Abstract
Background
The number of positive lymph nodes (LNs) has been proposed as a prognostic indicator in N1 non-small cell lung cancer (NSCLC). However, the number of positive LNs is confounded by the number of LNs resected during the surgery. The lymph node ratio (LNR; the ratio of the number of positive LNs divided by the number of LNs resected) can circumvent this limitation. The prognostic significance of the LNR has been demonstrated in elderly NSCLC patients. This study was done to evaluate whether a higher LNR is a marker of worse survival in NSCLC patients ≤65 years with N1 disease.
Methods
The Surveillance, Epidemiology, and End Results database was used to identify 4,004 patients who underwent resection for N1 NSCLC. Patients were classified into three LNR groups: ≤0.15, 0.16-0.5, and >0.5. The association of the LNR with lung cancer-specific and overall mortality was evaluated using the Kaplan Meier method. Stratified and Cox regression analyses were used to assess the relationship between the LNR and survival after adjusting for other prognostic factors.
Results
Unadjusted analysis showed that a higher LNR was associated with a worse lung cancer-specific (p <0.0001) and overall survival (p <0.0001). Stratified and multivariate analyses also showed that the LNR was an independent predictor of survival after controlling for potential confounders.
Conclusions
These results confirm that the LNR is an independent prognostic factor of survival in patients with N1 NSCLC. This information may be used to identify patients who are at higher risk of cancer recurrence.
Keywords: Non-Small Cell Lung Cancer; Lymph Node Metastasis; Lymph Node Ratio; Non-Small Cell Lung Cancer Staging; Surveillance, Epidemiology, and End Results Database
Introduction
The extent of lymph node (LN) involvement is a well established prognostic factor for patients with non-small cell lung cancer (NSCLC). Patients with N0 (no regional LN involvement) or N1 (involvement of ipsilateral intrapulmonary, peribronchial, or hilar LNs) disease are amenable to surgery with the possibility of achieving good long term outcomes 1, 2. Conversely, N2 (involvement of ipsilateral mediastinal or subcarinal LNs) and N3 (involvement of contralateral LNs) disease is usually treated with chemotherapy and/or radiation with 5 year survival rates of 15 to 30% 3.
NSCLC patients with N1 disease have variable outcomes. Risk of recurrence for patients with Stage II disease can range widely from 7% to 55% 4. This heterogeneity in outcomes has important implications for decisions about use of adjuvant treatments, post-operative surveillance, and makes prognosis discussions with patients and their families more difficult.
Prior studies have suggested that the number of positive LNs may provide independent prognostic data in patients with NSCLC 5-11. However, the maximal number of positive LNs is limited by the number of nodes sampled during surgery. Although the literature recommends resection of ≥10 LNs for accurate NSCLC staging, there is considerable practice variability, limiting the use of the actual number of positive LNs as a prognostic factor 11. This limitation can be addressed with the lymph node ratio (LNR), the ratio of the number of positive nodes to the total number of nodes resected during surgery. The LNR has been shown to predict survival in colon, esophageal, gastric, bladder, and breast cancer 12-17. A recent study showed that the LNR is also an independent predictor of survival in elderly patients (≥65 years) with N1 NSCLC 18. However, these results have not been validated in an independent population or in NSCLC patients ≤65 years of age. In this study, we used population based data to assess the prognostic value of the LNR in an independent group of patients ≤65 years of age with N1 NSCLC.
Methods
Study subjects were identified from the Surveillance, Epidemiology, and End Results (SEER) registry. SEER is a national registry that collects information on incidence, prevalence and survival of patients with cancer from geographic regions representing approximately 28% of the US population 19. All cases included in the study had undergone resection (lobectomy or pneumonectomy) and were found to have N1 NSCLC on pathological staging between 1988 and 2007. The study was limited to primary cases that were not diagnosed from death certificate data or autopsy 1. Patients who underwent preoperative radiation therapy (which can lead to down staging of LN involvement) and those with incomplete information on tumor size, tumor extension, and LN involvement were also excluded from the study. The final cohort consisted of 4,004 cases.
The SEER registry provides detailed information regarding the extent of LN involvement, the number of positive LNs and the number of LNs sampled during surgery. Using this information, the LNR was calculated for each patient. Consistent with prior studies, patients were classified into one of three LNR groups: ≤0.15, 0.16-0.5, and >0.518.
Sociodemographic characteristics (age, sex, race/ethnicity, and marital status) were obtained from the SEER registry. Cases were staged according to the 7th edition of the Tumor, Node, Metastasis (TNM) classification using SEER data on tumor extension and LN metastasis 20. Histology subtypes were classified as adenocarcinoma, squamous cell carcinoma, large cell carcinoma, bronchioalveolar carcinoma or other. Information regarding surgical treatment was ascertained from the site specific surgery codes available in SEER. Using these data, patients were classified as having undergone lobectomy (codes 20-45) or pneumonectomy (codes 50-70). Use of pre- and postoperative radiation therapy (external beam radiation) was also ascertained from SEER.
