and the luciferase activities were normalized to β-galactosidase activity.
Western Blot Analysis
Cells were seeded in DMEM/Ham's F-12 media containing 2.5% charcoal-stripped FBS for 24 hr and then treated with either the vehicle (DMSO) or the indicated compounds. Cells were collected by scraping in 150 μl high salt lysis buffer [50 mM HEPES, 0.5 M NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% (v/v) glycerol, 1% (v/v) Triton-X-100 and 5 μl/ml of Protease Inhibitor Cocktail (Sigma)]. The lysates were incubated on ice for one hour with intermittent vortexing followed by centrifugation at 20,000 g for 10 min at 4°C. Before electrophoresis, the samples were boiled for 3 min at 100°C, the amounts of protein were determined and 60 μg protein applied per lane. Samples were subjected to SDS-PAGE on 10% gel at 120 V for 3 to 4 hr. Proteins were transferred to polyvinylidene difluoride membrane (PVDF; Bio-Rad, Hercules, CA) at 0.9 amp for 90 min at 4°C in 1× transfer buffer (48 mM Tris-HCl, 39 mM glycine, and 0.025% SDS). The membranes were blocked for 30 min with 5% TBST-Blotto [10 mM Tris-HCl, 150 mM NaCl (pH 8.0), 0.05% Triton X-100 and 5% nonfat dry milk] and incubated in fresh 5% TBST-Blotto with primary antibody overnight with gentle shaking at 4°C. After washing with TBST for 10 min, the PVDF membrane was incubated with secondary antibody (1:5000) in 5% TBST-Blotto for 2–3 hr. The membrane was washed with TBST for 10 min and incubated with 10 ml of chemiluminiscence substrate (PerkinElmer Life Sciences) for 1.0 min and exposed to ImageTeK-H medical imaging film (Eastman American X-ray Supply, Inc.).
Reverse Transcriptase-PCR
Total RNA was extracted using RNeasy Mini Kit (Qiagen), and 1 μg RNA was used to synthesize cDNA using Reverse Transcription System (Promega). mRNA levels were normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal housekeeping gene. Primers obtained from IDT and used for amplification were PTTG-1 (sense 5'-ATG GCT ACT CTG ATC TAT-3' and antisense 5'-AAA ATC TAT GTC ACA GCA AAC-3'), bFGF (sense 5'-TTC TTC CTG CGC ATC CAC CC-3') and antisense 5'-CTC TTA GCA GAC ATT GGA AG-3') and GAPDH (sense 5'-ACG GAT TTG GTC GTA TTG GGC G-3' and antisense 5'-CTC CTG GAA GAT GGT GAT GG-3'). PCR products were electrophoresed on 1% agarose gels containing ethidium bromide and visualized under UV transillumination.
SiRNA Interference Assays
Small inhibitory RNAs were prepared by Dharmacon RNA Technologies (Chicago, IL). The iRNA complexes used in this study are indicated as follows:
Sp1 5'-AUC ACU CCA UGG AUG AAA UGA TT
Sp3 5'-GCG GCA GGU GGA GCC UUC ACU TT
Sp4 5'-GCA GUG ACA CAU UAG UGA GCT T
ARO thyroid cancer cells were seeded (1 × 105 per well) in 12-well plates in DMEM/Ham's F-12 medium supplemented with 2.5% charcoal-stripped FBS without antibiotic and left to attach for one day. The triple Sp SiRNA knockdown (iSp1, iSp3, iSp4 complex) (iSp) along with iLamin as control was performed using Lipofectamine 2000 transfection reagent per the manufacturer's instructions. For the cell proliferation studies, Sp knockdown by iSp was carried out. After 6 hr, media was changed and cells were counted after further incubation for 60 hr.
RESULTS
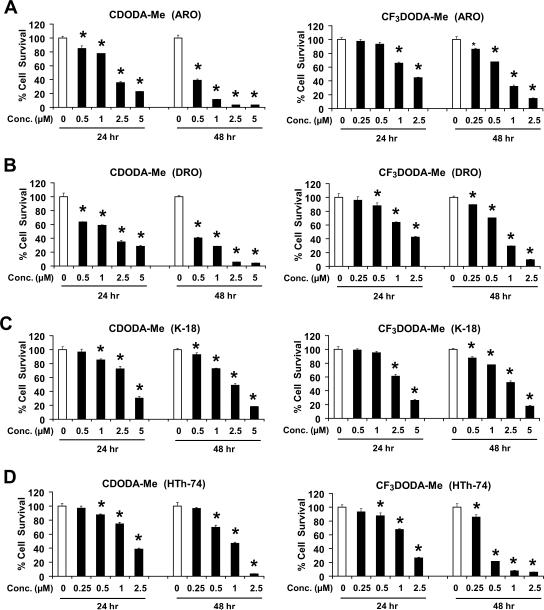
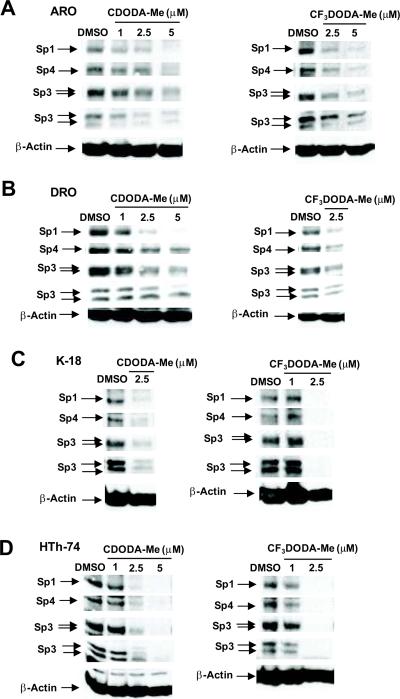
CDODA-Me and CF3DODA-Me inhibit growth of bladder and pancreatic cancer cells [42], and Figure 1 summarizes the effects of both compounds on survival of several thyroid cancer cell lines where 100% survival was designated for the DMSO control cells. CDODA-Me and CF3DODA-Me decreased survival of DRO, ARO, HTh-74 and K18 thyroid cancer cells after treatment for 24 hr, and the IC50 values for growth inhibition of these thyroid cancer cell lines after treatment for 6 days was < 1 μM for both compounds. Ongoing studies in other cancer cell lines indicate that the proapoptotic/growth inhibitory effects of CDODA-Me were due, in part, to repression of Sp proteins [40]. Figures 2A – 2D summarize the effects of CDODA-Me and CF3DODA-Me on Sp1, Sp3 and Sp4 protein levels in thyroid cancer cells. Sp proteins were highly expressed in all four thyroid cancer cell lines. Concentrations of 1 – 2.5 μM decreased levels of Sp1, Sp3 and Sp4 proteins and this corresponded with the effects of these compounds (and their concentrations) on cell survival (Fig. 1).
