Abstract
The amyloid hypothesis (AH) of Alzheimer’s disease (AD) posits that the fundamental cause of AD is the accumulation of the peptide amyloid beta (Aβ) in the brain. This hypothesis has been supported by observations that genetic defects in amyloid precursor protein (APP) and presenilin increase Aβ production and cause familial AD (FAD). The AH is widely accepted but does not account for important phenomena including recent failures of clinical trials to impact dementia in humans even after successfully reducing Aβ deposits.
Herein, the AH is viewed from the broader overarching perspective of the myelin model of the human brain that focuses on functioning brain circuits and encompasses white matter and myelin in addition to neurons and synapses. The model proposes that the recently evolved and extensive myelination of the human brain underlies both our unique abilities and susceptibility to highly prevalent age-related neuropsychiatric disorders such as late onset AD (LOAD). It regards oligodendrocytes and the myelin they produce as being both critical for circuit function and uniquely vulnerable to damage. This perspective reframes key observations such as axonal transport disruptions, formation of axonal swellings/sphenoids and neuritic plaques, and proteinaceous deposits such as Aβ and tau as by-products of homeostatic myelin repair processes. It delineates empirically testable mechanisms of action for genes underlying FAD and LOAD and provides “upstream” treatment targets. Such interventions could potentially treat multiple degenerative brain disorders by mitigating the effects of aging and associated changes in iron, cholesterol, and free radicals on oligodendrocytes and their myelin.
Keywords: Aging, Oligodendrocyte, Peroxisome, BACE, Neuregulin, Apolipoprotein, Prevention, Ubiquitin, α-Synuclein, TDP-43, FTLD
1. Introduction
The myelin model of the human brain proposes that the processes of myelin development, maintenance, and its eventual breakdown are essential to understanding our species’ unique cognitive and behavioral trajectories through life (Fig. 1). The model’s lifespan perspective delineates the interplay between the continuous developmental process of myelination and degenerative processes acting on several prominent vulnerabilities of oligodendrocytes and the myelin they sustain. These vulnerabilities make oligodendrocytes and their myelin the “weakest link” that will succumb to a variety of suboptimal genetic variants and environmental insults. The model proposes that the production, maintenance, and repair of the human brain’s pervasive myelin sheaths underlie our species’ unique vulnerability to highly prevalent neuropsychiatric disorders ranging from schizophrenia to degenerative disorders such as Alzheimer’s disease (AD) (Bartzokis, 2002, 2004a,b, 2005).
Fig. 1.
Quadratic (Inverted U) Trajectories of Human Brain Myelination Over the Lifespan. Myelination (Y-axis) versus age (X-axis) in frontal lobes of normal individuals. Left panel: in vivo MRI data (Bartzokis et al., 2001) using inversion recovery images that are most sensitive to the high cholesterol levels in myelin and are optimal for tracking myelination. Right panel: postmortem myelin stain data of frontal lobe cortex depicting intracortical myelination (from Kaes, 1907 adapted and reproduced in Kemper, 1994). The data were acquired 100 years apart yet the two samples of normal individuals show remarkably similar frontal lobe myelination trajectories, both reaching a peak at age 45. A very similar matching pattern (not shown—see Bartzokis, 2007) is observed in temporal lobe.
The myelin model helps integrate congruous as well as incongruous aspects of familial AD (FAD) and late onset AD (LOAD) phenomenology into a continuum of later-life cognitive decline that leads to the very high prevalence of AD observed in the older ages of the human lifespan. Aging- and disease-related myelin damage is viewed in the context of the brain’s continual homeostatic attempts to repair such damage. In what follows, the genetic defects that gave rise to the amyloid hypothesis (AH) of AD namely, amyloid precursor protein (APP) and γ-secretase complex and its presenilin (PS1 and PS2) mutations, will be examined from the perspective of myelin maintenance and repair processes. The roles of genes that affect the much more prevalent LOAD, namely β-site APP cleavage enzyme 1 (BACE1), Apolipoprotein E (ApoE) alleles, tau, ubiquitin, TAR DNA binding protein 43 (TDP-43), and iron-regulated proteins that include APP and α-synuclein (αSyn), will also be assessed from this same perspective.
By necessity this report will examine the often ignored, but ultimately inescapable, role of evolution. Evolution has shaped the human brain through the use of pre-existing genes and their protein products for multiple roles and functions (Jacob, 1977). This multiplicity of roles results in the dazzling complexity and redundancy on which normal brain function is based. Myelin, a relatively recent evolutionary development of the first vertebrates (fish), plays a critical role in the complex connectivity of the human brain (Bartzokis, 2004a,b). The model suggests that myelin maintenance and repair employ the same molecular processes involved in producing the proteinaceous lesions that define prevalent disorders such as AD, Parkinson’s diseases (PD), dementia with Lewy bodies (DLB), and frontotemporal lobar degeneration (FTLD). It asserts that the age-related increase in the need for maintenance and repair (Fig. 1), together with genetic variability in the efficiency and effectiveness of these processes, secondarily result in the production of the lesions that define these diseases (Figs. 2 and 3).
Fig. 2.
Myelin repair.
(A) Myelin is “cut” to reveal normal fast axonal transport (FAT).
(B) Myelin damage, FAT disruption, and axonal swelling phenotype of repair process.
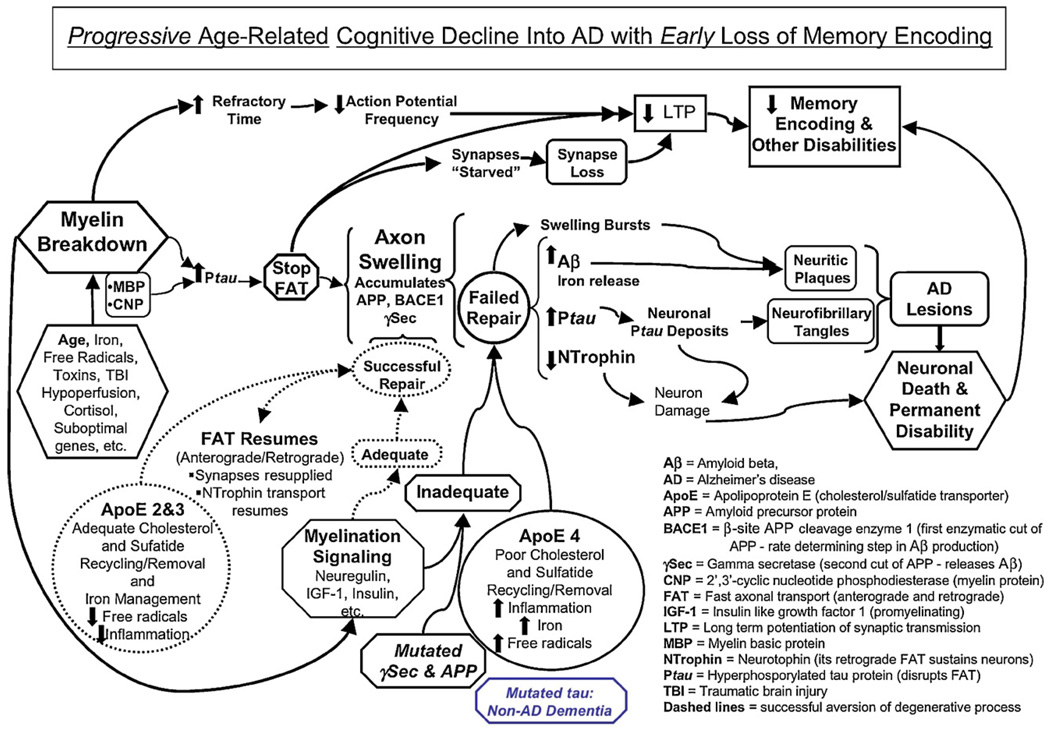
Fig. 3.
Progressive age-related cognitive decline into AD with early loss of memory encoding.
This wider evolutionary perspective serves to counterbalance the prevailing focus on Aβ as the core pathology of AD and reframes this key protein (as well as tau) as a byproduct of the myelin repair process rather than the principal cause of AD. The model helps explain why the first three large scale attempts to treat human AD by removing Aβ using active immunization (Holmes et al., 2008), reducing Aβ production by inhibiting the key γ-secretase enzymatic step (with Flurizam), and dissolving amyloid deposits (with Alzamed) have failed to impact the clinical syndrome, even though they may have succeeded in eliminating brain amyloid (Holmes et al., 2008). The model ultimately helps outline novel prevention and treatment interventions (Bartzokis and Altshuler, 2003; Bartzokis, 2004a, 2007), and cautions against several pitfalls associated with the massive pharmacological discovery effort focused on directly influencing the amyloidogenic pathway in the brain (Patton et al., 2006).
1.1. Why an overarching hypothesis of human brain “Alzheimerization”?
It has become increasingly apparent that the major age-related degenerative brain disorders represent co-deposition of several proteins such as Aβ, tau, αSyn, TDP43, etc. (Duyckaerts et al., 2009; Jellinger, 2009; Nelson et al., 2007; Schneider et al., 2007) (see Sections 6 and 7 below). At a minimum, AD itself represents a co-deposition of at least two proteins, Aβ and tau (reviewed in Duyckaerts et al., 2009; Jellinger, 2009). These multiple pathologies can reduce the relationship of any single lesion type to clinical symptoms (Nelson et al., 2007) and has resulted in calls for reconsidering the current pathologic diagnostic criteria (Jellinger, 2009). The myelin model considers brain circuits in their entirety (Figs. 2 and 3) and offers a viable mechanistic approach that complements and integrates the lesion-based pathologic approach as well as the genetic approach that forms the basis the AH.
The overarching mechanistic/evolution-based approach of the myelin model can be exemplified by briefly considering it in the context of recent modifications to the AH. Increased amyloid Aβ deposition is the ultimate manifestation of known forms of FAD and is an important process in the pathogenesis of AD (Hardy and Selkoe, 2002; Selkoe, 1999; Thal et al., 2002). The AH was modified to account for the weak association between neuritic plaque lesion load and AD symptoms (reviewed in Duyckaerts et al., 2009) as well as lack of correlation with rates of brain atrophy (Jack et al., 2009; Josephs et al., 2007). It also needed to account for the early manifestation of memory impairment and failure of amyloid plaque-removing treatments to alter dementia symptoms despite possibly successfully mitigating brain amyloid burden (Holmes et al., 2008). This modification suggests that before their deposition into neuritic plaques, soluble oligomeric forms of Aβ cause synapse loss and/or interfere with long-term potentiation (LTP) of synaptic transmission that underlies memory formation (Selkoe, 2002, 2008; Shankar et al., 2008; Townsend et al., 2006). It makes synaptic function paramount and draws support from the observation that the best-known correlate of cognition in AD seems to be synaptic number (DeKosky and Scheff, 1990; Terry et al., 1991).
The myelin model does not discount contribution of toxic species such as Aβ (as well as tau–see Section 6 below) to the overall pathology, but rather proposes overarching “upstream” mechanisms that trigger both Aβ and tau production, oligomerization, and deposition (Figs. 2 and 3). The new focus of the reformulated AH on synapses is rational given their essential role in cognition and memory and evidence that these structures can be lost when exposed to Aβ oligomers (Lacor et al., 2007; Selkoe, 2008; Shankar et al., 2008). Synapses are however extremely “plastic” (routinely lost and reacquired, McEwen et al., 2001; Radley et al., 2008), are lost in a variety of other disorders (Fiala et al., 2002), and are often sacrificed in old age (even in healthy individuals, Masliah et al., 1993; Peters et al., 2008a) in the attempt to repair myelin and axon damage/deafferentation (Fiala et al., 2002; Lacor et al., 2007) (see Sections 4 and 5 below, Figs. 2B and 3). Thus, synapse-based explanations of cognitive function have limited specificity. This is further exemplified by considering the process of brain maturation that builds functional circuits and includes axons and myelin in addition to synapses. The normal “pruning” (30–40% loss) of synapses in late childhood and teenage years (when AD is almost never observed) does not result in dementia. This profound loss of synapses can be re-conceptualized as a key permissive step for the myelination-driven cognitive and behavioral development of humans on their way to becoming healthy adults (Bartzokis, 2005). The pruning may well be necessitated by the fact that in late childhood the human skull becomes rigid, ending further brain expansion. Thus, in order for myelin expansion to continue into adulthood (Fig. 1), synapses and axons are sacrificed. This allows the brain to reach maturity in middle age when myelin content reaches its peak at approximately 25% of brain volume (Bartzokis, 2005; Bartzokis and Lu, 2009).
An overarching hypothesis should also help explain major clinical manifestations such as the primacy of memory impairment (Fig. 3) as well as the extreme prevalence of LOAD with age and its unique human manifestation. LOAD represents over 95% of all AD, exponentially increases after age 65 (doubling every 5 years), and becomes so prevalent that it afflicts >50% of the population over age 85. LOAD could therefore almost be considered a “physiologic” part of human brain aging, as age itself is its most important risk factor. This powerful age-dependent phenomenon of brain “Alzheimerization” (the deposition of protein aggregates (extracellular neuritic plaques containing Aβ and intracellular neurofibrillary tangles containing hyperphosphorylated tau)), which is the pathologic definition of AD (Duyckaerts et al., 2009), is by in large a process observed primarily in humans (Breteler et al., 1992; Walker and Cork, 1999). Brain Alzheimerization is not fully replicated in non-human primates in whom amyloid deposits are less prevalent/pronounced (Peters et al., 1991; Walker and Cork, 1999) and tau deposits are almost never observed (Lemere et al., 2008).
This unusual human predisposition to develop LOAD occurs despite the human species evolving new Apolipoprotein E (ApoE) alleles for brain cholesterol transport (namely ApoE3 and E2) that replaced (in >80% of the population) the ancestral (primate) ApoE4 allele (Fainman et al., 2007; Finch and Morgan, 2007; Mahley and Rall, 2000). ApoE4 is the sole allele present in non-human primates who nevertheless do not develop AD. Despite its presence in less than 20% of the human population however, ApoE4 accounts for as much as 50% of the genetic risk of AD (Ashford, 2004; Raber et al., 2004) and the great majority of LOAD cases with an onset before age 80 (Raber et al., 2004). In short, an overarching hypothesis that goes beyond the AH is needed to help explain why LOAD is essentially a uniquely human disease and why old age and alleles of the principal brain cholesterol transport protein (ApoE) are the first and second most powerful risk factors (Raber et al., 2004). Such a hypothesis should also help explain the common co-occurrence in FAD and LOAD of several other protein co-deposits such as tau, αSyn (the primary protein deposit in the Lewy bodies observed in DLB and PD), as well as other proteins (see Sections 6 and 7 below) (reviewed in Jellinger, 2009).
Many additional important AD phenomena should also be encompassed. These include the contribution of traumatic brain injury and vascular disease to the risk of LOAD, the prominence of axonal versus the relative scarcity of dendrite involvement in dystrophic neurites, and the largely cortical localization of Aβ containing neurites. Finally, it should help explain why myelinated projection cortico-cortical neurons are the ones primarily affected by the disease and why AD lesions spread across the brain in a bilateral and symmetric pattern. This bilateral spread pattern is central to the clinical devastation caused by the diseases as it overcomes the bilateral redundancy of brain neural circuitry. Braak and Braak (1996) pointed out that the spread pattern of AD lesion recapitulates the myelination pattern in reverse. Later-myelinating temporal and frontal lobe regions develop lesions first while early-myelinating regions such as the motor and sensory systems are spared until late disease stages (Bartzokis, 2004a; Bartzokis et al., 2004b, 2007b). This process of developmental recapitulation is echoed in the clinical progression of the cognitive, functional, and neurologic declines from later-acquired functions and memories to earlier ones (Reisberg et al., 2002).
1.2. The myelin model and Internet analogy of the human brain
The extensive scope and quantity of myelination is arguably the single-most important aspect in which the human brain differs from that of other species. This includes non-human primates (Schoenemann et al., 2005; Semendeferi et al., 2002) with whom we share all other brain components from cell types to neurotransmitters and synapses (Bartzokis, 2004a). The myelin model reframes many uniquely human developmental phenomena and disease manifestations within its context (Bartzokis, 2002, 2004a,b, 2005, 2007; Bartzokis et al., 2007a,b). It delineates testable hypotheses that could facilitate our understanding of the pathophysiology of multiple brain disorders by examining “endophenotypes” that represent more basic shared processes ranging from metabolism (Kadish et al., 2009; Ravera et al., 2009) to maintenance, transport, and repair processes (see Sections 4–6, Figs. 2 and 3). Such basic mechanisms are at the core of maintaining brain circuit function.
The model’s central premise is that the developmental trajectory of myelination, continual metabolic investment in its maintenance and repair, and its eventual age-related breakdown, forms the essence of our very uniqueness as a species across all life stages. The model frames the human lifespan in terms of seamless quadratic-like (inverted U) myelination trajectories of the many spatially distributed neural networks that underlie cognition and behavior (Fig. 1). This perspective cuts across current symptom-based classifications of neuropsychiatric diseases. It suggests that both developmental deficits in myelination of neural circuits (contributing to classic psychiatric disorders, Bartzokis, 2002, 2005), as well as degenerative breakdown and loss of myelin of the same circuits, can result in similar behavioral and cognitive symptoms despite entirely different etiologies (Bartzokis, 2004a,b). The case will be made herein that myelin maintenance and repair endophenotypes are upstream of and initiate pathophysiologic mechanisms that produce degenerative diseases such as AD. The current classification and diagnostic schema that use non-specific cognitive and behavioral syndromes as well as proteinaceous lesion-based postmortem definitions (reviewed in Jellinger, 2009) will be reconceptualized as end-products of this myelin-based pathophysiology (Figs. 2 and 3).
The myelination process is essential for many uniquely human brain functions such as language, higher cognition, and complex behaviors that are dependent on extremely fast information processing. These functions rely on high axonal conduction speed, wide “bandwidth” (highest frequency of action potentials that can be supported by the axons of a circuit, which is dependent on shorter refractory times achieved by myelin) and, when the entire circuit is myelinated in adolescence and adulthood, immediate “on line” (as opposed to “dial up”) connectivity. To a large extent all these properties depend on myelin. Together, they provide rapid, high frequency, and precisely timed action potentials over the spatially distributed networks of our brain’s “Internet” and make our unique abilities possible (Bartzokis et al., 2001; Bartzokis, 2004a,b, 2005).
Homeostatic mechanisms to produce, maintain, and repair myelin are considered herein in light of the unique vulnerabilities of oligodendrocytes and their myelin. The genes involved in controlling brain myelination have only recently begun to be identified. The regulation of myelin thickness through neuregulin 1 (neuregulin) signaling (Michailov et al., 2004; Taveggia et al., 2008) (reviewed in Nave and Salzer, 2006) influences transmission speed and refractory time (Nashmi and Fehlings, 2001) and is therefore of specific interest. This regulation involves cleavage of neuregulin and its receptors by the BACE1 and γ-secretase complexes (Taveggia et al., 2008) (reviewed in Nave and Salzer, 2006), the same two enzymes involved in cleaving APP to produce Aβ. Within the myelin model’s framework, the genetic defects that underlie FAD and the AH will be reframed as undermining the myelin repair process and thus promoting homeostatic responses that secondarily accelerate the manifestation of AD pathology (Aβ and tau deposits in plaques and tangles,Figs. 2 and 3). As a prelude to examining the relationship of myelin maintenance and repair to the genes associated with FAD and LOAD, a synopsis on the unique aspects of oligodendrocytes and human brain myelination will follow next.
1.3. The unique attributes of oligodendrocytes and the myelin they produce
Oligodendrocytes are unique in at least five ways that are directly pertinent to the model. First, brain cholesterol is synthesized almost exclusively de novo by glia (primarily oligodendrocytes and astrocytes) (Dietschy and Turley, 2001; Saher et al., 2005) and peripheral cholesterol does not enter the brain (Bjorkhem and Meaney, 2004). The human brain, which is approximately 2% of the body by weight, contains approximately 25% of the body’s membrane cholesterol (Dietschy and Turley, 2001; Morell and Jurevics, 1996) and up to 80% of brain cholesterol is in myelin (Muse et al., 2001). Cholesterol does not bind as much water as the polar phospholipids in membrane bilayers. Thus, cholesterol enrichment in the outer membrane of myelin membrane bilayers reduces water binding and allows the juxtaposition and tight packing of membranes achieved in myelin sheaths. Cholesterol and its derivatives such as sulfatide (a myelin-specific lipid that is almost exclusively produced by oligodendrocytes and subsumed herein under “cholesterol”) are thus indispensable for myelination (Marcus et al., 2006; Saher et al., 2005). With the aid of ApoE, cholesterol can be “exported” to neurons (Rouser et al., 1972; Wood et al., 1999) and contribute to a variety of brain processes (including synaptogenesis) that are dependent on this essential membrane ingredient (Goritz et al., 2005; Mauch et al., 2001). Cholesterol is metabolically expensive to synthesize and brain cholesterol is extensively recycled from broken down myelin (Ando et al., 2003) for use in repair/production of new myelin and other membranes (Bartzokis et al., 2006a, 2007a; Bjorkhem and Meaney, 2004). This recycling depends on interactions between ApoE, ATP-binding cassette transporter A1 that lipidates ApoE, low-density lipoproteins (LDL) with which ApoE associates, and LDL receptors. The efficiency of this recycling gives brain cholesterol a half-life measured in years (Bjorkhem et al., 2006). Myelin and its components such as cholesterol and myelin proteins are reduced in old age and are substantially further reduced in mild cognitive impairment (MCI) and AD (Bartzokis et al., 2004b; Gottfries et al., 1996; Han et al., 2002; House et al., 2006; Roher et al., 2002; Sjobeck et al., 2005).
Second, oligodendrocytes have the highest iron content of all brain cell types (reviewed in Todorich et al., 2008) and as much as 70% of brain iron may be associated with myelin (de los Monteros et al., 2000; Quintana et al., 2006). This is not surprising given that cholesterol and lipid synthesizing enzymes require iron to function (Cheepsunthorn et al., 2001). Increased intracellular iron is essential for oligodendrocyte differentiation (Sow et al., 2006). Inadequate dietary iron during early development can result in poor myelination and associated mental deficiencies in human infants (Connor and Menzies, 1996; Roncagliolo et al., 1998) and young adults (Murray-Kolb and Beard, 2007). Brain iron levels increase with age (Bartzokis et al., 2007e; Hallgren and Sourander, 1958) and are further increased in degenerative diseases of old age such as AD (Bartzokis et al., 2000, 2007b; Oakley et al., 2007; Quintana et al., 2006).
Third, the production and maintenance of the myelin sheath(s) that is up to 600× the surface area of oligodendrocyte soma membrane and 100× the weight of the soma (Morell and Toews, 1984; Wiggins, 1982) makes the energy requirements of oligodendrocytes 2–3 fold higher than other brain cells (Connor and Menzies, 1996). This metabolic stress increases oligodendrocyte susceptibility to a variety of insults ranging from hypoperfusion and toxic products of activated microglia, other free radicals, to heavy metals and excitotoxicity (for review see Bartzokis, 2004b; Bartzokis et al., 2004a) (Fig. 3). The metabolic demands are even higher for precursors and oligodendrocytes that are actively myelinating axon segments. Precursors produce three times their own weight in membrane lipids each day (Wiggins, 1982) and are even more exquisitely vulnerable than mature cells (Gerstner et al., 2008; Zatta et al., 2005) (reviewed in Bartzokis, 2004a).
