Abstract
Dendritic cells (DC) are “professional” bone marrow-derived antigen (Ag)-presenting cells of interest both as therapeutic targets and potential cellular vaccines due to their ability to regulate innate and adaptive immunity. Harnessing the inherent tolerogenicity of DC is a promising and incompletely explored approach to the prevention of allograft rejection. Previously, we and others have reported the ability of pharmacologically-modified DC that resist maturation to inhibit CD4+ T cell responses and prolong allograft survival. Here we evaluated the ability of murine myeloid DC conditioned with the immunosuppressive pro-drug rapamycin (RAPA) to acquire and directly present alloAg to syngeneic CD8+ T cells. RAPA-conditioned DC (RAPA-DC) pulsed with allogeneic splenocyte lysate acquired and expressed donor MHC class I and enhanced the apoptotic death of directly-reactive donor Ag-specific CD8+ T cells in vitro. Moreover, following their adoptive transfer, they reduced the survival of these T cells in vivo. The ability of RAPA-DC to inhibit the survival of alloAg-specific CD8+ T cells provides a potential mechanism by which host-derived DC may act as negative regulators of T cell alloreactivity and support donor-specific unresponsiveness. Adoptive cell therapy with alloAg-pulsed RAPA-DC may offer an effective approach to suppression of alloimmunity, with reduced dependence on systemic immunosuppression.
Keywords: rapamycin, dendritic cells, alloantigen presentation, T cells, apoptosis
1. Introduction
Dendritic cells (DC) are rare, uniquely well-equipped bone marrow (BM)-derived antigen (Ag)-presenting cells (APC), with both immunostimulatory and tolerogenic properties [1–3]. In the setting of transplantation, both donor- and recipient-derived DC are believed to play key roles in the instigation of host responses that lead to allograft rejection [4–8] or acceptance [9]. This functional dichotomy makes DC potentially important targets for therapeutic intervention.
Interstitial DC capture, then subsequently present Ag to T cell receptors (TCR) that recognize Ag fragments bound to major histocompatibility complex (MHC) proteins on the DC surface [10]. These peptide-binding proteins,- MHC class I and MHC class II, present Ag to CD8+ T cells and CD4+ T cells, respectively [1, 6, 10]. Following transplantation, MHC-bound alloAg is presented by donor DC to recipient T cells through what is known as the direct pathway of allorecognition. With adequate co-stimulation, this process leads to directly-reactive effector T cell responses. While donor DC are lost eventually to attrition, endogenous recipient DC also function as potent facilitators of anti-graft responses, since they capture and process alloAg presented to T cells via host MHC (the indirect pathway [11, 12]). Furthermore, presentation of acquired peptide/MHC class I and II, following their intercellular transfer to DC via cell-to-cell contact, has been described and termed the ‘semi-direct’ pathway of allorecognition [13, 14]. The process of DC acquisition and direct presentation of intact donor MHC has been demonstrated following adoptive DC transfer [15]. Also, transferred MHC class II/tumor Ag complexes are displayed on the surface of DC following their transfer from tumor cell lysates and during co-culture [16, 17]. These acquired tumor Ag/MHC class II complexes are functional and facilitate the activation of anti-tumor CD4+ T cells [16].
The ability of DC to induce immunity or tolerance is related to their state of maturation [1, 3, 9, 12, 18]. Maturation is triggered by numerous stimuli, including endogenous alarmins released by necrotic cells, pro-inflammatory cytokines, exogenous microbial products that bind to Toll-like receptors (TLRs), or other pattern recognition receptors, and by activated T cells expressing ligands (e.g., CD154) for costimulatory molecules of the tumor necrosis factor (TNF) receptor family [19, 20]. “Mature” DC express high levels of MHC and co-stimulatory molecules, migrate to secondary lymphoid tissue, and initiate effector T cell responses. By contrast, “immature” or steady-state DC that lack adequate T cell stimulatory capacity and suppress T cell responses via anergy, deletion, or regulatory T cell (Treg) enrichment [9, 21, 22]. Use of immature or “tolerogenic” donor- or recipient-derived DC to down-regulate T cell responses is a potential therapeutic option to inhibit allograft rejection (reviewed in [9]).
Rapamycin (RAPA), a macrocyclic triene antibiotic produced by Streptomyces hygroscopicus, exhibits potent immunosuppressive properties and is used in solid organ transplantation [23, 24]. RAPA inhibits DC maturation and their T cell stimulatory capacity in vitro and in vivo at clinically relevant levels [25–27]. Exposure of DC to RAPA confers resistance to maturation in response to LPS, pro-inflammatory cytokines or CD40 ligation [25, 28–30]. In mice, recipient-derived DC conditioned with RAPA (RAPA-DC) and pulsed with the cell-free lysate of donor splenocytes prolong MHC-mismatched organ allograft survival in an Ag-specific manner, an effect that is enhanced by multiple pulsed RAPA-DC infusions [26]. Thus, alloAg-pulsed RAPA-DC can block early direct T cell responses in the absence of immunosuppression [26]. It appears that multiple mechanism(s) may underlie how RAPA-DC regulate host responses to alloAg. Ours and others’ work [21, 27] suggests that RAPA-DC select for the survival of CD4+Foxp3+Treg, while enhancing apoptosis of allogeneic CD4+ effector T cells. However, it has not been determined whether RAPA-DC also directly regulate donor-reactive CD8+ T cell responses, which also play important roles in graft rejection. Here, we hypothesized that RAPA-DC, pulsed with donor alloAg, could attenuate anti-donor CD8+ T cell reactivity.
We first investigated the ability of RAPA-DC to capture intact donor MHC class I molecules from allogeneic cell lysates and to express these molecules on their surface. We hypothesized that this would give both RAPA-DC and control DC (not exposed to RAPA; CTR-DC), the ability to interact with CD8+ T cells through the direct pathway of alloAg recognition. As a source of directly donor-reactive CD8+ T cells, we used CD8+ T cells from 2C transgenic mice bred onto a C57BL/6 Rag−/− background (H-2b). These CD8+ T cells bear a transgene directed against the MHC class I H-2Ld molecule. Both in vitro and in vivo, RAPA-DC pulsed with lysate from BALB/c (H-2d) cells decreased the viability of alloAg-specific CD8+ T cells when compared to CTR-DC. These data suggest a mechanism whereby CD8+ T cell-mediated alloimmunity may be subverted by donor Ag-pulsed RAPA-DC.