Figure 1.
CDODA-Me and CF3DODA-Me decrease thyroid cancer cell survival. ARO (A), DRO (B), K-18 (C), and HTh-74 (D) were treated with CDODA-Me or CF3DODA-Me for 24 or 48 hr, and cells were counted as described in the Materials and Methods. Results are expressed as means ± SE for three replicate determinations for each treatment group. Significantly (p < 0.05) decreased cell survival compared to DMSO (solvent) control (set at 100%) is indicated by an asterisk.
Figure 2.
CDODA-Me and CF3DODA-Me decrease Sp protein expression. ARO (A), DRO (B), K-18 (C), and HTh-74 (D) were treated with CDODA-Me or CF3DODA-Me for 24 hr, and whole cell lysates were analyzed by western blots as described in the Materials and Methods. Similar results were observed in replicate (at least two) experiments and β-actin served as a loading control.
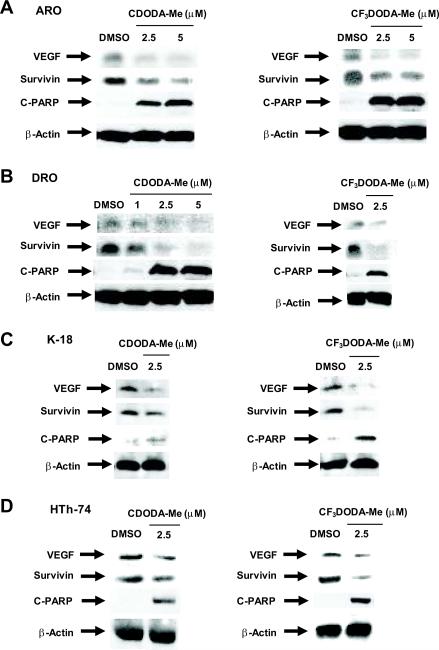
Drug-induced downregulation of Sp proteins decreases expression of multiple Sp-dependent genes including VEGF and survivin [38, 39, 41]. Figure 3 illustrates the effects of CDODA-Me and CF3DODA-Me on levels of VEGF and survivin proteins in thyroid cancer cells. Treatment of ARO, DRO, HTh-74 and K-18 cells with up to 7.5 μM CDODA-Me decreased VEGF and survivin levels, and similar results were observed in these same thyroid cancer cells treated with CF3DODA-Me. Since survivin inactivates caspases 3 and 7, we also examined the effects of CDODA-Me and CF3DODA-Me on caspase-dependent PARP cleavage, a well-characterized biochemical marker for apoptosis. The results show that CDODA-Me- and CF3DODA-Me-dependent downregulation of survivin expression was accompanied by increased PARP cleavage, suggesting that the decrease in Sp proteins and survivin contribute to CDODA-Me- and CF3DODA-Me-induced apoptosis in the thyroid cancer cells.
Figure 3.
CDODA-Me and CF3DODA-Me decrease expression of Sp-dependent proteins. ARO (A), DRO (B), K-18 (C), and HTh-74 (D) were treated with CDODA-Me or CF3DODA-Me for 24 hr, and whole cell lysates were analyzed by western blots as described in the Materials and Methods. Similar results were observed in replicate (at least two) experiments and β-actin served as a loading control.
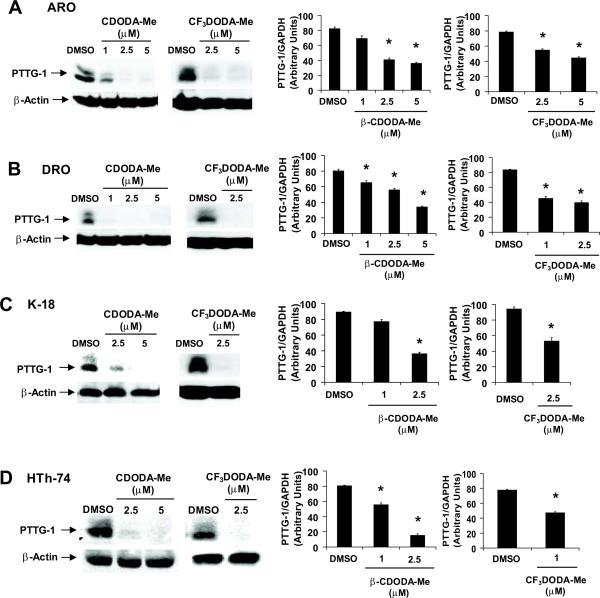
PTTG-1 plays an important pro-oncogenic role in thyroid cancer and endocrine tumorigenesis and there is evidence that in prostate cancers, basal expression of PTTG-1 is Sp1-dependent [43, 45]. Results in Figure 4A illustrate that both CDODA-Me and CF3DODA-Me decrease PTTG-1 protein expression in ARO cells and we also observed a decrease in PTTG-1 mRNA levels after treatment with the same compounds. The effects of CDODA-Me and CF3DODA-Me on PTTG-1 protein and mRNA levels were also determined in DRO, HTh-74 and K-18 cells (Figs. 4B – 4D) and decreased expression of PTTG-1 protein and mRNA was observed in these cell lines.