The vulnerability of myelin to toxins and especially oxidative damage has contributed to several adaptive compensations that mitigate those toxicities and the inflammation associated with them (Farooqui et al., 2007; Hirrlinger et al., 2002; Hulshagen et al., 2008). Oligodendrocytes are especially enriched in peroxisomes, organelles that help detoxify reactive oxygen species, and their peroxisomes may be superior to other brain cells at performing this function (Hirrlinger et al., 2002). Peroxisomes also produce plasmalogens (Pls) and myelin is especially enriched in Pls and the omega 3 fatty acids (especially docosahexaenoic acid (DHA)) they contain (Farooqui et al., 2007). Pls are unique phospholipids characterized by the presence of vinyl-ether bond at the sn-1 position of the glycerol backbone while the sn-2 bond is occupied by polyunsaturated fatty acid such as DHA (Brites et al., 2004; Khan et al., 2008; Van den Branden et al., 1990) whose synthesis from other omega-3 fatty acids is βoxidation-dependent and thus peroxisome-dependent (Goodenowe et al., 2007; Voss et al., 1991). The vinyl-ether bond acts as an endogenous antioxidant protecting DHA (Engelmann, 2004; Kuczynski and Reo, 2006) from oxidative damage (Engelmann, 2004; Sindelar et al., 1999). Furthermore, DHA metabolism generates neuroprotective/anti-inflammatory lipid byproducts that reduce myelin damage (Farooqui et al., 2007) and DHA may induce rate-limiting enzymes in Pl synthesis pathway (Andre et al., 2006b) and lipid transport (Leclercq et al., 2008). Peroxisome function and plasmalogen production is thus essential for adequate myelination (Hulshagen et al., 2008; Van den Branden et al., 1990). Given these properties it is not surprising that Pls seem most enriched in myelin (Farooqui et al., 2007). Brain myelin content appears to drive plasmalogen concentrations that peak in mid-life (Andre et al., 2006a; Rouser and Yamamoto, 1968; Weisser et al., 1997) following a similar quadratic lifespan trajectory as myelin (Bartzokis et al., 2001, 2003a; Kemper, 1994) and both decline in old age (Fig. 1) and are further reduced in AD (Farooqui et al., 1997; Favrelere et al., 2000; Ginsberg et al., 1995; Goodenowe et al., 2007; Guan et al., 1999; Murphy et al., 2000).
Fourth, oligodendrocytes are markedly heterogeneous based on when, during the protracted process of human brain development, they differentiated into myelin producing cells. Oligodendrocytes that differentiated late in life (Fig. 1) ensheath upwards of 50 smaller diameter axons as opposed to one oligodendrocyte myelinating a single axon segment of large, early-myelinating primary motor and sensory CNS neurons (Wood and Bunger, 1984). Compared to earlier-myelinating oligodendrocytes later-myelinating cells support thinner (Hof and Morrison, 1990;Lamantia and Rakic, 1990) and structurally more vulnerable myelin sheaths (Bartzokis et al., 2007b). In addition, later-differentiating oligodendrocytes have different lipid properties, myelin turnover, and reduced capacity for myelin repair than earlier differentiating cells (Bauer et al., 2002; Hildebrand et al., 1993; Nieuwenhuys, 1999; Power et al., 2002). Thus, later myelinating neurons of cortical association areas such as inferior temporal, frontal, and temporoparietal regions may be more susceptible to myelin breakdown (Kemper, 1994; Marner et al., 2003; Meyer, 1981b; Yakovlev and Lecours, 1967) and subsequent neuronal degeneration (see Sections 4 and 5 below) than early-myelinating neurons in the primary motor and visual areas (Bartzokis et al., 2007b). The thinner sheaths of these late-myelinating regions are differentially lost with age (27–45% reductions) in a pattern that is roughly bilateral and progressive (Bartzokis, 2004b; Bartzokis et al., 2006a; Kemper, 1994; Marner et al., 2003). In the context of the model, this developmentally-based continuum of increasing vulnerability may help explain the bilateral and progressive nature of AD lesion development and spread as well as the progression of functional impairments during aging described above (Bartzokis, 2004a; Bartzokis et al., 2004b, 2007b). Thus, during aging and gradual decline into AD, both the pathological proteinaceous lesions observed at postmortem (Braak and Braak, 1996) and clinical symptoms (Reisberg et al., 2002) roughly recapitulate the myelination pattern in reverse (Bartzokis, 2004a,b).
The fifth unique oligodendrocyte feature is especially important to degenerative diseases and attempts to reestablish homeostasis in the face of age-related myelin breakdown. Unlike neurons, whose numbers are essentially established at birth, in healthy primates oligodendrocytes continue to divide and increase their numbers across the lifespan by as much as 50% (O’Kusky and Colonnier, 1982; Peters and Sethares, 2004; Peters et al., 2008b) while remyelinating damaged or lost sheaths (Peters and Sethares, 2003; Peters et al., 2008b). The new myelin sheaths may however be more vulnerable in at least two ways. First, the sheath is thinner (Peters and Sethares, 2003; Peters et al., 2008b) and thus more susceptible to subsequent insults (Bartzokis, 2004a; Bartzokis et al., 2004b, 2007a). Second, the internodal length of repaired sheaths is shorter and thus the amount of perinodal myelin increases as myelin is repaired (Peters and Sethares, 2004; Peters et al., 2008b). Increased numbers of internodes (already higher in late- and thinly-myelinated cortical regions) further increase vulnerability (Sousa and Bhat, 2007) (see Figs. 1 and 2). Thus, the continual capacity of oligodendrocytes to repair and proliferate holds the promise of function restoration as well as the danger of increased vulnerability and toxicity as increased oligodendrocyte numbers are associated with increased iron levels (Bartzokis et al., 2007b,d). Increased numbers of oligodendrocytes and iron levels have been associated with aging (Bartzokis et al., 2007e; Hallgren and Sourander, 1958; O’Kusky and Colonnier, 1982; Peters and Sethares, 2004; Peters et al., 2008b) and degenerative diseases such as Huntington’s disease (reviewed in Bartzokis et al., 2007d). Elevated iron levels have also been observed in AD as well as PD (see Section 7) (Bartzokis et al., 2000, 2007b; Meadowcroft et al., 2009; Oakley et al., 2007; Quintana et al., 2006).
Continued oligogenesis and myelin repair may also “mask” the presence of myelin damage and the importance of repair mechanisms in age-related diseases such as AD. The dynamic nature of these homeostatic processes may help explain the under-appreciation of myelin breakdown and its consequences (such as interfering with axonal transport–see Section 5 and Fig. 2) (Praprotnik et al., 1996; Tsai et al., 2004) as an early event in the pathogenesis of degenerative brain diseases (reviewed in Bartzokis, 2004b; Bartzokis et al., 2004b, 2006b, 2007b,d). Before addressing these cellular changes however, the molecular basis of the AH and of Aβ production and the overlap with the genes and proteins underlying these homeostatic myelin repair processes will be examined next.
2. Aβ production and FAD genes in the context of the myelin model
2.1. Myelin and β-site APP cleavage enzyme 1
Aβ is produced by two acts of transmembrane cleavage of APP. The first step of amyloidogenic APP cleavage is carried out by a transmembrane aspartyl protease termed β-site APP cleavage enzyme 1. This required and rate-limiting step is followed by γ-secretase cleavage that results in the release of amyloidogenic forms of Aβ. The high neuronal levels of BACE1 activity coupled with low levels of anti-amyloidogenic BACE2 and α-secretase activity (that produces non-amyloidogenic APP cleavages) contribute to the accumulation of Aβ in the CNS, whereas other organs are spared (Laird et al., 2005). The exceptions to this general statement are muscles/neuromuscular junctions (Finch, 2006; Hirata et al., 2007; Lunemann et al., 2007) including those in blood vessel walls (Hellstrom-Lindahl et al., 2004; Qi et al., 2007). Both APP and its cleavage-related trophic signaling (through its breakdown products) are important in drosophila neuromuscular junctions (Torroja et al., 1999; Wang et al., 2005). This suggests that this signaling and adhesion system might have been “borrowed” from insects for an additional (non-synaptic) use in vertebrate myelination. Given the use of these same mechanisms in neuromuscular communication it is not surprising that Aβ accumulation is also associated with such different phenotypes as vascular amyloidosis (especially in APP mutation carriers) (Cabrejo et al., 2006) and inclusion body myositis (Finch, 2006) which will not be further considered herein.
In addition to APP, BACE1 cleaves neuregulin (Hu et al., 2008), a critical substrate involved in myelination signaling (Michailov et al., 2004; Taveggia et al., 2008) and possibly cholesterol synthesis (Pertusa et al., 2007). Like APP, neuregulin is a transmembrane protein. When cleaved by BACE1, neuregulin signals oligodendrocytes to myelinate (Hu et al., 2006; Nave and Salzer, 2006; Willem et al., 2006). The quantitative extent of myelination (thickness) seems to depend on BACE1 cleavage of neuregulin (Michailov et al., 2004; Taveggia et al., 2008) (reviewed in Nave and Salzer, 2006). The importance of BACE1 in myelination has been confirmed in BACE1-null mice (Hu et al., 2008; Savonenko et al., 2008) and the observation that BACE1 inhibitors (being developed to combat Aβ production) reduce myelin thickness (Hu et al., 2006; Willem et al., 2006).
The myelination of smaller and more thinly-myelinated fibers seems disproportionately dependent on neuregulin signaling compared to larger and more thickly-myelinated fibers (Michailov et al., 2004; Taveggia et al., 2008). This suggests that neuregulin signaling, and therefore BACE1 activation, may be especially important in repair of later- more thinly-myelinated regions where age-related myelin breakdown is most pronounced (Bartzokis et al., 2004b, 2006a, 2007a) (Fig. 1). In addition, when axons transition to an unmyelinated state, as occurs near neuronal bodies and synapses (both located exclusively in the gray matter), increased axonal membrane electrical capacitance can result in action potential failure. This problem can be overcome by shortening myelin segments in those regions (Nashmi and Fehlings, 2001). Shorter (and thinner) myelin segments are also produced by remyelination (Peters et al., 2008b) and may further increase vulnerability of intracortical myelin (Sousa and Bhat, 2007). Select regional vulnerability to myelin breakdown together with the BACE1/neuregulin homeostatic response to repair myelin can help explain why AD lesions first appear in later-myelinating intracortical locations (Bartzokis et al., 2007a,b; Braak and Braak, 1996). Recent data suggests that neuregulin also mediates cyclin-dependent kinase 5 and glycogen synthase kinase 3β (GSK3b) activity that increase tau phosphorylation and APP processing (Wen et al., 2008). As will be discussed in subsequent sections, this action is consistent with neuregulin’s pivotal role in myelin repair mechanisms that involve tau phosphorylation and APP processing (Figs. 2 and 3) in addition to its key function in the myelination signaling pathway described above.
2.2. BACE1 in development
The age-related changes in BACE1 brain levels further support its myelination signaling function. BACE1-null mice can develop into adults however, postnatal increases in BACE1 and APP are important for normal development of behavior and cognition (Ma et al., 2007). BACE1 has been shown to be vital in the development of cognitive, emotional, and synaptic functions such as LTP (Kobayashi et al., 2007; Laird et al., 2005) as well as motor and sensory function (Hu et al., 2006, 2008; Kobayashi et al., 2007). Although in some transgenic models of AD knocking out BACE1 ameliorated cognitive deficits (for review see Ohno et al., 2006), this observation is controversial since other studies show worsened memory function at earlier ages than would be expected (Kobayashi et al., 2007; Ma et al., 2007; Savonenko et al., 2008). In the context of the myelin model, these cognitive and behavioral deficits are regarded as secondary to reduced BACE1-dependent signaling that may stifle myelination.
Most importantly however, cognitive and behavioral deficits related to BACE1 inhibition should be substantially magnified in humans who, in the context of the model, are viewed as uniquely dependent on their more extensive myelination for normal function when compared to rodents and non-human primates (Bartzokis, 2004a, 2005; Bartzokis and Lu, 2009) (Fig. 1). This may help explain the absence of BACE1 mutations in FAD or LOAD despite its rate-determining role in Aβ production (McConlogue et al., 2007). The absence of AD-causing BACE1 mutations is especially striking when one considers the large number of genetic mutations (over 150) of other components of Aβ production (APP and γ-secretase presenilins) that are associated with FAD mutations (Wolfe, 2007) (see next section). Conversely, mutations affecting substrates of BACE1 that are involved in myelination (e.g., neuregulin) may contribute to developmental myelination deficits and are associated with uniquely human behavioral/cognitive disorders such as schizophrenia where myelination deficits are now well documented (Bartzokis et al., 2003b; Ho et al., 2003) (reviewed in Bartzokis et al., 2002; Dwork et al., 2007).
Given the importance of myelin, it is not surprising that the expression of BACE1, APP, and Aβ may be coordinated during the entire lifespan of mammals (Chiocco and Lamb, 2007; Marcinkiewicz and Seidah, 2000). The highest levels of these proteins occur in early post-natal development (Chiocco and Lamb, 2007; Loffler and Huber, 1992; Marcinkiewicz and Seidah, 2000) the period of maximal myelination (Muse et al., 2001; Willem et al., 2006). This suggests that all three proteins may have a role in the myelination process (see Sections 4 and 5). After this early developmentally-driven increase (2 fold), BACE1 protein levels decline to adult levels and then rise again in older age. In transgenic mice with elevated levels of human Aβ, only the older-age increases in these proteins are associated with Aβ deposition and plaque formation (Chiocco and Lamb, 2007; Marcinkiewicz and Seidah, 2000). These observations suggest that in addition to increased BACE activity and Aβ production, other age-related changes in brain (such as myelin breakdown and associated increases in iron levels—see Section 7) may be contributing to creating an environment in which Alzheimerization of the brain becomes increasingly likely (Bartzokis et al., 2007b,e; Roher et al., 2002).
The above observations help support the myelin model perspective of AD as a developmental disorder requiring myelination as an essential permissive step (Bartzokis, 2004a). This concept helps reframe the virtual absence of dementia and AD lesions before the third decade (even in FAD) as possibly consequent to insufficient vulnerable intracortical myelin for the Alzheimerization process to fully manifest (Bartzokis, 2004a; Bartzokis and Lu, 2009). After myelin levels peak in middle age (Bartzokis et al., 2001, 2003a; Benes et al., 1994; Kemper, 1994; Lintl and Braak, 1983), normal human aging is strongly associated with myelin breakdown and loss (Fig. 1) (Bartzokis et al., 2001, 2003a, 2007a; Benes et al., 1994; Kemper, 1994; Lintl and Braak, 1983; Marner et al., 2003). The “normal” aging-related myelin breakdown and loss can be detected with imaging in the fourth decade (Bartzokis et al., 2003a). This process is accelerated in presymptomatic individuals at increased genetic risk for FAD or LOAD (in ApoE4 carriers) (Bartzokis et al., 2006a, 2007a; Ringman et al., 2007). Thus, the most important risk factor for AD (age) and the second most important risk factor for LOAD (ApoE4) as well as genetic risk factors associated with FAD may all be associated with myelin breakdown (see Sections 2.3–4). The case is made herein that the subsequent compensatory myelin repair processes result in the pathologic manifestations of AD (Figs. 2 and 3). Imaging and postmortem studies suggest that unsuccessful repair processes are associated with the trajectory of decline into AD as both MCI and LOAD show more severe myelin damage than healthy older individuals (Bartzokis et al., 2003a; Chalmers et al., 2005; Chia et al., 1984; Englund and Brun, 1990; Han et al., 2002; House et al., 2006; Leow et al., 2009; Roher et al., 2002; Stricker et al., 2008; Terry et al., 1964) (reviewed in Bartzokis et al., 2004b).
2.3. Myelination and γ-secretase
The molecular effects on APP metabolism of the more than 150 FAD-causing γ-secretase (PS1 and PS2) mutations are not easy to predict resulting in controversy (Wolfe, 2007). Many FAD-causing PS1 mutations increase Aβ levels and easily “fit” the AH. An increase in the Aβ42/40 ratio has been used to explain the mechanism through which PS1 mutations that do not increase Aβ levels, can cause AD (Wolfe, 2007). This second explanation remains controversial as some PS1 mutations cause neither increased Aβ nor increased Aβ42/40 ratio (Dermaut et al., 2004; Maarouf et al., 2008; Shioi et al., 2007). The observation that different mutations cause different toxicities (Hashimoto et al., 2004; Maarouf et al., 2008) further undermines a unitary mechanistic explanation. Finally, a recent study suggests that an increased amount of β-site APP cleaved substrate (caused by the rate-limiting BACE1 cleavage of APP described above) drives the increased Aβ42/40 ratio observed in both PS1 mutations as well as in wild type PS1 (Yin et al., 2007) and reinforces the preeminence of the BACE1 cleavage of APP in the production of Aβ (McConlogue et al., 2007). Reexamination of these issues from the perspective of γ-secretase effects on myelination provides important insights.
In addition to neuregulin, myelination-related signaling involves several other proteins such as neuregulin receptors (ErbB4), insulin-like growth factor 1 (IGF-1), and membrane bound reticulon family members such as Nogo and Notch (Kasuga et al., 2007; Lee et al., 2002; McElroy et al., 2007; Nicolay et al., 2007). The relationship described in the previous section whereupon BACE1 has both APP and neuregulin as substrates, also exists between γ-secretase and these additional myelination-related proteins. They all seem to be γ-secretase substrates. The efficiency of myelination/remyelination could therefore be influenced by γ-secretase interacting with these proteins (see below). This is analogous to the situation described in the prior sections whereupon BACE1 interacting with its neuregulin substrate may influence the quantity of myelin deposited (Fig. 3). Recent examination of gene transcription patterns for both aging and AD showed a high co-expression of PS1 and canonical myelin proteins (Miller et al., 2008), supporting a possible association between PS1 and myelin repair (Figs. 2 and 3).
Disruptions/inefficiencies in myelin-related signaling caused by the many γ-secretase mutations are proposed to decrease the efficiency of myelination and/or myelin repair through multiple possible mechanisms. For example, ErbB4 undergoes a series of proteolytic steps, including γ-secretase cleavage (Lee et al., 2002) that releases the ErbB4 intracellular domain. This domain translocates to the nucleus (Lee et al., 2002; Ni et al., 2001) and may promote myelin formation (Lai and Feng, 2004). Inadequate myelination/myelin repair signaling (due to PS1 mutations for example) could be expected to undermine repair/remyelination attempts, trigger compensatory upregulation of BACE1, and thus increase amyloidogenic APP processing. This could increase the substrate for the subsequent γ-secretase processing of APP and thus further increase Aβ and Aβ42/40 ratio (Yin et al., 2007). Indirectly supporting this scenario, transgenic mouse models have demonstrated that FAD-associated PS1 mutations increase oligodendrocyte vulnerability to a variety of toxic and nutritional insults (Pak et al., 2003a,b) as well as promote myelin and axonal damage (Delatour et al., 2006; Wirths et al., 2006). Furthermore, decreased γ-secretase function may interfere with endocytosis of lipoprotein particles (Tamboli et al., 2008) that could reduce debris clearance needed in myelin repair (see next section).
The possibility that inefficient γ-secretase function disrupts myelination is further reinforced by observations that insulin-like growth factor 1 (IGF-1) receptor as well as the structurally homologous insulin receptor are also γ-secretase substrates (Kasuga et al., 2007; McElroy et al., 2007). IGF-1 is a powerful promoter of myelination (Carson et al., 1993; Goddard et al., 1999) and inhibitor of oligodendrocyte apoptosis during primary demyelination (Mason et al., 2000). Most if not all myelination-related actions of IGF-1 are mediated by its receptor (Mason et al., 2003; Zeger et al., 2007). As was the case with neuregulin signaling (Michailov et al., 2004; Taveggia et al., 2008) the myelination of smaller and more thinly-myelinated fibers may be disproportionately dependent on IGF-1 signaling compared to larger and more thickly-myelinated fibers (Ye et al., 1995).
The promyelination effects of IGF-1 (Zeger et al., 2007) are observed both in white matter tracts such as the corpus callosum and in cortical and hippocampal gray matter regions, and are evident even in the face of myelotoxins (Wood et al., 2007) as well as nutritional deficiencies known to reduce myelination (Chowen et al., 2002; Ye et al., 2000) (reviewed in Chesik et al., 2008). Conversely, dysregulated IGF-1 processing has deleterious consequences and results in marked hypomyelination (Beck et al., 1995). IGF-1 receptor triggered mechanisms are essential for myelination, remyelination, and their timing (Freude et al., 2008; Mason et al., 2003; Zeger et al., 2007). Dysregulation of these pathways are associated with poor neurocognitive performance in functions impacted by age (Dik et al., 2003; van Dam and Aleman, 2004), and reduced serum IGF-1 levels seem to correlate with aging-associated cognitive decline (van Dam and Aleman, 2004). This is not surprising since peripheral infusion of IGF-1 has been shown to enter the brain and may increase cortical oligodendrocytes (Aberg et al., 2007).
The molecular-level elucidation of γ-secretase effects on myelination remains incomplete (Jurynczyk et al., 2008; Watkins et al., 2008). Given its influence in multiple aspects of myelination signaling however, different mutations can be expected to have different effects on myelin repair and could help explain the inconsistent effects γ-secretase mutations have on Aβ production summarized above (Dermaut et al., 2004; Maarouf et al., 2008; Shioi et al., 2007; Wolfe, 2007).
The proposed myelin-based hypothesis for the association between PS1 mutations and premature manifestations of degenerative diseases such as occurs in FAD is also indirectly supported by human imaging data. Several examples of white matter abnormalities in symptomatic PS1 mutation carriers have been published (Dermaut et al., 2004; Marrosu et al., 2006; O’Riordan et al., 2002). Furthermore, a recent study of presymptomatic PS1 FAD mutation carriers who were compared to their non-carrier family members demonstrated that PS1 carriers have lower white matter integrity in late-myelinating regions and lower white matter track volumes compared to their non-mutation carrier relatives (Ringman et al., 2007). At this early presymptomatic stage of the disease process, neither brain nor hippocampal atrophy (structural biomarkers for AD) was observed (Ringman et al., 2007). This supports the suggestion that myelin breakdown and loss precedes brain atrophy (Bartzokis et al., 2003a; Bartzokis, 2004a). Decreased white matter integrity parallels similar findings in LOAD subjects as well as asymptomatic subjects at increased risk for LOAD on the basis of ApoE4 genotype presence (Bartzokis et al., 2006a, 2007a; Persson et al., 2006) (see next section).
3. Membrane cholesterol and LOAD genes: ApoE and low-density lipoprotein receptors (LDLr)
After age itself, ApoE is the second most important risk factor for LOAD and by far its single most important genetic factor (Ashford, 2004; Raber et al., 2004). ApoE is a secreted glycoprotein that associates with lipoproteins and mediates uptake of these lipid-rich particles into cells via receptor-mediated endocytosis by the LDL receptor family (Rebeck et al., 2006). In brain, ApoE is the primary transporter of endogenously produced brain lipids such as cholesterol and sulfatide (Dietschy and Turley, 2004; Poirier, 2003; Puglielli et al., 2003; Vos et al., 1994) that are essential for myelin production, function, and integrity (Dietschy and Turley, 2004; Marcus et al., 2006; Saher et al., 2005; Vos et al., 1994).
ApoE may be the key to the process of “recycling” lipids released in degrading damaged myelin or other membranes for reuse in rapid membrane biogenesis during remyelination (Boyles et al., 1989; Goodrum, 1991; Kitagawa et al., 2001; Posse De Chaves et al., 2000). Recycling of cholesterol and other essential lipid membrane building blocks may depend on transport by ApoE molecules (Boyles et al., 1989; Goodrum, 1991; Kitagawa et al., 2001; Rebeck et al., 1998).
Individuals with ApoE4 alleles (ApoE4+) have fewer ApoE molecules compared to those without an ApoE4 allele (ApoE4−) (Larson et al., 2000; Poirier, 2005). A similar quantitative gradient (ApoE2 > ApoE3 >ApoE4) is observed in CSF of transgenic mice that had these human ApoE alleles knocked in (Riddell et al., 2008). This quantitative difference may be further magnified by more effective delivery of cholesterol by ApoE3 compared to ApoE4 (Gong et al., 2002). In the face of a generalized and accelerating age-related myelin breakdown process (Bartzokis et al., 2003a; Peters et al., 1996; Peters, 2002) (Fig. 1), a reduced capacity to mobilize these essential lipids and recycle them into myelin repair processes may be a critical disadvantage for human ApoE4+ allele carriers. This impaired myelin repair is especially critical for the maximally myelinated middle-aged human brain (Fig. 1). Impaired myelin repair was proposed to mediate the increased age-related decline of myelin integrity and cognitive performance of healthy ApoE4+ carriers and result in their increased rates of AD (Bartzokis et al., 2001, 2003a, 2006a, 2007a, 2008) (Fig. 3).
