Abstract
The exocyst -- an octameric protein complex mediating vesicle tethering at the plasma membrane for exocytosis -- is a downstream effector of the Rab proteins Rab8 and Rab11, which are key regulators of membrane trafficking from the trans-Golgi network and recycling endosome to the plasma membrane. Rab11 and Rab8 coordinate their actions via Rabin8, the guanine nucleotide exchange factor of Rab8. A cascade of protein-protein interactions involving the Rabs and the exocyst complex couples the generation of secretory vesicles at donor compartments to their docking and fusion at the plasma membrane. Here, we discuss recent work implicating Rab proteins and the exocyst in primary ciliogenesis and epithelial lumenogenesis. In addition, we discuss early work in the budding yeast Saccharomyces cerevisiae, which provided initial insight into the molecular mechanisms of polarized exocytosis.
Polarized exocytosis
Cells are not created equal. The diversity of cell structure and function is vital for most organisms. Despite this diversity, however, common principles are often found to operate within cells during various processes. One such common principle concerns polarized exocytosis, in which secretory vesicles carrying proteins such as receptors and ion channels are generated from the trans-Golgi network (TGN) or recycling endosomes, and transported to and eventually fused with specific areas of the plasma membrane. Several proteins play key roles in exocytosis. The exocyst, a multi-protein complex consisting of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84, mediates the tethering of secretory vesicles to the plasma membrane before SNARE-mediated fusion [1,2]. The Rab family of small GTPases are master regulators in exocytosis. In their GTP-bound form, Rab proteins interact with downstream effectors, thereby controlling various steps of exocytosis [1,3]. Polarized exocytosis is important for a wide range of processes from the asymmetric growth of yeast cells to neurite branching and tumor invasion. Recently, several papers published in quick succession highlight the role of membrane trafficking in primary ciliogenesis and epithelial tube formation. We will discuss the role of Rabs and the exocyst in these processes and trace back to early work in yeast, which provided initial insights into the molecular basis of polarized exocytosis.
Primary Ciliogenesis
Most cells have a single microtubule-based membrane projection called the primary cilium. This cell surface “antenna” is enriched with important molecules such as G-protein coupled receptors (GPCRs) like Smoothened, somatostatin receptors, and rhodopsin, which are central to several signaling pathways. Defects in primary ciliogenesis have been implicated in a wide array of pathological disorders ranging from retinal degeneration to Bardet-Biedl syndrome. The generation of primary cilia involves microtubule organization and polarized membrane trafficking. A classic electron microscopy study provided sequential snapshots of the transport of secretory vesicles to the site of ciliogenesis [4]. The small GTPase Rab8 is involved in membrane trafficking from the TGN and recycling endosome to the plasma membrane [5,6]. Disruption of Rab8 in frog photoreceptor cells blocks rhodopsin transport and results in an accumulation of tubulo-vesicular structures at the base of the retinal rod outer segment, a specialized form of cilia [7,8]. Studies using cultured human retinal pigment epithelium (hRPE) cells have further demonstrated that Rab8 and its guanine nucleotide exchange factor (GEF) Rabin8 play important roles in primary ciliogenesis [9,10]. Rabin8 interacts with BBS1, a component of the BBSome, which is a multi-protein complex implicated in Bardet-Biedl syndrome and is involved in cargo transport to the primary cilia [10,11]. Rabin8 is a direct downstream effector of Rab11 [12], which mediates vesicle transport from the TGN and recycling endosomes [13,14]. The GTP-bound form of Rab11 interacts with Rabin8 and kinetically stimulates its GEF activity toward Rab8. This effect is specific, as other Rab GTPases such as Rab3 and Rab5 do not exhibit any stimulatory effect on Rabin8 activity [12]. Fluorescence microscopic studies revealed that Rab11 is localized near the base of the primary cilia, and its disruption inhibits ciliogenesis [12]. It was therefore proposed that Rab11 modulates Rab8 function by activating Rabin8. This cascade of Rab activation couples cargo transport from the TGN and recycling endosomes to vesicle docking and fusion at the plasma membrane. In another interesting study, it was shown that Rab11, Rabin8, and Rab8 are involved in the de novo generation of primary cilium [15]. Using time-lapse video microscopy, the authors observed the process of Rab8 recruitment into the ciliary membrane during primary ciliogenesis in live cells, and found that Rab8 ciliary membrane localization was preceded by the trafficking of Rabin8 to the centrosome, which in turn was dependent on Rab11.