Figure 4.
CDODA-Me and CF3DODA-Me decrease PTTG-1 expression in thyroid cancer cells. ARO (A), DRO (B), K-18 (C), and HTh-74 (D) were treated with CDODA-Me or CF3DODA-Me for 24 hr, and protein and RNA extracts were obtained and analyzed by western blot or RT-PCR, respectively, as described in the Materials and Methods. Similar results were observed in replicate (at least two) experiments and β-actin served as loading control. The RT-PCR results for PTTG-1 mRNA levels were replicated (3×) and results are presented as means ± SE (relative to the DMSO control and normalized to GAPDH). Significant (p < 0.05) decreases in PTTG-1 mRNA levels are indicated (*).
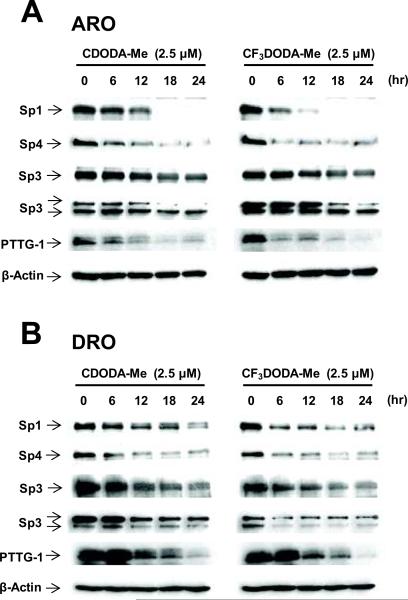
We also investigated the time-course effects of CDODA-Me and CF3DODA-Me on expression of Sp1, Sp3, Sp4 and PTTG-1 proteins in ARO (Fig. 5A) and DRO (Fig. 5B) cells. A decrease in the proteins showed some variability with respect to the two different compounds and cell context. In ARO cells treated with CDODA-Me, the pattern of Sp1, Sp4 and PTTG-1 protein degradation was similar. Small decreases were observed within 12 hr and levels decreased dramatically 12–18 hr after treatment. Similar results were observed for Sp1, Sp4 and PTTG-1 in ARO cells treated with CF3DODA-Me; however, expression was decreased at earlier time points (0–6 hr). In contrast, the rate of Sp3 degradation was more gradual over the 24 hr treatment with both compounds. A different pattern was observed in DRO cells (Fig. 5B). Treatment with CDODA-Me resulted in a gradual decrease in Sp1, Sp3 and PTTG-1 over the 24 hr period, whereas Sp4 protein levels decreased rapidly after 6 hr. In contrast, treatment with CF3DODA-Me resulted in a rapid decrease in Sp1, Sp3 and Sp4 after 0–6 hr, but decreased PTTG-1 was not observed until 6–12 hr. These results clearly link Sp1, Sp3 and Sp4 expression with PTTG-1; however, this does not exclude a role for other CDODA-Me-/CF3DODA-Me-induced pathways in regulation of PTTG-1.
Figure 5.
Time-dependent decrease in Sp proteins and PTTG-1 in cells treated with CDODA-Me or CF3DODA-Me. (A) ARO cells. Cells were treated with 2.5 μM CDODA-Me or 2.5 μM CF3DODA-Me for 0 (DMSO, control), 6, 12, 18 or 24 hr, and whole cell lysates were analyzed by western blots as outlined in the Materials and Methods. (B) DRO cells. This experiment was carried out as described in (A) using DRO thyroid cancer cells. Similar results (A and B) were observed in at least 2 separate experiments.