2. Objective
This study was conducted to ascertain the ability of RAPA-conditioned, conventional myeloid DC to acquire MHC class I alloAg and their impact on activation and survival of alloAg-specific CD8+ effector T cells in vitro and in vivo.
3. Materials and methods
3.1. Mice
Eight- to 12-week-old C57BL/6 (B6; H2b), C3H/HeJ (C3H; H2k) and BALB/c (H2d) male mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in the specific pathogen-free Central Animal Facility of the University of Pittsburgh School of Medicine. 2C TCR-transgenic C57BL/6 Rag−/− mice were kindly provided by Dr. Geetha Chalasani, Starzl Transplantation Institute, Department of Surgery, University of Pittsburgh. Experiments were conducted under an institutional animal care and use committee-approved protocol, and in accordance with National Institutes of Health-approved guidelines.
3.2. Bone marrow (BM)-derived DC generation
BM-derived myeloid DC were propagated as described [31, 32]. Briefly, BM was flushed from the femurs and tibias of B6 and BALB/c mice and erythrocytes removed by hypotonic lysis. The cells were cultured for 7 days in RPMI-1640 with 10% v/v heat-inactivated fetal calf serum, L-glutamine, non-essential amino acids, sodium pyruvate, penicillin-streptomycin, HEPES (N-2-hydroxyethylpiperazine-N’-2-ethane-sulfonic acid), 2-mercaptoethanol (all from Life Technologies, Gaithersburg, MD), 1000 U/ml recombinant (r) murine granulocyte-macrophage colony-stimulating factor (GM-CSF; Schering-Plough, Kenilworth, NJ) and 1000 U/ml r murine IL-4 (R&D Systems, Minneapolis, MN). On day 2, 10 ng/ml RAPA (Sigma, St. Louis, MO) was added as described [21]. Every 2 days, 75% of the culture supernatant was replaced with fresh, cytokine-containing medium (with or without RAPA). On day 4, non-adherent cells were removed. On day 6, the culture supernatant was again replaced with fresh media. By day 7, ≥95% of the non-adherent cells expressed CD11c, regardless of treatment with RAPA.
3.3. AlloAg pulsing of DC
Allogeneic cell lysates were prepared and used to pulse DC as described [26], with minor modifications. Spleens were harvested from either BALB/c or C3H mice, macerated, and passed through 22 µm nylon cell strainers (BD Biosciences, San Jose, CA) with hypotonic lysis of erythrocytes. Following centrifugation, the cell pellets were resuspended in a small volume (200–300 µl) of PBS and frozen in liquid nitrogen. The cells were then thawed in a 37°C water bath. This process was repeated two more times, and the resultant lysate (avoiding visibly clumped debri) was added to CD11c+ immunomagnetic bead (Miltenyi Biotech, Auburn, CA)-purified** CTR- or RAPA-DC. The DC were incubated with the splenocyte lysates at a DC:splenocyte equivalent ratio of 1:10 for 18 hours at 37° C.
3.4. Flow cytometry
Cell surface Ag expression was analyzed on days 7 or 8, as described [21]. FITC-, PE-, or PE-Cy5-conjugated, or biotinylated monoclonal antibodies (mAbs) were used to detect the expression of CD11c (HL3), CD80 (16-10A1), CD86 (GL1), I-Ab, and Ld (30-5-7; a kind gift from Dr. Joyce Solheim, University of Nebraska Medical Center, Omaha, NE). Appropriately conjugated, isotype-matched IgGs were used as negative controls. All mAbs, as well as isotype- control IgGs and streptavidin-PE-Cy5, were from BD PharMingen, San Jose, CA, unless otherwise specified. Data were acquired using a BD LSR II flow cytometer (BD ImmunoCytometry Systems, San Jose, CA) and analyzed using FlowJo 8.1.1 (Tree Star, Inc., Ashland, OR) software.
3.5. Immunofluorescence staining of alloAg-pulsed DC
Either unpulsed or alloAg-pulsed CTR- or RAPA-DC were analyzed for the expression of MHC Ag by immunofluorescence staining and confocal microscopy. DC were stained for CD11c and the presence of the Ld Ag on the cell surface, both with and without alloAg pulsing. DC were propagated as described above and, following CD11c bead purification on day 7 of culture, they were incubated overnight with BALB/c lysate. After alloAg pulsing, the DC were resuspended in 500 µl PBS and placed on poly-lysine-coated glass slides in 24-well plates. After 2 hours at 4°C, the adherent cells were washed and fixed with 2% paraformaldehyde in PBS for 1 hour. The cells were washed 3 times with PBS, then permeablized with 0.1% Triton-X-100 in PBS for 15 min. Cells were washed in incubation buffer (PBG: 0.15% glycine, 5% BSA in PBS) and blocked with 5% v/v normal goat serum in PBG for 45 min. To block non**-specific mouse IgG binding, Fc receptors were blocked for 1 hour using rat anti-mouse CD16/32 (1:100 dilution in PBG; invitrogen, Carlsbad, CA, clone FCR-4G8). Cells were stained for CD11c (Armenian hamster mAb, 1:100 dilution in PBG, BD Biosciences) and anti-Ld (1:100 dilution mouse anti-mouse 30-5-7 mAb in PBG; BD Biosciences) for 1 hour at room temperature. Following several washes with PBG, the cells were stained with anti-hamster Cy3 (1:1000 in PBG, Jackson ImmunoResearch, West Grove, PA) and anti-mouse IgG Fab1 fragments conjugated to Alexa 488 (1:500 in PBG, Invitrogen). Nuclear staining was performed just prior to coverslipping with Gelvatol (23 g polyvinyl alcohol 2000, 50 ml glycerol, 0.1% sodium azide to 100 ml PBS) using 0.01% Hoechst Dye (bis benzimide) in PBS. Single optical slice confocal microscopy images were taken on an Olympus Fluoview 1000 microscope (Center Valley, PA) using an oil immersion 100× UPlan S Apo objective, NA= 1.40.