This same reduced myelin repair ability may help explain the poor outcome of ApoE4+ individuals to a wide variety of brain insults. ApoE4 genotype seems to be associated with worse outcomes in immune disorders such as MS, brain infections such as HIV, strokes (ischemic and hemorrhagic), traumatic brain injuries (reviewed in Bartzokis et al., 2006a, 2007a), as well as age-related neurodegenerative diseases such as AD, PD, DLB, and amyotrophic lateral sclerosis (ALS) (Borroni et al., 2006; Corder et al., 1993; Li et al., 2004; Zareparsi et al., 2002). These associations are supported by murine ApoE knockout models of similar injuries and disorders (reviewed in Laskowitz and Vitek, 2007). These animal models have also provided evidence that the newly evolved human alleles (ApoE 3 and 2) are advantageous for brain repair when compared to ApoE4 (Crawford et al., 2009; Maezawa et al., 2006; Yao et al., 2004). Finally, in AD transgenic mice, reduced brain Alzheimerization is observed when human ApoE 3 and 2 is “knocked in” compared to ApoE4 knockins and wild type mice (Fagan et al., 2002; Holtzman et al., 2000).
With its focus on myelin repair, the model helps provide a parsimonious explanation for the phenomena described in this section. It was proposed that these findings relate to the differential abilities (poor in ApoE4+ and enhanced in ApoE2+ individuals) to recycle lipid debris for reuse in myelin repair (Bartzokis et al., 2006a). The removal of myelin sulfatide debris is also ApoE-dependent (Cheng et al., 2008b) and critically important for successful repair since this particular myelin lipid is highly inflammatory (Halder et al., 2007; Kanter et al., 2006). The difference in repair efficiency is hypothesized to mitigate the different cognitive decline trajectories associated with normal aging (Bartzokis et al., 2007a; Blacker et al., 2007; Packard et al., 2007). This repair difference may also help explain the epidemiologic observation that prevalence of the ApoE2 alleles in healthy individuals increase in older age concomitant with a decreased prevalence of healthy individuals with ApoE4 as the vast majority of the latter develop AD before age 85 (Raber et al., 2004). In short, the myelination of brain cognitive circuitry remains most functional in ApoE2 carriers partially independent of the increasing plaque and tangle load (Bartzokis et al., 2007a; Berlau et al., 2009) that may accumulate as a byproduct of the continual process of myelin repair (see Sections 5–6 and Figs. 2 and 3). Finally, the protective effect of ApoE2 (Bartzokis et al., 2006a, 2007a; Blacker et al., 2007) is observed despite an apparent increased risk for vascular ischemic changes associated with this allele (Lemmens et al., 2007; Schmidt et al., 1997) that would tend to “mask” its beneficial effects on myelin repair and cognition.
4. The roles of APP and its processing in the context of the myelin model
The complex architecture, synaptic requirements, and lack of protein synthesis in axons and synapses render neuronal cells extremely dependent on fast axonal transport (FAT), a bidirectional process powered by energy-requiring motors (kinesins for anterograde and dyneins for retrograde transport). Almost everything from mitochondria (for energy) to neurotransmitter vesicles must be anterogradely transported down axons to synapses. Conversely, damaged mitochondria destined for destruction, and products such as neurotrophin signaling molecules that are essential for neuronal survival, need to be retrogradely transported from synapses back to neuron body (Dorsey et al., 2006; Goritz et al., 2005; Mauch et al., 2001; Salehi et al., 2006) (Fig. 2A). Genetic or environmental disruptions of the anterograde kinesin as well as the retrograde dynein protein motors directly contribute to a range of neurodegenerative diseases such as hereditary spastic paraplegia (Connell et al., 2008) and Charcot-Marie-Tooth disease (Duncan and Goldstein, 2006). FAT disruptions are also likely involved in age-related neurodegenerative diseases such as AD (Morfini et al., 2005; Wishart et al., 2006) (see next section). The dependence of synapses on FAT also means that synaptic deficits, often observed with normal aging (Masliah et al., 1993; Peters et al., 2008a) as well as very early in the process of several degenerative diseases including AD (DeKosky and Scheff, 1990; Terry et al., 1991), could be secondary to FAT disruption (Fiala et al., 2002; Wishart et al., 2006) (Figs. 2B and 3).
Although the functions of APP remain to be fully elucidated, APP has an adhesion role in neuromuscular junctions (Wang et al., 2005). The initial evolution of APP may have been related to synapse adhesion and cholinergic transmission at neuromuscular junctions in both invertebrates (Torroja et al., 1999) and later in vertebrates (Wang et al., 2005). APP also has a key adhesion role in the FAT process itself. APP adheres the vesicles transported by FAT to the energy-requiring motors that propel them down axons on microtubule “tracks” towards the synapses (Kamal et al., 2001) (Fig. 2). BACE1 and PS1 are also transported in these APP-anchored vesicles (Kamal et al., 2001; Sheng et al., 2003). Additional roles of APP in membrane adhesion may involve maintenance of both pre- and post-synaptic adhesion as well as axonal-myelin adherence at perinodal regions (Ikeda and Tomonaga, 1990; Sapirstein et al., 1994; Wang et al., 2005; Wang and Ha, 2004; Yang et al., 2005; Zheng and Koo, 2006) that are highly vulnerable structures (Sousa and Bhat, 2007). Thus, changes associated with mutated APP could degrade its function/availability independently of Aβ production (Stokin et al., 2008) and could undermine vulnerable perinodal regions as well as the efficiency of FAT transport that is required in maintenance and repair processes (see next two sections).
Increased production of wild type APP (as occurs in Trisomy 21 and other APP gene triplications) can degrade axonal transport and cognition in several animal models (Dorsey et al., 2006; Rusu et al., 2007; Salehi et al., 2006; Stokin et al., 2008) and increased intracellular oligomeric Aβ could also directly contribute (Pigino et al., 2009). Deficits in myelin development are observed in trisomy 21 (Vlkolinsky et al., 2001). The timeline of the trisomy 21 axonal endophenotype is roughly consistent with a myelin repair deficit. It begins with axonal sphenoids and dystrophic neurites in the second decade followed by evidence of myelin degeneration in young adults with both sphenoids and neurites increasing with age (Dickson et al., 1990; Mattiace et al., 1991; Migheli et al., 1992). Not surprisingly the impact is greatest in late-myelinating regions that seem to have low levels of myelin-specific proteins in trisomy 21 as well as LOAD (Vlkolinsky et al., 2001) (see next section).
In the context of the myelin model, the co-transport by FAT of three components of the myelination machinery (BACE1, PS1, and APP) is suggestive of a necessary and coordinated delivery to specific axonal regions in need of myelin repair. As described above, signaling for myelination may depend on BACE1 and PS1 while adhesion processes of the newly formed myelin onto axons and “repair supply” vesicles may depend partly on APP (Wang and Ha, 2004; Zheng and Koo, 2006) (Fig. 2). The exact axon-glial signaling through which specific regions of axon are selected for myelin repair may involve myelin-specific proteins (see next section). Substantial evidence has accumulated that the mechanism through which the myelin repair process is executed involves stopping/slowing axonal transport mechanisms and especially FAT. Since axon and synapse maintenance depend on FAT, the slowing/interruption of FAT to repair myelin suggest that, in organisms that lay down myelin (vertebrates), evolution may have selected myelin health/function over synaptic health/function (Figs. 2B and 3). Thus, myelin receives the highest “repair priority” reinforcing the suggestion that myelin holds a preeminent role in information fidelity and functional connectivity of vertebrate CNS (Bartzokis, 2004b, 2005).
5. Fast axon transport and axon-glial communication in the myelin repair process
Oligodendrocyte and myelin abnormalities can slow or stop axonal FAT (Edgar et al., 2004b; Garbern et al., 2002; Kassmann et al., 2007; Kirkpatrick et al., 2001; Lappe-Siefke et al., 2003; Rasband et al., 2005; Yin et al., 2006). Such observations suggest that the axon-glial communication needed to repair specific myelin segments, occurs at least in part by slowing axonal transport and involves canonical myelin proteins. Some AD transgenic models suggest that reduced axonal transport rate occurs before plaque formation (Smith et al., 2007). When FAT slows or stops, the axons form swellings (which when enlarged are also called sphenoids) proximal to the region with the myelin abnormality (Fig. 2B). These swellings are commonly referred to as axonal “defects” and considered evidence of axonopathy. As will be reviewed below, these structural abnormalities may initially be manifestations of myelin repair attempts. If the repair is slow or fails and the swellings persist, the distal axon and synapses are “starved” of their metabolic requirements by the arrested FAT (Figs. 2B and 3). This can result in synaptic loss and eventually axonal degeneration (for review see Edgar and Garbern, 2004; Fiala et al., 2002). Animal models have demonstrated that myelin abnormalities may precede evidence of axonopathies (Desai et al., 2008; Dumser et al., 2007) and many types of myelin abnormalities disrupt FAT in the underlying axons.
Canonical myelin proteins such as 2′,3′-cyclic nucleotide phosphodiesterase (CNP) and myelin basic protein (MBP) are themselves microtubule-associated proteins (Bifulco et al., 2002; Hill et al., 2005). They are able to bind tubulin, induce microtubule polymerization, and help direct the formation of process outgrowth in both glia and neurons (Lee et al., 2005). Deficiencies (Lee et al., 2005) or overproduction (Gravel et al., 1996; Lee et al., 2005; Yin et al., 1997) of these proteins cause a breakdown in myelin integrity that is phenotypically very similar to the breakdown observed in aged primates (Hinman et al., 2006). Age-related accumulations of partially degraded (ubiquitinated) CNP are suspected to contribute to age-related myelin disruption (Hinman et al., 2008) and subsequent axonal pathology (Edgar et al., 2004b; Lappe-Siefke et al., 2003; Rasband et al., 2005). Interestingly, decreased levels of CNP are reported in late-myelinating regions in AD as well as trisomy 21 (Vlkolinsky et al., 2001). Since these myelin proteins are capable of interacting with the microtubule transport system in both axons and oligodendrocytes they could be involved in the key axon-glial signaling needed to maintain and continually repair the vast expanses of myelinated circuits on which brain function depends (Hinman et al., 2008; Rasband et al., 2005). The hypothesis put forth herein is that these myelin proteins evolved at least in part to trigger the stopping/slowing of FAT that seems necessary to effect myelin repair at specific places along the axons.
This hypothesis is supported by human and experimental evidence demonstrating the importance of myelin proteins to the health and morphology of the underlying axon. A common endophenotype seems to develop when homeostasis of these different myelin proteins is disturbed; as occurs for example when CNP is knocked out from oligodendrocytes (Edgar et al., 2004b; Lappe-Siefke et al., 2003; Rasband et al., 2005). This missing myelin component modulates FAT resulting in axonal swellings and, when this process is prolonged or the repair is unsuccessful, leads to synaptic and ultimately axonal degeneration (for review see Edgar and Garbern, 2004). This scenario occurs even when the overlying myelin appears grossly normal (Edgar et al., 2004a; Kassmann et al., 2007). Furthermore, even when these myelin proteins are normal but their metabolism is altered, similar endophenotypes can result (Bizzozero et al., 2001; Klugmann et al., 1997). Another myelin protein that may play a similar role is the major CNS myelin protein proteolipid protein 1 (PLP1). Humans lacking PLP1 develop a very similar axonal degeneration phenotype in the absence of demyelination or inflammation (Garbern et al., 2002). Similar observations are made in PLP1 knockout mouse models. In both species, absence of PLP1 in the overlying myelin, or even the replacement of PLP1 with the integral membrane protein Po (the equivalent to PLP in peripheral nerves) (Yin et al., 2006), can result in similar axonal swelling phenotypes (Edgar et al., 2004b).
Maturation of axonal cytoskeleton requires formation of compact myelin (Brady et al., 1999). Evidence from mutant mice suggests that myelination regulates both the expression and phosphorylation of neurofilament proteins, and is essential for the cytoplasmic organization of myelinated axons (Gotow et al., 1999). Local oligodendrocytes can influence neurofilament accumulation during radial growth of myelinating axons (Sanchez et al., 2000). Absence of MBP can result in reduced or absent compact myelin in shiverer mice (Hu et al., 2008; Rosenbluth, 1980). Compact myelin is an important and sensitive regulator of axonal microtubules. It can increase microtubule density and reduce microtubule stability even when myelin is present and only its quantity is reduced (Kirkpatrick et al., 2001).
The above data are interpreted within the framework of the myelin model to suggest that along axons, repair of specific myelin segments is accomplished by reducing or stopping FAT at that specific damaged segment. The mechanism seems to involve canonical myelin proteins themselves (e.g., CNP and MBP) interacting with the axonal cytoskeleton. The very evolution of these myelin proteins may be related to this repair/maintenance function in addition to other myelin-specific functions such as compaction of myelin lamellae. Additional myelin proteins may also be involved. For example, myelin-associated glycoprotein (MAG) has recently been suggested to interact with axonal receptors and have protective effects upon underlying axons (Nguyen et al., 2009; Schnaar and Lopez, 2009). Another possible protective/supportive effect of myelin is suggested by the recent report that myelin membrane may itself produce ATP without mitochondria (Ravera et al., 2009) and could help explain reports of surprisingly low brain mitochondria concentrations compared to other organs (Veltri et al., 1990).
A microtubule-dependent repair and remodeling molecular mechanism has been recently suggested. The mechanism involves tethering specifically oriented microtubules to cell–cell adhesion sites (such as the ones between axons and oligodendrocytes) that then promote localized and targeted delivery of signaling molecules, additional adhesion molecules, and mitochondria for energy (Ligon and Holzbaur, 2007). Furthermore, recent studies suggest that a direct molecular link between myelin and axonal transport may exist. Phospholipids and sulfatide (a myelin-specific lipid) have been observed to stimulate autophosphorylation of GSK-3β as well as the GSK-3β phosphorylation of both MBP and tau (Kawakami et al., 2008; Suzuki et al., 2009) (see Section 6). GSK3b may primarily stop anterograde transport (Pigino et al., 2009) and would ensure the localized delivery of repair “supplies” such as mitochondria from the cell body. Localized delivery of mitochondria may be especially important since the damage and loss/dysfunction of myelin may be a critical deficit given its possible role as a source of energy (ATP) (Ravera et al., 2009).
Increasing phosphorylation of the microtubule-associated protein tau can stop FAT (Mandelkow et al., 2003) (for review see Edgar and Garbern, 2004). Tau hyperphosphorylation destabilizes microtubule “tracks” that FAT depends on and results in the accumulation of the ingredients transported by FAT at the location of the myelin disturbance (Fig. 2B). The accumulation of FAT-transported ingredients such as BACE1, PS1, and APP could increase the amount of local axon-glial signaling that promotes myelin repair. This process can occur even when the myelin appears grossly normal (Edgar et al., 2004a; Kassmann et al., 2007) and may make myelin repair processes difficult to detect. If successful, the repair process could “mask” the initial myelin problem and may thus contribute to an underappreciation of the importance of myelin vulnerability and repair/remyelination processes in CNS disease. This active and time-limited repair process has been directly assessed using dynamic probes (Praprotnik et al., 1996; Tsai et al., 2004). These probes suggest that successful repair can result in the resolution of swellings and neuritic abnormalities (Tsai et al., 2004) with the Aβ eventually transported down the axon and released at synapses (Lazarov et al., 2002; Sheng et al., 2002; Stokin et al., 2005) (Fig. 3). Conversely, when unsuccessful, these swellings may break (Tsai et al., 2004) and Aβ could be released at the axonal swellings. Released Aβ aggregates can bind to receptors (that may mediate synaptic toxicity, Lauren et al., 2009; Selkoe, 2008; Shankar et al., 2008) as well as lipids and cholesterol (Avdulov et al., 1997; Small et al., 2007) and thus may also be toxic to myelin given its extreme cholesterol content (Muse et al., 2001). This scenario could increase toxicity to synapses as well as surrounding myelin, axons, and dendrites, and result in additional neurite formation with the eventual development of a mature neuritic plaque (Phinney et al., 1999; Tsai et al., 2004; Wirths et al., 2007).
Consistent with the concept that age-related myelin breakdown increases the need to repair myelin (see Section 1), axonal swellings and sphenoids seem to increase with age (Dickson et al., 1990; Geula et al., 2008; Kawarabayashi et al., 1993; Migheli et al., 1992). The possibility that the proposed myelin repair mechanism is generalized is supported by the apparent presence of these swellings in many major CNS disorders including traumatic brain injury (Uryu et al., 2007), multiple sclerosis (Aboul-Enein et al., 2006), and ischemic damage (Akiguchi et al., 2004). All these conditions require myelin repair, and myelin is the white matter component hypothesized to be most vulnerable (Akiguchi et al., 2004; Bartzokis, 2004a).
Hyperphosphorylated tau may eventually deposit as neurofibrillary tangles (Figs. 2B and 3) that increase with age in the human brain (reviewed in Duyckaerts et al., 2009) and is proposed herein to be an indirect manifestation of the generalized age-related myelin repair process. Thus, similar to the process of Aβ production and deposition described above, the production and deposition of hyperphosphorylated tau observed in AD neurofibrillary tangles are proposed to also represent a by-product of the myelin repair process and its associated FAT disruption. A relatively “pure” tauopathy is observed in trauma-related (e.g., repair-related) dementia called dementia pugilistica (Geddes et al., 1999). Further indirect support of the involvement of myelin repair processes in tau deposits is provided by the observation that unlike adults, very young infants sustaining brain trauma do not seem to develop tau deposits (Geddes et al., 2001). The infant brain is not extensively myelinated and would not be expected to undergo extensive myelin-repair. In the context of the model, this reduced need for myelin repair is consistent with the lack of extensive tau deposits (reviewed in Bartzokis, 2004a).
Multiple local disturbances could be corrected by slowing/stopping FAT resulting in axonal swellings. This myelin repair mechanism provides a parsimonious explanation for the plethora of imaging, animal model, or postmortem data suggesting white matter involvement in AD (Adalbert et al., 2007; Bartzokis et al., 2001; Bartzokis, 2004a) and many disorders that have not necessarily been considered directly related to myelin until recently (Bartzokis, 2002, 2005; Bartzokis et al., 2004b). These include but are not limited to genetically-influenced disorders such as AD (Bartzokis et al., 2006a; Ringman et al., 2007; Wirths et al., 2007), Huntington’s disease (Bartzokis et al., 2007d; Fennema-Notestine et al., 2004), amyotrophic lateral sclerosis (Abe et al., 1997; Mori et al., 2007), schizophrenia (Bartzokis et al., 2002; Haroutunian and Davis, 2007), insult-based disorders such as TBI (Kraus et al., 2007; Liu et al., 2006) and toxic substance exposure (Bartzokis et al., 2002; Pfefferbaum et al., 2006), as well as myelin-based inflammatory disorders such as multiple sclerosis (Aboul-Enein et al., 2006). Although daunting, this diversity is less surprising in the context of the model that hypothesizes a “weakest link”/“highest repair priority” status for vertebrate myelin especially in human CNS (Bartzokis, 2004a,b, 2005).
In the specific case of AD, the prevalence of axonal (as opposed to dendritic) swellings, even in dendrite-rich cortex (Duyckaerts et al., 2009; Grutzendler et al., 2007), is interpreted in the context of the model to reinforce the vulnerability of intracortical axons of late-myelinating regions (see Section 1) (Bartzokis, 2007; Bartzokis et al., 2007b; Kemper, 1994; Meyer, 1981a). As shown in Fig. 1 intracortical myelin is very susceptible to breakdown and loss. This may contribute to the primarily intracortical nature of the Alzheimerization of the brain. In fact recent stereological measures of subcortical myelin show no difference between healthy elderly and subjects with severe AD (Jorgensen et al., 2008). Intracortical myelin may be unique not only in its vulnerability but also in its enhanced ability to repair/remyelinate as shown in animal models and humans with multiple sclerosis (Albert et al., 2007; Merkler et al., 2006; Skripuletz et al., 2008) (reviewed in Franklin and Kotter, 2008). Although remyelination attempts continue into old age as demonstrated by striking increasing numbers (doubling) of intracortical oligodendrocytes (O’Kusky and Colonnier, 1982; Peters and Sethares, 2004; Peters et al., 2008b), successful remyelination slows with age (Shen et al., 2008; Shields et al., 1999). Unsuccessful attempts could increase the risk of permanent loss in function since remyelination failures predispose underlying axons and neurons to death (Irvine and Blakemore, 2008; Kornek et al., 2000) (see next section and Figs. 2B and 3).
5.1. Consequences of retrograde FAT disruptions
The preceding section focused primarily on myelin repair and the anterograde FAT on which it depends. It is however expected that by hyperphosphorylating tau and disrupting microtubule tracts, the myelin repair process could also stop retrograde FAT that uses the same microtubule tracts. When FAT stops, retrograde transport of neurotrophins also stops (Fig. 2). Neurotrophins signal the cell body that it is part of a functioning circuit, and their absence may cause neuron loss (Dorsey et al., 2006; Lazarov et al., 2007; Salehi et al., 2006) and permanent disability (Yin et al., 2004) (Fig. 3).
Why would such a dangerous repair process evolve? If myelin repair fails, the information carried in the pattern and timing of action potentials could be degraded (Rasminsky and Sears, 1972; Shrager, 1993). Miscoding of information could hypothetically be more detrimental than having the damaged circuit turned “off” altogether. Reducing or halting retrograde FAT may be a reasonable solution that would allow conservation of resources (devoted to maintenance of synapses and axons of malfunctioning circuits) while minimizing incorrect information processing. There would be no “reason” for retrograde neurotrophin transport to support further production of synaptic vesicles unless myelin repair was successful (Miller et al., 2008).
The scenario of bidirectional FAT interruption that also conserves resources, could provide reasonable explanations for several observations about LOAD. For example, brain axonal transport (Blalock et al., 2004), synaptic vesicle trafficking (Blalock et al., 2004; Yao et al., 2003), and microtubule genes (Blalock et al., 2004; Cash et al., 2003) are apparently downregulated in LOAD. Neuronal resources could thus be conserved and sent down another axon and could account for age-related axonal loss in the absence of measurable cortical neuronal loss (Peters et al., 1998; Peters and Sethares, 2002).
FAT disruption can also interfere with synaptic long-term potentiation (LTP) of synaptic transmission that may be the basis of memory encoding. Like most other synaptic needs, the molecular components underlying LTP depend on anterograde FAT (Pinheiro et al., 2007). It is therefore quite possible that memory impairments in aging, MCI, and AD may be related to myelin breakdown in at least two powerful interdependent ways. Myelin breakdown would degrade the ability of circuits to support the high action potential frequencies needed to trigger LTP and memory storage (Rasminsky and Sears, 1972) (reviewed in Bartzokis et al., 2008). In addition, FAT reductions could impair/delay the arrival of necessary proteins to promote the establishment of LTP both directly and indirectly by blocking anterograde and retrograde FAT, respectively. Such potentially powerful consequences of myelin repair attempts could help explain why memory impairment is the key and persistent clinical manifestation of decline into AD, years before the diagnosis can be made (Fig. 3).
6. Tauopathies and other frontotemporal lobar degenerations (FTLD)
A detailed review of the complex and quickly changing field of FTLD (Mackenzie et al., 2009) is beyond the scope of this report. However, a general assessment of how such disorders could mechanistically “fit” the context of the myelin model is important to consider for three principal reasons. First, co-deposition of proteins associated with different disorders (AD, DLB, PD, and FTLD) in the same brain is common (see below and next section). Second, compelling arguments can be made that tau rather than Aβ pathology best correlates with clinical symptoms of AD (recently reviewed in Duyckaerts et al., 2009; Iqbal et al., 2009). Third is the conundrum of dementia-causing tau mutations. Tau mutations can manifest with similar intraneuronal tau deposits required for the pathologic diagnosis of AD however, they cause only non-AD dementia phenotypes. These seemingly incongruous observations may be reconciled from the mechanistic prospective of the myelin model’s implications for overall neural circuitry maintenance (Figs. 2 and 3).
The previous sections delineated how like Aβ deposits, AD tau deposits may be byproducts of the myelin repair processes which depend on phosphorylation of wild type tau in order to stop FAT (Figs. 2 and 3). On the other hand, mutations of the tau protein itself can directly cause this key protein to malfunction. Such malfunctions could affect basic FAT-dependent “maintenance” processes and, in addition to neurons with long axons, may be expected to impact other cells such as oligodendrocytes that themselves have substantial (long range) transport needs (Maier et al., 2008). Consistent with the generally earlier ages of onset of many FTLD disorders, a basic deficit in an important maintenance function such as transport may be relatively independent of and predate myelin repair needs that trigger FAT slowing in AD (see above). Tau mutation-based deficits could thus disrupt the routine maintenance of axons and synapses (anterograde FAT) and neurons (retrograde FAT) (Fig. 3) as well as non-neuronal cells with high transport needs and thus precipitate FTLD syndromes.