The primary outcome was lung cancer-specific survival, as it allows for controlling for unrelated causes of death. We performed secondary analyses assessing the association of the LNR with all cause mortality. To estimate lung cancer-specific survival, patients dying from causes other than lung cancer were classified as censored at the date of death. Cause of death information in SEER is provided by the National Center for Health Statistics and obtained from state death certificates. Survival was determined as the interval from the date of diagnosis to the date of death or the last follow-up date available in the SEER registry (December 31, 2007).
Statistical Analysis
Differences in the baseline characteristics across the three LNR groups were evaluated using the chi-squared test. Survival curves were estimated for patients in the three LNR groups using the Kaplan Meier method and compared with the log-rank test. Survival was also estimated for the three LNR groups after stratifying by age, gender, race, histology, tumor site and status, type of surgery, and use of radiation therapy to assess if prognostic differences across the three LNR groups remained significant after controlling for these confounders. Survival figures are shown up to 15 years after diagnosis to avoid reporting survival estimates based on a small number of observations. Cox regression analysis was used to assess the association between the LNR and survival, after adjusting for other prognostic factors. The assumption of proportionality of hazards was evaluated using log-log plots of survival curves. All analyses were performed with SPSS statistical package (IBM, Chicago, Illinois) using two sided p-values.
Results