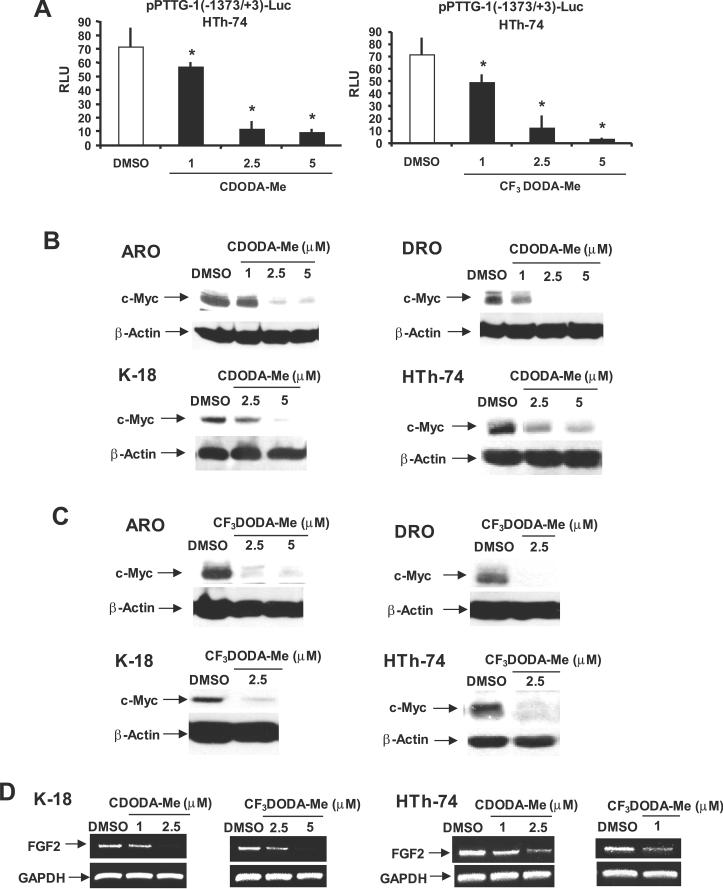
We also examined the effects of CDODA-Me and CF3DODA-Me on luciferase activity in thyroid cancer cells transfected with pPTTG-1(−1373/+3)luc which contains the −1373 to +3 region of the PTTG-1 promoter linked to a luciferase reporter gene [45]. The results obtained in HTh-74 cells (Fig. 6A) show that CDODA-Me and CF3DODA-Me decreased luciferase activity and similar results were observed in K-18 cells and in ARO and DRO cells for CF3DODA-Me (data not shown). CF3-DODA-Me and CDODA-Me also decrease luciferase activity in colon (RKO and SW480) and pancreatic (MiaPaca-2 and L3.6p1) cancer cells transfected with pPTTG-1(−1373/+3)-Luc (data not shown). In contrast, CDODA-Me induced luciferase activity in ARO and DRO cells transfected with PTTG-1(−1373/+3)-Luc, even though this compound decreased PTTG-1 mRNA and protein expression (Figs. 4A and 4B). Differences between CDODA-Me and CF3-DODA-Me in modulating luciferase activity in ARO and DRO cells transfected with the PTTG-1 promoter construct may be due to their differences in structure (2-CN vs. 2-CF3 groups) or some limitations in the length of the promoter insert and these are currently being investigated. PTTG-1 is a transcription factor that regulates expression of several genes including c-Myc and FGF2 [2, 3]. Figure 6B shows that treatment with CDODAMe decreased c-Myc protein levels in ARO, DRO, K-18 and HTh-74 cells. CF3DODAMe also decreased Myc expression in the thyroid cancer cells (Fig. 6C) and this demonstrates that both compounds not only decrease PTTG-1 but also a PTTG-dependent gene. FGF2 is also regulated by PTTG-1 and preliminary studies show that low to non-detectable levels of FGF2 mRNA are expressed in ARO or DRO cells (data not shown). However, FGF2 is expressed in both K-18 and HTh-74 cells (Fig. 5D) and treatment with CDODA-Me or CF3DODA-Me decreased FGF2 mRNA levels in both cell lines.
Figure 6.
CDODA-Me and CF3DODA-Me decrease PTTG-1 and PTTG-1-dependent responses. (A) Decreased luciferase activity. HTh-74 cells were transfected with pPTTG-1(−1371/+3)luc, treated with CDODA-Me or CF3DODA-Me, and luciferase activity was determined as described in the Materials and Methods. Results are expressed as means ± SE for three replicate experiments. Significantly (p < 0.05) decreased activity is indicated (*). Decreased expression of Myc protein in cells treated with CDODA-Me (B) or CF3DODA-Me (C). Thyroid cancer cells were treated with CDODA-Me or CF3DODA-Me for 24 hr, and whole cell lysates were analyzed for expression of Myc by western blots as outlined in the Materials and Methods. (D) Decreased expression of FGF2 mRNA levels. K-18 and HTh-74 cells were treated with CDODA-Me or CF3DODA-Me for 24 hr, and FGF2 mRNA levels were determined by RT-PCR as described in the Materials and Methods.
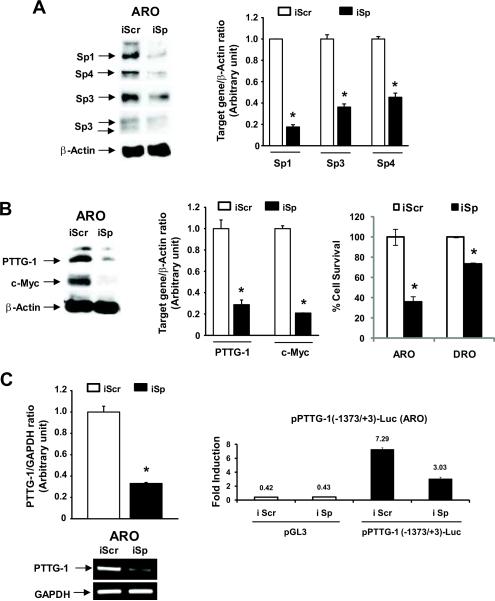
CDODA-Me and CF3DODA-Me coordinately decrease PTTG-1 (Fig. 4), Sp1, Sp3 and Sp4 (Fig. 2) in thyroid cancer cells and the role of Sp proteins in regulation of PTTG-1 was investigated by RNA interference. In this study, we used a cocktail (iSp) of small inhibitory RNAs for Sp1, Sp3 and Sp4 as previously described [34, 41] and the results in Figure 7A demonstrate that after transfection of ARO cells with iSp, there was a significant decrease in Sp1, Sp3 and Sp4 protein expression (Fig. 7A). Knockdown of Sp proteins was also accompanied by decreased expression of PTTG-1 protein levels and Sp knockdown also resulted in inhibition of ARO and DRO cell growth (Fig. 7B). Sp knockdown also decreased PTTG-1 mRNA levels, and luciferase activity (Fig. 7C) in ARO cells transfected with the pPTTG-1(−1373/+3)luc construct. These results demonstrate that PTTG-1 is an Sp-regulated gene in thyroid cancer cells and that compounds such as CDODA-Me and CF3DODA-Me that downregulate Sp protein levels coordinately decrease the pro-oncogenic PTTG-1 gene.
