Abstract
Human papillomavirus type 16 (HPV16) infection has been linked to the development of cervical and anal dysplasia and cancer. One hallmark of persistent infection is the synthesis of the viral E7 protein in cervical epithelial cells. The expression of E7 in dysplastic and transformed cells and its recognition by the immune system as a foreign antigen make it an ideal target for immunotherapy. Utilizing the E7-expressing murine tumor cell line, TC-1, as a model of cervical carcinoma, an immunotherapy based on the administration of an adjuvant-free fusion protein comprised of Mycobacterium bovis BCG Hsp65 linked to HPV16 E7 (HspE7) has been developed. Initial in vitro analyses indicate that immunization with HspE7 results in the induction of a type 1 immune response based on the pattern of secreted cytokines and the presence of cytolytic activity following antigenic recall. It has been previously shown that prophylactic immunization with HspE7 protected mice against challenge with TC-1 cells and that these tumor-free animals are also protected against rechallenge with TC-1 cells. The present report shows that a single therapeutic immunization with HspE7 induces regression of palpable tumors, confers protection against tumor rechallenge, and is associated with long-term survival (>253 days). In vivo studies using mice with targeted mutations in CD8 or MHC class II or depleted of CD8 or CD4 lymphocyte subsets demonstrate that tumor regression following therapeutic HspE7 immunization is CD8 dependent and CD4 independent. These studies extend previous observations on the induction of CTL by Hsp fusion proteins and are consistent with the clinical application of HspE7 as an immunotherapy for human cervical and anal dysplasia and cancer.
INTRODUCTION
Among the approximately 100 different genotypes of human papillomavirus (HPV), it is the presence of HPV16 that is most frequently associated with the appearance of high-grade cervical intraepithelial neoplasia (CIN) (Olsen et al 1995) as well as anal (Frisch et al 1999) and cervical cancer (Bosch et al 1995). It is believed that the induction of cell-mediated immunity by the host contributes to limiting the progression from HPV infection and low-grade CIN to high-grade CIN and cancer (Tsukui et al 1996). E7 (an early viral protein of HPV16) is continuously expressed by the target epithelial cell on viral integration and cellular transformation. It is highly conserved in amino acid sequence among HPV genotypes and is antigenic in man. Immunotherapeutic strategies to enhance the endogenous response to this tumor-specific antigen for the treatment of HPV-associated disease are being developed (Murakami et al 1999).
The unusual immunogenicity of heat shock proteins (Hsp), originally observed in the context of microbial infection, has prompted researchers to exploit these properties in the development of infectious disease vaccines and cancer immunotherapies (reviewed in Mizzen 1998). It has been previously demonstrated that adjuvant-free immunization of mice with a recombinant fusion protein consisting of Mycobacterium bovis BCG Hsp65 and portions of the nucleoprotein (NP) antigen of influenza virus elicits MHC class I–restricted, NP-specific CTL (Anthony et al 1998). Based on these and other results demonstrating induction of CD8+ cytotoxic T lymphocytes (CTLs) by mycobacterial Hsp fusions (Suzue et al 1997; Yamakazi et al 1999; Cho et al 2000; Huang et al 2000), the potential utility of a recombinant fusion protein comprised of M bovis BCG Hsp65 and HPV16 E7 (HspE7) has been evaluated for the immunotherapy of an E7-expressing murine tumor cell line, TC-1. TC-1 is tumorigenic in syngeneic, immunocompetent mice and has been characterized as a model for human cervical carcinoma (Lin et al 1996).
The present study demonstrates that immunization of tumor-bearing mice with HspE7 leads to tumor regression and long-term survival. Using mice genetically deficient in CD8 or MHC class II Aβ chain or mice depleted of CD4+ or CD8+ T lymphocyte subsets in vivo by antibody administration, it is shown that tumor regression following HspE7 immunization is dependent on CD8+ T cells but independent of CD4+ T cells.