The syndromes classified under frontotemporal lobar degeneration (FTLD) manifest initially with three major types of clinical deficits before progressing to full dementia. These can be roughly classified into deficits of behavioral inhibition/control (e.g., Pick’s disease) and language abnormalities (e.g., primary progressive aphasias and semantic dementia) as well as some (e.g., progressive supranuclear palsy and corticobasilar degeneration) that may manifest initially with motor abnormalities (for review see Kumar-Singh and Van Broeckhoven, 2007). Unlike AD, where late- and thinly-myelinated circuitry is affected initially, the circuits controlling the three functions most often associated with FTLD (movement, language, and behavioral inhibition) may contain fibers that would make them especially vulnerable to more fundamental maintenance deficits. The model would suggest that the circuitry supporting these functions contain earlier-myelinating, high-workload fibers that may often support high-frequency impulse trains (Anderson et al., 1999; Bartzokis et al., 2008; Jinno et al., 2007; Missal et al., 2002). Such circuits would be expected to have higher functional demands and higher maintenance requirements specifically making them most vulnerable to derangements in basic maintenance functions such as transport.
Mutations, suboptimal genetic variants, or malfunctions in other proteins involved in key basic maintenance functions would also be expected to preferentially and initially impact these same highly vulnerable circuits. Within the conceptual framework of high-maintenance networks being specifically vulnerable to deficient maintenance processes, it is not surprising that such disorders could cause different pathological markers yet present with similar if not identical symptoms/phenotypes as tau mutation transport deficits. Non-tauopathy FTLD syndromes are sometimes more common than the classic tau mutation FTLD syndromes and they are often pathologically identified by ubiquitin deposits. Ubiquitination of proteins marks them for destruction, incorporation into endosomes (Fig. 2), and subsequent transport to the neuron body for lysozomal metabolism (Filimonenko et al., 2007; Katzmann et al., 2001). Several genetic defects have been identified (e.g., VCP, CHMP2B, progranulin, dynactin, for review see Kumar-Singh and Van Broeckhoven, 2007) that may interact with and disrupt or overwhelm the protein “clean up” aspect of maintenance contributing to intracellular ubiquitin deposits. A recently identified protein that forms intracellular deposits and is often co-deposited with other proteins is TAR DNA binding protein 43 (TDP-43) (Connell et al., 2008; Filimonenko et al., 2007; Lee et al., 2007b; Rusten and Simonsen, 2008; Tamai et al., 2008). Ubiquitin and TDP-43 are currently the major proteinopathies associated with non-tauopathy FTLD (Kumar-Singh and Van Broeckhoven, 2007). TDP-43 together with progranulin may be involved in other basic maintenance functions such as inflammatory response to cell damage (Ahmed et al., 2007; Kojima et al., 2009; Moisse et al., 2009) that is essential for adequate myelin repair (see Section 1). Additional mutations or suboptimal genetic variants that disrupt basic maintenance and repair functions remain to be discovered and may potentially help account for the FTLD phenotypes in which a specific pathology has yet to be identified (Puls et al., 2005).
In older age, the process of age-related myelin breakdown could be expected to stress and possibly sometime overwhelm the capacity of maintenance processes to cope. In the most vulnerable circuits this could trigger limited manifestations of the FTLD proteinopathy mechanisms. Interactions between repair and maintenance processes could help account for the commonly observed co-deposition of protein precipitates containing ubiquitin and TDP 43 in diseases such as AD and DLB (Higashi et al., 2007; Rohn, 2008) as well as FTLD (Geser et al., 2009). The exact mechanisms and vulnerabilities that would cause these hypothetical interactions remain to be identified. The general framework of maintenance and repair mechanisms of the myelin model may provide a hypothesis-generating approach that could help direct experimental efforts.
7. Brain iron and co-deposition of iron-regulated proteins
As previously described, oligodendrocytes need iron to differentiate (Sow et al., 2006) and have the highest iron levels amongst brain cells (reviewed in Todorich et al., 2008). Age-related myelin breakdown and subsequent remyelination (Peters and Sethares, 2003) may contribute to substantial increases in cortical oligodendrocyte numbers observed in healthy aging (O’Kusky and Colonnier, 1982; Peters and Sethares, 2004; Peters et al., 2008b). Many vertebrates show similar age-related brain iron increases (El Tannir El Tayara et al., 2006; Hallgren and Sourander, 1958; Klintworth, 1973; Maynard et al., 2002; Meguro et al., 2008). Consistent with its proportionately higher myelination, human brain may have higher iron levels than other species (Meadowcroft et al., 2009) (reviewed in Bartzokis et al., 2007b). By increasing oligodendrocyte numbers, remyelination/repair processes may in part underlie the age-related increase in brain iron observed in humans (Bartzokis et al., 2007b,e; Hallgren and Sourander, 1958) as well as the further increase in iron levels observed in many degenerative diseases (Bartzokis et al., 2007d; Bush, 2003; Kaur et al., 2006; Ke and Ming Qian, 2003; Turnbull et al., 2003; Zecca et al., 2004) (Fig. 3) although other processes such as microhemorrhages could also contribute (Frackowiak et al., 2009; Hardy, 2007).
As much as 70% of brain iron may be associated with myelin (de los Monteros et al., 2000; Quintana et al., 2006). Age-related myelin breakdown can potentially release this iron and thus promote free radical damage and aggregation of proteins including Aβ. The recent demonstration of higher brain iron levels in healthy men compared to women suggests that peripheral iron (higher in men) could contribute to earlier ages at disease onset in men (Bartzokis et al., 2007e). Men have a younger age at onset of PD, DLB, and trisomy 21 (Downs syndrome) (reviewed in Bartzokis et al., 2007e). These observations have been interpreted to suggest that increased iron levels may contribute to the age risk-factor of developing these diseases (for review see Bartzokis et al., 1993, 2007e; Bartzokis, 2004a,b; Oakley et al., 2007) as well as acceleration of onset in males (Bartzokis et al., 1999, 2007e). Finally, prevalent genetic defects that increase iron absorption may also influence risk of AD especially by lowering onset age (Alizadeh et al., 2007; Connor and Lee, 2006; Lehmann et al., 2006; van Rensburg et al., 2000).
Importantly, iron management is achieved through both transcription of genes that code for ferritin and transferrin as well as an unusual and important second layer of homeostatic post-transcriptional controls in cytoplasm. The additional control is provided by iron responsive elements (IREs) on the mRNA of transferrin and ferritin that respond to intracellular iron levels (reviewed in Rogers et al., 2008; Zecca et al., 2004). These IREs are also present on APP and αSyn mRNAs (Rogers et al., 2008). Binding of iron regulatory protein to the IRE of APP mRNA may be even tighter than to ferritin IRE. This suggests that levels of ferritin as well as APP and αSyn may be responsive to age-related increases in brain iron. Thus, the observed age-related increase in ferritin iron levels (Bartzokis et al., 2007e) as well as APP and αSyn levels (Moir et al., 1998; Preece et al., 2004; Sandbrink et al., 1994; Tanaka et al., 1992) may share increased intracellular iron as a common driving force.
Iron may also promote protein oligomerization (Frackowiak et al., 2009; Kostka et al., 2008) supporting suggestions that abnormal protein aggregations in general may be dependent on the promotion of protein oligomerization and cytotoxic effects of transition metals such as iron (for review see Bartzokis, 2004a; Bartzokis et al., 2007e). A mechanism of toxicity of proteinaceous deposits involving dysregulated iron management has recently been described. Normal cell surface proteins called prions can transform into β-sheet isoform deposits (Singh et al., 2009a). Iron sequestration within the protein deposits apparently creates a state of intracellular iron deficiency resulting in inappropriate increased cellular iron uptake (Singh et al., 2009b). If generalized, such a mechanism could help explain the common observation of increased iron levels in a variety of proteinaceous lesions (Frackowiak et al., 2009; Meadowcroft et al., 2009; Rajendran et al., 2009; Singh et al., 2009a) and could possibly contribute to their toxicity (Frackowiak et al., 2009; Kostka et al., 2008).
The observations summarized above help support the hypothesis that age-related increases in brain iron levels, promoted in part by myelin repair/remyelination processes, may drive the age-related risk for the major degenerative diseases (AD, PD, and DLB) as well as earlier manifestation of these diseases in men compared to women (Bartzokis et al., 2007b,e). Furthermore, the presence of IREs on the 5′ end of both APP and αSyn mRNA could help explain the highly prevalent co-occurrence of Aβ and αSyn deposits in these three major degenerative diseases as well as FAD (Barker et al., 2002; Rosenberg et al., 2000; Snider et al., 2005) (reviewed in Bartzokis et al., 2007e; Jellinger, 2009).
8. Future directions and treatment implications
The dearth of AD animal model studies that include direct examination of myelin (Desai et al., 2008) may be seriously impeding progress in unraveling disease mechanisms. Studies that integrate assessments of myelin pathology in addition to neuron and synaptic changes are needed in order to help clarify molecular mechanisms (Peters et al., 2008a,b). The substantial quantitative differences in myelin and iron levels between humans and other species may be important to consider when extrapolating animal model results to humans (Bartzokis et al., 2007b; Meadowcroft et al., 2009). Differences in myelination, cholesterol, and iron may help explain the striking dichotomy of numerous positive/curative interventions in transgenic animal models of AD and their failure in human trials (Becker and Greig, 2008; Holmes et al., 2008).
Many of the hypotheses derived from the myelin model need further assessment in prospective studies that can help determine causality (Jack et al., 2009). The tools (imaging, genetic, clinical, etc,) to perform such studies in humans (including effects of treatment interventions) are available or being developed at a rapid pace. For example, Aβ and tau deposits, the development and breakdown of myelin, as well as important risk factors such as ferritin iron levels can be indirectly measured with increasing specificity in vivo using non-invasive imaging methods (Bartzokis et al., 2004b, 2007e; Kochunov et al., 2007) (reviewed in Hampel et al., 2008; Nordberg, 2007) (Fig. 1).
The greatest promise of the myelin model of the human brain is its application to therapeutics. The model cautions against approaches that may hinder myelin repair such as indiscriminate inhibition of BACE1 and γ-secretase, key therapeutic targets in the framework of the AH. In counter distinction to this focus on reducing Aβ, the myelin model suggests entirely different approaches to treatment and prevention of AD that precede (are “upstream” of) significant Aβ and tau formation (Figs. 2 and 3). It expands the range of therapeutic targets to include interventions that maintain myelin health, resilience, and repair/regeneration efficiency. This approach provides opportunities for novel therapies with potentially wide spectra of efficacy (Bartzokis, 2004a) as well as reexamination of potentially under-explored uses of existing treatments (Ballard et al., 2007; Bartzokis and Altshuler, 2003; Bartzokis, 2007; Bartzokis et al., 2007c; Chen et al., 2007; He et al., 2007; Xiao et al., 2007).
Enhanced efficiency of myelin repair and myelin protection from both environmental and physiologic toxins are key treatment directions. This approach could include management of age-related increases in iron and other metals (Bartzokis et al., 2007e; Stankiewicz et al., 2007) and have potential as treatment and prevention (Bartzokis et al., 2007e; Lehmann et al., 2006; Mandel et al., 2007). Novel chelator and antioxidant combination molecules may be helpful in controlling iron-related free radical toxicity (Avramovich-Tirosh et al., 2007; Mandel et al., 2007) and build on an early suggestion of efficacy in human trials that used more toxic first generation chelators (McLachlan et al., 1991).
The much higher human brain iron levels compared to rodent together with the age-related increase in iron may catalyze free radical reactions (Bartzokis et al., 2007b,e; Meadowcroft et al., 2009). These reactions can potentially consume vulnerable plasmalogens (Pls) that are highly concentrated in myelin (Farooqui et al., 2007). Reduced Pls in human brain can be detected after age 70 (Weisser et al., 1997). The species-related difference in iron levels could help explain the substantially greater reduction of Pls in AD brain than in transgenic mouse AD models (Han et al., 2001) and possibly the human pattern of myelin breakdown and amyloid deposition (Bartzokis et al., 2007b; Meadowcroft et al., 2009). In addition to being a building block for myelin, Pls are important in fusion of membranes such as vesicles during neurotransmission (reviewed in Goodenowe et al., 2007). Such fusion events may be important for other much less explored functions such as delivery of membranes for building and repair, endosome-lysozome fusion, etc. (see Fig. 2).
The myelin model perspective provides a framework for considering several clinically available treatment approaches that could positively influence these age-related declines in myelin health. Peroxisome proliferators activated receptors (PPAR) are nuclear transcription factors that control lipid metabolism. Two PPARγs (rosiglitazone and pioglitazone) are marketed for diabetes type 2 because of their insulin sensitizing properties. These have shown efficacy in a variety of diseases and disease models that involve lipid and myelin changes including AD, PD, ALS, and HD (Kumar et al., 2009; Lovett-Racke et al., 2004; Quintanilla et al., 2008; Santos et al., 2005; Schintu et al., 2009; Schutz et al., 2005; Watson et al., 2005). Similar suggestions of improved function have been made with DHA supplementation (Martinez and Vazquez, 1998; Salvati et al., 2008) (a crucial Pl component—see Section 1). Several studies suggest that some of the anti-inflammatory effects of PPARs are associated with DHA metabolism (Guillot et al., 2008; Musiek et al., 2008) and may provide additive beneficial affects (Zapata-Gonzalez et al., 2008). High-affinity interaction of PPARγ, PPARα, and DHA (Gani and Sylte, 2008; Kumar et al., 2009; Schintu et al., 2009) may underlie such additive effects. PPARγ activation may also protect from direct Aβ oligomer toxicity and glucocorticoid toxicity (De Felice et al., 2009; Escribano et al., 2009). The PPAR activator action seems necessary since metformin, another diabetes treatment with insulin sensitizing effects that lacks this action, when used as monotherapy may be detrimental and increase BACE1 and APP processing into Aβ (Chen et al., 2009).
Directly increasing brain ApoE levels is an especially attractive approach as it would take advantage of physiologic repair pathways (Laskowitz and Vitek, 2007). Pharmaceutical stimulation of ligand-activated transcription factor named liver X receptor (LXR) increases expression of lipid metabolism genes including ApoE as well as the principal protein involved in ApoE’s lipidation, ATP-binding cassette transporter A1 (Koldamova et al., 2005; Lefterov et al., 2007; Repa et al., 2007; Riddell et al., 2007). This intervention may be especially effective by promoting both efficient cholesterol recycling and removal of inflammation-promoting myelin debris (Lefterov et al., 2007; Zelcer et al., 2007) that may help combat age-related reduction of successful remyelination (Shen et al., 2008; Shields et al., 1999). Such interventions may have serious systemic side effects however (Cheng et al., 2008a; Davies et al., 2008).
Important in considering therapeutic interventions is the option of direct delivery of proteins and other large molecules into the brain. The safe delivery of a variety of treatments including ApoE, metal chelators (including apotransferrin), neurotrophins that have promyelinating effect (such as IGF1, nerve growth factor, and insulin) (Liang et al., 2007; Zeger et al., 2007) (reviewed in Chesik et al., 2008) may be possible through intranasal administration (Benedict et al., 2004; De Rosa et al., 2005; Reger et al., 2006, 2008; Thorne et al., 2004; Yamada et al., 2007). Small pilot studies have demonstrated that insulin delivery through this method may be efficacious in treatment of AD and MCI while avoiding major systemic side effects (Reger et al., 2006, 2008). In the context of the model and the cross-reactivity of IGF-1 and insulin receptors (Carson et al., 1993) one could speculate that promyelinating effects may be responsible (Aberg et al., 2007; Carson et al., 1993) possibly by increasing survival of newborn oligodendrocytes (Aberg et al., 2007).
These interventions could be combined with each other in a “cocktail” as well as lower-risk non-pharmacologic approaches such as physical exercise. Exercise is a behavioral intervention that also sensitizes insulin receptors (Zhang et al., 2007) and has been shown to improve cognition (Hillman et al., 2008). Physical exercise may also improve cognition through stimulation of growth factor production (such as IGF1) (Llorens-Martin et al., 2008) and may be underemphasized as a preventive approach to age-related cognitive decline. The minimal risks and well known cardiovascular benefits should make this intervention more highly promoted to the general population before the need for medical interventions as well as in concert with them.
The above interventions could also promote myelination indirectly by decreasing myelotoxic factors such as hypercortisolemia (Benedict et al., 2004; Escribano et al., 2009) that is associated with greater and more rapid cognitive decline (Csernansky et al., 2006; Lee et al., 2007a). Chronic elevation of glucocorticoid stress hormones may decrease myelination (Alonso, 2000) and, by interfering with efficient myelin repair, could promote Alzheimerization of the brain (Green et al., 2006). In the framework of the model, interventions that would reduce detrimental effects on myelination (such as cortisol and inflammation) and promote its development/repair (as described above) could also potentially prove helpful for both treatment and prevention.
9. Conclusions
Brain aging and the aging-related processes of myelin production as well as its subsequent maintenance, breakdown, and repair is relevant to understanding the relationships between human brain development and prevalent age-related brain disorders such as AD. The myelin model of the human brain incorporates the known physiological roles of FAD and LOAD gene products into a unifying pathophysiology of human brain aging and the various trajectories of decline of its circuitry into highly prevalent dementing disorders. This model suggests that at its earliest stages, highly prevalent diseases such as AD are rooted in an age-related increase in myelotoxicity that is in part driven by the extensive human brain myelination process itself (Fig. 1). The damage to vulnerable late-myelinating regions and circuits increasingly enlists dynamic homeostatic processes that strive to repair myelin and restore optimal function. Pathologic manifestations of these diseases are reconceptualized as by-products of myelin repair processes (e.g., Aβ and tau deposition, and synaptic/axonal/neuronal losses; Figs. 2 and 3). Clinical manifestations are also reconceptualized as primarily associated with the success or failure of these processes (e.g., failure to maintain myelin and axon integrity resulting in synapse and eventually neuronal loss) (Bartzokis, 2004a,b). The model suggests that efforts to eliminate Aβ may be focused on a late point in the degenerative process that is fraught with dangers of disturbing homeostatic repair mechanisms (e.g., BACE1 and γ-secretase inhibitors) or promoting inflammation and possibly iron release from Aβ deposits (Aβ immunization strategies). The disappointing failure of the recent large trials aimed at clearing or disrupting production of amyloid have thus far supported these predictions (Holmes et al., 2008; Patton et al., 2006) and invite reexamination of accepted constructs (Hardy, 2007; Jellinger, 2009).
This myelin model of the human brain represents a “scaffold” that attempts to bind the various levels of the research endeavor from molecular genetics through mechanism, cells, circuits, and clinical manifestations in the context of their largely hidden evolutionary connections. It focuses on oligodendrocytes and the myelin they produce not because these structures are the basic unit of our CNS, the neurons that form our circuits are this basic unit despite their meager proportion (approximately 10% of human CNS cells). Rather, as the last evolved component of CNS circuitry and our species’ unique dependence on it, myelin represents our “weakest link” in the chain of interdependent cells and processes that blossom over decades into our complex cognition and behavior. Many aspects of the model’s framework may require modification as new data becomes available that can clarify important connections between research levels.
-
-
Molecular: What other prevalent gene variants (like ApoE4) influence maintenance and repair of CNS circuitry and what other molecular mechanisms (e.g., epigenetic) influence our myelination trajectory through life?
-
-
Mechanistic: What metabolic pathways (energy, lipid, metals, protein) and processes (transport, debris removal, cell division, etc.) are most vulnerable in CNS maintenance and repair?
-
-
Cellular: To what extent do oligodendrocytes continue to divide in humans and what aspects of intracellular communication and division of labor (energy and material (e.g., cholesterol) production, inter- and extra-cellular repair and debris removal, etc.) are most vulnerable?
-
-
Pathologic: What aspects of pathognomonic lesions are key toxic entities as opposed to protective or innocent bystanders?
-
-
Circuit: What aspects of action potential (propagation speed, frequency, maintenance of oscillations between regions, etc.) most profoundly influence cognitive function and what makes some circuits uniquely/especially vulnerable?
-
-
Clinical: What biomarkers or combination thereof (genetic, imaging, proteinacious, lipid, metal, etc.) are best at predicting circuitry malfunction and repair efforts as well as subsequent clinical changes?
-
-
Epidemiologic: What modifiable insults (toxic, nutritional, psychological, etc.) and protective factors (nutritional, educational, physical exercise, etc.) interact most effectively with aspects of circuit development, maintenance, and repair?
The list could be longer and additional, yet undiscovered elements are surely to be added through continuing research. The answers will help modify and refine the model and help fulfill its fundamental purpose of helping identify optimal targets and timing for therapies. As with other models of human brain disease its ultimate falsification will hinge on its ability or failure to aid in the development of useful prevention and treatment interventions (Hardy, 2007). More than twenty years elapse between the onset of brain Alzheimerization as manifested by the appearance of the first amyloid and tau deposits (reviewed in Duyckaerts et al., 2009) in the absence of appreciable neuronal loss and cognitive changes suggestive of early AD (Price et al., 2001). The myelin model helps develop hypothesis-driven “roadmaps” of novel myelin-centered approaches to treatment interventions. Imaging biomarker technology has emerged that is safe, repeatable, widely available, and able to track the dynamic changes in brain myelin as well as associated changes in iron levels over the age-span. It makes it possible to prospectively test the model in human beings as well as accelerate medication development by identifying high-risk individuals and tracking treatment effects. This provides the opportunity to begin treatments earlier, possibly even before differentiation from the healthiest brain aging of ApoE2 allele carriers (Bartzokis et al., 2006a). Myelin-centered interventions with relatively benign side-effect profiles may already be available. With early initiation, such interventions could potentially substantially reduce the increasingly catastrophic burden of dementia.
Acknowledgments
Part of this work was supported by NIH grants (MH066029 and AG027342); the RCS Alzheimer’s Foundation; and the Department of Veterans Affairs Research and Psychiatry Services.
Footnotes
Conflict of interest
There are no actual or potential conflicts of interest.