Rab proteins perform their functions through their downstream effectors. The exocyst was shown to be a downstream effector of the exocytic Rabs; the exocyst subunit Sec15 directly interacts with the GTP-bound Rab proteins including Rab8 and Rab11 [16–19]. Components of the exocyst are localized at the base of the cilia [20–22]. In Madin-Darby Canine Kidney (MDCK) cells, knockdown of the exocyst component Sec10 led to shorter cilia, whereas over expression of Sec10 led to elongated cilia [21]. Together, these studies implicate a role for the exocyst in ciliogenesis [20–24]. We propose that a series of protein interactions from Rab11 to the exocyst control the polarized transport and docking of vesicles carrying ciliary proteins and possibly the basal body to the plasma membrane for primary ciliogenesis (Figure 1).
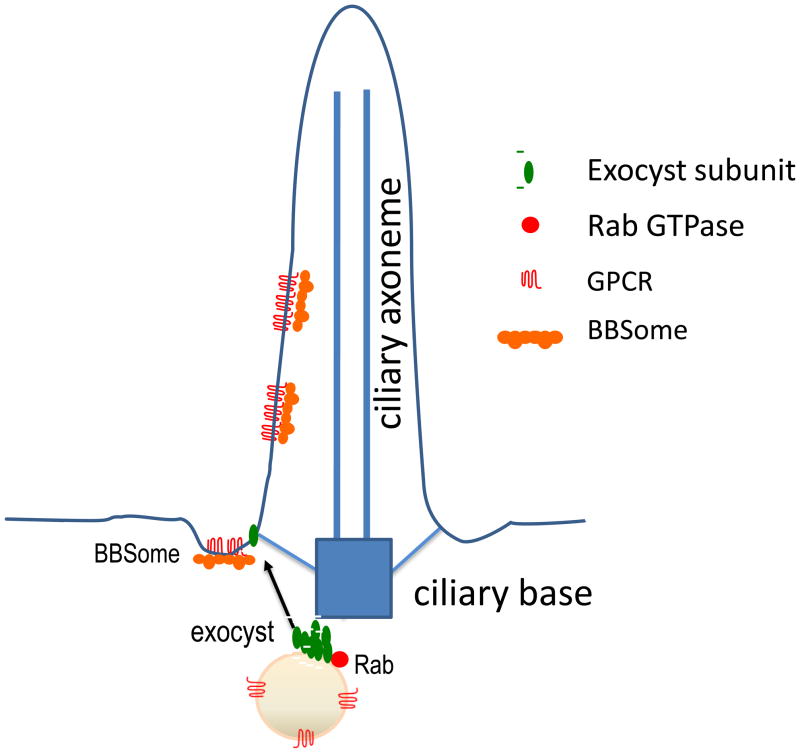
Figure 1. Transport of proteins to the primary cilia.
Ciliary membrane proteins such as GPCRs are delivered from TGN or recycling endosomes to plasma membrane near the base of the primary cilia via tubulo-vesicular carriers (shown as vesicles for simplicity). The exocyst subunits (shown in green) are distributed on the tubulo-vesicular carriers and the plasma membrane. The assembly of the exocyst tethers vesicles to the plasma membrane for fusion, which leads to the incorporation of transmembrane proteins such as GPCRs to the plasma membrane. The Rab proteins (shown in red) on the vesicles regulate assembly of the exocyst complex. Once the cargos are incorporated to the plasma membrane, the BBSome further transports the cargos into the cilia. For simplicity, the intraflagellar transport (IFT) particles are not shown here.