We identified 4,004 cases of N1 NSCLC from the SEER database. Overall, 34%, 43% and 23% of the study patients had an LNR of ≤0.15, 0.16-0.5, and >0.5, respectively. There were differences in the distribution of sociodemographic (sex, race/ethnicity, marital status) and tumor (histology, location, status, size) characteristics between the LNR groups (p<0.05 for all comparisons; Table 1). Additionally, patients with the highest LNR group were more likely to have adenocarcinoma (p<0.0001), an upper lobe tumor (p<0.0001), had undergone lobectomy, (p<0.0001) or received postoperative radiation therapy (p<0.0001).
Table 1.
Baseline Characteristics of Patients ≤65 years of Age with N1 Lymph Node Involvement
| Characteristic | Lymph Node Ratio | P-value | ||
|---|---|---|---|---|
| ≤0.15 (N=1,346) |
0.16-0.50 (N=1,737) |
≥0.5 (N=921) |
||
| Age, years, N (%) | ||||
| ≤50 | 255 (19) | 305 (18) | 160 (18) | 0.48 |
| 51-60 | 609 (45) | 838 (48) | 427 (46) | |
| ≥60 | 482 (36) | 594 (34) | 334 (36) | |
| Female, N (%) | 526 (39) | 707 (41) | 427 (46) | 0.002 |
| Race/Ethnicity, N (%) | ||||
| White | 1110 (83) | 1363 (79) | 661 (72) | <0.0001 |
| African American | 111 (8) | 200 (11) | 131 (14) | |
| Hispanic | 52 (4) | 60 (3) | 36 (4) | |
| Other | 73 (5) | 114 (7) | 93 (10) | |
| Married, N (%) | 908 (68) | 1112 (64) | 571 (62) | 0.02 |
| Histology, N (%) | ||||
| Adenocarcinoma | 646 (48) | 890 (51) | 546 (59) | <0.0001 |
| Squamous cell carcinoma | 498 (37) | 568 (33) | 214 (23) | |
| Large cell carcinoma | 71 (5) | 99 (6) | 60 (7) | |
| Bronchioalveolar cell carcinoma | 58 (4) | 89 (5) | 53 (6) | |
| Other | 73 (6) | 91 (5) | 48 (5) | |
| Tumor Location, N (%) | ||||
| Upper lobe | 772 (58) | 1001 (58) | 507 (55) | <0 .0001 |
| Middle lobe | 43 (3) | 76 (4) | 52 (6) | |
| Lower lobe | 404 (30) | 525 (30) | 315 (34) | |
| Other location | 127 (9) | 135 (8) | 47 (5) | |
| Tumor Status, N (%) | ||||