References
- Abe K, Fujimura H, Kobayashi Y, Fujita N, Yanagihara T. Degeneration of the pyramidal tracts in patients with amyotrophic lateral sclerosis. A premortem and postmortem magnetic resonance imaging study. J. Neuroimaging. 1997;7:208–212. doi: 10.1111/jon199774208. [DOI] [PubMed] [Google Scholar]
- Aberg ND, Johansson UE, Aberg MA, Hellstrom NA, Lind J, Bull C, Isgaard J, Anderson MF, Oscarsson J, Eriksson PS. Peripheral infusion of insulin-like growth factor-i increases the number of newborn oligodendrocytes in the cerebral cortex of adult hypophysectomized rats. Endocrinology. 2007;148:3765–3772. doi: 10.1210/en.2006-1556. [DOI] [PubMed] [Google Scholar]
- Aboul-Enein F, Weiser P, Hoftberger R, Lassmann H, Bradl M. Transient axonal injury in the absence of demyelination: a correlate of clinical disease in acute experimental autoimmune encephalomyelitis. Acta Neuropathol. 2006;111:539–547. doi: 10.1007/s00401-006-0047-y. [DOI] [PubMed] [Google Scholar]
- Adalbert R, Gilley J, Coleman MP. Abeta, tau and apoe4 in alzheimer’s disease: the axonal connection. Trends Mol. Med. 2007;13:135–142. doi: 10.1016/j.molmed.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Mackenzie IR, Hutton ML, Dickson DW. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J. Neuroinflammation. 2007;4:7. doi: 10.1186/1742-2094-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiguchi I, Tomimoto H, Wakita H, Kawamoto Y, Matsuo A, Ohnishi K, Watanabe T, Budka H. Topographical and cytopathological lesion analysis of the white matter in binswanger’s disease brains. Acta Neuropathol. 2004;107:563–570. doi: 10.1007/s00401-004-0850-2. [DOI] [PubMed] [Google Scholar]
- Albert M, Antel J, Bruck W, Stadelmann C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17:129–138. doi: 10.1111/j.1750-3639.2006.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh BZ, Njajou OT, Millan MR, Hofman A, Breteler MM, van Duijn CM. Hfe variants, apoe and alzheimer’s disease: findings from the population-based rotterdam study. Neurobiol. Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Anderson B, Southern BD, Powers RE. Anatomic asymmetries of the posterior superior temporal lobes: a postmortem study. Neuropsychiatry Neuropsychol. Behav. Neurol. 1999;12:247–254. [PubMed] [Google Scholar]
- Ando S, Tanaka Y, Toyoda Y, Kon K. Turnover of myelin lipids in aging brain. Neurochem. Res. 2003;28:5–13. doi: 10.1023/a:1021635826032. [DOI] [PubMed] [Google Scholar]
- Andre A, Chanseaume E, Dumusois C, Cabaret S, Berdeaux O, Chardigny JM. Cerebral plasmalogens and aldehydes in senescence-accelerated mice p8 and r1: a comparison between weaned, adult and aged mice. Brain Res. 2006a;1085:28–32. doi: 10.1016/j.brainres.2006.02.067. [DOI] [PubMed] [Google Scholar]
- Andre A, Juaneda P, Sebedio JL, Chardigny JM. Plasmalogen metabolism-related enzymes in rat brain during aging: influence of n-3 fatty acid intake. Biochimie. 2006b;88:103–111. doi: 10.1016/j.biochi.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Ashford JW. Apoe genotype effects on alzheimer’s disease onset and epidemiology. J. Mol. Neurosci. 2004;23:157–166. doi: 10.1385/JMN:23:3:157. [DOI] [PubMed] [Google Scholar]
- Avdulov NA, Chochina SV, Igbavboa U, Warden CS, Vassiliev AV, Wood WG. Lipid binding to amyloid beta-peptide aggregates: preferential binding of cholesterol as compared with phosphatidylcholine and fatty acids. J. Neurochem. 1997;69:1746–1752. doi: 10.1046/j.1471-4159.1997.69041746.x. [DOI] [PubMed] [Google Scholar]
- Avramovich-Tirosh Y, Amit T, Bar-Am O, Zheng H, Fridkin M, Youdim MB. Therapeutic targets and potential of the novel brain-permeable multifunctional iron chelator-monoamine oxidase inhibitor drug, m-30, for the treatment of alzheimer’s disease. J. Neurochem. 2007;100:490–502. doi: 10.1111/j.1471-4159.2006.04258.x. [DOI] [PubMed] [Google Scholar]
- Ballard CG, Chalmers KA, Todd C, McKeith IG, O’Brien JT, Wilcock G, Love S, Perry EK. Cholinesterase inhibitors reduce cortical abeta in dementia with lewy bodies. Neurology. 2007;68:1726–1729. doi: 10.1212/01.wnl.0000261920.03297.64. [DOI] [PubMed] [Google Scholar]
- Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, Waters C, Jimison P, Shepherd E, Sevush S, Graff-Radford N, Newland D, Todd M, Miller B, Gold M, Heilman K, Doty L, Goodman I, Robinson B, Pearl G, Dickson D, Duara R. Relative frequencies of alzheimer disease, lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the state of florida brain bank. Alzheimer Dis. Assoc. Disord. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Aravagiri M, Oldendorf WH, Mintz J, Marder SR. Field dependent transverse relaxation rate increase may be a specific measure of tissue iron stores. Magn. Reson. Med. 1993;29:459–464. doi: 10.1002/mrm.1910290406. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings J, Perlman S, Hance DB, Mintz J. Increased basal ganglia iron levels in huntington disease. Arch. Neurol. 1999;56:569–574. doi: 10.1001/archneur.56.5.569. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Cummings BJ, Holt LE, Hance DB, Henderson VW, Mintz J. In vivo evaluation of brain iron in alzheimer’s disease and normal controls using magnetic resonance imaging. Arch. Gen. Psychiatry. 2000;57:47–53. doi: 10.1001/archpsyc.57.1.47. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men:a magnetic resonance imaging study. Arch. Gen. Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Schizophrenia: breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharmacology. 2002;27:672–683. doi: 10.1016/S0893-133X(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P, Mintz J. Brain maturation may be arrested in chronic cocaine addicts. Biol. Psychiatry. 2002;51:605–611. doi: 10.1016/s0006-3223(02)01315-x. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Altshuler LL. Biological underpinnings of treatment resistance in schizophrenia: an hypothesis. Psychopharmacol. Bull. 2003;37:5–7. [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with alzheimer disease:a magnetic resonance imaging study. Arch. Neurol. 2003a;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: a magnetic resonance imaging study. Biol. Psychiatry. 2003b;53:412–421. doi: 10.1016/s0006-3223(02)01835-8. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and alzheimer’s disease. Neurobiol. Aging. 2004a;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Quadratic trajectories of brain myelin content: unifying construct for neuropsychiatric disorders. Neurobiol. Aging. 2004b;25:49–62. [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Bridge P, Mintz J. Cortical gray matter volumes are associated with subjective responses to cocaine infusion. Am. J. Addict. 2004a;13:64–73. doi: 10.1080/10550490490265352. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings J. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “Disconnection” In aging and alzheimer’s disease. Neurobiol. Aging. 2004b;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Brain myelination in prevalent neuropsychiatric developmental disorders: primary and comorbid addiction. Adolescent Psychiatry. 2005;29:55–96. [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Geschwind DH, Edwards N, Mintz J, Cummings JL. Apolipoprotein e genotype and age-related myelin breakdown in healthy individuals: implications for cognitive decline and dementia. Arch. Gen. Psychiatry. 2006a;63:63–72. doi: 10.1001/archpsyc.63.1.63. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tishler TA, Perlman S. In vivo assessment of iron in huntington’s disease and other age-related degenerative brain diseases. In: Sigel A, Sigel H, Sigel RKO, editors. Metal Ions in Life Sciences. vol. 1. Chichester: Wiley & Sons; 2006b. pp. 151–177. [Google Scholar]
- Bartzokis G. Acetylcholinesterase inhibitors may improve myelin integrity. Biol. Psychiatry. 2007;62:294–301. doi: 10.1016/j.biopsych.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Geschwind D, Tingus K, Huang D, Mendez MF, Edwards N, Mintz J. Apolipoprotein e affects both myelin breakdown and cognition: implications for age-related trajectories of decline into dementia. Biol. Psychiatry. 2007a;62:1380–1387. doi: 10.1016/j.biopsych.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Mintz J. Human brain myelination and amyloid beta deposition in alzheimer’s disease. Alzheimer’s Dementia: J. Alzheimer’s Assoc. 2007b;3:122–125. doi: 10.1016/j.jalz.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Nuechterlein KH, Gitlin M, Doi C, Edwards N, Lieu C, Altshuler LL, Mintz J. Differential effects of typical and atypical antipsychotics on brain myelination in schizophrenia. Schizophr. Res. 2007c;93:13–22. doi: 10.1016/j.schres.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tishler TA, Fong SM, Oluwadara B, Finn JP, Huang D, Bordelon Y, Mintz J, Perlman S. Myelin breakdown and iron changes in huntington’s disease: pathogenesis and treatment implications. Neurochem. Res. 2007d;32:1655–1664. doi: 10.1007/s11064-007-9352-7. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M, Huang D, Edwards N, Mintz J. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol. Aging. 2007e;28:414–423. doi: 10.1016/j.neurobiolaging.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, Thompson PM, Mintz J. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH. Brain volume: age-related changes. In: Squire LR, editor. Encyclopedia of Neuroscience. vol. 2. Oxford: Academic Press; 2009. pp. 417–447. [Google Scholar]
- Bauer J, Bradl M, Klein M, Leisser M, Deckwerth TL, Wekerle H, Lassmann H. Endoplasmic reticulum stress in plp-overexpressing transgenic rats: gray matter oligodendrocytes are more vulnerable than white matter oligodendrocytes. J. Neuropathol. Exp. Neurol. 2002;61:12–22. doi: 10.1093/jnen/61.1.12. [DOI] [PubMed] [Google Scholar]
- Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F. Igf1 gene disruption results in reduced brain size, cns hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995;14:717–730. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- Becker RE, Greig NH. Alzheimer’s disease drug development in 2008 and beyond: problems and opportunities. Curr. Alzheimer Res. 2008;5:346–357. doi: 10.2174/156720508785132299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch. Gen. Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, Corrada MM, Head E, Kawas CH. Apoe epsilon2 is associated with intact cognition but increased alzheimer pathology in the oldest old. Neurology. 2009;72:829–834. doi: 10.1212/01.wnl.0000343853.00346.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifulco M, Laezza C, Stingo S, Wolff J. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase: a membrane-bound, microtubule-associated protein and membrane anchor for tubulin. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1807–1812. doi: 10.1073/pnas.042678799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzozero OA, Bixler HA, Davis JD, Espinosa A, Messier AM. Chemical deacylation reduces the adhesive properties of proteolipid protein and leads to decompaction of the myelin sheath. J. Neurochem. 2001;76:1129–1141. doi: 10.1046/j.1471-4159.2001.00116.x. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I, Heverin M, Leoni V, Meaney S, Diczfalusy U. Oxysterols and alzheimer’s disease. Acta Neurol. Scand. Suppl. 2006;185:43–49. doi: 10.1111/j.1600-0404.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, Moss M, Albert M. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch. Neurol. 2007;64:862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Grassi M, Costanzi C, Archetti S, Caimi L, Padovani A. Apoe genotype and cholesterol levels in lewy body dementia and alzheimer disease: investigating genotype-phenotype effect on disease risk. Am. J. Geriatr. Psychiatry. 2006;14:1022–1031. doi: 10.1097/01.JGP.0000225088.29353.08. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Zoellner CD, Anderson LJ, Kosik LM, Pitas RE, Weisgraber KH, Hui DY, Mahley RW, Gebicke-Haerter PJ, Ignatius MJ, et al. A role for apolipoprotein e, apolipoprotein a-i, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J. Clin. Invest. 1989;83:1015–1031. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Development of alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol. (Berl.) 1996;92:197–201. doi: 10.1007/s004010050508. [DOI] [PubMed] [Google Scholar]
- Brady ST, Witt AS, Kirkpatrick LL, de Waegh SM, Readhead C, Tu PH, Lee VM. Formation of compact myelin is required for maturation of the axonal cytoskeleton. J. Neurosci. 1999;19:7278–7288. doi: 10.1523/JNEUROSCI.19-17-07278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breteler MM, Claus JJ, van Duijn CM, Launer LJ, Hofman A. Epidemiology of alzheimer’s disease. Epidemiol. Rev. 1992;14:59–82. doi: 10.1093/oxfordjournals.epirev.a036092. [DOI] [PubMed] [Google Scholar]
- Brites P, Waterham HR, Wanders RJ. Functions and biosynthesis of plasmalogens in health and disease. Biochim. Biophys. Acta. 2004;1636:219–231. doi: 10.1016/j.bbalip.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Bush AI. The metallobiology of alzheimer’s disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- Cabrejo L, Guyant-Marechal L, Laquerriere A, Vercelletto M, De la Fourniere F, Thomas-Anterion C, Verny C, Letournel F, Pasquier F, Vital A, Checler F, Frebourg T, Campion D, Hannequin D. Phenotype associated with app duplication in five families. Brain. 2006;129:2966–2976. doi: 10.1093/brain/awl237. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulinlike growth factor i increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–740. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- Cash AD, Aliev G, Siedlak SL, Nunomura A, Fujioka H, Zhu X, Raina AK, Vinters HV, Tabaton M, Johnson AB, Paula-Barbosa M, Avila J, Jones PK, Castellani RJ, Smith MA, Perry G. Microtubule reduction in alzheimer’s disease and aging is independent of tau filament formation. Am. J. Pathol. 2003;162:1623–1627. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers K, Wilcock G, Love S. Contributors to white matter damage in the frontal lobe in alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2005;31:623–631. doi: 10.1111/j.1365-2990.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- Cheepsunthorn P, Palmer C, Menzies S, Roberts RL, Connor JR. Hypoxic/ischemic insult alters ferritin expression and myelination in neonatal rat brains. J. Comp. Neurol. 2001;431:382–396. doi: 10.1002/1096-9861(20010319)431:4<382::aid-cne1077>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Chen S, Owens GC, Crossin KL, Edelman DB. Serotonin stimulates mitochondrial transport in hippocampal neurons. Mol. Cell Neurosci. 2007;36:472–483. doi: 10.1016/j.mcn.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao FF. Antidiabetic drug metformin (glucophager) increases biogenesis of alzheimer’s amyloid peptides via up-regulating bace1 transcription. Proc. Natl. Acad. Sci. U.S.A. 2009;106:3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Kim WS, Garner B. Regulation of alpha-synuclein expression by liver x receptor ligands in vitro. Neuroreport. 2008a;19:1685–1689. doi: 10.1097/WNR.0b013e32831578b2. [DOI] [PubMed] [Google Scholar]
- Cheng H, Zhou Y, Holtzman DM, Han X. Apolipoprotein e mediates sulfatide depletion in animal models of alzheimer’s disease. Neurobiol. Aging. 2008b doi: 10.1016/j.neurobiolaging.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesik D, De Keyser J, Wilczak N. Insulin-like growth factor system regulates oligodendroglial cell behavior: therapeutic potential in cns. J. Mol. Neurosci. 2008 doi: 10.1007/s12031-008-9041-2. [DOI] [PubMed] [Google Scholar]
- Chia LS, Thompson JE, Moscarello MA. X-ray diffraction evidence for myelin disorder in brain from humans with alzheimers disease. Biochem. Biophys. Acta. 1984;775:308–312. doi: 10.1016/0005-2736(84)90185-8. [DOI] [PubMed] [Google Scholar]
- Chiocco MJ, Lamb BT. Spatial and temporal control of age-related app processing in genomic-based beta-secretase transgenic mice. Neurobiol. Aging. 2007;28:75–84. doi: 10.1016/j.neurobiolaging.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowen JA, Goya L, Ramos S, Busiguina S, Garcia-Segura LM, Argente J, Pascual-Leone AM. Effects of early undernutrition on the brain insulin-like growth factor-i system. J. Neuroendocrinol. 2002;14:163–169. doi: 10.1046/j.0007-1331.2001.00758.x. [DOI] [PubMed] [Google Scholar]
- Connell JW, Lindon C, Luzio JP, Reid E. Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic. 2008 doi: 10.1111/j.1600-0854.2008.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Connor JR, Lee SY. Hfe mutations and alzheimer’s disease. J. Alzheimers Dis. 2006;10:267–276. doi: 10.3233/jad-2006-102-311. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein e type 4 allele and the risk of alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Crawford F, Wood M, Ferguson S, Mathura V, Gupta P, Humphrey J, Mouzon B, Laporte V, Margenthaler E, O’Steen B, Hayes R, Roses A, Mullan M. Apolipoprotein e-genotype dependent hippocampal and cortical responses to traumatic brain injury. Neuroscience. 2009;159:1349–1362. doi: 10.1016/j.neuroscience.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with alzheimer-type dementia. Am. J. Psychiatry. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JS, Kotokorpi P, Lindahl U, Oscarsson J, Wells T, Mode A. Effects of the synthetic liver x receptor agonist t0901317 on the growth hormone and thyroid hormone axes in male rats. Endocrine. 2008;33:196–204. doi: 10.1007/s12020-008-9067-9. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. Protection of synapses against alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of abeta oligomers. Proc. Natl. Acad. Sci. U.S.A. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Monteros AE, Korsak RA, Tran T, Vu D, de Vellis J, Edmond J. Dietary iron and the integrity of the developing rat brain:a study with the artificially-reared rat pup. Cell Mol. Biol. (Noisy-le-grand) 2000;46:501–515. [PubMed] [Google Scholar]
- De Rosa R, Garcia AA, Braschi C, Capsoni S, Maffei L, Berardi N, Cattaneo A. Intranasal administration of nerve growth factor (ngf) rescues recognition memory deficits in ad11 anti-ngf transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3811–3816. doi: 10.1073/pnas.0500195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Delatour B, Guegan M, Volk A, Dhenain M. In vivo mri and histological evaluation of brain atrophy in app/ps1 transgenic mice. Neurobiol. Aging. 2006;27:835–847. doi: 10.1016/j.neurobiolaging.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Dermaut B, Kumar-Singh S, Engelborghs S, Theuns J, Rademakers R, Saerens J, Pickut BA, Peeters K, van den Broeck M, Vennekens K, Claes S, Cruts M, Cras P, Martin JJ, Van Broeckhoven C, De Deyn PP. A novel presenilin 1 mutation associated with pick’s disease but not beta-amyloid plaques. Ann. Neurol. 2004;55:617–626. doi: 10.1002/ana.20083. [DOI] [PubMed] [Google Scholar]
- Desai MK, Sudol KL, Janelsins MC, Mastrangelo MA, Frazer ME, Bowers WJ. Triple-transgenic alzheimer’s disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia. 2008 doi: 10.1002/glia.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Wertkin A, Kress Y, Ksiezak-Reding H, Yen SH. Ubiquitin immunoreactive structures in normal human brains. Distribution and developmental aspects. Lab. Invest. 1990;63:87–99. [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Dik MG, Pluijm SM, Jonker C, Deeg DJ, Lomecky MZ, Lips P. Insulin-like growth factor i (igf-i) and cognitive decline in older persons. Neurobiol. Aging. 2003;24:573–581. doi: 10.1016/s0197-4580(02)00136-7. [DOI] [PubMed] [Google Scholar]
- Dorsey SG, Renn CL, Carim-Todd L, Barrick CA, Bambrick L, Krueger BK, Ward CW, Tessarollo L. In vivo restoration of physiological levels of truncated trkb. T1 receptor rescues neuronal cell death in a trisomic mouse model. Neuron. 2006;51:21–28. doi: 10.1016/j.neuron.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Dumser M, Bauer J, Lassmann H, Berger J, Forss-Petter S. Lack of adrenoleukodystrophy protein enhances oligodendrocyte disturbance and microglia activation in mice with combined abcd1/mag deficiency. Acta Neuropathol. (Berl.) 2007 doi: 10.1007/s00401-007-0288-4. [DOI] [PubMed] [Google Scholar]
- Duncan JE, Goldstein LS. The genetics of axonal transport and axonal transport disorders. PLoS Genet. 2006;2:e124. doi: 10.1371/journal.pgen.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of alzheimer disease. Acta Neuropathol. 2009 doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- Dwork AJ, Mancevski B, Rosoklija G. White matter and cognitive function in schizophrenia. Int. J. Neuropsychopharmacol. 2007:1–24. doi: 10.1017/S1461145707007638. [DOI] [PubMed] [Google Scholar]
- Edgar JM, Garbern J. The myelinated axon is dependent on the myelinating cell for support and maintenance: molecules involved. J. Neurosci. Res. 2004;76:593–598. doi: 10.1002/jnr.20063. [DOI] [PubMed] [Google Scholar]
- Edgar JM, McLaughlin M, Barrie JA, McCulloch MC, Garbern J, Griffiths IR. Age-related axonal and myelin changes in the rumpshaker mutation of the plp gene. Acta Neuropathol. (Berl.) 2004a;107:331–335. doi: 10.1007/s00401-003-0808-9. [DOI] [PubMed] [Google Scholar]
- Edgar JM, McLaughlin M, Yool D, Zhang SC, Fowler JH, Montague P, Barrie JA, McCulloch MC, Duncan ID, Garbern J, Nave KA, Griffiths IR. Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. J. Cell Biol. 2004b;166:121–131. doi: 10.1083/jcb.200312012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Tannir El Tayara N, Delatour B, Le Cudennec C, Guegan M, Volk A, Dhenain M. Age-related evolution of amyloid burden, iron load, and mr relaxation times in a transgenic mouse model of alzheimer’s disease. Neurobiol. Dis. 2006;22:199–208. doi: 10.1016/j.nbd.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Engelmann B. Plasmalogens: targets for oxidants and major lipophilic antioxidants. Biochem. Soc. Trans. 2004;32:147–150. doi: 10.1042/bst0320147. [DOI] [PubMed] [Google Scholar]
- Englund E, Brun A. White matter changes in dementia of alzheimer’s type: the difference in vulnerability between cell compartments. Histopathology. 1990;16:433–439. doi: 10.1111/j.1365-2559.1990.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Escribano L, Simon AM, Perez-Mediavilla A, Salazar-Colocho P, Del Rio J, Frechilla D. Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an alzheimer’s disease mouse model. Biochem. Biophys. Res. Commun. 2009;379:406–410. doi: 10.1016/j.bbrc.2008.12.071. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine apoe markedly alters a beta metabolism before and after plaque formation in a mouse model of alzheimer’s disease. Neurobiol. Dis. 2002;9:305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- Fainman J, Eid MD, Ervin FR, Palmour RM. A primate model for alzheimer’s disease: investigation of the apolipoprotein e profile of the vervet monkey of st Kitts. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007;144B:818–819. doi: 10.1002/ajmg.b.30276. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Rapoport SI, Horrocks LA. Membrane phospholipid alterations in alzheimer’s disease: deficiency of ethanolamine plasmalogens. Neurochem. Res. 1997;22:523–527. doi: 10.1023/a:1027380331807. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J. Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- Favrelere S, Stadelmann-Ingrand S, Huguet F, De Javel D, Piriou A, Tallineau C, Durand G. Age-related changes in ethanolamine glycerophospholipid fatty acid levels in rat frontal cortex and hippocampus. Neurobiol. Aging. 2000;21:653–660. doi: 10.1016/s0197-4580(00)00170-6. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Archibald SL, Jacobson MW, Corey-Bloom J, Paulsen JS, Peavy GM, Gamst AC, Hamilton JM, Salmon DP, Jernigan TL. In vivo evidence of cerebellar atrophy and cerebral white matter loss in huntington disease. Neurology. 2004;63:989–995. doi: 10.1212/01.wnl.0000138434.68093.67. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res. Brain Res. Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerod L, Fisher EM, Isaacs A, Brech A, Stenmark H, Simonsen A. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J. Cell Biol. 2007;179:485–500. doi: 10.1083/jcb.200702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE. A perspective on sporadic inclusion-body myositis: the role of aging and inflammatory processes. Neurology. 2006;66:S1–S6. doi: 10.1212/01.wnl.0000192259.34541.e4. [DOI] [PubMed] [Google Scholar]