3.6. CD8+ T cell purification
CD8+ T cells were purified from spleen and lymph node cell suspensions by negative selection. Thus, mononuclear cells were labeled with anti-CD11b, anti-TER-119, anti-Gr-1, anti-I-A/I-E, anti-B220, anti-CD32/16, anti-CD4, and anti-Gr-1 mAbs (all from BD PharMingen). Following incubation with Mouse Depletion Dynabeads® (Dynal Biotech, Oslo, Norway) bead-bound cells were removed by magnetic isolation. Purity was consistently ≥95%.
3.7. MLR using 2C CD8+ T cells as responders
Graded numbers of γ-irradiated (20 Gy) B6 or BALB/c DC were used as stimulators of purified 2C splenic T cells (2×105/well) in 72 hour MLR using 96-well, round-bottom plates. For the final 16–18 hours, individual wells were pulse-labeled with 1 µCi 3[H] thymidine. Radioisotope incorporation was determined using a β scintillation counter. Results are expressed as mean counts per minute (c.p.m.) ± 1 SEM calculated from triplicate wells.
3.8. Analysis of 2C T cell apoptosis in vitro and in vivo
2C CD8+ T cells stimulated with irradiated CTR-DC, RAPA-DC or BALB/c splenocytes at a 10:1 ratio were harvested on day 4 of MLR and labeled with PE-Cy7-conjugated anti-CD8 and PE-conjugated anti-CD62L mAbs. The incidences of viable cells and early and late apoptotic cells were determined using the Annexin V-FITC apoptosis detection kit (BD PharMingen) in accordance with the manufacturer’s instructions. Following staining of externalized phosphatidylserine with Annexin-V-FITC, data were acquired and analyzed as described above.
2C CD8+ T cells (5–6 × 106) were also injected i.v. via the lateral tail vein into B6 mice (syngeneic with 2C mice). After 2 days, 2 × 106 alloAg-pulsed B6 CTR- or RAPA-DC or unpulsed BALB/c CTR-DC were injected i.v. Four days later, the mice were euthanized and their splenocytes and lymph node cells analyzed by flow cytometry. 2C T cells were defined by APC-conjugated staining for CD8, and Pacific Blue-conjugated anti-Thy1.1. Cy7-conjugated anti-CD62L and 7-AAD staining was performed, as described above.
3.9. Statistics
Results are expressed as means ± 1SEM. The significances of differences between means were determined using Student's 't' test and the JMP IN 4.04 Statistical Package (SAS Institute Inc., Cary, NC). A 'P' value < 0.05 was considered significant.
4. Results
AlloAg-pulsed RAPA-DC are capable of allo-MHC class I and II surface expression
Murine BM cells from B6 mice were cultured for 7 days GM-CSF and IL-4, with or without RAPA (10 ng/ml) to generate myeloid CTR- and RAPA-DC, respectively. Based on CD11c staining, the purity of the in vitro-propagated RAPA- and CTR-DC was ≥95%, following purification as described in the Materials and Methods. As described previously [21], RAPA-DC exhibited lower cell surface levels of endogenous MHC class I (H2-Kb), and lower surface levels of MHC class II and costimulatory molecules when compared to CTR-DC (data not shown).
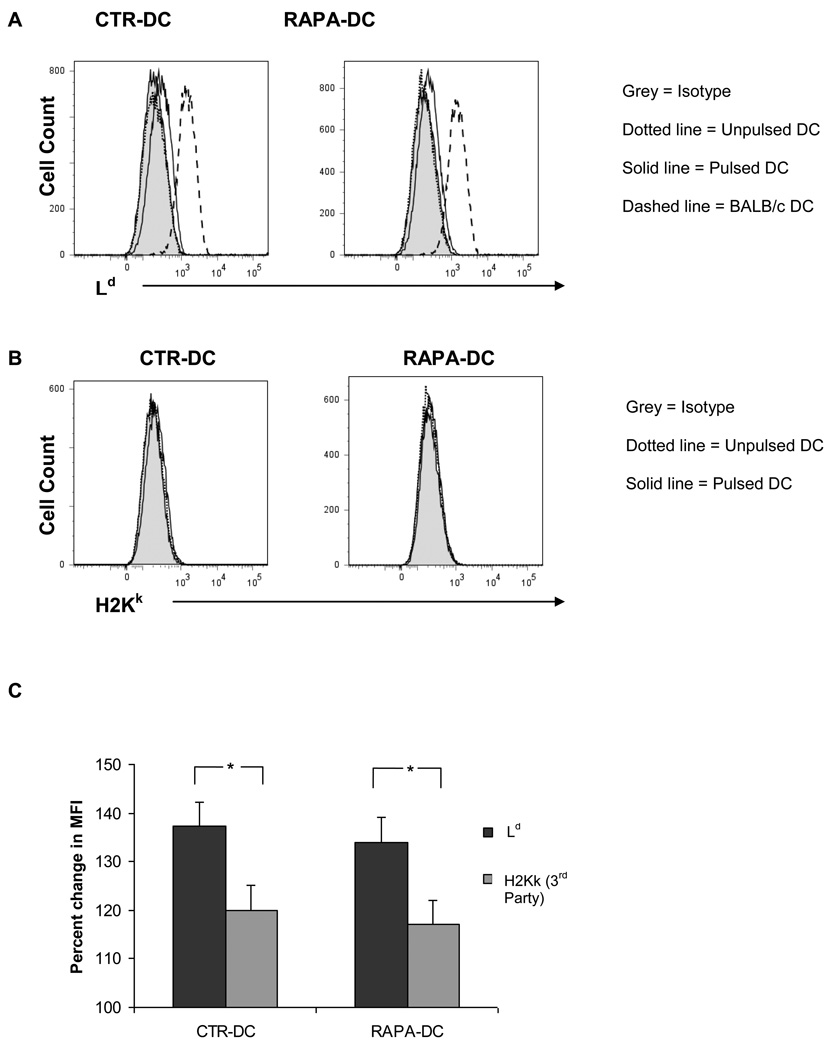
Purified RAPA- and CTR-DC pulsed for 18 hours (the period we have found previously to be optimal [26]) with cell-free lysate of BALB/c splenocytes (DC: splenocyte equivalent of 1:10), as described in the Materials and Methods, were analyzed for the expression of allogeneic H-2d MHC class I molecules on their surface. Fig. 1A shows the expression of the BALB/c MHC class I molecule, Ld, on the surface of alloAg-pulsed CTR- and RAPA-DC after pulsing for 18 hours. Expression of third party MHC class I molecule (H2Kk) was also evaluated, but no significant staining was observed compared to that for the pulsed Ag (Fig. 1B). The expression of Ld relative to H2Kk for both CTR-DC and RAPA-DC is shown in Fig. 1C. Similar results were obtained pulsing the DC for 24 hours (data not shown). The expression of acquired alloAg by lysate-pulsed DC is similar to that described under similar conditions [16, 17] for ‘cross-dressed’ DC that activate tumor-specific T cells.