Figure 7.
Effects of decreased Sp1, Sp3 and Sp4 by RNA interference on PTTG-1 and Myc expression. Effects of iSp on Sp proteins (A) and PTTG-1 and Myc proteins and cell proliferation (B). ARO cells were transfected with iSp (small inhibitory RNAs for Sp1, Sp3 and Sp4 combined), and whole cell lysates were analyzed by western blots as described in the Materials and Methods and cell proliferation was determined after a total of 66 hr. Results are expressed as means ± SE for three replicate determinations. Significantly (p < 0.05) decreased responses in the iSp group compared to the non-specific iScr group (set at 1.0) are indicated (*). (C) iSp decreases PTTG-1 gene expression. ARO cells were transfected with PTTG-1(−1373/+3)luc and iScr or iSp, and luciferase activity was determined as described in the Materials and Methods. ARO cells were also transfected with iSp alone and after 24 hr, PTTG-1 mRNA levels were determined by RT-PCR as described in the Materials and Methods. Results are expressed as means ± SE for three replicate determinations for each group. Significant decreases by iSp are indicated (*).
DISCUSSION
Sp transcription factors are overexpressed in many human tumors and cancer cell lines [25–31] and a recent study identified Sp1 as a biomarker for highly aggressive pancreatic ductal adenocarcinomas [31]. Recent studies have reported that in addition to Sp1, breast, colon, pancreatic and prostate cancer cells also overexpress Sp3 and Sp4 proteins [30, 32–34, 38, 39]; however, their use as prognostic or diagnostic markers have not been investigated. Moreover, results of gel mobility shift and antibody supershift assays using 32P-labeled GC-rich oligonucleotides suggest that Sp1, Sp3 and Sp4 are the major proteins expressed that bind GC-rich motifs [39, 46]. However, this does not exclude a role for other Sp/KLF proteins and, in ongoing western blot assays, we are continually screening lysates for other GC-binding proteins. Results of this investigation show that Sp1, Sp3 and Sp4 are also highly expressed in thyroid cancer cell lines (Fig. 2). RNA interference studies suggest that pro-oncogenic genes such as VEGF, VEGFR1, VEGFR2, bcl-2, cyclin D1 and survivin may be coregulated by Sp1, Sp3 and Sp4 in cancer cells [32, 34, 38, 39]. Overexpression of Sp proteins in tumors and cancer cells coupled with their regulation of survival, growth promoting and angiogenic genes suggests that these transcription factors may be important targets for cancer chemotherapy [47]. Use of such drugs is supported by the age-dependent decrease in expression and function of Sp proteins in humans [48], the low expression of Sp protein in immortalized vs. transformed cell lines (breast) [30], and the low expression of Sp proteins in non-tumor tissue in animal models [30, 38, 39].
Recent studies have identified agents such as cyclooxygenase inhibitors (celecoxib and tolfenamic acid) and the anticancer drugs betulinic acid and curcumin that act, in part, by downregulation of Sp proteins [33, 38, 39, 46, 49]. Moreover, ongoing studies in colon cancer cells also show that CDODA-Me decreases expression of Sp proteins both in vitro and in vivo [40]. Previous reports have demonstrated that PTTG-1 exhibits oncogenic activity and is overexpressed in many tumors and cell lines [1–3] and, in human prostate and hepatoma cancer cells, there is evidence that PTTG-1 is an Sp-dependent gene [43, 45]. The major objective of this study was to determine the effects of CDODA-Me and a new analog, CF3DODA-Me on Sp protein expression in thyroid cancer cells and to investigate the role of these agents and Sp proteins in regulating expression of PTTG-1 in highly aggressive undifferentiated thyroid cancer cell lines.
Both CDODA-Me and CF3DODA-Me decreased proliferation of thyroid cancer cells (Fig. 1) and these results were consistent with the inhibition of pancreatic and bladder cancer cell growth by the same two compounds [42] and also inhibition of prostate and colon cancer cells by CDODA-Me [40, 50]. The overall cytotoxicity of CDODA-Me in these cancer cell lines has been associated with both growth inhibition and induction of apoptosis and the induction of caspase-dependent PARP cleavage and downregulation of survivin is consistent with the proapoptotic effects of CDODA-me and CF3DODA-Me in thyroid cancer cells (Fig. 3). Like curcumin, tolfenamic acid and the triterpenoid natural product betulinic acid [33, 38, 39, 46, 49], CDODA-Me decreased expression of Sp1, Sp3 and Sp4 proteins and Sp-dependent gene products in colon cancer cells [40]. Figure 2 demonstrates that thyroid cancer cells also express Sp1, Sp3 and Sp4 proteins and treatment of these cell lines with CDODA-Me or CF3DODA-Me decreases expression of all three Sp transcription factors. Moreover, CDODA-Me and CF3DODA-Me also decreased survivin and VEGF (Fig. 3), two prototypical Sp-dependent genes in ARO,DRO, K-18 and HTh-711 thyroid cancer cells.