- Finch CE, Morgan TE. Systemic inflammation, infection, apoe alleles, and alzheimer disease: a position paper. Curr. Alzheimer Res. 2007;4:185–189. doi: 10.2174/156720507780362254. [DOI] [PubMed] [Google Scholar]
- Frackowiak J, Potempska A, Mazur-Kolecka B. Formation of amyloid-beta oligomers in brain vascular smooth muscle cells transiently exposed to iron-induced oxidative stress. Acta Neuropathol. 2009;117:557–567. doi: 10.1007/s00401-009-0497-0. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Kotter MR. The biology of cns remyelination: the key to therapeutic advances. J. Neurol. 2008;255 Suppl. 1:19–25. doi: 10.1007/s00415-008-1004-6. [DOI] [PubMed] [Google Scholar]
- Freude S, Leeser U, Muller M, Hettich MM, Udelhoven M, Schilbach K, Tobe K, Kadowaki T, Kohler C, Schroder H, Krone W, Bruning JC, Schubert M. Irs-2 branch of igf-1 receptor signaling is essential for appropriate timing of myelination. J. Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05631.x. [DOI] [PubMed] [Google Scholar]
- Gani OA, Sylte I. Molecular recognition of docosahexaenoic acid by peroxisome proliferator-activated receptors and retinoid-x receptor alpha. J. Mol. Graph. Model. 2008;27:217–224. doi: 10.1016/j.jmgm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Garbern JY, Yool DA, Moore GJ, Wilds IB, Faulk MW, Klugmann M, Nave KA, Sistermans EA, van der Knaap MS, Bird TD, Shy ME, Kamholz JA, Griffiths IR. Patients lacking the major cns myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain. 2002;125:551–561. doi: 10.1093/brain/awf043. [DOI] [PubMed] [Google Scholar]
- Geddes JF, Vowles GH, Nicoll JA, Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. (Berl.) 1999;98:171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- Geddes JF, Vowles GH, Hackshaw AK, Nickols CD, Scott IS, Whitwell HL. Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain. 2001;124:1299–1306. doi: 10.1093/brain/124.7.1299. [DOI] [PubMed] [Google Scholar]
- Gerstner B, DeSilva TM, Genz K, Armstrong A, Brehmer F, Neve RL, Felderhoff-Mueser U, Volpe JJ, Rosenberg PA. Hyperoxia causes maturation-dependent cell death in the developing white matter. J. Neurosci. 2008;28:1236–1245. doi: 10.1523/JNEUROSCI.3213-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Martinez-Lage M, Kwong LK, Lee VM, Trojanowski JQ. Amyotrophic lateral sclerosis, frontotemporal dementia and beyond: the tdp-43 diseases. J. Neurol. 2009 doi: 10.1007/s00415-009-5069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula C, Nagykery N, Nicholas A, Wu CK. Cholinergic neuronal and axonal abnormalities are present early in aging and in alzheimer disease. J. Neuropathol. Exp. Neurol. 2008;67:309–318. doi: 10.1097/NEN.0b013e31816a1df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg L, Rafique S, Xuereb JH, Rapoport SI, Gershfeld NL. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in alzheimer’s disease brain. Brain Res. 1995;698:223–226. doi: 10.1016/0006-8993(95)00931-f. [DOI] [PubMed] [Google Scholar]
- Goddard DR, Berry M, Butt AM. In vivo actions of fibroblast growth factor-2 and insulin-like growth factor-i on oligodendrocyte development and myelination in the central nervous system. J. Neurosci. Res. 1999;57:74–85. doi: 10.1002/(SICI)1097-4547(19990701)57:1<74::AID-JNR8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Gong JS, Kobayashi M, Hayashi H, Zou K, Sawamura N, Fujita SC, Yanagisawa K, Michikawa M. Apolipoprotein e (apoe) isoform-dependent lipid release from astrocytes prepared from human apoe3 and apoe4 knock-in mice. J. Biol. Chem. 2002;277:29919–29926. doi: 10.1074/jbc.M203934200. [DOI] [PubMed] [Google Scholar]
- Goodenowe DB, Cook LL, Liu J, Lu Y, Jayasinghe DA, Ahiahonu PW, Heath D, Yamazaki Y, Flax J, Krenitsky KF, Sparks DL, Lerner A, Friedland RP, Kudo T, Kamino K, Morihara T, Takeda M, Wood PL. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in alzheimer’s disease and dementia. J. Lipid Res. 2007;48:2485–2498. doi: 10.1194/jlr.P700023-JLR200. [DOI] [PubMed] [Google Scholar]
- Goodrum JF. Cholesterol from degenerating nerve myelin becomes associated with lipoproteins containing apolipoprotein e. J. Neurochem. 1991;56:2082–2086. doi: 10.1111/j.1471-4159.1991.tb03469.x. [DOI] [PubMed] [Google Scholar]
- Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a cns neuron. Mol. Cell Neurosci. 2005;29:190–201. doi: 10.1016/j.mcn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Gotow T, Leterrier JF, Ohsawa Y, Watanabe T, Isahara K, Shibata R, Ikenaka K, Uchiyama Y. Abnormal expression of neurofilament proteins in dysmyelinating axons located in the central nervous system of jimpy mutant mice. Eur. J. Neurosci. 1999;11:3893–3903. doi: 10.1046/j.1460-9568.1999.00820.x. [DOI] [PubMed] [Google Scholar]
- Gottfries CG, Karlsson I, Svennerholm L. Membrane components separate early-onset alzheimer’s disease from senile dementia of the alzheimer type. Int. Psychogeriatr. 1996;8:365–372. doi: 10.1017/s1041610296002736. [DOI] [PubMed] [Google Scholar]
- Gravel M, Peterson J, Yong VW, Kottis V, Trapp B, Braun PE. Overexpression of 2′,3′-cyclic nucleotide 3′-phosphodiesterase in transgenic mice alters oligodendrocyte development and produces aberrant myelination. Mol. Cell Neurosci. 1996;7:453–466. doi: 10.1006/mcne.1996.0033. [DOI] [PubMed] [Google Scholar]
- Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of alzheimer’s disease. J. Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzendler J, Helmin K, Tsai J, Gan WB. Various dendritic abnormalities are associated with fibrillar amyloid deposits in alzheimer’s disease. Ann. N. Y. Acad. Sci. 2007;1097:30–39. doi: 10.1196/annals.1379.003. [DOI] [PubMed] [Google Scholar]
- Guan Z, Wang Y, Cairns NJ, Lantos PL, Dallner G, Sindelar PJ. Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with alzheimer disease. J. Neuropathol. Exp. Neurol. 1999;58:740–747. doi: 10.1097/00005072-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Guillot N, Debard C, Calzada C, Vidal H, Lagarde M, Vericel E. Effects of docosahexaenoic acid on some megakaryocytic cell gene expression of some enzymes controlling prostanoid synthesis. Biochem. Biophys. Res. Commun. 2008;372:924–928. doi: 10.1016/j.bbrc.2008.05.155. [DOI] [PubMed] [Google Scholar]
- Halder RC, Jahng A, Maricic I, Kumar V. Mini review: immune response to myelin-derived sulfatide and cns-demyelination. Neurochem. Res. 2007;32:257–262. doi: 10.1007/s11064-006-9145-4. [DOI] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J. Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Hampel H, Burger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of alzheimer’s disease. Alzheimers Dement. 2008;4:38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Han X, Holtzman DM, McKeel DW., Jr. Plasmalogen deficiency in early alzheimer’s disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 2001;77:1168–1180. doi: 10.1046/j.1471-4159.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- Han X, Holtzman DM, McKeel DW, Jr., Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early alzheimer’s disease: potential role in disease pathogenesis. J. Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy J. Does abeta 42 have a function related to blood homeostasis? Neurochem. Res. 2007;32:833–835. doi: 10.1007/s11064-006-9221-9. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Davis KL. Introduction to the special section: myelin and oligodendrocyte abnormalities in schizophrenia. Int. J. Neuropsychopharmacol. 2007;10:499–502. doi: 10.1017/S1461145706007449. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Tsukamoto E, Niikura T, Yamagishi Y, Ishizaka M, Aiso S, Takashima A, Nishimoto I. Amino- and carboxyl-terminal mutants of presenilin 1 cause neuronal cell death through distinct toxic mechanisms: study of 27 different presenilin 1 mutants. J. Neurosci. Res. 2004;75:417–428. doi: 10.1002/jnr.10861. [DOI] [PubMed] [Google Scholar]
- He J, Luo H, Yan B, Yu Y, Wang H, Wei Z, Zhang Y, Xu H, Tempier A, Li X, Li XM. Beneficial effects of quetiapine in a transgenic mouse model of alzheimer’s disease. Neurobiol. Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Mousavi M, Ravid R, Nordberg A. Reduced levels of abeta 40 and abeta 42 in brains of smoking controls and alzheimer’s patients. Neurobiol. Dis. 2004;15:351–360. doi: 10.1016/j.nbd.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H. Concurrence of tdp-43, tau and alpha-synuclein pathology in brains of alzheimer’s disease and dementia with lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Remahl S, Persson H, Bjartmar C. Myelinated nerve fibres in the cns. Prog. Neurobiol. 1993;40:319–384. doi: 10.1016/0301-0082(93)90015-k. [DOI] [PubMed] [Google Scholar]
- Hill CM, Libich DS, Harauz G. Assembly of tubulin by classic myelin basic protein isoforms and regulation by post-translational modification. Biochemistry. 2005;44:16672–16683. doi: 10.1021/bi050646+. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hinman JD, Peters A, Cabral H, Rosene DL, Hollander W, Rasband MN, Abraham CR. Age-related molecular reorganization at the node of ranvier. J. Comp. Neurol. 2006;495:351–362. doi: 10.1002/cne.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman JD, Chen CD, Oh SY, Hollander W, Abraham CR. Age-dependent accumulation of ubiquitinated 2′,3′-cyclic nucleotide 3′-phosphodiesterase in myelin lipid rafts. Glia. 2008;56:118–133. doi: 10.1002/glia.20595. [DOI] [PubMed] [Google Scholar]
- Hirata M, Sakuma K, Okajima S, Fujiwara H, Inashima S, Yasuhara M, Kubo T. Increased expression of neuregulin-1 in differentiating muscle satellite cells and in motoneurons during muscle regeneration. Acta Neuropathol. (Berl.) 2007 doi: 10.1007/s00401-007-0198-5. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Resch A, Gutterer JM, Dringen R. Oligodendroglial cells in culture effectively dispose of exogenous hydrogen peroxide: comparison with cultured neurones, astroglial and microglial cells. J. Neurochem. 2002;82:635–644. doi: 10.1046/j.1471-4159.2002.00999.x. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch. Gen. Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in alzheimer’s disease: II. Primary and secondary visual cortex. J. Comp. Neurol. 1990;301:55–64. doi: 10.1002/cne.903010106. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of abeta42 immunisation in alzheimer’s disease: follow-up of a randomised, placebo-controlled phase i trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein e isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House MJ, St Pierre TG, Foster JK, Martins RN, Clarnette R. Quantitative mr imaging r2 relaxometry in elderly participants reporting memory loss. AJNR Am. J. Neuroradiol. 2006;27:430–439. [PMC free article] [PubMed] [Google Scholar]
- Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 2006 doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, Trapp BD, Yan R. Genetic deletion of bace1 in mice affects remyelination of sciatic nerves. Faseb J. 2008 doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshagen L, Krysko O, Bottelbergs A, Huyghe S, Klein R, Van Veld-hoven PP, De Deyn PP, D’Hooge R, Hartmann D, Baes M. Absence of functional peroxisomes from mouse cns causes dysmyelination and axon degeneration. J. Neurosci. 2008;28:4015–4027. doi: 10.1523/JNEUROSCI.4968-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Tomonaga M. Alzheimer’s disease amyloid precursor protein is present in the myelin sheath of central nervous system in rat. Brain Res. 1990;527:140–144. doi: 10.1016/0006-8993(90)91072-o. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, Alonso AD, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009 doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008 doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC. Serial pib and mri in normal, mild cognitive impairment and alzheimer’s disease: implications for sequence of pathological events in alzheimer’s disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F. Evolution and tinkering. Science. 1977;196:1161–1166. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Criteria for the neuropathological diagnosis of dementing disorders: routes out of the swamp? Acta Neuropathol. 2009;117:101–110. doi: 10.1007/s00401-008-0466-z. [DOI] [PubMed] [Google Scholar]
- Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, Fuentealba P, Bushong EA, Henze D, Buzsaki G, Somogyi P. Neuronal diversity in gabaergic long-range projections from the hippocampus. J. Neurosci. 2007;27:8790–8804. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen AM, Marner L, Pakkenberg B. No change in total length of white matter fibers in alzheimer’s disease. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.06.075. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Ahmed Z, Shiung MM, Weigand SD, Knopman DS, Boeve BF, Parisi JE, Petersen RC, Dickson DW, Jack CR., Jr. Beta-amyloid burden is not associated with rates of brain atrophy. Ann. Neurol. 2007 doi: 10.1002/ana.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurynczyk M, Jurewicz A, Bielecki B, Raine CS, Selmaj K. Overcoming failure to repair demyelination in eae: gamma-secretase inhibition of notch signaling. J. Neurol. Sci. 2008;265:5–11. doi: 10.1016/j.jns.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J. Neurosci. 2009;29:1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaes T. Die Grosshirnrinde des Menschen in ihren Massen und in ihrem Fasergehalt. Jena: Gustav Fisher; 1907. [Google Scholar]
- Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires app. Nature. 2001;414:643–648. doi: 10.1038/414643a. [DOI] [PubMed] [Google Scholar]
- Kanter JL, Narayana S, Ho PP, Catz I, Warren KG, Sobel RA, Steinman L, Robinson WH. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat. Med. 2006;12:138–143. doi: 10.1038/nm1344. [DOI] [PubMed] [Google Scholar]
- Kassmann CM, Lappe-Siefke C, Baes M, Brugger B, Mildner A, Werner HB, Natt O, Michaelis T, Prinz M, Frahm J, Nave KA. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 2007;39:969–976. doi: 10.1038/ng2070. [DOI] [PubMed] [Google Scholar]
- Kasuga K, Kaneko H, Nishizawa M, Onodera O, Ikeuchi T. Generation of intracellular domain of insulin receptor tyrosine kinase by gamma-secretase. Biochem. Biophys. Res. Commun. 2007;360:90–96. doi: 10.1016/j.bbrc.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, escrt-i. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- Kaur D, Peng J, Chinta SJ, Rajagopalan S, Di Monte DA, Cherny RA, Andersen JK. Increased murine neonatal iron intake results in parkinson-like neurodegeneration with age. Neurobiol. Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kawakami F, Yamaguchi A, Suzuki K, Yamamoto T, Ohtsuki K. Biochemical characterization of phospholipids, sulfatide and heparin as potent stimulators for autophosphorylation of gsk-3beta and the gsk-3beta-mediated phosphorylation of myelin basic protein in vitro. J. Biochem. 2008;143:359–367. doi: 10.1093/jb/mvm228. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Shoji M, Yamaguchi H, Tanaka M, Harigaya Y, Ishiguro K, Hirai S. Amyloid beta protein precursor accumulates in swollen neurites throughout rat brain with aging. Neurosci. Lett. 1993;153:73–76. doi: 10.1016/0304-3940(93)90080-5. [DOI] [PubMed] [Google Scholar]
- Ke Y, Ming Qian Z. Iron misregulation in the brain: a primary cause of neurodegenerative disorders. Lancet Neurol. 2003;2:246–253. doi: 10.1016/s1474-4422(03)00353-3. [DOI] [PubMed] [Google Scholar]
- Kemper T. Neuroanatomical and neuropathological changes during aging and dementia. In: Albert M, Knoefel J, editors. Clinical Neurology of Aging. 2nd Edition. New York: Oxford University Press; 1994. pp. 3–67. [Google Scholar]
- Khan M, Singh J, Singh I. Plasmalogen deficiency in cerebral adrenoleukodystrophy and its modulation by lovastatin. J. Neurochem. 2008;106:1766–1779. doi: 10.1111/j.1471-4159.2008.05513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick LL, Witt AS, Payne HR, Shine HD, Brady ST. Changes in microtubule stability and density in myelin-deficient shiverer mouse cns axons. J. Neurosci. 2001;21:2288–2297. doi: 10.1523/JNEUROSCI.21-07-02288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Kuwabara K, Ohtsuki T, Hori M. Delayed, but marked, expression of apolipoprotein e is involved in tissue clearance after cerebral infarction. J. Cereb. Blood Flow Metab. 2001;21:1199–1207. doi: 10.1097/00004647-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Klintworth GK. Huntington’s chorea—morphologic contributions of a century. In: Barbeau A, Paulson GW, Chase TN, editors. Advances in Neurology, v. 1: Huntington’s Chorea, 1872–1972. New York: Raven Press; 1973. pp. 353–368. [Google Scholar]
- Klugmann M, Schwab MH, Puhlhofer A, Schneider A, Zimmermann F, Griffiths IR, Nave KA. Assembly of cns myelin in the absence of proteolipid protein. Neuron. 1997;18:59–70. doi: 10.1016/s0896-6273(01)80046-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Zeller M, Cole T, Buttini M, McConlogue L, Sinha S, Freedman S, Morris RG, Chen KS. Bace1 gene deletion: impact on behavioral function in a model of alzheimer’s disease. Neurobiol. Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, Royall DR, Laird A, Fox PT. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Ono K, Inoue K, Takagi Y, Kikuta KI, Nishimura M, Yoshida Y, Nakashima Y, Matsumae H, Furukawa Y, Mikuni N, Nobuyoshi M, Kimura T, Kita T, Tanaka M. Progranulin expression in advanced human atherosclerotic plaque. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Koldamova R, Staufenbiel M, Lefterov I. Lack of abca1 considerably decreases brain apoe level and increases amyloid deposition in app23 mice. J. Biol. Chem. 2005;280:43224–43235. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, Linington C, Schmidbauer M, Lassmann H. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 2000;157:267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostka M, Hogen T, Danzer KM, Levin J, Habeck M, Wirth A, Wagner R, Glabe CG, Finger S, Heinzelmann U, Garidel P, Duan W, Ross CA, Kretzschmar H, Giese A. Single-particle characterization of iron-induced pore-forming alpha-synuclein oligomers. J. Biol. Chem. 2008 doi: 10.1074/jbc.M709634200. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007 doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Kuczynski B, Reo NV. Evidence that plasmalogen is protective against oxidative stress in the rat brain. Neurochem. Res. 2006;31:639–656. doi: 10.1007/s11064-006-9061-7. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kaundal RK, More S, Sharma SS. Beneficial effects of pioglitazone on cognitive impairment in mptp model of parkinson’s disease. Behav. Brain Res. 2009;197:398–403. doi: 10.1016/j.bbr.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Kumar-Singh S, Van Broeckhoven C. Frontotemporal lobar degeneration: current concepts in the light of recent advances. Brain Pathol. 2007;17:104–114. doi: 10.1111/j.1750-3639.2007.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in alzheimer’s disease. J. Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Feng L. Implication of gamma-secretase in neuregulin-induced maturation of oligodendrocytes. Biochem. Biophys. Res. Commun. 2004;314:535–542. doi: 10.1016/j.bbrc.2003.12.131. [DOI] [PubMed] [Google Scholar]
- Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, Borchelt DR, Price DL, Lee HK, Wong PC. Bace1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J. Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamantia AS, Rakic P. Cytological and quantitative characteristics of four cerebral commissures in the rhesus monkey. J. Comp. Neurol. 1990;291:520–537. doi: 10.1002/cne.902910404. [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Larson IA, Ordovas JM, DeLuca C, Barnard JR, Feussner G, Schaefer EJ. Association of apolipoprotein (apo)e genotype with plasma apo e levels. Atherosclerosis. 2000;148:327–335. doi: 10.1016/s0021-9150(99)00280-4. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Vitek MP. Apolipoprotein e and neurological disease: therapeutic potential and pharmacogenomic interactions. Pharmacogenomics. 2007;8:959–969. doi: 10.2217/14622416.8.8.959. [DOI] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Lee M, Peterson DA, Sisodia SS. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J. Neurosci. 2002;22:9785–9793. doi: 10.1523/JNEUROSCI.22-22-09785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Morfini GA, Pigino G, Gadadhar A, Chen X, Robinson J, Ho H, Brady ST, Sisodia SS. Impairments in fast axonal transport and motor neuron deficits in transgenic mice expressing familial alzheimer’s disease-linked mutant presenilin 1. J. Neurosci. 2007;27:7011–7020. doi: 10.1523/JNEUROSCI.4272-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Skrzypski J, Courvoisier A, Gondcaille C, Bonnetain F, Andre A, Chardigny JM, Bellenger S, Bellenger J, Narce M, Savary S. Effect of dietary polyunsaturated fatty acids on the expression of peroxisomal abc transporters. Biochimie. 2008;90:1602–1607. doi: 10.1016/j.biochi.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS. Associations of salivary cortisol with cognitive function in the baltimore memory study. Arch. Gen. Psychiatry. 2007a;64:810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Jung KM, Huang YZ, Bennett LB, Lee JS, Mei L, Kim TW. Presenilin-dependent gamma-secretase-like intramembrane cleavage of erbb4. J. Biol. Chem. 2002;277:6318–6323. doi: 10.1074/jbc.M110371200. [DOI] [PubMed] [Google Scholar]
- Lee J, Gravel M, Zhang R, Thibault P, Braun PE. Process outgrowth in oligodendrocytes is mediated by cnp, a novel microtubule assembly myelin protein. J. Cell. Biol. 2005;170:661–673. doi: 10.1083/jcb.200411047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Beigneux A, Ahmad ST, Young SG, Gao FB. Escrt-iii dysfunction causes autophagosome accumulation and neurodegeneration. Curr. Biol. 2007b;17:1561–1567. doi: 10.1016/j.cub.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Lefterov I, Bookout A, Wang Z, Staufenbiel M, Mangelsdorf D, Koldamova R. Expression profiling in app23 mouse brain: inhibition of abeta amyloidosis and inflammation in response to lxr agonist treatment. Mol. Neurodegener. 2007;2:20. doi: 10.1186/1750-1326-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann DJ, Worwood M, Ellis R, Wimhurst VL, Merryweather-Clarke AT, Warden DR, Smith AD, Robson KJ. Iron genes, iron load and risk of alzheimer’s disease. J. Med. Genet. 2006;43:e52. doi: 10.1136/jmg.2006.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Oh J, Stanish HA, Peng Y, Pepivani I, Fagan AM, Yamaguchi H, Westmoreland SV, Mansfield KG. Cerebral amyloid-beta protein accumulation with aging in cotton-top tamarins: a model of early alzheimer’s disease? Rejuvenation Res. 2008;11:321–332. doi: 10.1089/rej.2008.0677. [DOI] [PubMed] [Google Scholar]
- Lemmens R, Gorner A, Schrooten M, Thijs V. Association of apolipoprotein e epsilon2 with white matter disease but not with microbleeds. Stroke. 2007;38:1185–1188. doi: 10.1161/01.STR.0000259816.31370.44. [DOI] [PubMed] [Google Scholar]
- Leow AD, Yanovsky I, Parikshak N, Hua X, Lee S, Toga AW, Jack CR, Jr., Bernstein MA, Britson PJ, Gunter JL, Ward CP, Borowski B, Shaw LM, Trojanowski JQ, Fleisher AS, Harvey D, Kornak J, Schuff N, Alexander GE, Weiner MW, Thompson PM. Alzheimer’s disease neuroimaging initiative: a one-year follow up study using tensor-based morphometry correlating degenerative rates, biomarkers and cognition. Neuroimage. 2009;45:645–655. doi: 10.1016/j.neuroimage.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Pericak-Vance MA, Haines JL, Siddique N, McKenna-Yasek D, Hung WY, Sapp P, Allen CI, Chen W, Hosler B, Saunders AM, Dellefave LM, Brown RH, Siddique T. Apolipoprotein e is associated with age at onset of amyotrophic lateral sclerosis. Neurogenetics. 2004;5:209–213. doi: 10.1007/s10048-004-0193-0. [DOI] [PubMed] [Google Scholar]