Fig. 1.
RAPA-DC pulsed with alloAg express acquired cell surface MHC class I alloAg. RAPA-DC and CTR-DC, generated from B6 (H2b) mice as described in the Materials and Methods, were pulsed with alloAg from lysed BALB/c (H2d) splenocytes for 18 hours, after which flow cytometric analysis was performed. (A), DC stained with Ab against the Ld Ag (30-5-7) show a distinguishable increase in cell surface expression of the molecule following Ag pulsing when compared to baseline. Ld was readily detected on BALB/c DC (positive control). (B), To control for non-specific staining, mAb staining against third-party MHC class I Ag (H2-Kk) was also performed. (C), the average percentage increase in MFI was calculated and compared showing increased Ld surface expression on the pulsed DC compared with third party (C3H) DC. Data are representative of at least 5 experiments. Shaded profile = isotype control; dotted line = unpulsed DC; solid line = alloAg-pulsed DC; dashed line = BALB/c DC. *, p<0.01.
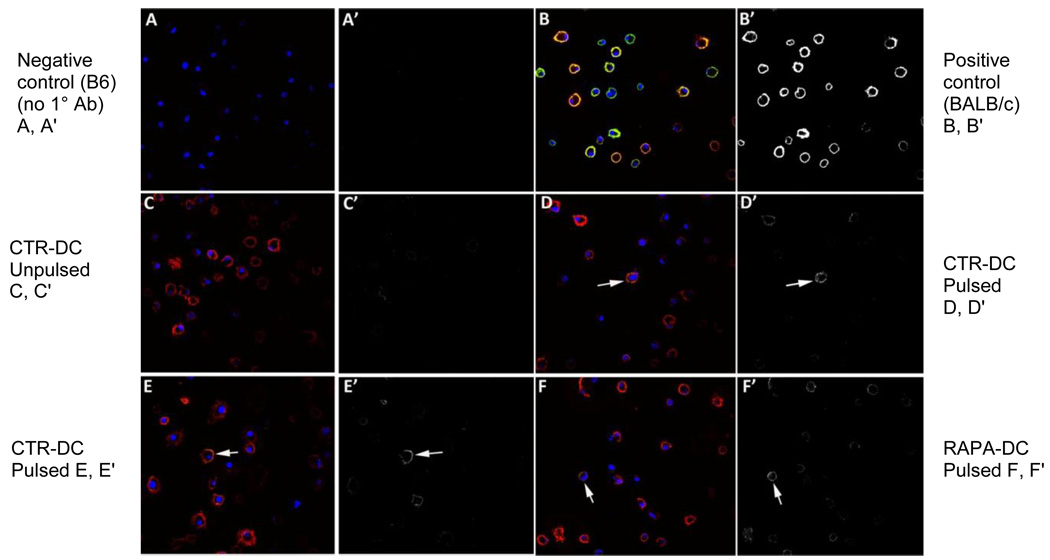
To confirm the expression of acquired alloAg detected by flow cytometry, pulsed and unpulsed DC were also examined by confocal microscopy. The CTR- and RAPA-DC were cultured as described above, then incubated overnight (18 hours) with BALB/c lysate on day 7 of culture, then stained for alloAg expression. Expression of transferred intact Ld peptide was determined using the 30-5-7 mAb, followed by Alexa Fluor 488 anti-mouse IgG secondary Ab staining. The mAb 30-5-7 binds specifically to the α2 domain of peptide + folded Ld only, and its reactivity is lost upon dissociation of the peptide or β2 microglobulin from the MHC class I heavy chain [33, 34]. The results are shown in Fig. 2. DC displaying surface Ld were detected in both the allogeneic lysate-pulsed CTR- and RAPA-DC populations, with only a mild degree of background staining for the unpulsed populations. BALB/c DC were used as positive controls for the expression of Ld, and showed uniform cell surface staining for the Ag.
Fig. 2.
Visualization of acquired alloAg (Ld) on RAPA-DC by confocal microscopy. B6 (H2b) RAPA-DC and CTR-DC were prepared as described in the Materials and Methods, as were DC from (positive) control BALB/c (H2d) mice. After 18 hours incubation with alloAg, the DC were transferred to glass coverslips in 24-well plates. After 2 hours incubation in PBS on melting ice, the cells were stained with mAb against CD11c (red), MHC class I Ld Ag (30-5-7; green) and nuclear stain (blue). The images were acquired using a Fluoview 1000 confocal microscope. The images for each channel were collected sequentially, such that no bleed-through was evident between the red and green channels. The RGB (red green blue) images were opened in Photoshop and the red and blue channels were removed, allowing only the green channel to be visible. The green channel was then converted to monochrome (black and white), but no other adjustments were made to the images other than the change in color (no level or contrast changes). The apparent absence of green signal in the merged panels is because the red signal is so high it overpowers the green. Thus, in order to show that the signal is indeed there, panels with only the green staining are included. Green signal is also shown separately as a black and white image in the letter prime panels (e.g. B') to the right of the representative 3-color panels. Confocal analysis of the cells revealed Ld staining (arrows) of pulsed B6 CTR- and RAPA-DC ****(CTR-DC pulsed: D, D’, E, E’; RAPA-DC pulsed: F, F’), with strong staining of positive control BALB/c DC (H2d; cells that constitutively express the Ld epitope (B, B’). Unpulsed DC (C, C’) showed minimal staining for Ld, but did not reach the intensity displayed by pulsed DC (D, D’, E, E’). B6 CTR-DC stained with secondary Ab alone are shown in the top left (A, A’) as a control. Data are representative of 2 separate experiments. All images taken at 1000×.