Epithelial lumenogenesis
Similar to polarized trafficking to the primary cilia, the Rabs and exocyst also mediate biogenesis of the epithelial lumen. Epithelial cells form tubes such as the kidney and liver tracts. A functional lumen can be generated de novo when the apical domains of a group of epithelial cells are generated and aligned to face a common hollow space. Lumenogenesis requires polarized exocytosis, through which proteins such as polarity complexes and ion channels are transported from recycling endosomes or TGN to the apical domain of the plasma membrane [25]. In a recent paper [26], the Rab11-Rabin8-Rab8 cascade was shown to be important for proper lumenogenesis. Depletion of any of these proteins led to the formation of multiple rudimentary lumens. Inactive Rab11a, an isoform of Rab11, prevents the vesicular recruitment of Rabin8 or Rab8 and disrupts unilumenogenesis. The authors further demonstrated that the Rab GTPases regulate lumenogenesis through the exocyst complex. Knockdown of the exocyst components Sec15 or Sec10 led to inhibition of single lumen formation. Expression of a Sec15 mutant that is defective in binding Rab11 disrupts unilumenogenesis. The authors proposed a model in which the exocyst transports apical cargos, such as podocalyxin, via Rab11a-positive endosomes to create the polarized site for exocytosis (“AMIS”-apical membrane initiation site) (Figure 2). This study provides important insights into the early stages of lumenogenesis, which is pivotal for organogenesis. Thus, Rab proteins and the exocyst regulate the asymmetric distribution of proteins in epithelial cells to generate polarized tissue architecture.
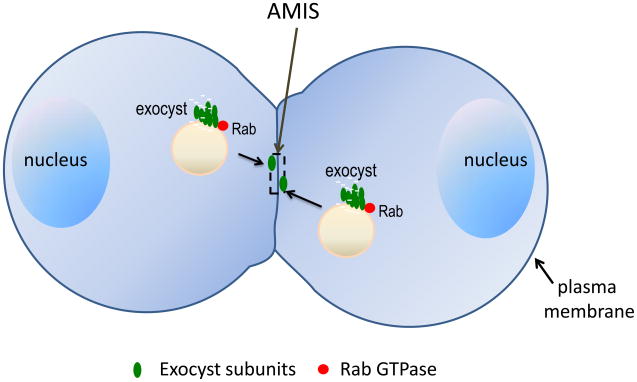
Figure 2. Rab proteins and exocyst mediate membrane trafficking during the early stages of epithelial lumenogenesis.
Cargos such as podocalyxins and apical polarity complexes are delivered to the nascent apical membrane initiation site (AMIS). The Rab proteins (red) on the vesicles regulate assembly of the exocyst complex (green) for the tethering of tubulo-vesicular carriers at the AMIS. The AMIS later gives rise to apical surfaces that face the luminal space. Junctional proteins and polarity proteins are not shown in the diagram.
Conserved themes in Rab and exocyst function
While primary ciliogenesis and lumenogenesis are distinct cellular processes, both require the functions of the Rab11-Rabin8-Rab8 cascade and the exocyst. Cell biological discoveries of fundamental importance often came from studies in simpler model systems. Both Rabs and exocyst were first identified in the budding yeast Saccharomyces cerevisiae [27–29]. Genetic and biochemical studies first revealed that the exocyst component Sec15p is a direct downstream effector of Sec4p, the Rab protein that regulates post-Golgi trafficking in yeast [16]. Also, the yeast work first led to the Rab cascade model: Sec4p is activated by its guanine nucleotide exchange factor Sec2p, which in turn is controlled by GTP-bound Ypt31p and Ypt32p, the Rab proteins that regulate vesicle budding from the TGN [30,31] (Figure 3). The mammalian homologues of Sec4p, Sec2p, and Ypt31/32p are Rab8, Rabin8, and Rab11, respectively. In yeast, Ypt32p was proposed to recruit Sec2p to the secretory vesicles. In mammalian cells, Rab11 is not only required for Rabin8 localization, but also kinetically stimulates the GEF activity of Rabin8 towards Rab8 [12,15,26]. The recruitment model and the kinetic activation model are not mutually exclusive. Future studies may reveal that both mechanisms operate in the same cells for optimal outputs. The Rab cascade is not only conserved in exocytic trafficking, but may also exist in other stages of membrane trafficking such as the early to late endosome conversion [32,33]. Altogether, these studies suggest that a highly choreographed series of Rab activation coordinates various stages of transport and may confer directionality to membrane trafficking.
Figure 3. The Rab cascade model.