| T1A | 147 (11) | 187 (11) | 121 (13) | 0.01 |
| T1B | 207 (15) | 265 (15) | 174 (19) | |
| T2 | 154 (11) | 192 (11) | 112 (12) | |
| T2A | 415 (31) | 598 (35) | 284 (31) | |
| T2B | 173 (13) | 215 (12) | 103 (11) | |
| T3 | 250 (19) | 280 (16) | 127 (14) | |
| Type of Surgery, N (%) | ||||
| Lobectomy | 937 (70) | 1286 (74) | 770 (84) | <0.0001 |
| Pneumonectomy | 409 (30) | 451 (26) | 151 (16) | |
| Postoperative Radiation Therapy, N (%) | ||||
| Yes | 384 (28) | 670 (39) | 438 (48) | <0.0001 |
| No | 962 (72) | 1067 (61) | 483 (52) | |
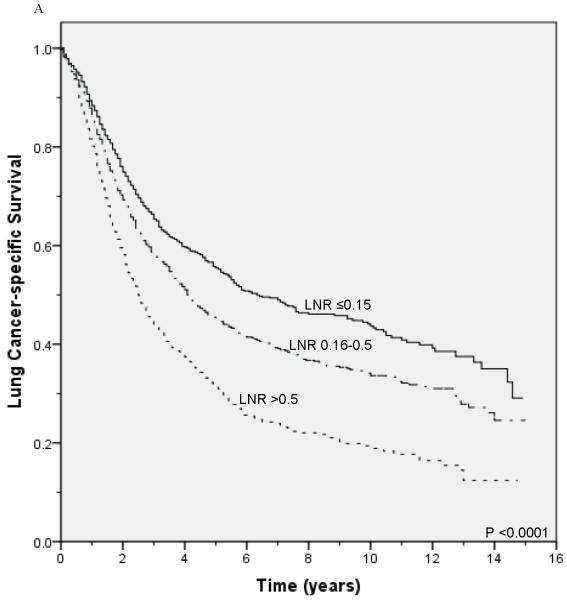
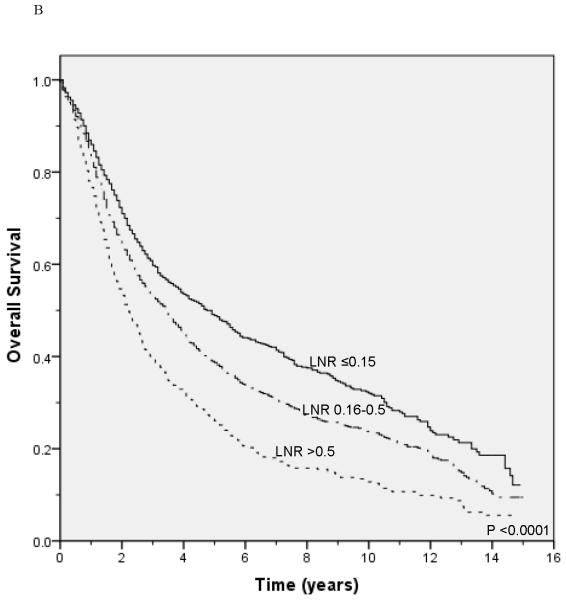
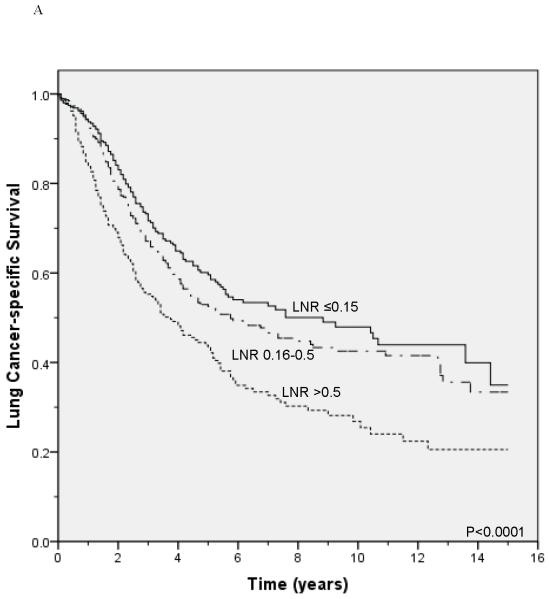
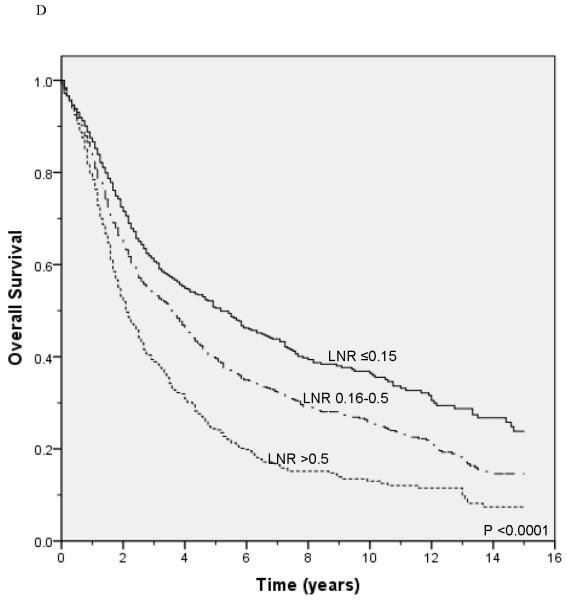
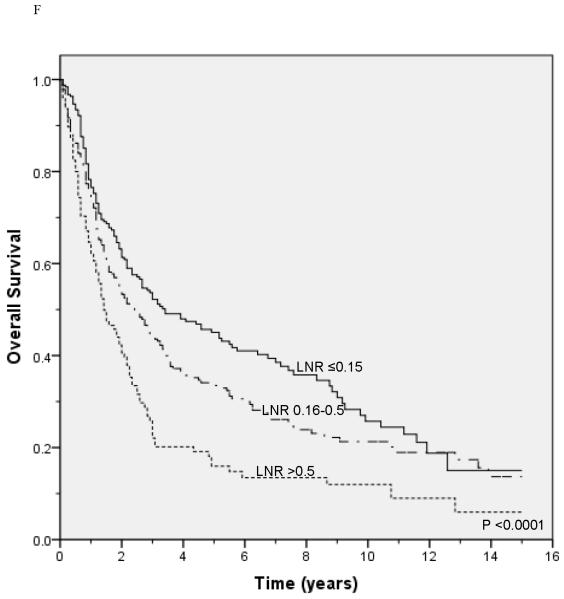
Unadjusted survival analysis showed that a higher LNR group was associated with worse lung cancer-specific survival (p <0.0001; Figure 1A) and overall survival (p <0.0001; Figure 1B). Median lung cancer-specific survival was 7.2 years, 4.3 years and 2.6 years for patients in the 0.15, 0.16-0.5, and >0.5 LNR groups, respectively. Stratification by tumor status, an established prognostic factor, also showed a significant association between a higher LNR and worse lung cancer-specific and overall survival (Figures 2A-2F). Similar results were obtained in stratified analyses by age, sex, marital status, tumor size, type of surgery, and use of radiation therapy (Table 2).
Figure 1.
Lung cancer-specific (A) and overall survival (B) according to the lymph node ratio
Figure 2.
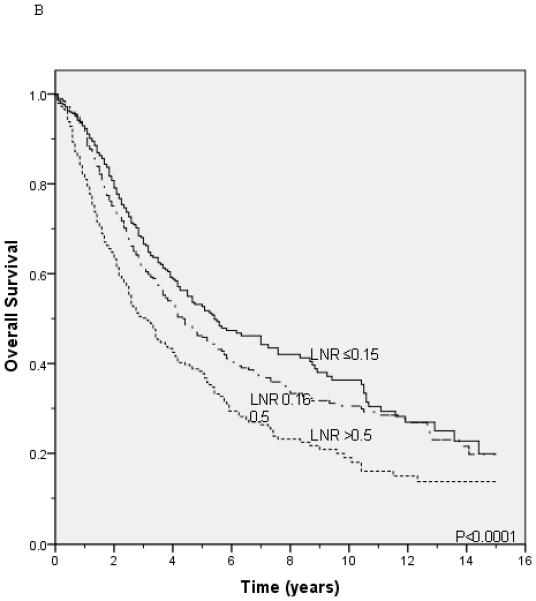
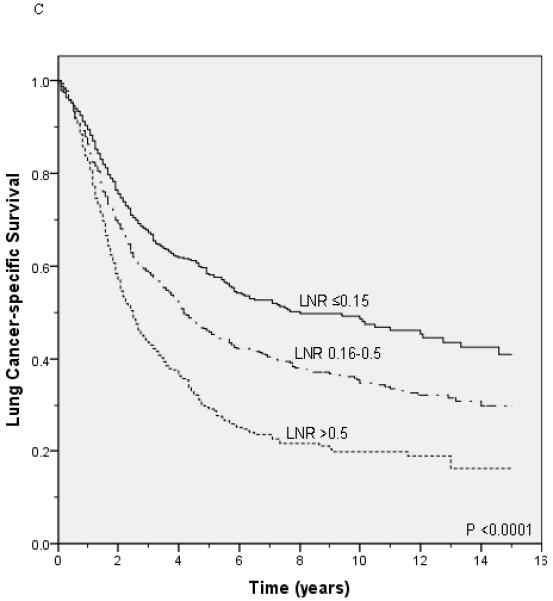
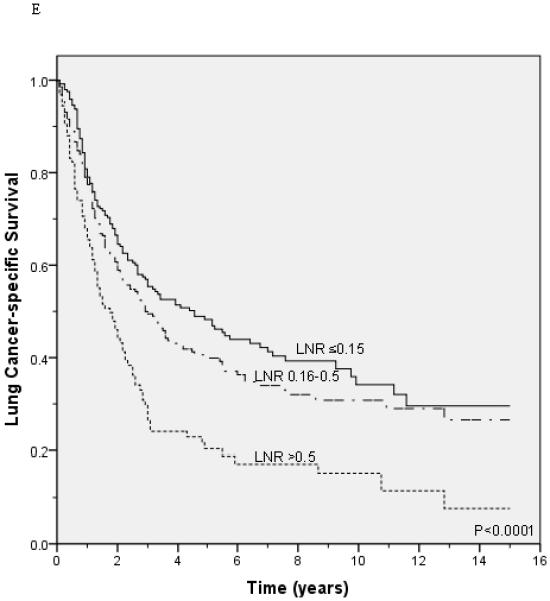
Lung cancer-specific and overall survival according to the lymph node ratio among patients with T1 (A and B), T2 (C and D), and T3 (E and F) disease.