The relationship between Sp proteins and PTTG-1 expression is complex and dependent on tumor type. In prostate cancer cells, basal expression of PTTG-1 was dependent on Sp1 expression [43, 45]. In HepG2 cells overexpressing the thyroid hormone receptor, treatment with triiodothyronine (T3) decreased Sp1, minimally decreased Sp3 (< 30%), and did not affect Sp4 proteins and this was accompanied by decreased PTTG-1 protein and mRNA [45]. Similar results were observed in other hepatocellular carcinoma cell lines transfected with the thyroid hormone receptor; however, in non-transfected cells T3 did not affect Sp1 or PTTG-1 expression. In contrast, overexpression of Sp1 or Sp1 knockdown by RNA interference did not affect PTTG-1 expression in human choriocarcinoma JEG-3 cells [6], demonstrating that regulation of PTTG-1 by Sp1 was dependent on the tumor type. Since PTTG-1 plays an important role in thyroid tumorigenesis [1–3], we also examined PTTG-1 expression in ARO, DRO, K-18 and HTh-74 cells and PTTG-1 protein was detected in all four cell lines (Fig. 4). CDODA-Me and CF3DODA-Me decreased expression of PTTG-1 protein and mRNA (Fig. 4) and luciferase activity in HTh-74 cells transfected with pPTTG-1(−1373/+3)luc (Fig. 6A). Confirmation that CDODA-Me/CF3DODA-Me-dependent downregulation of PTTG-1 was due to decreased expression of Sp1, Sp3 and Sp4 was determined by RNA interference studies in which simultaneous knockdown of Sp1, Sp3 and Sp4 by RNA interference in thyroid cancer cells was accompanied by decreased expression of PTTG-1 (Fig. 7A). However, although time course studies on CDODA-Me/CF3DODA-Me-dependent downregulation of Sp1, Sp3, Sp4 and PTTG-1 were similar (Fig. 5), there were some differences that suggest that other compound-induced pathways could also contribute to PTTG-1 downregulation. During the course of this study, it was reported that the widely used ARO and DRO cells may be derived from melanoma cells [44]; nevertheless, similar responses were observed in all cell lines and ongoing studies in other cancer cell lines show that PTTG-1 is an Sp-regulated gene.
PTTG-1 is overexpressed in multiple tumor types and regulates multiple genes associated with tumor growth and angiogenesis [2, 3]. This gene is particularly important in pituitary and thyroid cancers, and development of agents that decrease PTTG-1 expression may provide a novel and clinically-relevant chemotherapeutic approach for treating these cancers [3]. PTTG-1 protects against pituitary tumor development in mouse models [9, 10] and the oncogenic activity of this transcription factor is due, in part, to PTTG-1-dependent regulation of genes such as FGF-2 and the protooncogene c-Myc [4, 5]. Thus, agents such as CDODA-Me and CF3DODA-Me that downregulate Sp transcription factors and Sp-dependent genes such as PTTG-1 also modulate expression of PTTG-1-dependent genes including FGF-2 and c-Myc (Fig. 6). Previous reports show that other Sp-dependent genes including survivin, bcl-2, VEGF, VEGFR1 and the hepatocyte growth factor receptor (c-met) can be repressed by drugs targeting Sp transcription factors [32–34, 39–42, 49], and results of this study demonstrate for the first time that PTTG-1 is also an Sp-dependent gene in thyroid cancer cells and is downregulated by CDODA-Me and CF3-DODA-Me. The mechanisms associated with decreased Sp proteins in thyroid cancer cells treated with CDODA-Me and CF3DODA-Me are complex and involve proteasome-dependent and -independent pathways and microRNAs, and these are currently being investigated. In summary, our results identify for the first time agents that indirectly target PTTG-1 through downregulation of Sp transcription factors and drugs such as CDODA-Me and CF3-DODA-Me are currently being developed for treatment of thyroid cancer and other endocrine neoplasias.
Acknowledgments
Funding: This research was supported by funding from the National Institutes of Health (R01-CA108718 and R01-CA112337) and Texas AgriLife.
Abbreviations
- β-Gal
β-galactosidase
- ATC
anaplastic thyroid cancer
- CDODA-Me
methyl 2- cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate
- FGF-2
fibroblast growth factor-2
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- PTTG-1
pituitary tumor-transforming gene-1
- PVDF
polyvinylidene difluoride
- Sp
specificity protein
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
REFERENCES
- 1.Dominguez A, Ramos-Morales F, Romero F, et al. hpttg, a human homologue of rat pttg, is overexpressed in hematopoietic neoplasms. Evidence for a transcriptional activation function of hPTTG. Oncogene. 1998;17:2187–2193. doi: 10.1038/sj.onc.1202140. [DOI] [PubMed] [Google Scholar]
- 2.Kim DS, Fong J, Read ML, McCabe CJ. The emerging role of pituitary tumour transforming gene (PTTG) in endocrine tumourigenesis. Mol Cell Endocrinol. 2007;278:1–6. doi: 10.1016/j.mce.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Vlotides G, Eigler T, Melmed S. Pituitary tumor-transforming gene: physiology and implications for tumorigenesis. Endocr Rev. 2007;28:165–186. doi: 10.1210/er.2006-0042. [DOI] [PubMed] [Google Scholar]
- 4.Pei L. Identification of c-myc as a down-stream target for pituitary tumor-transforming gene. J Biol Chem. 2001;276:8484–8491. doi: 10.1074/jbc.M009654200. [DOI] [PubMed] [Google Scholar]