AlloAg-pulsed RAPA-DC enhance apoptosis of alloAg-specific CD8+ T cells in vitro
RAPA-DC have been shown to enhance apoptosis of allogeneic CD4+ T effector cell populations during MLR [21]. To evaluate the influence of alloAg-pulsed and unpulsed B6 RAPA-DC on syngeneic CD8+ T cells (2C expressing TCR specific for the BALB/c MHC class I Ld Ag) we used 2C T cells from mice bred with B6 Rag−/−Thy 1.1 mice to create B6 mice whose T cells are CD8+, Ld-specific and detectable using anti-Thy1.1 (CD90) mAb.
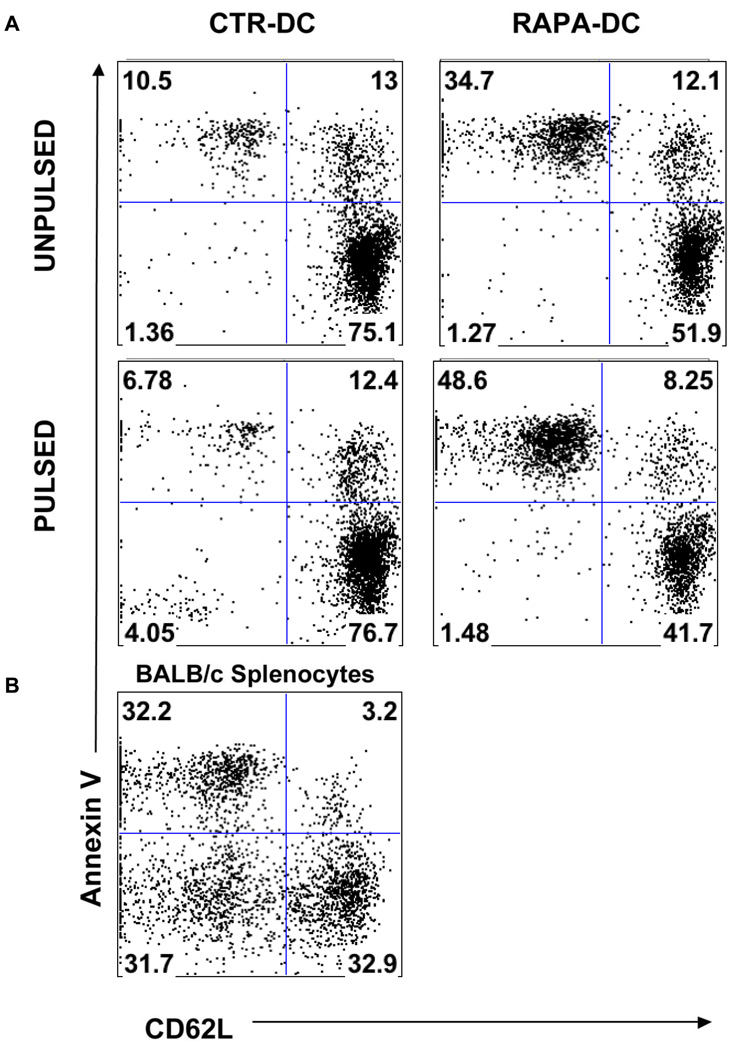
Following 4 days co culture of BALB/c alloAg-pulsed CTR- or RAPA-DC with 2C T cells, apoptosis was assessed by staining the T cells with Annexin-V. L-selectin (CD62L) staining was performed simultaneously to assess T cell activation, as levels of CD62L diminish with T cell activation [35, 36]. As shown in Fig. 3, an overall increase in the incidence of Annexin V+ 2C cells was observed following incubation with B6 RAPA-DC when compared to those 2C cells incubated with CTR-DC. Specifically, apoptosis was enhanced by both unpulsed and pulsed RAPA-DC (suggesting the role of a non-specific mechanism that may reflect poor co-stimulatory ability). However, apoptosis was consistently greater in the CD8+ T cells stimulated with the alloAg-pulsed population. Importantly, an activated, viable (CD62Llo Annexin V−) 2C population was observed when T cells were incubated with alloAg-pulsed CTR-DC, but with neither of the RAPA-DC populations, confirming the failure of alloAg-pulsed RAPA-DC to induce significant CD8+ T cell activation. As anticipated, a pronounced CD62Llo Annexinlo (activated and live) B6 T cell population was prominent among CD8+ 2C T cells incubated with allogeneic Ld+ BALB/c splenocytes (Fig 3).
Fig. 3.
AlloAg-pulsed RAPA-DC enhance apoptosis of alloAg-specific CD8+ T cells in vitro. (A) When BALB/c alloAg-pulsed B6 RAPA-DC were incubated with 2C CD8+ T cells in 72-hour MLR, increased apoptosis of the 2C cells was observed. In the upper left panel, B6 CTR-DC (unpulsed) were incubated with 2C cells. The 2C cells show minimal levels of activation (reduced CD62L expression) or apoptosis (Annexin V+). When incubated with unpulsed B6 RAPA-DC (upper right), 2C cells induced higher baseline levels of apoptosis compared to T cells incubated with unpulsed CTR-DC. When the DC were pulsed, however, a population of CD62Llo 2C cells was present after incubation with pulsed CTR-DC, but not after incubation with BALB/c alloAg-pulsed RAPA-DC. Furthermore, a greater incidence of 2C cells underwent apoptosis after incubation with pulsed B6 RAPA-DC (lower right) than with unpulsed RAPA-DC. Results are representative of 2 separate experiments. (B) Following interaction with BALB/c splenocytes (H2-Ld), a large population of 2C T cells displayed reduced CD62L and were Annexin V−.