(a) In yeast, GTP-bound Ypt32p directly interacts with Sec2p, the GEF for Sec4p. This interaction helps to recruit Sec2p to the secretory vesicles in close proximity to Sec4p for its activation. The GTP-bound Sec4p directly interacts with the exocyst to regulate vesicle tethering at the daughter cell plasma membrane. GTP-Sec4p is later switched to its inactive GDP-bound form by GTP hydrolysis facilitated by its GTPase Activating Proteins (GAPs). (b) In mammalian cells, Rab11, in its GTP-bound form, directly interacts with Rabin8. Rab11 stimulates the GEF activity of Rabin8 towards Rab8 and may also help to recruit Rabin8 to the transport carriers. The activated Rab8 regulates membrane trafficking to the plasma membrane through interaction with its downstream effectors, such as the exocyst. The GTP-bound Rab11 was also shown to interact with the exocyst subunit Sec15. GTP-Rab8 is later switched to its inactive GDP-bound form by GTP hydrolysis facilitated by its GAPs.
Location, location, location
Membrane trafficking is intimately linked to cell polarity and unilumenogenesis concerns both exocytosis and cell polarity. The intimate connection between exocytosis machinery and polarity regulators is well demonstrated in the context of epithelial lumenogenesis [26]. It was shown that the Par3/aPKC complex and the exocyst complex have a mutual dependence for their localization to the AMIS. In addition, Cdc42, a major regulator of polarity, associates with Rab8/Rab11a-positive vesicles and may act downstream of Rab8. Knockdown of Rab8 led to decreased activation of Cdc42 in cells. This effect is probably through Tuba, a GEF for Cdc42 [26]. Similarly, the polarity factors also operate in the primary cilium. As the lonely projection at the cell surface, it is intriguing how this remarkable membrane asymmetry is achieved. It was previously shown that Crumbs3 and Par3 are involved in primary ciliogenesis [34,35]. Also, it was found that the exocyst co-immunoprecipitated with Par3 in cells; knockdown of exocyst components affects ciliary localization of Par3 [21]. It is very likely that the molecular network that drives lumenogenesis described above functions during primary ciliogenesis. In yeast, the exocyst component Sec3 is a direct downstream effector of Cdc42, which spatially and kinetically regulates exocyst function during asymmetric daughter cell growth (“budding”) [36,37]. Cdc42 polarization, in turn, is in part controlled by membrane trafficking [38–40]. As such, a positive feedback loop functions for the establishment and maintenance of yeast cell polarity. It will be interesting to see whether similar positive feedback loops exist in more complex processes such as ciliogenesis and lumenogenesis in higher eukaryotes.
Future perspectives
While the Rab cascade and the exocyst are critical components in the exocytic pathway, they are clearly not the only players. Recently, it was shown that Arf4, Rab11, FIP3, and the Arf GTPase-activating protein ASAP1 are important for the transport of rhodopsins to the retina outer segments [41]. The Arf4-based protein complex is probably involved in the selection and packaging of specific cargos, including GPCRs, for their delivery to the cilia. It will be interesting to know how vesicle packaging and budding are coupled to subsequent transport and docking at the plasma membrane. Once incorporated into the plasma membrane, ciliary cargos are collected by the BBSome, which acts as a planar coat that transports proteins to the cilia [11]. It will be important to determine how vesicle fusion is connected to subsequent cargo entry to the cilia, and how the BBSome is coupled to intraflagellar transport particles (IFTs) for cargo movement within the cilia. The field also awaits a better understanding of the connections between the secretory machinery and cell polarity regulators. While functional analyses [26,34,35] and immunoprecipitation experiments [21] have demonstrated a connection between Rabs and exocyst with Cdc42 and Par proteins, future experiments are called for to elucidate the molecular interactions among these proteins.
The regulation of membrane trafficking by Rab GTPases and the exocyst has profound implications in human physiology and diseases. For example, both the Rab proteins and the exocyst are implicated in tumorigenesis and cancer dissemination [42–48]. Also, bacterial pathogens can hijack Rab and exocyst for their invasion and proliferation [49,50]. Elucidation of the molecular mechanism of polarized exocytosis will help us better understand many complex cell biological processes, which should ultimately result in improved treatments of human diseases.