- 5.Boelaert K, Yu R, Tannahill LA, et al. PTTG's C-terminal PXXP motifs modulate critical cellular processes in vitro. J Mol Endocrinol. 2004;33:663–677. doi: 10.1677/jme.1.01606. [DOI] [PubMed] [Google Scholar]
- 6.Tong Y, Tan Y, Zhou C, Melmed S. Pituitary tumor transforming gene interacts with Sp1 to modulate G1/S cell phase transition. Oncogene. 2007;26:5596–5605. doi: 10.1038/sj.onc.1210339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Yu R, Melmed S. Mice lacking pituitary tumor transforming gene show testicular and splenic hypoplasia, thymic hyperplasia, thrombocytopenia, aberrant cell cycle progression, and premature centromere division. Mol Endocrinol. 2001;15:1870–1879. doi: 10.1210/mend.15.11.0729. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Moro E, Kovacs K, Yu R, Melmed S. Pituitary tumor transforming gene-null male mice exhibit impaired pancreatic beta cell proliferation and diabetes. Proc Natl Acad Sci U S A. 2003;100:3428–3432. doi: 10.1073/pnas.0638052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesnokova V, Kovacs K, Castro AV, Zonis S, Melmed S. Pituitary hypoplasia in Pttg−/− mice is protective for Rb+/− pituitary tumorigenesis. Mol Endocrinol. 2005;19:2371–2379. doi: 10.1210/me.2005-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbud RA, Takumi I, Barker EM, et al. Early multipotential pituitary focal hyperplasia in the alpha-subunit of glycoprotein hormone-driven pituitary tumor-transforming gene transgenic mice. Mol Endocrinol. 2005;19:1383–1391. doi: 10.1210/me.2004-0403. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Horwitz GA, Heaney AP, et al. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocrinol Metab. 1999;84:761–767. doi: 10.1210/jcem.84.2.5432. [DOI] [PubMed] [Google Scholar]
- 12.Saez C, Japon MA, Ramos-Morales F, et al. hpttg is over-expressed in pituitary adenomas and other primary epithelial neoplasias. Oncogene. 1999;18:5473–5476. doi: 10.1038/sj.onc.1202914. [DOI] [PubMed] [Google Scholar]
- 13.Boelaert K, McCabe CJ, Tannahill LA, et al. Pituitary tumor transforming gene and fibroblast growth factor-2 expression: potential prognostic indicators in differentiated thyroid cancer. J Clin Endocrinol Metab. 2003;88:2341–2347. doi: 10.1210/jc.2002-021113. [DOI] [PubMed] [Google Scholar]
- 14.Solbach C, Roller M, Fellbaum C, Nicoletti M, Kaufmann M. PTTG mRNA expression in primary breast cancer: a prognostic marker for lymph node invasion and tumor recurrence. Breast. 2004;13:80–81. doi: 10.1016/j.breast.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Thompson AD, III, Kakar SS. Insulin and IGF-1 regulate the expression of the pituitary tumor transforming gene (PTTG) in breast tumor cells. FEBS Lett. 2005;579:3195–3200. doi: 10.1016/j.febslet.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Rehfeld N, Geddert H, Atamna A, et al. The influence of the pituitary tumor transforming gene-1 (PTTG-1) on survival of patients with small cell lung cancer and non-small cell lung cancer. J Carcinog. 2006;5:4. doi: 10.1186/1477-3163-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hlubek F, Pfeiffer S, Budczies J, et al. Securin (hPTTG1) expression is regulated by beta-catenin/TCF in human colorectal carcinoma. Br J Cancer. 2006;94:1672–1677. doi: 10.1038/sj.bjc.6603155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saez C, Martinez-Brocca MA, Castilla C, et al. Prognostic significance of human pituitary tumor-transforming gene immunohistochemical expression in differentiated thyroid cancer. J Clin Endocrinol Metab. 2006;91:1404–1409. doi: 10.1210/jc.2005-2532. [DOI] [PubMed] [Google Scholar]
- 19.Ogbagabriel S, Fernando M, Waldman FM, Bose S, Heaney AP. Securin is overexpressed in breast cancer. Mod Pathol. 2005;18:985–990. doi: 10.1038/modpathol.3800382. [DOI] [PubMed] [Google Scholar]
- 20.Shibata Y, Haruki N, Kuwabara Y, et al. Expression of PTTG (pituitary tumor transforming gene) in esophageal cancer. Jpn J Clin Oncol. 2002;32:233–237. doi: 10.1093/jjco/hyf058. [DOI] [PubMed] [Google Scholar]
- 21.Heaney AP, Singson R, McCabe CJ, et al. Expression of pituitary-tumour transforming gene in colorectal tumours. Lancet. 2000;355:716–719. doi: 10.1016/S0140-6736(99)10238-1. [DOI] [PubMed] [Google Scholar]
- 22.Cho-Rok J, Yoo J, Jang YJ, et al. Adenovirus-mediated transfer of siRNA against PTTG1 inhibits liver cancer cell growth in vitro and in vivo. Hepatology. 2006;43:1042–1052. doi: 10.1002/hep.21137. [DOI] [PubMed] [Google Scholar]
- 23.Kim DS, Franklyn JA, Stratford AL, et al. Pituitary tumor-transforming gene regulates multiple downstream angiogenic genes in thyroid cancer. J Clin Endocrinol Metab. 2006;91:1119–1128. doi: 10.1210/jc.2005-1826. [DOI] [PubMed] [Google Scholar]
- 24.Kakar SS, Malik MT. Suppression of lung cancer with siRNA targeting PTTG. Int J Oncol. 2006;29:387–395. [PubMed] [Google Scholar]
- 25.Wang L, Wei D, Huang S, et al. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371–6380. [PubMed] [Google Scholar]
- 26.Yao JC, Wang L, Wei D, et al. Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res. 2004;10:4109–4117. doi: 10.1158/1078-0432.CCR-03-0628. [DOI] [PubMed] [Google Scholar]
- 27.Shi Q, Le X, Abbruzzese JL, et al. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61:4143–4154. [PubMed] [Google Scholar]