AlloAg-pulsed RAPA-DC reduce survival of alloAg-specific CD8+ T cells in vivo
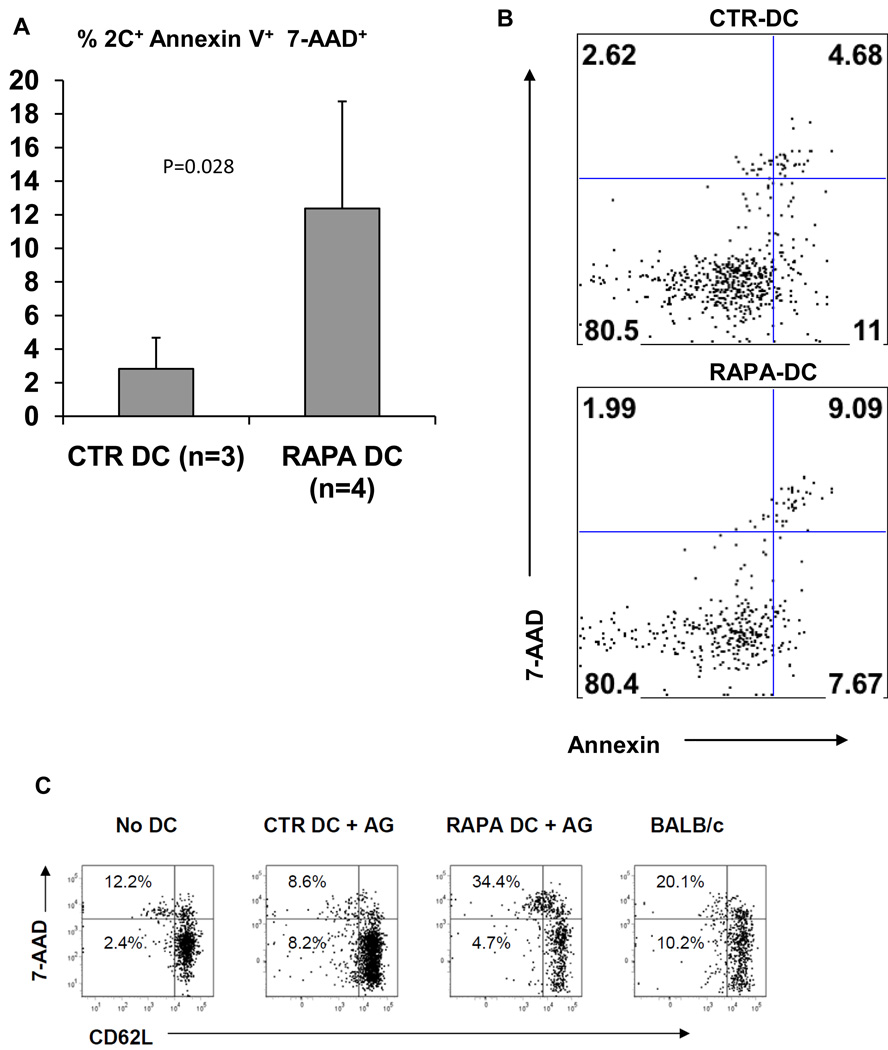
To further examine the ability of alloAg-pulsed RAPA-DC to inhibit directly-reactive CD8+ T cell responses, we evaluated the activation and survival of alloAg-specific 2C TCRtg T cells harvested from the lymphoid tissues of B6 mice injected systemically with either pulsed or unpulsed B6 CTR- or RAPA-DC. Six million 2C CD8+ T cells were injected i.v. into normal syngeneic B6 mice. After 48 hours, the 2C-infused B6 mice were injected i.v. with 2 × 106 BALB/c Ag-pulsed or unpulsed B6 RAPA- or CTR-DC, or third party BALB/c DC. After 4 days, splenocytes and lymph node lymphocytes were isolated and stained for CD8, Thy1.1 and CD62L expression. In addition, 7-AAD staining was used to quantitate the resultant levels of 2C cell survival (7-AAD is excluded by viable cells, but penetrates cell membranes of dying or dead cells). The results are shown in Fig. 4. Consistent with our in vitro findings, alloAg-pulsed RAPA-DC decreased the survival of viable, alloAg-specific CD8+ T cells when compared to allo-Ag-pulsed CTR-DC. The 2C cells in mice that were not injected with DC displayed a very small population (approx 2%) of viable CD62Llo T cells (Fig. 4A). Conversely, 2C cells from positive control mice injected with normal BALB/c DC demonstrated a larger population of CD62Llo, 7-AAD−/lo (viable and activated) T cells. Importantly, mice injected with alloAg-pulsed RAPA-DC displayed a significantly reduced population of CD62Llo, 7AAD−/lo 2C cells when compared to their CTR-DC-injected counterparts (Fig. 4B), indicating the ability of alloAg-pulsed RAPA-DC to reduce the survival of directly-reactive CD8+ T cells in vivo, presumably as a consequence of semi-direct alloAg presentation of H-2Ld. Fig. 4C provides confirmatory evidence that the survival of alloAg-specific CD8+ T cells (Thy1.1+) was reduced in mice infused with alloAg-pulsed RAPA-DC.
Fig. 4.
CD8+ T cells exposed to alloAg-pulsed RAPA-DC in vivo display reduced activation and survival. Data were acquired from flow analysis of CD8+ 2C T cells after their in vivo exposure to alloAg-pulsed B6 CTR-DC, B6 RAPA-DC, or BALB/c DC. Normal B6 mice received i.v. injections of 6 × 106 2C CD8+ T cells (Thy 1.1+). Forty-eight hours later, they were given 2 × 106 DC i.v. After 4 days, lymph nodes and spleens were harvested and cell populations analyzed. (A and B) significantly higher levels of death (Annexin V+7-AAD+) of 2C cells were observed after infusion of alloAg-pulsed RAPA-DC when compared to 2C cells exposed in vivo to alloAg-pulsed CTR-DC. In (C), the percentage of CD62Llo7-AAD− (activated, live) 2C cells is compared for each type of DC. The data show the percentage of T cells recovered from mice that were both CD8+ and Thy 1.1+. Mice infused with alloAg-pulsed RAPA-DC displayed a lower percentage of recoverable, activated 2C cells than those infused with CTR-DC. 2C cells from mice infused with vehicle alone showed little reduction in CD62L expression, whereas mice infused with allogeneic BALB/c DC exhibited a significant loss of CD62L expression, indicating their activation. Data are from at least 3 separate experiments (A) or representative of 3 separate experiments (B and C).
5. Discussion
In the context of transplantation, both donor- and recipient-derived DC play important roles in the generation of immune responses that lead to allograft rejection [4–8]. Under inflammatory conditions, donor DC-mediated direct alloAg presentation, together with appropriate costimulation, activates host T cells, leading to vigorous T cell proliferation and effector T cell responses. As donor-derived DC are eventually lost to attrition, recipient DC that can convey and present donor alloAg indirectly to host T cells [10, 12] eventually supplant them. While the presence of both the direct and indirect pathways of Ag recognition in transplantation is well-recognized [37], the exact contribution of each pathway to the rejection (or acceptance) of an organ allograft is still not well understood. Thus, the ability of stably-immature, alloAg-pulsed host RAPA-DC to inhibit early graft rejection in the absence of immunosuppression [21, 26] suggests that such pharmacologically-modified DC have the ability to affect the host immune response to direct alloAg recognition, in addition to their presumed effects on the indirect pathway of allorecognition.