Acknowledgments
We are grateful to Dr. Peter Novick (University of California, San Diego) for insightful discussions and suggestions. We also thank people in Wei Guo’s laboratory including Drs. Shanshan Feng, Kelly Orlando, and John Schmidt for their helpful comments. The work in Wei Guo’s laboratory has been supported by the National Institutes of Health, Pew Scholars Program in Biomedical Sciences, and American Heart Association. Amlan Das is supported by the National Kidney Foundation post-doctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- 3.Grosshans BL, et al. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–7. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber LA, et al. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang AL, et al. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deretic D, et al. Rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995;108:215–224. doi: 10.1242/jcs.108.1.215. [DOI] [PubMed] [Google Scholar]
- 8.Moritz OL, et al. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12:2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimura S, et al. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 11.Jin H, et al. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knödler A, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ullrich O, et al. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, et al. Rab11 is required for trans-Golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell. 1998;9:3241–3257. doi: 10.1091/mbc.9.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westlake CJ, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. 2011;108:2759–64. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo W, et al. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang XM, et al. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem. 2004;279:43027–43034. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
- 18.Wu S, et al. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol. 2005;12:879–885. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- 19.Oztan A, et al. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol Biol Cell. 2007;18:3978–3992. doi: 10.1091/mbc.E07-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers KK, et al. The exocyst localizes to the primary cilium in MDCK cells. Biochem Biophys Res Commun. 2004;319:138–143. doi: 10.1016/j.bbrc.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 21.Zuo X, et al. The Exocyst Protein Sec10 Is Necessary for Primary Ciliogenesis and Cystogenesis In Vitro. Mol Biol Cell. 2009;20:2522–2529. doi: 10.1091/mbc.E08-07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park TJ, et al. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overgaard CE, et al. Deciliation is associated with dramatic remodeling of epithelial cell junctions and surface domains. Mol Biol Cell. 2009;20:102–113. doi: 10.1091/mbc.E08-07-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazelova J, et al. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci. 2009;122:2003–2013. doi: 10.1242/jcs.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryant DM, et al. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novick P, et al. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- 28.Salminen A, Novick PJ. A ras-like protein for post-Golgi event in yeast secretion. Cell. 1987;49:527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- 29.TerBush DR, Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortiz D, et al. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizuno-Yamasaki E, et al. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell. 2010;18:828–840. doi: 10.1016/j.devcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rink J, et al. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 33.Poteryaev D, et al. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Fan S, et al. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin beta interactions. J Cell Biol. 2007;178:387–398. doi: 10.1083/jcb.200609096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sfakianos J, et al. Par3 functions in the biogenesis of the primary cilium in polarized epithelial cells. J Cell Biol. 2007;179:1133–1140. doi: 10.1083/jcb.200709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, et al. Cdc42 interacts with the exocyst and regulates polarized secretion. J Biol Chem. 2001;276:46745–46750. doi: 10.1074/jbc.M107464200. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, et al. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol. 2008;180:145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wedlich-Soldner R, et al. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- 39.Zajac A, et al. Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth. Mol Biol Cell. 2005;16:1500–1512. doi: 10.1091/mbc.E04-10-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlando K, et al. Exo-endocytic trafficking and the septin-based diffusion barrier are required for the maintenance of Cdc42p polarization during budding yeast asymmetrical growth. Mol Biol Cell. 2011;22:624–33. doi: 10.1091/mbc.E10-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazelova J, et al. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng KW, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nature Med. 2004;10:1251–1256. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 43.Caswell PT, et al. Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Bravo-Cordero JJ, et al. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007;26:1499–1510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chien Y, et al. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127:157–170. doi: 10.1016/j.cell.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 46.Sakurai-Yageta M, et al. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, et al. The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol Biol Cell. 2009;20:3763–3771. doi: 10.1091/mbc.E08-09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Issaq SH, et al. Sec5 and Exo84 foster oncogenic ras-mediated tumorigenesis. Mol Cancer Res. 2010;8:223–231. doi: 10.1158/1541-7786.MCR-09-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guichard A, et al. Anthrax toxins cooperatively inhibit endocytic recycling by the Rab11/Sec15 exocyst. Nature. 2010;467:854–858. doi: 10.1038/nature09446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nichols CD, Casanova JE. Salmonella-directed recruitment of new membrane to invasion foci via the host exocyst complex. Curr Biol. 2010;20:1316–1320. doi: 10.1016/j.cub.2010.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]