Table 2.
Stratified Comparisons of Median Lung Cancer-Specific Survival Time to Lymph Node Ratio Group
| Characteristic | Lymph Node Ratio | P-value | ||
|---|---|---|---|---|
| ≤0.15 | 0.16-0.50 | ≥0.5 | ||
| Median Lung Cancer-Specific Survival (years) | ||||
| Age, years | ||||
| ≤50 | 10.08 | 4.08 | 3.00 | <0.0001 |
| 51-60 | 7.25 | 4.33 | 2.75 | <0.0001 |
| ≥60 | 5.50 | 4.33 | 2.25 | <0.0001 |
| Female | 7.50 | 4.75 | 2.83 | <0.0001 |
| Race/Ethnicity | ||||
| White | 7.08 | 4.33 | 2.50 | <0.0001 |
| African American | 5.58 | 4.17 | 2.92 | 0.01 |
| Hispanic | 3.58 | 3.42 | 0.15 | |
| Other | 7.58 | 4.92 | 2.92 | 0.005 |
| Married | 7.25 | 4.50 | 2.67 | <0.0001 |
| Histology | ||||
| Adenocarcinoma | 5.75 | 4.17 | 2.58 | <0.0001 |
| Squamous cell carcinoma | 11.00 | 4.67 | 2.42 | <0.0001 |
| Large cell carcinoma | 3.17 | 2.42 | 2.50 | 0.73 |
| Bronchioalveolar cell carcinoma | 11.17 | 3.92 | 3.58 | 0.07 |
| Other | 7.33 | 5.00 | 2.67 | <0.0001 |
| Tumor Location | ||||
| Upper lobe | 9.25 | 5.25 | 2.67 | <0.0001 |
| Middle lobe | 10.08 | 4.92 | 4.25 | 0.13 |
| Lower lobe | 5.75 | 3.58 | 2.17 | <0.0001 |
| Other location | 5.00 | 3.33 | 2.92 | 0.47 |
| Tumor Status | ||||
| T1A | 6.75 | 4.42 | <0.0001 | |
| T1B | 5.58 | 5.17 | 3.33 | 0.001 |
| T2 | 8.00 | 5.75 | 2.42 | <0.0001 |
| T2A | 13.33 | 4.33 | 2.58 | <0.0001 |
| T2B | 5.92 | 2.50 | 2.25 | 0.001 |
| T3 | 4.58 | 2.92 | 1.75 | <0.0001 |
| Type of Surgery | ||||
| Lobectomy | 8.00 | 4.50 | 2.83 | <0.0001 |
| Pneumonectomy | 5.17 | 3.58 | 1.75 | <0.0001 |
| Postoperative Radiation Therapy | ||||
| Yes | 7.00 | 4.08 | 2.50 | <0.0001 |
| No | 7.17 | 4.58 | 2.75 | <0.0001 |
The LNR was also an independent predictor of lung cancer-specific and overall survival in Cox regression analysis adjusting for potential confounders (Table 3). Compared to patients in the lowest LNR group (≤0.15), there was a 1.28 (95% CI: 1.15-1.43) and 1.96 (95% CI: 1.74-2.22) increased hazard of lung cancer mortality in the 0.16-0.5 and ≥0.5 groups, respectively. A similar pattern was observed in analyses using all cause mortality as an outcome; compared with the ≤0.15 LNR category, an increased hazard of lung cancer mortality of 1.25 (95% CI: 1.14-1.38) and 1.82 (95% CI: 1.63-2.03) were observed among patients in the 0.16-0.5 and ≥0.5 LNR groups, respectively.
Table 3.