- Liang G, Cline GW, Macica CM. Igf-1 stimulates de novo fatty acid biosynthesis by schwann cells during myelination. Glia. 2007;55:632–641. doi: 10.1002/glia.20496. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Holzbaur EL. Microtubules tethered at epithelial cell junctions by dynein facilitate efficient junction assembly. Traffic. 2007;8:808–819. doi: 10.1111/j.1600-0854.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Lintl P, Braak H. Loss of intracortical myelinated fibers:a distinctive age-related alteration in the human striate area. Acta Neuropathol. (Berl.) 1983;61:178–182. doi: 10.1007/BF00691983. [DOI] [PubMed] [Google Scholar]
- Liu MC, Akle V, Zheng W, Kitlen J, O’Steen B, Larner SF, Dave JR, Tortella FC, Hayes RL, Wang KK. Extensive degradation of myelin basic protein isoforms by calpain following traumatic brain injury. J. Neurochem. 2006;98:700–712. doi: 10.1111/j.1471-4159.2006.03882.x. [DOI] [PubMed] [Google Scholar]
- Llorens-Martin M, Torres-Aleman I, Trejo JL. Growth factors as mediators of exercise actions on the brain. Neuromolecular Med. 2008 doi: 10.1007/s12017-008-8026-1. [DOI] [PubMed] [Google Scholar]
- Loffler J, Huber G. Beta-amyloid precursor protein isoforms in various rat brain regions and during brain development. J. Neurochem. 1992;59:1316–1324. doi: 10.1111/j.1471-4159.1992.tb08443.x. [DOI] [PubMed] [Google Scholar]
- Lovett-Racke AE, Hussain RZ, Northrop S, Choy J, Rocchini A, Matthes L, Chavis JA, Diab A, Drew PD, Racke MK. Peroxisome proliferator-activated receptor alpha agonists as therapy for autoimmune disease. J. Immunol. 2004;172:5790–5798. doi: 10.4049/jimmunol.172.9.5790. [DOI] [PubMed] [Google Scholar]
- Lunemann JD, Schmidt J, Schmid D, Barthel K, Wrede A, Dalakas MC, Munz C. Beta-amyloid is a substrate of autophagy in sporadic inclusion body myositis. Ann. Neurol. 2007;61:476–483. doi: 10.1002/ana.21115. [DOI] [PubMed] [Google Scholar]
- Ma H, Lesne S, Kotilinek L, Steidl-Nichols JV, Sherman M, Younkin L, Younkin S, Forster C, Sergeant N, Delacourte A, Vassar R, Citron M, Kofuji P, Boland LM, Ashe KH. Involvement of beta-site app cleaving enzyme 1 (bace1) in amyloid precursor protein-mediated enhancement of memory and activity-dependent synaptic plasticity. Proc. Natl. Acad. Sci. U.S.A. 2007;104:8167–8172. doi: 10.1073/pnas.0609521104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarouf CL, Daugs ID, Spina S, Vidal R, Kokjohn TA, Patton RL, Kalback WM, Luehrs DC, Walker DG, Castano EM, Beach TG, Ghetti B, Roher AE. Histopathological and molecular heterogeneity among individuals with dementia associated with presenilin mutations. Mol. Neurodegener. 2008;3:20. doi: 10.1186/1750-1326-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, Ince PG, Kamphorst W, Revesz T, Rozemuller AJ, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Dickson DW, Trojanowski JQ, Mann DM. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Zaja-Milatovic S, Milatovic D, Stephen C, Sokal I, Maeda N, Montine TJ, Montine KS. Apolipoprotein e isoform-dependent dendritic recovery of hippocampal neurons following activation of innate immunity. J. Neuroinflammation. 2006;3:21. doi: 10.1186/1742-2094-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr. Apolipoprotein e: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Maier O, Hoekstra D, Baron W. Polarity development in oligodendrocytes: sorting and trafficking of myelin components. J.Mol. Neurosci. 2008;35:35–53. doi: 10.1007/s12031-007-9024-8. [DOI] [PubMed] [Google Scholar]
- Mandel S, Amit T, Bar-Am O, Youdim MB. Iron dysregulation in alzheimer’s disease: multimodal brain permeable iron chelating drugs, possessing neuroprotective-neurorescue and amyloid precursor protein-processing regulatory activities as therapeutic agents. Prog. Neurobiol. 2007 doi: 10.1016/j.pneurobio.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Stamer K, Vogel R, Thies E, Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol. Aging. 2003;24:1079–1085. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz M, Seidah NG. Coordinated expression of beta-amyloid precursor protein and the putative beta-secretase bace and alpha-secretase adam10 in mouse and human brain. J. Neurochem. 2000;75:2133–2143. doi: 10.1046/j.1471-4159.2000.0752133.x. [DOI] [PubMed] [Google Scholar]
- Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL. Sulfatide is essential for the maintenance of cns myelin and axon structure. Glia. 2006;53:372–381. doi: 10.1002/glia.20292. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J. Comp. Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Marrosu MG, Floris G, Costa G, Schirru L, Spinicci G, Cherchi MV, Mura M, Mascia MG, Cocco E. Dementia, pyramidal system involvement, and leukoencephalopathy with a presenilin 1 mutation. Neurology. 2006;66:108–111. doi: 10.1212/01.wnl.0000191360.08881.12. [DOI] [PubMed] [Google Scholar]
- Martinez M, Vazquez E. Mri evidence that docosahexaenoic acid ethyl ester improves myelination in generalized peroxisomal disorders. Neurology. 1998;51:26–32. doi: 10.1212/wnl.51.1.26. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43:192–197. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- Mason JL, Ye P, Suzuki K, D’Ercole AJ, Matsushima GK. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. J. Neurosci. 2000;20:5703–5708. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (igf) signaling through type 1 igf receptor plays an important role in remyelination. J. Neurosci. 2003;23:7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiace LA, Kress Y, Davies P, Ksiezak-Reding H, Yen SH, Dickson DW. Ubiquitin-immunoreactive dystrophic neurites in down’s syndrome brains. J. Neuropathol. Exp. Neurol. 1991;50:547–559. doi: 10.1097/00005072-199109000-00003. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. Cns synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Maynard CJ, Cappai R, Volitakis I, Cherny RA, White AR, Beyreuther K, Masters CL, Bush AI, Li QX. Overexpression of alzheimer’s disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J. Biol. Chem. 2002;277:44670–44676. doi: 10.1074/jbc.M204379200. [DOI] [PubMed] [Google Scholar]
- McConlogue L, Buttini M, Anderson JP, Brigham EF, Chen KS, Freedman SB, Games D, Johnson-Wood K, Lee M, Zeller M, Liu W, Motter R, Sinha S. Partial reduction of bace1 has dramatic effects on alzheimer plaque and synaptic pathology in app transgenic mice. J. Biol. Chem. 2007 doi: 10.1074/jbc.M611687200. [DOI] [PubMed] [Google Scholar]
- McElroy B, Powell JC, McCarthy JV. The insulin-like growth factor 1 (igf-1) receptor is a substrate for gamma-secretase-mediated intramembrane proteolysis. Biochem. Biophys. Res. Commun. 2007 doi: 10.1016/j.bbrc.2007.05.062. [DOI] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan DRC, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W, Andrews DF. Intramuscular desferrioxamine in patients with alzheimer’s disease. Lancet. 1991;337:1304–1308. doi: 10.1016/0140-6736(91)92978-b. [DOI] [PubMed] [Google Scholar]
- Meadowcroft MD, Connor JR, Smith MB, Yang QX. Mri and histological analysis of beta-amyloid plaques in both human alzheimer’s disease and app/ps1 transgenic mice. J. Magn. Reson. Imaging. 2009;29:997–1007. doi: 10.1002/jmri.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro R, Asano Y, Odagiri S, Li C, Shoumura K. Cellular and subcellular localizations of nonheme ferric and ferrous iron in the rat brain: a light and electron microscopic study by the perfusion-perls and -turnbull methods. Arch. Histol. Cytol. 2008;71:205–222. doi: 10.1679/aohc.71.205. [DOI] [PubMed] [Google Scholar]
- Merkler D, Ernsting T, Kerschensteiner M, Bruck W, Stadelmann C. A new focal eae model of cortical demyelination: multiple sclerosis-like lesions with rapid resolution of inflammation and extensive remyelination. Brain. 2006;129:1972–1983. doi: 10.1093/brain/awl135. [DOI] [PubMed] [Google Scholar]
- Meyer A. Paul flechsig’s system of myelogenetic cortical localization in the light of recent research in neuroanatomy and neurophysiology. Part I. Can. J. Neurol. Sci. 1981a;8:1–6. doi: 10.1017/s031716710004275x. [DOI] [PubMed] [Google Scholar]
- Meyer A. Paul flechsig’s system of myelogenetic cortical localization in the light of recent research in neuroanatomy and neurophysiology part ii. Can. J. Neurol. Sci. 1981b;8:95–104. doi: 10.1017/s0317167100042980. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Migheli A, Attanasio A, Pezzulo T, Gullotta F, Giordana MT, Schiffer D. Age-related ubiquitin deposits in dystrophic neurites: an immunoelectron microscopic study. Neuropathol. Appl. Neurobiol. 1992;18:3–11. doi: 10.1111/j.1365-2990.1992.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Miller JA, Oldham MC, Geschwind DH. Asystems level analysis of transcriptional changes in alzheimer’s disease and normal aging. J. Neurosci. 2008;28:1410–1420. doi: 10.1523/JNEUROSCI.4098-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missal M, Coimbra A, Lefevre P, Olivier E. A quantitative analysis of the correlations between eye movements and neural activity in the pretectum. Exp. Brain Res. 2002;143:373–382. doi: 10.1007/s00221-002-0999-7. [DOI] [PubMed] [Google Scholar]
- Moir RD, Lynch T, Bush AI, Whyte S, Henry A, Portbury S, Multhaup G, Small DH, Tanzi RE, Beyreuther K, Masters CL. Relative increase in alzheimer’s disease of soluble forms of cerebral abeta amyloid protein precursor containing the kunitz protease inhibitory domain. J. Biol. Chem. 1998;273:5013–5019. doi: 10.1074/jbc.273.9.5013. [DOI] [PubMed] [Google Scholar]
- Moisse K, Volkening K, Leystra-Lantz C, Welch I, Hill T, Strong MJ. Divergent patterns of cytosolic tdp-43 and neuronal progranulin expression following axotomy: implications for tdp-43 in the physiological response to neuronal injury. Brain Res. 2009;1249:202–211. doi: 10.1016/j.brainres.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Morell P, Toews AD. In vivo metabolism of oligodendroglial lipids. In: Norton WT, editor. Oligodendroglia. New York: Plenium Press; 1984. pp. 47–86. [Google Scholar]
- Morell P, Jurevics H. Origin of cholesterol in myelin. Neurochem. Res. 1996;21:463–470. doi: 10.1007/BF02527711. [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Brady ST. Polyglutamine expansion diseases: failing to deliver. Trends Mol. Med. 2005;11:64–70. doi: 10.1016/j.molmed.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Mori H, Yagishita A, Takeda T, Mizutani T. Symmetric temporal abnormalities on mr imaging in amyotrophic lateral sclerosis with dementia. AJNR Am. J. Neuroradiol. 2007;28:1511–1516. doi: 10.3174/ajnr.A0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EJ, Schapiro MB, Rapoport SI, Shetty HU. Phospholipid composition and levels are altered in down syndrome brain. Brain Res. 2000;867:9–18. doi: 10.1016/s0006-8993(00)02205-8. [DOI] [PubMed] [Google Scholar]
- Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am. J. Clin. Nutr. 2007;85:778–787. doi: 10.1093/ajcn/85.3.778. [DOI] [PubMed] [Google Scholar]
- Muse ED, Jurevics H, Toews AD, Matsushima GK, Morell P. Parameters related to lipid metabolism as markers of myelination in mouse brain. J. Neurochem. 2001;76:77–86. doi: 10.1046/j.1471-4159.2001.00015.x. [DOI] [PubMed] [Google Scholar]
- Musiek ES, Brooks JD, Joo M, Brunoldi E, Porta A, Zanoni G, Vidari G, Blackwell TS, Montine TJ, Milne GL, McLaughlin B, Morrow JD. Electrophilic cyclopentenone neuroprostanes are anti-inflammatory mediators formed from the peroxidation of the omega-3 polyunsaturated fatty acid docosahexaenoic acid. J. Biol. Chem. 2008;283:19927–19935. doi: 10.1074/jbc.M803625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Fehlings MG. Mechanisms of axonal dysfunction after spinal cord injury: with an emphasis on the role of voltage-gated potassium channels. Brain Res. Brain Res. Rev. 2001;38:165–191. doi: 10.1016/s0165-0173(01)00134-5. [DOI] [PubMed] [Google Scholar]
- Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR. Clinicopathologic correlations in a large alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “Do count” When staging disease severity. J. Neuropathol. Exp. Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Mehta NR, Conant K, Kim KJ, Jones M, Calabresi PA, Melli G, Hoke A, Schnaar RL, Ming GL, Song H, Keswani SC, Griffin JW. Axonal protective effects of the myelin-associated glycoprotein. J. Neurosci. 2009;29:630–637. doi: 10.1523/JNEUROSCI.5204-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni CY, Murphy MP, Golde TE, Carpenter G. Gamma-secretase cleavage and nuclear localization of erbb-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- Nicolay DJ, Doucette JR, Nazarali AJ. Transcriptional control of oligodendrogenesis. Glia. 2007 doi: 10.1002/glia.20540. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. Structure and organization of fibre systems (chapter 3) In: Nieuwenhuys R, Tendokelaar JH, Nicholson C, editors. The Central Nervous System of Vertebrates. vol. 1. Berlin: Springer; 1999. pp. 113–157. [Google Scholar]
- Nordberg A. Amyloid imaging in Alzheimer’s disease. Curr. Opin. Neurol. 2007;20:398–402. doi: 10.1097/WCO.0b013e3281a47744. [DOI] [PubMed] [Google Scholar]
- O’Kusky J, Colonnier M. Postnatal changes in the number of astrocytes, oligodendrocytes, and microglia in the visual cortex (area 17) of the macaque monkey: a stereological analysis in normal and monocularly deprived animals. J. Comp. Neurol. 1982;210:307–315. doi: 10.1002/cne.902100309. [DOI] [PubMed] [Google Scholar]
- O’Riordan S, McMonagle P, Janssen JC, Fox NC, Farrell M, Collinge J, Rossor MN, Hutchinson M. Presenilin-1 mutation (e280g), spastic paraparesis, and cranial mri white-matter abnormalities. Neurology. 2002;59:1108–1110. doi: 10.1212/wnl.59.7.1108. [DOI] [PubMed] [Google Scholar]
- Oakley AE, Collingwood JF, Dobson J, Love G, Perrott HR, Edwardson JA, Elstner M, Morris CM. Individual dopaminergic neurons show raised iron levels in parkinson disease. Neurology. 2007;68:1820–1825. doi: 10.1212/01.wnl.0000262033.01945.9a. [DOI] [PubMed] [Google Scholar]
- Ohno M, Chang L, Tseng W, Oakley H, Citron M, Klein WL, Vassar R, Disterhoft JF. Temporal memory deficits in alzheimer’s mouse models: rescue by genetic deletion of bace1. Eur. J. Neurosci. 2006;23:251–260. doi: 10.1111/j.1460-9568.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- Packard CJ, Westendorp RG, Stott DJ, Caslake MJ, Murray HM, Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Jolles J, Perry IJ, Sweeney BJ, Twomey C. Association between apolipoprotein e4 and cognitive decline in elderly adults. J. Am. Geriatr. Soc. 2007;55:1777–1785. doi: 10.1111/j.1532-5415.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- Pak K, Chan SL, Mattson MP. Presenilin-1 mutation sensitizes oligodendrocytes to glutamate and amyloid toxicities, and exacerbates white matter damage and memory impairment in mice. Neuromolecular Med. 2003a;3:53–64. doi: 10.1385/NMM:3:1:53. [DOI] [PubMed] [Google Scholar]
- Pak KJ, Chan SL, Mattson MP. Homocysteine and folate deficiency sensitize oligodendrocytes to the cell death-promoting effects of a presenilin-1 mutation and amyloid beta-peptide. Neuromolecular Med. 2003b;3:119–128. doi: 10.1385/NMM:3:2:119. [DOI] [PubMed] [Google Scholar]
- Patton RL, Kalback WM, Esh CL, Kokjohn TA, Van Vickle GD, Luehrs DC, Kuo YM, Lopez J, Brune D, Ferrer I, Masliah E, Newel AJ, Beach TG, Castano EM, Roher AE. Amyloid-beta peptide remnants in an-1792-immunized alzheimer’s disease patients: a biochemical analysis. Am. J. Pathol. 2006;169:1048–1063. doi: 10.2353/ajpath.2006.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Cruts M, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. Altered brain white matter integrity in healthy carriers of the apoe epsilon4 allele: a risk for ad? Neurology. 2006;66:1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48. [DOI] [PubMed] [Google Scholar]
- Pertusa M, Morenilla-Palao C, Carteron C, Viana F, Cabedo H. Transciptional control of cholesterol biosynthesis in schwann cells by axonal neuregulin 1. J. Biol. Chem. 2007 doi: 10.1074/jbc.M701878200. [DOI] [PubMed] [Google Scholar]
- Peters A, Josephson K, Vincent SL. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat. Rec. 1991;229:384–398. doi: 10.1002/ar.1092290311. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J. Neuropathol. Exp. Neurol. 1996;55:861–874. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Are neurons lost from the primate cerebral cortex during normal aging? Cereb. Cortex. 1998;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Peters A. Structural changes in the normally aging cerebral cortex of primates. Prog. Brain Res. 2002;136:455–465. doi: 10.1016/s0079-6123(02)36038-2. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J. Comp. Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Is there remyelination during aging of the primate central nervous system? J. Comp. Neurol. 2003;460:238–254. doi: 10.1002/cne.10639. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Oligodendrocytes, their progenitors and other neuroglial cells in the aging primate cerebral cortex. Cereb. Cortex. 2004;14:995–1007. doi: 10.1093/cercor/bhh060. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008a;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Verderosa A, Sethares C. The neuroglial population in the primary visual cortex of the aging rhesus monkey. Glia. 2008b doi: 10.1002/glia.20686. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol. Psychiatry. 2006;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Phinney A, Deller T, Stalder M, Calhoun M, Frotscher M, Sommer B, Staufenbiel M, Jucker M. Cerebral amyloid induces aberrant axonal sprouting and ectopic terminal formation in amyloid precursor protein transgenic mice. J. Neurosci. 1999;19:8552–8559. doi: 10.1523/JNEUROSCI.19-19-08552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, LaDu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR, Malva JO, Heinemann SF, Mulle C. Glur7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12181–12186. doi: 10.1073/pnas.0608891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein e and cholesterol metabolism in the pathogenesis and treatment of alzheimer’s disease. Trends Mol. Med. 2003;9:94–101. doi: 10.1016/s1471-4914(03)00007-8. [DOI] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein e, cholesterol transport and synthesis in sporadic alzheimer’s disease. Neurobiol. Aging. 2005;26:355–361. doi: 10.1016/j.neurobiolaging.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Posse De Chaves EI, Vance DE, Campenot RB, Kiss RS, Vance JE. Uptake of lipoproteins for axonal growth of sympathetic neurons. J. Biol. Chem. 2000;275:19883–19890. doi: 10.1074/jbc.275.26.19883. [DOI] [PubMed] [Google Scholar]
- Power J, Mayer-Proschel M, Smith J, Noble M. Oligodendrocyte precursor cells from different brain regions express divergent properties consistent with the differing time courses of myelination in these regions. Dev. Biol. 2002;245:362–375. doi: 10.1006/dbio.2002.0610. [DOI] [PubMed] [Google Scholar]
- Praprotnik D, Smith MA, Richey PL, Vinters HV, Perry G. Filament heterogeneity within the dystrophic neurites of senile plaques suggests blockage of fast axonal transport in alzheimer’s disease. Acta Neuropathol. 1996;91:226–235. doi: 10.1007/s004010050420. [DOI] [PubMed] [Google Scholar]
- Preece P, Virley DJ, Costandi M, Coombes R, Moss SJ, Mudge AW, Jazin E, Cairns NJ. Amyloid precursor protein mrna levels in alzheimer’s disease brain. Brain Res. Mol. Brain Res. 2004;122:1–9. doi: 10.1016/j.molbrainres.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and ca1 in preclinical alzheimer disease. Arch. Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nat. Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- Puls I, Oh SJ, Sumner CJ, Wallace KE, Floeter MK, Mann EA, Kennedy WR, Wendelschafer-Crabb G, Vortmeyer A, Powers R, Finnegan K, Holzbaur EL, Fischbeck KH, Ludlow CL. Distal spinal and bulbar muscular atrophy caused by dynactin mutation. Ann. Neurol. 2005;57:687–694. doi: 10.1002/ana.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Nordberg A, Xiu J, Guan ZZ. The consequences of reducing expression of the alpha7 nicotinic receptor by rna interference and of stimulating its activity with an alpha7 agonist in sh-sy5y cells indicate that this receptor plays a neuroprotective role in connection with the pathogenesis of alzheimer’s disease. Neurochem. Int. 2007 doi: 10.1016/j.neuint.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Quintana C, Bellefqih S, Laval JY, Guerquin-Kern JL, Wu TD, Avila J, Ferrer I, Arranz R, Patino C. Study of the localization of iron, ferritin, and hemosiderin in alzheimer’s disease hippocampus by analytical microscopy at the subcellular level. J. Struct. Biol. 2006;153:42–54. doi: 10.1016/j.jsb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Quintanilla RA, Jin YN, Fuenzalida K, Bronfman M, Johnson GV. Rosiglitazone treatment prevents mitochondrial dysfunction in mutant huntingtin expressing cells: possible role of ppargamma in the pathogenesis of huntington disease. J. Biol. Chem. 2008 doi: 10.1074/jbc.M804291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Huang Y, Ashford JW. Apoe genotype accounts for the vast majority of ad risk and ad pathology. Neurobiol. Aging. 2004;25:641–650. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comp. Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran R, Minqin R, Ynsa MD, Casadesus G, Smith MA, Perry G, Halliwell B, Watt F. A novel approach to the identification and quantitative elemental analysis of amyloid deposits—insights into the pathology of alzheimer’s disease. Biochem. Biophys. Res. Commun. 2009;382:91–95. doi: 10.1016/j.bbrc.2009.02.136. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Tayler J, Kaga Y, Yang Y, Lappe-Siefke C, Nave KA, Bansal R. Cnp is required for maintenance of axon-glia interactions at nodes of ranvier in the cns. Glia. 2005;50:86–90. doi: 10.1002/glia.20165. [DOI] [PubMed] [Google Scholar]
- Rasminsky M, Sears TA. Internodal conduction in undissected demyelinated nerve fibres. J. Physiol. 1972;227:323–350. doi: 10.1113/jphysiol.1972.sp010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravera S, Panfoli I, Calzia D, Aluigi MG, Bianchini P, Diaspro A, Mancardi G, Morelli A. Evidence for aerobic atp synthesis in isolated myelin vesicles. Int. J. Biochem. Cell Biol. 2009;41:1581–1591. doi: 10.1016/j.biocel.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Alonzo NC, Berezovska O, Harr SD, Knowles RB, Growdon JH, Hyman BT, Mendez AJ. Structure and functions of human cerebrospinal fluid lipoproteins from individuals of different apoe genotypes. Exp. Neurol. 1998;149:175–182. doi: 10.1006/exnr.1997.6710. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, LaDu MJ, Estus S, Bu G, Weeber EJ. The generation and function of soluble apoe receptors in the cns. Mol. Neurodegener. 2006;1:15. doi: 10.1186/1750-1326-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Frey 2nd WH, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by apoe genotype. Neurobiol. Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early ad. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Franssen EH, Souren LE, Auer SR, Akram I, Kenowsky S. Evidence and mechanisms of retrogenesis in alzheimer’s and other dementias: management and treatment import. Am. J. Alzheimers Dis. Other Demen. 2002;17:202–212. doi: 10.1177/153331750201700411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Li H, Frank-Cannon TC, Valasek MA, Turley SD, Tansey MG, Dietschy JM. Liver x receptor activation enhances cholesterol loss from the brain, decreases neuroinflammation, and increases survival of the npc1 mouse. J. Neurosci. 2007;27:14470–14480. doi: 10.1523/JNEUROSCI.4823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Comery TA, Kouranova E, Lo CF, Warwick HK, Ring RH, Kirksey Y, Aschmies S, Xu J, Kubek K, Hirst WD, Gonzales C, Chen Y, Murphy E, Leonard S, Vasylyev D, Oganesian A, Martone RL, Pangalos MN, Reinhart PH, Jacobsen JS. The lxr agonist to901317 selectively lowers hippocampal abeta42 and improves memory in the tg2576 mouse model of alzheimer’s disease. Mol. Cell Neurosci. 2007;34:621–628. doi: 10.1016/j.mcn.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, Wagner E, Parratt A, Xu J, Li Z, Zaleska MM, Jacobsen JS, Pangalos MN, Reinhart PH. Impact of apolipoprotein e (apoe) polymorphism on brain apoe levels. J. Neurosci. 2008;28:11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman J, O’Neill J, Geschwind D, Medina L, Apostolova L, Rodriguez Y, Schaffer B, Varpetian A, Tseng B, Ortiz F, Fitten J, Cummings JL, Bartzokis G. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial alzheimer’s disease mutations. Brain. 2007;130:1767–1776. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Bush AI, Cho HH, Smith DH, Thomson AM, Friedlich AL, Lahiri DK, Leedman PJ, Huang X, Cahill CM. Iron and the translation of the amyloid precursor protein (app) and ferritin mrnas: riboregulation against neural oxidative damage in alzheimer’s disease. Biochem. Soc. Trans. 2008;36:1282–1287. doi: 10.1042/BST0361282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Weiss N, Kokjohn TA, Kuo YM, Kalback W, Anthony J, Watson D, Luehrs DC, Sue L, Walker D, Emmerling M, Goux W, Beach T. Increased abeta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in alzheimer’s disease. Biochemistry. 2002;41:11080–11090. doi: 10.1021/bi026173d. [DOI] [PubMed] [Google Scholar]