RESULTS
Splenocytes from C57BL/6 mice immunized with HspE7 produced IFN-γ but no detectable IL-5 on restimulation with (h)E7 (a histidine-tagged version of E7). In contrast, cells derived from mice immunized with equimolar amounts of (h)E7 released IL-5 only (Chu et al 2000). In other experiments, only splenocytes from HspE7-immunized mice displayed specific lysis of 51Cr-labeled TC-1 cells. These in vitro studies using primed cells support the notion that fusion of the BCG Hsp65 moiety to E7 creates an immunogen now capable of inducing a type 1 immune response. To evaluate the efficacy of HspE7 immunization in vivo, the TC-1 tumor cell line (obtained from T.C. Wu of John Hopkins University) capable of inducing tumor in immunocompetent, syngeneic C57BL/6 mice was used. Because implantation of the tumor cells leads to the formation of a discrete subcutaneous (SC) nodule readily detected by visual observation, palpation, and caliper measurement, the TC-1 model is a convenient readout for assessing preclinical efficacy of therapeutic agents. Immunization with HspE7, either as prophylaxis against tumor implantation or as treatment for pre-existing tumor, ultimately results in the complete rejection of TC-1 tumor in 80% to 100% of the immunized animals. Furthermore, as a demonstration of the persistence of the immune response, tumor-free animals are capable of rejecting a second tumor challenge.
A single therapeutic immunization with HspE7 induces TC-1 tumor regression and promotes long-term survival
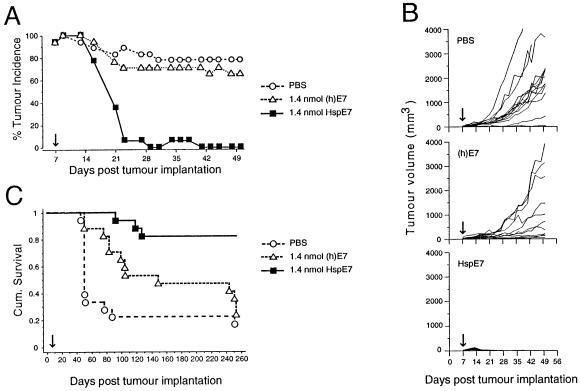
Animals used in the previously described experiments had received 2 immunizations with HspE7. However, it was observed that a decrease in tumor incidence was already apparent in the HspE7-treated animals prior to the time of the second immunization. Therefore, a single immunization regimen was investigated. The therapeutic efficacy of a single HspE7 immunization was compared to that of an equimolar amount of (h)E7 protein alone in mediating tumor regression. As shown in Figure 1A, a palpable SC tumor is apparent in all mice by 9 days postimplantation. Within 3 weeks following a single therapeutic immunization with HspE7, there is nearly complete tumor rejection. In contrast, over this same period, tumor incidence in PBS-treated and (h)E7-treated animals remains at 80% and 70%, respectively. For comparison, tumor volumes from individual mice are shown in Figure 1B. By the conclusion of the 50-day measurement period, average tumor volume in PBS-treated mice is 1200 mm3, and a majority of animals have been become moribund and have to be sacrificed. In (h)E7-treated animals, tumor progression is observed in a majority of animals and, by day 50 average tumor volume is 800 mm3. However, in HspE7-treated animals, tumor progression is either absent or transient, and by day 28 postimplantation, 100% of animals are tumor free. To determine the effect of HspE7 therapy on long-term survival, these animals are followed for a period of 253 days. The Kaplan-Meier survival plot (Fig 1C) shows that 66% of PBS-treated animals are moribund by day 56, and only 3/18 (17%) animals survived to day 253. For the (h)E7-treated group, 53% of animals are moribund by day 154, and only 4/17 (24%) survived to day 253. In contrast, survival in the HspE7-treated group is 82% (14/17) over this 8-month period and is statistically significant relative to the PBS- and (h)E7-treated cohorts (P < 0.0001).
Fig 1.
A single therapeutic immunization with HspE7 induces TC-1 tumor regression and promotes long-term survival. C57BL/6 mice were injected SC in the hind flank with 1.3 × 105 TC-1 cells (day 0). Seven days postimplantation, when measurable tumor (2 × 2-mm minimum) was apparent in all animals, mice were arbitrarily assigned into groups (17 to 18 animals per group) and immunized SC in the scruff of the neck with either PBS, 1.4 nmol of (h)E7, or 1.4 nmol HspE7 (arrow). The same cohorts of PBS (O), (h)E7 (▵), or HspE7 (▪)-treated mice were used to produce the data displayed in (A), (B), and (C). (A) Percentage tumor incidence in mice receiving HspE7 therapy. Mice were scored for the presence or absence of a palpable SC tumor nodule for 50 days postimplantation. (B) Individual tumor volumes from mice receiving HspE7 therapy. Tumor volumes of SC nodules were assessed for 50 days postimplantation. (C) Long-term survival in mice receiving HspE7 therapy. Mice were monitored for survival over a 253-day period postimplantation. Mice that became moribund because of tumor burden were sacrificed. Time to death is plotted on a Kaplan-Meier survival curve (reprinted with permission; see author's note).