Adjusted Association between the Lymph Node Ratio and Survival of Patients with N1 Disease
| Variable | Lung Cancer-specific Survival Hazard Ratio (95% CI1) |
Overall Survival Hazard Ratio (95% CI) |
|---|---|---|
| Lymph Node Ratio | ||
| ≤0.15 | Reference | Reference |
| 0.16-0.5 | 1.28 (1.15-1.43) | 1.25 (1.14-1.38) |
| >0.5 | 1.96 (1.74-2.22) | 1.82 (1.63-2.03) |
| Age, years | 1.02 (1.00-1.04) | 1.02 (1.01-1.04) |
| Female | 0.87 (0.80-0.96) | 0.86 (0.79-0.93) |
| Race/Ethnicity | ||
| White | Reference | Reference |
| Black | 1.02 (0.88-1.18) | 1.11 (0.98-1.26) |
| Hispanic | 0.90 (0.71-1.16) | 1.00 (0.81-1.24) |
| Other | 0.87 (0.73-1.04) | 0.88 (0.74-1.03) |
| Married | 0.89 (0.81-0.97) | 0.84 (0.78-0.92) |
| Histology | ||
| Adenocarcinoma | Reference | Reference |
| Squamous cell carcinoma | 0.73 (0.65-0.82) | 0.81 (0.74-0.89) |
| Large cell carcinoma | 1.09 (0.91-1.31) | 1.12 (0.96-1.32) |
| Bronchioalveolar carcinoma | 0.85 (0.68-1.05) | 0.92 (0.76-1.11) |
| Other | 0.94 (0.78-1.15) | 0.91 (0.76-1.10) |
| Tumor Status | ||
| T1A | Reference | Reference |
| T1B | 1.31 (1.09-1.57) | 1.29 (1.10-1.52) |
| T2 | 1.34 (1.10-1.63) | 1.30 (1.09-1.55) |
| T2A | 1.38 (1.17-1.62) | 1.35 (1.17-1.56) |
| T2B | 1.67 (1.38-2.02) | 1.59 (1.34-1.88) |
| T3 | 1.91 (1.59-2.29) | 1.72 (1.46-2.02) |
| Tumor Location | ||
| Upper lobe | Reference | Reference |
| Middle lobe | 1.00 (0.80-1.25) | 0.98 (0.80-1.20) |
| Lower lobe | 1.20 (1.09-1.33) | 1.24 (1.13-1.35) |
| Other location | 1.06 (0.89-1.26) | 1.12 (0.96-1.31) |
| Type of surgery | ||
| Lobectomy | Reference | Reference |
| Pneumonectomy | 1.22 (1.09-1.37) | 1.20 (1.09-1.33) |
| Postoperative Radiation Therapy | 1.07 (0.97-1.17) | 1.05 (0.97-1.14) |
Discussion
Patients with N1 NSCLC have heterogeneous outcomes. A recent study showed that the LNR is a prognostic factor in elderly patients with N1 disease 18. In this study, we validated these findings in a large, population-based cohort of patients ≤65 with N1 NSCLC. We found a consistent increased risk of mortality with higher LNRs after controlling for other prognostic factors included in current lung cancer staging classifications. Moreover, the increased hazard of death among patients with a LNR of ≥0.5 was equivalent to that of T3 disease, an established prognostic factor. These results suggest that the LNR can be used to stratify patients with N1 disease into subgroups with different risks of lung cancer recurrence following resection.
The LNR has shown to be an important prognostic factor in several malignancies, including breast, bladder, colon, esophageal, and gastric cancer 12-17. Similarly, Bria et al. showed an association between the LNR and lung cancer outcomes in a cohort of 415 patients with N1 and N2 disease 11. More recently, a study using the SEER-Medicare registry found that the LNR was independently associated with overall and lung cancer-specific survival in a large cohort of elderly patients 18. While these results support the usefulness of the LNR, there is a need to validate these findings in an independent population before they can be adopted in clinical practice. Moreover, elderly lung cancer patients may be less likely to experience cancer recurrence as they have a higher chance of dying from competing risks. Thus, it is also important to replicate these findings among younger patients with N1 NSCLC.
Our results support future adoption in clinical practice of the LNR as a prognostic factor for N1 NSCLC. The LNR provides physicians independent information that should lead to more accurate staging and assist physicians to target adjuvant treatment for their NSCLC patients. Patients with a higher LNR appear to be at an increased risk of recurrence and should be considered for aggressive postoperative treatment in order to improve their long-term outcomes. Current treatment guidelines for N1 NSCLC recommend surgical resection followed by adjuvant platinum-based chemotherapy 21. However, there is a risk of acute toxicity and long term adverse effects from adjuvant chemotherapy 22. Patients with advanced age, multiple comorbidities, or poor performance status may not be able to tolerate or experience the long term benefits of adjuvant chemotherapy. Thus, physicians may consider withholding adjuvant therapy for these patients if they are found to have a LNR <0.15. While validated biomarkers may be available in the future, the prognostic value of the LNR can help treating physicians provide patients with more accurate information regarding their chances of recurrence, regardless of the number of nodes they had dissected. This information can assist patients and their families make important treatment decisions and long-term plans.