Classically, the direct pathway is represented in transplantation by donor APC that, display allogeneic intact MHC/peptide to host T cells specific for those Ags. In general, donor-reactive T cells are comparatively plentiful in the recipient, and the result is the proliferation of effector CTL against the graft. The semi-direct pathway, first described by Herrera et al [38], ascribes to DC the ability to display intact Ag on their surface after contact with both cells and cell lysate, a property termed “cross-dressing” [16, 17]. The ability of recipient DC to display donor MHC on their surface would effectively allow these DC to participate in the direct pathway of T cell activation. Donor-reactive CD8+ T cells would also be able to recognize intact donor MHC class I and peptide on the surface of recipient DC, allowing the latter to modulate the response of donor-reactive CTL.
In this study, following alloAg pulsing with the lysate of donor splenocytes, both phenotypically mature CTR-DC and stably-immature RAPA-DC acquired the ability to display donor MHC on their surface. This observation parallels previous findings suggesting that DC have the ability to acquire preformed MHC class I or II molecules from tumor cells that have undergone membrane disruption through repeated freeze-thawing or osmotic lysis [16, 17]. Based on the data we have obtained, and in line with the observations of other groups [13, 14, 16, 17], the expression of these allogeneic MHC is not robust, but sufficient to allow DC to modulate the extent of donor-reactive CD8+ T cell activation and survival, both in vivo and in vitro.
Whereas it has been shown previously that transferred MHC class I:ovalbumin complexes can induce Ag-specific CD8+ T cell proliferation [38], our report is the first to describe increased levels of apoptosis in alloAg-specific CD8+ T cells stimulated by alloAg-pulsed, pharmacologically-modified “immature”/maturation-resistant DC (RAPA-DC). Not only were B6 RAPA-DC (H2b) able to capture intact MHC:peptide molecules from the lysate of BALB/c splenocytes (H2d), but compared with similarly-pulsed CTR-DC, they enhanced apoptosis of CD8+ T cells expressing TCR specific for the BALB/c MHC class I Ld Ag. This contrasts with the fate of T cells stimulated by BALB/c DC, that constitutively expressed the MHC class I Ld Ag for which the 2C TCR is specific, and that exhibited an activated phenotype, with significantly fewer apoptotic cells. The findings are consistent with our previous demonstration that allogeneic RAPA-DC (C3H; H2k) induce increased apoptosis of directly-reactive B10 (H2b) CD4+ T cells [26].
The efficacy of alloAg transfer that we have demonstrated is similar to the previously reported ability of DC to acquire functional MHC class I/peptide complexes via direct intercellular contact [38] or through the uptake of exosomes [39]. We did not assess which specific pathways may account for our findings, but a combination of mechanisms might be responsible and worthy of further investigation. Reduced expression of co-stimulatory molecules on the surface of RAPA-DC diminishes T cell activation, but promotes their apoptosis-inducing ability [21]. Previously, we have reported that costimulatory molecule and death ligand expression on myeloid DC provide counter-regulatory signals for T cell survival and proliferation [40]. Investigation of the role of pathways of apoptosis induction by RAPA-DC, including the Fas (CD95)-FasL pathway and the TNF family of receptors and their ligands, may prove an especially fruitful area of future study.
In conclusion, RAPA-conditioned, alloAg-pulsed DC can present acquired MHC-peptide complexes from donor cells and modulate directly-reactive CD8+ T cell function. These DC enhance Ag-specific CD8+ T cell apoptosis in vitro, and diminish their survival in vivo. In addition to shedding additional light on the impact of mTOR inhibition on DC function, our demonstration of the capacity of RAPA-DC to promote diminished T cell responsiveness to donor Ag supports the concept of an autologous cell-based “negative vaccine” approach to allograft tolerance [26, 41–44], that may help reduce dependence on systemic immunosuppressive drug therapy [45].
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R01 AI67541 and P01 AI081678 (AWT). HRT is supported by NIH K99/R00 HL97155. RTF was supported by NIH T32 DK71492. We thank Ms. Lisa Mathews and Dr. Elizabeth Stenger for skilled assistance with experimental procedures and Ms. Miriam Freeman for administrative support.
Abbreviations
- Ag
antigen
- BM
bone marrow
- CTR-DC
control dendritic cells
- DC(s)
dendritic cells
- MHC
major histocompatibility complex
- MLR
mixed leukocyte reaction
- RAPA
rapamycin
- RAPA-DC
rapamycin-conditioned dendritic cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 4.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould DS, Auchincloss H., Jr Direct and indirect recognition: the role of MHC antigens in graft rejection. Immunol Today. 1999;20:77–82. doi: 10.1016/s0167-5699(98)01394-2. [DOI] [PubMed] [Google Scholar]
- 7.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 8.Lechler RI, Ng WF, Steinman RM. Dendritic cells in transplantation - friend or foe? Immunity. 2001;14:357–368. doi: 10.1016/s1074-7613(01)00116-9. [DOI] [PubMed] [Google Scholar]
- 9.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 10.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 11.Rogers NJ, Lechler RI. Allorecognition. Am J Transplant. 2001;1:97–102. [PubMed] [Google Scholar]
- 12.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Smyth LA, Afzali B, Tsang J, Lombardi G, Lechler RI. Intercellular transfer of MHC and immunological molecules: molecular mechanisms and biological significance. Am J Transplant. 2007;7:1442–1449. doi: 10.1111/j.1600-6143.2007.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smyth LA, Herrera OB, Golshayan D, Lombardi G, Lechler RI. A novel pathway of antigen presentation by dendritic and endothelial cells: Implications for allorecognition and infectious diseases. Transplantation. 2006;82:S15–S18. doi: 10.1097/01.tp.0000231347.06149.ca. [DOI] [PubMed] [Google Scholar]