- Rohn TT. Caspase-cleaved tar DNA-binding protein-43 is a major pathological finding in alzheimer’s disease. Brain Res. 2008 doi: 10.1016/j.brainres.2008.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brainstem responses. Am. J. Clin. Nutr. 1998;68:683–690. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- Rosenberg CK, Pericak-Vance MA, Saunders AM, Gilbert JR, Gaskell PC, Hulette CM. Lewy body and alzheimer pathology in a family with the amyloid-beta precursor protein app717 gene mutation. Acta Neuropathol. (Berl.) 2000;100:145–152. doi: 10.1007/s004019900155. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Peripheral myelin in the mouse mutant shiverer. J. Comp. Neurol. 1980;193:729–739. doi: 10.1002/cne.901930310. [DOI] [PubMed] [Google Scholar]
- Rouser G, Yamamoto A. Curvilinear regression course of human brain lipid composition changes with age. Lipids. 1968;3:284–287. doi: 10.1007/BF02531202. [DOI] [PubMed] [Google Scholar]
- Rouser G, Kritchevsky G, Yamamoto A, Baxter CF. Lipids in the nervous system of different species as a function of age: brain, spinal cord, peripheral nerve, purified whole cell preparations, and subcellular particulates: regulatory mechanisms and membrane structure. Adv. Lipid Res. 1972;10:261–360. doi: 10.1016/b978-0-12-024910-7.50013-0. [DOI] [PubMed] [Google Scholar]
- Rusten TE, Simonsen A. Escrt functions in autophagy and associated disease. Cell Cycle. 2008;7:1166–1172. doi: 10.4161/cc.7.9.5784. [DOI] [PubMed] [Google Scholar]
- Rusu P, Jansen A, Soba P, Kirsch J, Lower A, Merdes G, Kuan YH, Jung A, Beyreuther K, Kjaerulff O, Kins S. Axonal accumulation of synaptic markers in app transgenic drosophila depends on the npty motif and is paralleled by defects in synaptic plasticity. Eur. J. Neurosci. 2007;25:1079–1086. doi: 10.1111/j.1460-9568.2007.05341.x. [DOI] [PubMed] [Google Scholar]
- Saher G, Brugger B, Lappe-Siefke C, Mobius W, Tozawa R, Wehr MC, Wieland F, Ishibashi S, Nave KA. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005;8:468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, Xia W, Villar A, Campbell WA, Kulnane LS, Nixon RA, Lamb BT, Epstein CJ, Stokin GB, Goldstein LS, Mobley WC. Increased app expression in a mouse model of down’s syndrome disrupts ngf transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Salvati S, Natali F, Attorri L, Di Benedetto R, Leonardi F, Di Biase A, Ferri F, Fortuna S, Lorenzini P, Sanchez M, Ricceri L, Vitelli L. Eicosapentaenoic acid stimulates the expression of myelin proteins in rat brain. J. Neurosci. Res. 2008;86:776–784. doi: 10.1002/jnr.21537. [DOI] [PubMed] [Google Scholar]
- Sanchez I, Hassinger L, Sihag RK, Cleveland DW, Mohan P, Nixon RA. Local control of neurofilament accumulation during radial growth of myelinating axons in vivo. Selective role of site-specific phosphorylation. J. Cell Biol. 2000;151:1013–1024. doi: 10.1083/jcb.151.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbrink R, Masters CL, Beyreuther K. App gene family: unique age-associated changes in splicing of alzheimer’s betaa4-amyloid protein precursor. Neurobiol. Dis. 1994;1:13–24. doi: 10.1006/nbdi.1994.0003. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Quintanilla RA, Toro A, Grandy R, Dinamarca MC, Godoy JA, Inestrosa NC. Peroxisomal proliferation protects from beta-amyloid neurodegeneration. J. Biol. Chem. 2005;280:41057–41068. doi: 10.1074/jbc.M505160200. [DOI] [PubMed] [Google Scholar]
- Sapirstein VS, Durrie R, Berg MJ, Marks N. Amyloid precursor protein is enriched in axolemma and periaxolemmal-myelin and associated clathrin-coated vesicles. J. Neurosci. Res. 1994;37:348–358. doi: 10.1002/jnr.490370307. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of bace1-dependent nrg1/erbb4 signaling and schizophrenia-like phenotypes in bace1-null mice. Proc. Natl. Acad. Sci. U.S.A. 2008 doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR. Ppar-gamma-mediated neuroprotection in a chronic mouse model of parkinson’s disease. Eur. J. Neurosci. 2009;29:954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Fazekas F, Schumacher M, Niederkorn K, Kapeller P, Weinrauch V, Kostner GM. Apolipoprotein e polymorphism and silent microangiopathy-related cerebral damage. Results of the austrian stroke prevention study. Stroke. 1997;28:951–956. doi: 10.1161/01.str.28.5.951. [DOI] [PubMed] [Google Scholar]
- Schnaar RL, Lopez PH. Myelin-associated glycoprotein and its axonal receptors. J. Neurosci. Res. 2009 doi: 10.1002/jnr.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schoenemann PT, Sheehan MJ, Glotzer LD. Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nat. Neurosci. 2005;8:242–252. doi: 10.1038/nn1394. [DOI] [PubMed] [Google Scholar]
- Schutz B, Reimann J, Dumitrescu-Ozimek L, Kappes-Horn K, Landreth GE, Schurmann B, Zimmer A, Heneka MT. The oral antidiabetic pioglitazone protects from neurodegeneration and amyotrophic lateral sclerosis-like symptoms in superoxide dismutase-g93a transgenic mice. J. Neurosci. 2005;25:7805–7812. doi: 10.1523/JNEUROSCI.2038-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Translating cell biology into therapeutic advances in alzheimer’s disease. Nature. 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav. Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nat. Neurosci. 2002;5:272–276. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJ, Casaccia-Bonnefil P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 2008 doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Price DL, Koliatsos VE. Disruption of corticocortical connections ameliorates amyloid burden in terminal fields in a transgenic model of abeta amyloidosis. J. Neurosci. 2002;22:9794–9799. doi: 10.1523/JNEUROSCI.22-22-09794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Price DL, Koliatsos VE. The beta-amyloid-related proteins presenilin 1 and bace1 are axonally transported to nerve terminals in the brain. Exp. Neurol. 2003;184:1053–1057. doi: 10.1016/j.expneurol.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Shields SA, Gilson JM, Blakemore WF, Franklin RJ. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced cns demyelination. Glia. 1999;28:77–83. doi: 10.1002/(sici)1098-1136(199910)28:1<77::aid-glia9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Shioi J, Georgakopoulos A, Mehta P, Kouchi Z, Litterst CM, Baki L, Robakis NK. Fad mutants unable to increase neurotoxic abeta 42 suggest that mutation effects on neurodegeneration may be independent of effects on abeta. J. Neurochem. 2007;101:674–681. doi: 10.1111/j.1471-4159.2006.04391.x. [DOI] [PubMed] [Google Scholar]
- Shrager P. Axonal coding of action potentials in demyelinated nerve fibers. Brain Res. 1993;619:278–290. doi: 10.1016/0006-8993(93)91622-y. [DOI] [PubMed] [Google Scholar]
- Sindelar PJ, Guan Z, Dallner G, Ernster L. The protective role of plasmalogens in iron-induced lipid peroxidation. Free Radic. Biol. Med. 1999;26:318–324. doi: 10.1016/s0891-5849(98)00221-4. [DOI] [PubMed] [Google Scholar]
- Singh A, Isaac AO, Luo X, Mohan ML, Cohen ML, Chen F, Kong Q, Bartz J, Singh N. Abnormal brain iron homeostasis in human and animal prion disorders. PLoS Pathog. 2009a;5:e1000336. doi: 10.1371/journal.ppat.1000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Mohan ML, Isaac AO, Luo X, Petrak J, Vyoral D, Singh N. Prion protein modulates cellular iron uptake: a novel function with implications for prion disease pathogenesis. PLoS ONE. 2009b;4:e4468. doi: 10.1371/journal.pone.0004468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjobeck M, Haglund M, Englund E. Decreasing myelin density reflected increasing white matter pathology in alzheimer’s disease—a neuropathological study. Int. J. Geriatr. Psychiatry. 2005;20:919–926. doi: 10.1002/gps.1384. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Lindner M, Kotsiari A, Garde N, Fokuhl J, Linsmeier F, Trebst C, Stangel M. Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. Am. J. Pathol. 2008;172:1053–1061. doi: 10.2353/ajpath.2008.070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DH, Maksel D, Kerr ML, Ng J, Hou X, Chu C, Mehrani H, Unabia S, Azari MF, Loiacono R, Aguilar MI, Chebib M. The beta-amyloid protein of alzheimer’s disease binds to membrane lipids but does not bind to the alpha7 nicotinic acetylcholine receptor. J. Neurochem. 2007;101:1527–1538. doi: 10.1111/j.1471-4159.2006.04444.x. [DOI] [PubMed] [Google Scholar]
- Smith KD, Kallhoff V, Zheng H, Pautler RG. In vivo axonal transport rates decrease in a mouse model of alzheimer’s disease. Neuroimage. 2007;35:1401–1408. doi: 10.1016/j.neuroimage.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider BJ, Norton J, Coats MA, Chakraverty S, Hou CE, Jervis R, Lendon CL, Goate AM, McKeel DW, Jr., Morris JC. Novel presenilin 1 mutation (s170f) causing alzheimer disease with lewy bodies in the third decade of life. Arch. Neurol. 2005;62:1821–1830. doi: 10.1001/archneur.62.12.1821. [DOI] [PubMed] [Google Scholar]
- Sousa AD, Bhat MA. Cytoskeletal transition at the paranodes: the achilles’ heel of myelinated axons. Neuron Glia Biol. 2007;3:169–178. doi: 10.1017/S1740925X07000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow A, Lamant M, Bonny JM, Larvaron P, Piaud O, Lecureuil C, Fontaine I, Saleh MC, Garcia Otin AL, Renou JP, Baron B, Zakin M, Guillou F. Oligodendrocyte differentiation is increased in transferrin transgenic mice. J. Neurosci. Res. 2006;83:403–414. doi: 10.1002/jnr.20741. [DOI] [PubMed] [Google Scholar]
- Stankiewicz J, Panter SS, Neema M, Arora A, Batt CE, Bakshi R. Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics. 2007;4:371–386. doi: 10.1016/j.nurt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, Goldstein LS. Axonopathy and transport deficits early in the pathogenesis of alzheimer’s disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- Stokin GB, Almenar-Queralt A, Gunawardena S, Rodrigues EM, Falzone T, Kim J, Lillo C, Mount SL, Roberts EA, McGowan E, Williams DS, Goldstein LS. Amyloid precursor protein-induced axonopathies are independent of amyloid-{beta}peptides. Hum. Mol. Genet. 2008 doi: 10.1093/hmg/ddn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, Frank LR, Salmon DP, Bondi MW. Decreased white matter integrity in late-myelinating fiber pathways in alzheimer’s disease supports retrogenesis. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kawakami F, Sasaki H, Maruyama H, Ohtsuki K. Biochemical characterization of tau protein and its associated syndapin 1 and protein kinase cvarepsilon for their functional regulation in rat brain. Biochim. Biophys. Acta. 2009;1790:188–197. doi: 10.1016/j.bbagen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Tamai K, Toyoshima M, Tanaka N, Yamamoto N, Owada Y, Kiyonari H, Murata K, Ueno Y, Ono M, Shimosegawa T, Yaegashi N, Watanabe M, Sugamura K. Loss of hrs in the central nervous system causes accumulation of ubiquitinated proteins and neurodegeneration. Am. J. Pathol. 2008;173:1806–1817. doi: 10.2353/ajpath.2008.080684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamboli IY, Prager K, Thal DR, Thelen KM, Dewachter I, Pietrzik CU, St George-Hyslop P, Sisodia SS, De Strooper B, Heneka MT, Filippov MA, Muller U, van Leuven F, Lutjohann D, Walter J. Loss of gamma-secretase function impairs endocytosis of lipoprotein particles and membrane cholesterol homeostasis. J. Neurosci. 2008;28:12097–12106. doi: 10.1523/JNEUROSCI.2635-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Liu L, Kimura J, Shiojiri S, Takahashi Y, Kitaguchi N, Nakamura S, Ueda K. Age-related changes in the proportion of amyloid precursor protein mrnas in alzheimer’s disease and other neurological disorders. Brain Res. Mol. Brain Res. 1992;15:303–310. doi: 10.1016/0169-328x(92)90122-r. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type iii neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56:284–293. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- Terry RD, Gonatas NK, Weiss M. Ultrastructural studies in alzheimer’s presenile dementia. Am. J. Pathol. 1964;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of a beta-deposition in the human brain and its relevance for the development of ad. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, Frey 2nd WH. Delivery of insulin-like growth factor-i to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2008 doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- Torroja L, Packard M, Gorczyca M, White K, Budnik V. The drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J. Neurosci. 1999;19:7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J. Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- Turnbull S, Tabner BJ, Brown DR, Allsop D. Generation of hydrogen peroxide from mutant forms of the prion protein fragment prp121-231. Biochemistry. 2003;42:7675–7681. doi: 10.1021/bi030036e. [DOI] [PubMed] [Google Scholar]
- Uryu K, Chen XH, Martinez D, Browne KD, Johnson VE, Graham DI, Lee VM, Trojanowski JQ, Smith DH. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp. Neurol. 2007 doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam PS, Aleman A. Insulin-like growth factor-i, cognition and brain aging. Eur. J. Pharmacol. 2004;490:87–95. doi: 10.1016/j.ejphar.2004.02.047. [DOI] [PubMed] [Google Scholar]
- Van den Branden C, Leeman J, Dacremont G, Collumbien R, Roels F. Experimental inhibition of peroxisomal beta-oxidation in rats: influence on brain myelination. Glia. 1990;3:458–463. doi: 10.1002/glia.440030604. [DOI] [PubMed] [Google Scholar]
- van Rensburg SJ, Potocnik FC, De Villiers JN, Kotze MJ, Taljaard JJ. Earlier age of onset of alzheimer’s disease in patients with both the transferrin c2 and apolipoprotein e-epsilon 4 alleles. Ann. N. Y. Acad. Sci. 2000;903:200–203. doi: 10.1111/j.1749-6632.2000.tb06369.x. [DOI] [PubMed] [Google Scholar]
- Veltri KL, Espiritu M, Singh G. Distinct genomic copy number in mitochondria of different mammalian organs. J. Cell Physiol. 1990;143:160–164. doi: 10.1002/jcp.1041430122. [DOI] [PubMed] [Google Scholar]
- Vlkolinsky R, Cairns N, Fountoulakis M, Lubec G. Decreased brain levels of 2′,3′-cyclic nucleotide-3′-phosphodiesterase in down syndrome and alzheimer’s disease. Neurobiol. Aging. 2001;22:547–553. doi: 10.1016/s0197-4580(01)00218-4. [DOI] [PubMed] [Google Scholar]
- Vos JP, Lopes-Cardozo M, Gadella BM. Metabolic and functional aspects of sulfogalactolipids. Biochim. Biophys. Acta. 1994;1211:125–149. doi: 10.1016/0005-2760(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Voss A, Reinhart M, Sankarappa S, Sprecher H. The metabolism of 7,10,13,16,19-docosapentaenoic acid to 4,7,10,13,16,19-docosahexaenoic acid in rat liver is independent of a 4-desaturase. J. Biol. Chem. 1991;266:19995–20000. [PubMed] [Google Scholar]
- Walker LC, Cork LC. The neurobiology of aging in non-human primates. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer’s Disease. Philadelphia: Lippincott, Williams and Wilkins; 1999. pp. 233–243. [Google Scholar]
- Wang P, Yang G, Mosier DR, Chang P, Zaidi T, Gong YD, Zhao NM, Dominguez B, Lee KF, Gan WB, Zheng H. Defective neuromuscular synapses in mice lacking amyloid precursor protein (app) and app-like protein 2. J. Neurosci. 2005;25:1219–1225. doi: 10.1523/JNEUROSCI.4660-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ha Y. The x-ray structure of an antiparallel dimer of the human amyloid precursor protein e2 domain. Mol. Cell. 2004;15:343–353. doi: 10.1016/j.molcel.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Watkins TA, Emery B, Mulinyawe S, Barres BA. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating cns coculture system. Neuron. 2008;60:555–569. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am. J. Geriatr. Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- Weisser M, Vieth M, Stolte M, Riederer P, Pfeuffer R, Leblhuber F, Spiteller G. Dramatic increase of alpha-hydroxyaldehydes derived from plasmalogens in the aged human brain. Chem. Phys. Lipids. 1997;90:135–142. doi: 10.1016/s0009-3084(97)00089-3. [DOI] [PubMed] [Google Scholar]
- Wen Y, Planel E, Herman M, Figueroa HY, Wang L, Liu L, Lau LF, Yu WH, Duff KE. Interplay between cyclin-dependent kinase 5 and glycogen synthase kinase 3beta mediated by neuregulin signaling leads to differential effects on tau phosphorylation and amyloid precursor protein processing. J. Neurosci. 2008;28:2624–2632. doi: 10.1523/JNEUROSCI.5245-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins RC. Myelin development and nutritional insufficiency. Brain Res. 1982;257:151–175. doi: 10.1016/0165-0173(82)90016-9. [DOI] [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase bace1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- Wirths O, Weis J, Szczygielski J, Multhaup G, Bayer TA. Axonopathy in an app/ps1 transgenic mouse model of alzheimer’s disease. Acta Neuropathol. (Berl.) 2006;111:312–319. doi: 10.1007/s00401-006-0041-4. [DOI] [PubMed] [Google Scholar]
- Wirths O, Weis J, Kayed R, Saido TC, Bayer TA. Age-dependent axonal degeneration in an alzheimer mouse model. Neurobiol. Aging. 2007;28:1689–1699. doi: 10.1016/j.neurobiolaging.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Wishart TM, Parson SH, Gillingwater TH. Synaptic vulnerability in neurodegenerative disease. J. Neuropathol. Exp. Neurol. 2006;65:733–739. doi: 10.1097/01.jnen.0000228202.35163.c4. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. When loss is gain: reduced presenilin proteolytic function leads to increased abeta42/abeta40. Talking point on the role of presenilin mutations in alzheimer disease. EMBO Rep. 2007;8:136–140. doi: 10.1038/sj.embor.7400896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P, Bunger RP. The biology of the oligodendrocyte. In: Norton WT, editor. Oligodendroglia. New York: Plenum Press; 1984. pp. 1–46. [Google Scholar]
- Wood TL, Loladze V, Altieri S, Gangoli N, Levison SW, Brywe KG, Mallard C, Hagberg H. Delayed igf-1 administration rescues oligodendrocyte progenitors from glutamate-induced cell death and hypoxic-ischemic brain damage. Dev. Neurosci. 2007;29:302–310. doi: 10.1159/000105471. [DOI] [PubMed] [Google Scholar]
- Wood WG, Schroeder F, Avdulov NA, Chochina SV, Igbavboa U. Recent advances in brain cholesterol dynamics: transport, domains, and alzheimer’s disease. Lipids. 1999;34:225–234. doi: 10.1007/s11745-999-0357-9. [DOI] [PubMed] [Google Scholar]
- Xiao L, Xu H, Zhang Y, Wei Z, He J, Jiang W, Li X, Dyck LE, Devon RM, Deng Y, Li XM. Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol. Psychiatry. 2007 doi: 10.1038/sj.mp.4002064. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. Regional Development of the Brain in Early Life. Boston: Blackwell Scientific Publications; 1967. pp. 3–70. [Google Scholar]
- Yamada M, Chiba T, Sasabe J, Terashita K, Aiso S, Matsuoka M. Nasal colivelin treatment ameliorates memory impairment related to alzheimer’s disease. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301591. [DOI] [PubMed] [Google Scholar]
- Yang G, Gong YD, Gong K, Jiang WL, Kwon E, Wang P, Zheng H, Zhang XF, Gan WB, Zhao NM. Reduced synaptic vesicle density and active zone size in mice lacking amyloid precursor protein (app) and app-like protein 2. Neurosci. Lett. 2005;384:66–71. doi: 10.1016/j.neulet.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Yao J, Petanceska SS, Montine TJ, Holtzman DM, Schmidt SD, Parker CA, Callahan MJ, Lipinski WJ, Bisgaier CL, Turner BA, Nixon RA, Martins RN, Ouimet C, Smith JD, Davies P, Laska E, Ehrlich ME, Walker LC, Mathews PM, Gandy S. Aging, gender and apoe isotype modulate metabolism of alzheimer’s abeta peptides and f-isoprostanes in the absence of detectable amyloid deposits. J. Neurochem. 2004;90:1011–1018. doi: 10.1111/j.1471-4159.2004.02532.x. [DOI] [PubMed] [Google Scholar]
- Yao PJ, Zhu M, Pyun EI, Brooks AI, Therianos S, Meyers VE, Coleman PD. Defects in expression of genes related to synaptic vesicle trafficking in frontal cortex of alzheimer’s disease. Neurobiol. Dis. 2003;12:97–109. doi: 10.1016/s0969-9961(02)00009-8. [DOI] [PubMed] [Google Scholar]
- Ye P, Carson J, D’Ercole AJ. Insulin-like growth factor-i influences the initiation of myelination: studies of the anterior commissure of transgenic mice. Neurosci. Lett. 1995;201:235–238. doi: 10.1016/0304-3940(95)12194-3. [DOI] [PubMed] [Google Scholar]
- Ye P, Lee KH, D’Ercole AJ. Insulin-like growth factor-i (igf-i) protects myelination from undernutritional insult: studies of transgenic mice overexpressing igf-i in brain. J. Neurosci. Res. 2000;62:700–708. doi: 10.1002/1097-4547(20001201)62:5<700::AID-JNR9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Yin X, Peterson J, Gravel M, Braun PE, Trapp BD. Cnp overexpression induces aberrant oligodendrocyte membranes and inhibits mbp accumulation and myelin compaction. J. Neurosci. Res. 1997;50:238–247. doi: 10.1002/(SICI)1097-4547(19971015)50:2<238::AID-JNR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Yin X, Kidd GJ, Pioro EP, McDonough J, Dutta R, Feltri ML, Wrabetz L, Messing A, Wyatt RM, Balice-Gordon RJ, Trapp BD. Dysmyelinated lower motor neurons retract and regenerate dysfunctional synaptic terminals. J. Neurosci. 2004;24:3890–3898. doi: 10.1523/JNEUROSCI.4617-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Baek RC, Kirschner DA, Peterson A, Fujii Y, Nave KA, Macklin WB, Trapp BD. Evolution of a neuroprotective function of central nervous system myelin. J. Cell Biol. 2006;172:469–478. doi: 10.1083/jcb.200509174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin YI, Bassit B, Zhu L, Yang X, Wang C, Li YM. {gamma}-secretase substrate concentration modulates the abeta42/abeta40 ratio: implications for alzheimer disease. J. Biol. Chem. 2007;282:23639–23644. doi: 10.1074/jbc.M704601200. [DOI] [PubMed] [Google Scholar]
- Zapata-Gonzalez F, Rueda F, Petriz J, Domingo P, Villarroya F, Diaz-Delfin J, de Madariaga MA, Domingo JC. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through ppar{gamma}:Rxr heterodimers: comparison with other polyunsaturated fatty acids. J. Leukoc. Biol. 2008;84:1172–1182. doi: 10.1189/jlb.1007688. [DOI] [PubMed] [Google Scholar]
- Zareparsi S, Camicioli R, Sexton G, Bird T, Swanson P, Kaye J, Nutt J, Payami H. Age at onset of parkinson disease and apolipoprotein e genotypes. Am. J. Med. Genet. 2002;107:156–161. doi: 10.1002/ajmg.10111. [DOI] [PubMed] [Google Scholar]
- Zatta P, Zambenedetti P, Musicco M, Adorni F. Metallothionein-i-ii and gfap positivity in the brains from frontotemporal dementia patients. J. Alzheimers Dis. 2005;8:109–116. doi: 10.3233/jad-2005-8203. discussion 209–115. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zeger M, Popken G, Zhang J, Xuan S, Lu QR, Schwab MH, Nave KA, Rowitch D, D’Ercole AJ, Ye P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55:400–411. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Khanlou N, Clare R, Jiang Q, Reed-Geaghan EG, Landreth GE, Vinters HV, Tontonoz P. Attenuation of neuroinflammation and alzheimer’s disease pathology by liver x receptors. Proc. Natl. Acad. Sci. U.S.A. 2007;104:10601–10606. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QJ, Li QX, Zhang HF, Zhang KR, Guo WY, Wang HC, Zhou Z, Cheng HP, Ren J, Gao F. Swim training sensitizes myocardial response to insulin: role of akt-dependent enos activation. Cardiovasc. Res. 2007;75:369–380. doi: 10.1016/j.cardiores.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Zheng H, Koo EH. The amyloid precursor protein: beyond amyloid. Mol. Neurodegener. 2006;1:5. doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]