The use of post-operative radiation therapy (PORT) for lung cancer patients with LN involvement remains controversial 23, 24. The PORT meta-analysis found no clear benefit from post-operative radiation therapy 23. Similarly, a retrospective study conducted using the SEER database showed no significant improvement in survival with PORT in patients with N1 NSCLC24. In our study, patients with a higher LNR were more likely to receive PORT. However, a higher LNR was associated with worse survival in analysis stratifying and adjusting for PORT use. Future studies evaluating the role of PORT should control for the LNR, a potential confounder of the association between radiation therapy use and survival.
The number of N1 LNs has also been proposed as a prognostic factor for resected patients with NSCLC. Marra et al. found an association between the number of positive nodes and survival in a cohort of patients with resected N1 NSCLC 25. Similarly, a study in Japan showed that a higher number of positive nodes resulted in worse survival in patients with N1 and N2 disease 26. However, the maximal number of positive LNs is determined by the number of LNs sampled during surgery. As many lung cancer patients (>50%) undergo sampling of <10 LNs, this criteria may not be applicable to all cases in routine clinical practice. While future efforts should focus on adequate sampling of LNs for patients undergoing lung cancer resection, the LNR can be used for predicting risk of recurrence regardless of the extent of LN sampling.
There are some limitations to this study. There was a significant difference in baseline characteristics of patients in the three LNR groups. As several of these factors are established prognostic indicators for resected NSCLC, there is a possibility of confounding. However, stratified and multivariate analysis showed a consistent association between the LNR and survival, suggesting that the LNR is an independent prognostic factor.
Assessment of lung cancer-specific survival requires accurate information about the cause of death. The SEER program determines the cause of death from death certificates, a potential source of misclassification. However, the underlying cause of patients dying from lung cancer progression was found to be relatively accurate in a study using a larger registry 27. Additionally, we confirmed our results in secondary analyses using all cause mortality, which should not be subject to misclassification. SEER does not include information on chemotherapy; however, our study excluded patients who had undergone neoadjuvant radiation therapy, which is usually given in conjunction with neoadjuvant chemotherapy, decreasing the possibility that these patients were included in our cohort. Although less detailed than analyses from single institutions, an advantage of using registry data include the large sample size and the improved generalization of the study findings.
The LNR may provide useful prognostic information to make important treatment decisions for patients with N1 NSCLC. Our findings show that the LNR is an independent prognostic factor in patients ≤65 with resected N1 NSCLC, validating findings from prior studies. Patients with a higher LNR had worse outcomes and should be considered for more aggressive treatment and increased surveillance for recurrence. Our results provide support for incorporation of the LNR into future lung cancer staging systems.
Acknowledgments
Funding: The study was funded by the Doris Duke Foundation for Clinical Research and in part, by the National Cancer Institute (5R01CA131348-03).
Footnotes
Sirisha Jonnalagadda, Jacqueline Arcinega and Cardinale Smith have no financial disclosures. Juan Wisnivesky is a member of the EHE research board, has received lecture honorarium from Novartis Pharmaceuticals, and a COPD research grant from Glaxo-Smith-Kline.
References
- 1.Wisnivesky JP, Henschke CI, Swanson S, et al. Limited Resection for the Treatment of Patients With Stage IA Lung Cancer. Ann Surg. 2010;251(3):550–54. doi: 10.1097/SLA.0b013e3181c0e5f3. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto T, Cassivi S, Yang P, et al. Completely resected N1 non–small cell lung cancer: Factors affecting recurrence and long-term survival. J Thorac Cardiovasc Surg. 2006;132(3):499–506. doi: 10.1016/j.jtcvs.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Luzzi L, Paladini P, Ghiribelli C, et al. Assessing the prognostic value of the extent of mediastinal lymph node infiltration in surgically-treated non-small cell lung cancer (NSCLC) Lung Cancer. 2000;30(2):99–105. doi: 10.1016/s0169-5002(00)00133-1. [DOI] [PubMed] [Google Scholar]
- 4.Kelsey CR, Marks LB, Hollis D, et al. Local recurrence after surgery for early stage lung cancer. Cancer. 2009;115(22):5218–27. doi: 10.1002/cncr.24625. [DOI] [PubMed] [Google Scholar]