- 15.de Heusch M, Blocklet D, Egrise D, Hauquier B, Vermeersch M, Goldman S, et al. Bidirectional MHC molecule exchange between migratory and resident dendritic cells. J Leukoc Biol. 2007;82:861–868. doi: 10.1189/jlb.0307167. [DOI] [PubMed] [Google Scholar]
- 16.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Tumor-specific CD4+ T cells are activated by "cross-dressed" dendritic cells presenting peptide-MHC class II complexes acquired from cell-based cancer vaccines. J Immunol. 2006;176:1447–1455. doi: 10.4049/jimmunol.176.3.1447. [DOI] [PubMed] [Google Scholar]
- 17.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177:6018–6024. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- 18.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nouri-Shirazi M, Thomson AW. Dendritic cells as promoters of transplant tolerance. Expert Opin Biol Ther. 2006;6:325–339. doi: 10.1517/14712598.6.4.325. [DOI] [PubMed] [Google Scholar]
- 20.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 21.Turnquist H, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 22.Fazekasova H, Golshayan D, Read J, Tsallios A, Tsang JY, Dorling A, et al. Regulation of rat and human T-cell immune response by pharmacologically modified dendritic cells. Transplantation. 2009;87:1617–1628. doi: 10.1097/TP.0b013e3181a5504c. [DOI] [PubMed] [Google Scholar]
- 23.Kahan BD, Camardo JS. Rapamycin: clinical results and future opportunities. Transplantation. 2001;72:1181–1193. doi: 10.1097/00007890-200110150-00001. [DOI] [PubMed] [Google Scholar]
- 24.Chueh SC, Kahan BD. Clinical application of sirolimus in renal transplantation: an update. Transpl Int. 2005;18:261–277. doi: 10.1111/j.1432-2277.2004.00039.x. [DOI] [PubMed] [Google Scholar]
- 25.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, et al. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 26.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce Ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5:228–236. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 27.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turnquist HR, Thomson AW. Taming the lions: manipulating dendritic cells for use as negative cellular vaccines in organ transplantation. Curr Opin Organ Transplant. 2008;13:350–357. doi: 10.1097/MOT.0b013e328306116c. [DOI] [PubMed] [Google Scholar]
- 29.Turnquist HR, Cardinal J, Macedo C, Rosborough BR, Sumpter TL, Geller DA, et al. mTOR and GSK-3 shape the CD4+ T cell stimulatory and differentiation capacity of myeloid DC following exposure to LPS. Blood. 2010;115:4758–4769. doi: 10.1182/blood-2009-10-251488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monti P, Mercalli A, Leone BE, Valerio DC, Allavena P, Piemonti L. Rapamycin impairs antigen uptake of human dendritic cells. Transplantation. 2003;75:137–145. doi: 10.1097/00007890-200301150-00025. [DOI] [PubMed] [Google Scholar]
- 31.Morelli AE, Zahorchak AF, Larregina AT, Colvin BL, Logar AJ, Takayama T, et al. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512–1523. doi: 10.1182/blood.v98.5.1512. [DOI] [PubMed] [Google Scholar]
- 32.Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood. 2002;100:1084–1087. doi: 10.1182/blood.v100.3.1084. [DOI] [PubMed] [Google Scholar]
- 33.Ozato K, Hansen TH, Sachs DH. Monoclonal antibodies to mouse MHC antigens. II. Antibodies to the H-2Ld antigen, the products of a third polymorphic locus of the mouse major histocompatibility complex. J Immunol. 1980;125:2473–2477. [PubMed] [Google Scholar]
- 34.Smith JD, Lie WR, Gorka J, Myers NB, Hansen TH. Extensive peptide ligand exchange by surface class I major histocompatibility complex molecules independent of exogenous beta 2-microglobulin. Proc Natl Acad Sci U S A. 1992;89:7767–7771. doi: 10.1073/pnas.89.16.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galkina E, Tanousis K, Preece G, Tolaini M, Kioussis D, Florey O, et al. L-selectin shedding does not regulate constitutive T cell trafficking but controls the migration pathways of antigen-activated T lymphocytes. J Exp Med. 2003;198:1323–1335. doi: 10.1084/jem.20030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamann A, Klugewitz K, Austrup F, Jablonski-Westrich D. Activation induces rapid and profound alterations in the trafficking of T cells. Eur J Immunol. 2000;30:3207–3218. doi: 10.1002/1521-4141(200011)30:11<3207::AID-IMMU3207>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 37.Benichou G, Thomson AW. Direct versus indirect allorecognition pathways: on the right track. Am J Transplant. 2009;9:655–656. doi: 10.1111/j.1600-6143.2009.02572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, et al. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173:4828–4837. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 39.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 40.Lu L, Qian S, Hershberger PA, Rudert WA, Lynch DH, Thomson AW. Fas ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. J Immunol. 1997;158:5676–5684. [PubMed] [Google Scholar]
- 41.Horibe EK, Sacks J, Unadkat J, Raimondi G, Wang Z, Ikeguchi R, et al. Rapamycin-conditioned, alloantigen-pulsed dendritic cells promote indefinite survival of vascularized skin allografts in association with T regulatory cell expansion. Transpl Immunol. 2008;18:307–318. doi: 10.1016/j.trim.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Ikeguchi R, Sacks JM, Unadkat JV, Solari M, Horibe EK, Thomson AW, et al. Long-term survival of limb allografts induced by pharmacologically conditioned, donor alloantigen-pulsed dendritic cells without maintenance immunosuppression. Transplantation. 2008;85:237–246. doi: 10.1097/TP.0b013e31815e870e. [DOI] [PubMed] [Google Scholar]
- 43.Chaussabel D, Banchereau J. Dendritic cells, therapeutic vectors of immunity and tolerance. Am J Transplant. 2005;5:205–206. doi: 10.1111/j.1600-6143.2004.00784.x. [DOI] [PubMed] [Google Scholar]
- 44.Mirenda V, Berton I, Read J, Cook T, Smith J, Dorling A, et al. Modified dendritic cells coexpressing self and allogeneic major histocompatibility complex molecules: an efficient way to induce indirect pathway regulation. J Am Soc Nephrol. 2004;15:987–997. doi: 10.1097/01.asn.0000119575.98696.1d. [DOI] [PubMed] [Google Scholar]
- 45.McCurry KR, Colvin BL, Zahorchak AF, Thomson AW. Regulatory dendritic cell therapy in organ transplantation. Transpl Int. 2006;19:525–538. doi: 10.1111/j.1432-2277.2006.00306.x. [DOI] [PubMed] [Google Scholar]