- 28.Zannetti A, Del VS, Carriero MV, et al. Coordinate up-regulation of Sp1 DNA-binding activity and urokinase receptor expression in breast carcinoma. Cancer Res. 2000;60:1546–1551. [PubMed] [Google Scholar]
- 29.Chiefari E, Brunetti A, Arturi F, et al. Increased expression of AP2 and Sp1 transcription factors in human thyroid tumors: a role in NIS expression regulation? BMC Cancer. 2002;2:35. doi: 10.1186/1471-2407-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein (Sp) transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 31.Jiang NY, Woda BA, Banner BF, et al. Sp1, a new biomarker that identifies a subset of aggressive pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:1648–1652. doi: 10.1158/1055-9965.EPI-07-2791. [DOI] [PubMed] [Google Scholar]
- 32.Abdelrahim M, Smith R, III, Burghardt R, Safe S. Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res. 2004;64:6740–6749. doi: 10.1158/0008-5472.CAN-04-0713. [DOI] [PubMed] [Google Scholar]
- 33.Abdelrahim M, Baker CH, Abbruzzese JL, et al. Regulation of vascular endothelial growth factor receptor-1 (VEGFR1) expression by specificity proteins 1, 3 and 4 in pancreatic cancer cells. Cancer Res. 2007;67:3286–3294. doi: 10.1158/0008-5472.CAN-06-3831. [DOI] [PubMed] [Google Scholar]
- 34.Higgins KJ, Abdelrahim M, Liu S, Yoon K, Safe S. Regulation of vascular endothelial growth factor receptor-2 expression in pancreatic cancer cells by Sp proteins. Biochem Biophys Res Commun. 2006;345:292–301. doi: 10.1016/j.bbrc.2006.04.111. [DOI] [PubMed] [Google Scholar]
- 35.Finkenzeller G, Sparacio A, Technau A, Marme D, Siemeister G. Sp1 recognition sites in the proximal promoter of the human vascular endothelial growth factor gene are essential for platelet-derived growth factor-induced gene expression. Oncogene. 1997;15:669–676. doi: 10.1038/sj.onc.1201219. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Xie M, Yang J, et al. The expression of antiapoptotic protein survivin is transcriptionally upregulated by DEC1 primarily through multiple sp1 binding sites in the proximal promoter. Oncogene. 2006;25:3296–3306. doi: 10.1038/sj.onc.1209363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J, Ling X, Pan D, et al. Molecular mechanism of inhibition of survivin transcription by the GC-rich sequence-selective DNA binding antitumor agent, hedamycin: evidence of survivin down-regulation associated with drug sensitivity. J Biol Chem. 2005;280:9745–9751. doi: 10.1074/jbc.M409350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chintharlapalli S, Papineni S, Ramaiah SK, Safe S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007;67:2816–2823. doi: 10.1158/0008-5472.CAN-06-3735. [DOI] [PubMed] [Google Scholar]
- 39.Abdelrahim M, Baker CH, Abbruzzese JL, Safe S. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst. 2006;98:855–868. doi: 10.1093/jnci/djj232. [DOI] [PubMed] [Google Scholar]
- 40.Chintharlapalli S, Papineni S, Abdelrahim M, et al. Oncogenic microRNA-27a is a target for anticancer agent methyl 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate in colon cancer cells. Int J Cancer. 2009;125:1965–1974. doi: 10.1002/ijc.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chadalapaka G, Jutooru I, Chintharlapalli S, et al. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–5354. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chadalapaka G, Jutooru I, McAlees A, Stefanac T, Safe S. Structure-dependent inhibition of bladder and pancreatic cancer cell growth by 2-substituted glycyrrhetinic and ursolic acid derivatives. Bioorg Med Chem Lett. 2008;18:2633–2639. doi: 10.1016/j.bmcl.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clem AL, Hamid T, Kakar SS. Characterization of the role of Sp1 and NF-Y in differential regulation of PTTG/securin expression in tumor cells. Gene. 2003;322:113–121. doi: 10.1016/j.gene.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen RN, Huang YH, Yeh CT, Liao CH, Lin KH. Thyroid hormone receptors suppress pituitary tumor transforming gene 1 activity in hepatoma. Cancer Res. 2008;68:1697–1706. doi: 10.1158/0008-5472.CAN-07-5492. [DOI] [PubMed] [Google Scholar]
- 46.Abdelrahim M, Safe S. Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expession in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Mol Pharmacol. 2005;68:317–329. doi: 10.1124/mol.105.011825. [DOI] [PubMed] [Google Scholar]
- 47.Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. Eur J Cancer. 2005;41:2438–2448. doi: 10.1016/j.ejca.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Oh JE, Han JA, Hwang ES. Downregulation of transcription factor, Sp1, during cellular senescence. Biochem Biophys Res Commun. 2007;353:86–91. doi: 10.1016/j.bbrc.2006.11.118. [DOI] [PubMed] [Google Scholar]
- 49.Papineni S, Chintharlapalli S, Abdelrahim M, et al. Tolfenamic acid inhibits esophageal cancer through repression of specificity proteins and c-Met. Carcinogenesis. 2009;30:1193–1201. doi: 10.1093/carcin/bgp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papineni S, Chintharlapalli S, Safe S. Methyl 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate is a peroxisome proliferator-activated receptor γ agonist that induces receptor-independent apoptosis in LNCaP prostate cancer cells. Mol Pharmacol. 2008;73:553–565. doi: 10.1124/mol.107.041285. [DOI] [PubMed] [Google Scholar]