- 5.Osaki T, Nagashima A, Yoshimatsu T, Tashima Y, Yasumoto K. Survival and characteristics of lymph node involvement in patients with N1 non-small cell lung cancer. Lung Cancer. 2004;43:151–57. doi: 10.1016/j.lungcan.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Demir A, Turna A, Kocaturk C, et al. Prognostic Significance of Surgical-Pathologic N1 Lymph Node Involvement in Non-Small Cell Lung Cancer. Ann Thorac Surg. 2009;87:1014–22. doi: 10.1016/j.athoracsur.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 7.Sayar A, Turna A, Kilicgun A, Solak O, Urer N, Gurses A. Prognostic significance of surgical-pathologic multiple-station N1 disease in non-small cell carcinoma of the lung. Eur J Cardiothorac Surg. 2004;25:434–38. doi: 10.1016/j.ejcts.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Yoshino I, Nakanishi R, Osaki T, et al. Unfavorable Prognosis of Patients with Stage II Non-small Cell Lung Cancer Associated With Macroscopic Nodal Metastases. Chest. 1999;116:144–49. doi: 10.1378/chest.116.1.144. [DOI] [PubMed] [Google Scholar]
- 9.Fukui T, Mori S, Yokoi K, Mitsudomi T. Significance of the Number of Positive Lymph Nodes in Resected Non-small cell Lung Cancer. J Thorac Oncol. 2006;1(2):220–25. [PubMed] [Google Scholar]
- 10.Lee JG, Lee CY, Park IK, et al. Number of Metastatic Lymph Nodes in Resected Non-Small Cell Lung Cancer Predicts Patient Survival. Ann Thorac Surg. 2008;85:211–15. doi: 10.1016/j.athoracsur.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Bria E, Milella M, Sperduti I, et al. A novel clinical prognostic score incorporating the number of resected lymph-nodes to predict recurrence and survival in non-small-cell lung cancer. Lung Cancer. 2009;66:365–71. doi: 10.1016/j.lungcan.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Berger AC. Colon Cancer Survival Is Associated With Decreasing Ratio of Metastatic to Examined Lymph Nodes. J Clin Oncol. 2005;23(34):8706–12. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 13.Greenstein A, Litle V, Swanson S, Divino C, Packer S, Wisnivesky J. Prognostic Significance of the Number of Lymph Node Metastases in Esophageal Cancer. J Am Coll Surg. 2008;206(2):239–46. doi: 10.1016/j.jamcollsurg.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Herr H. Superiority of Ratio Based Lymph Node Staging for Bladder Cancer. J Urol. 2003;169(3):943–45. doi: 10.1097/01.ju.0000032474.22093.06. [DOI] [PubMed] [Google Scholar]
- 15.Lee H-Y, Choi H-J, Park K-J, et al. Prognostic Significance of Metastatic Lymph Node Ratio in Node-Positive Colon Carcinoma. Ann Surg Oncol. 2007;14(5):1712–17. doi: 10.1245/s10434-006-9322-3. [DOI] [PubMed] [Google Scholar]
- 16.Nitti D, Marchet A, Olivieri M, et al. Ratio Between Metastatic and Examined Lymph Nodes Is an Independent Prognostic Factor After D2 Resection for Gastric Cancer: Analysis of a Large European Monoinstitutional Experience. Ann Surg Oncol. 2003;10(9):1077–85. doi: 10.1245/aso.2003.03.520. [DOI] [PubMed] [Google Scholar]
- 17.Vinh-Hung V, Verschraegen C, Promish DI, et al. Ratios of involved nodes in early breast cancer. Breast Cancer Res. 2004;6(6):R680–R88. doi: 10.1186/bcr934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wisnivesky J, Arciniega J, Mhango G, Halm E. Lymph node ratio as a prognostic factor in elderly patients with pathological N1 non-small cell lung cancer. Thorax. 2010 doi: 10.1136/thx.2010.148601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altekruse S, Kosary C, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute; Bethesda, MD: 2010. [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. Springer; New York: 2010. p. 646. [Google Scholar]
- 21.The NCCN Practice Guidelines in Oncology: Non-small cell lung cancer (version v.I.2010).
- 22.Arriagada R, Dunant A, Pignon J-P, et al. Long Term Results of the International Adjuvant Lung cancer Trial Evaluating Adjuvant Cisplatin-Based Chemotherapy in Resected Lung Cancer. J Clin Oncol. 2010;28(1):35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]
- 23.Group PM-aT Postoperative radiation therapy for non-small cell lung cancer. Cochrane Database of Systematic Review. 2005;(2) doi: 10.1002/14651858.CD005221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lally BE. Postoperative Radiotherapy for Stage II or III Non-Small-Cell Lung Cancer Using the Surveillance, Epidemiology, and End Results Database. J Clin Oncol. 2006;24(19):2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- 25.Marra A, Hillejan L, Zaboura G, Fujimoto T, Greschuchna D, Stamatis G. Pathologic N1 non-small cell lung cancer: Correlation between pattern of lymphatic spread and prognosis. J Thorac Cardiovasc Surg. 2003;125(3):543–53. doi: 10.1067/mtc.2003.322. [DOI] [PubMed] [Google Scholar]
- 26.Ueda K, Kaneda Y, Sakano H, et al. Independent Predictive Value of the Overall Number of Metastatic N1 and N2 Stations in Lung Cancer. Jpn J Thorac Cardiovasc Surg. 2003;51:297–301. doi: 10.1007/BF02719381. [DOI] [PubMed] [Google Scholar]
- 27.Percy C, Stanek E, 3rd, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981;71(3):242–50. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]