Tumor regression following HspE7 immunization is CD8 dependent and CD4 independent
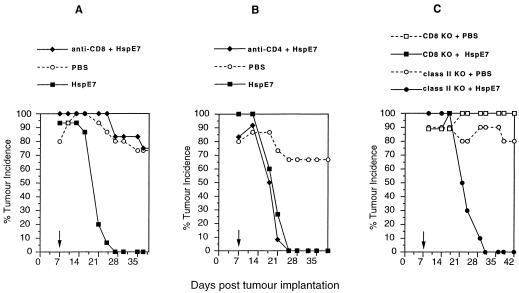
To assess the role of CD8+ and CD4+ T cells in tumor regression in mice receiving therapeutic HspE7 immunization, in vivo antibody depletion experiments were performed. Mice are depleted of CD8+ or CD4+ lymphocyte subsets by the chronic administration of the Mabs 53.6-72 or GK1.5, respectively, then challenged with TC-1 cells. At 7 days postimplantation, lymphocyte depleted and nondepleted cohorts are immunized with HspE7, and tumor incidence is followed for 39 days. The data in Figure 2A,B indicate that whereas CD8 depletion abolishes the efficacy of HspE7 immunization, CD4 depletion has virtually no effect. These results indicate that CD8+ but not CD4+ T cells are required for tumor regression in HspE7-immunized mice. To corroborate these findings, tumor regression following HspE7 immunization was tested in mice with a targeted mutation in either the CD8α gene (CD8 knockout [KO] KO) or the β chain of the Ab molecule (class II KO). Figure 2C shows that HspE7 immunization is ineffective in CD8 KO mice, whereas complete tumor regression is observed in class II KO mice. Therefore, data from in vivo antibody depletion and gene “knockout” approaches are consistent with a CD8 dependent, CD4 independent mechanism for tumor regression in HspE7-immunized animals.
Fig 2.
Tumor regression following HspE7 immunization is CD8 dependent and CD4 independent. (A) and (B) Antibody-depleted or nondepleted mice were injected SC in the hind flank with 1.3 × 105 TC-1 cells (day 0). Seven days postimplantation (arrow), a depleted and nondepleted group of mice (12 to 15 animals per group) were treated with 1.4 nmol HspE7, as indicated in the legend. A third group was treated with PBS. The presence of SC tumor was monitored for 37 or 39 days. Data are presented as percent tumor incidence per group. (A) CD8+ T cell–depleted mice using MoAb 53.6-72; (B) CD4+ T cell-depleted mice using MoAb GK1.5. (C) Mice with a targeted mutation in CD8α (CD8 KO) or the β chain of Ab (class II KO) were implanted with 1.3 × 105 TC-1 cells SC in the hind flank. Seven days later (arrow), the CD8 KO and class II KO cohorts were divided into 2 groups (9 to 10 per group) and immunized with PBS or 1.4 nmol HspE7, as indicated in the legend. The presence of SC tumor was monitored for 42 days. Data are presented as percentage tumor incidence per group (reprinted with permission; see author's note)
DISCUSSION
Adjuvant-free immunization of C57BL/6 mice with a BCG Hsp65-HPV16 E7 fusion protein (HspE7) is efficacious in the prophylaxis and therapy of an E7-expressing murine tumor cell line, TC-1. Notably, tumor regression and long-term survival occurs in mice receiving a single HspE7 immunization. Consistent with induction of CTL by other Hsp fusion proteins (Suzue et al 1997; Anthony et al 1998; Yamakazi et al 1999; Cho et al 2000; Huang et al 2000), regression of TC-1 tumors following HspE7 immunization is a feature of the intact fusion protein only (data not shown). This study represents the first instance of tumor therapy with an Hsp fusion protein and provides the scientific basis for the clinical application of HspE7 in the treatment of HPV-associated human cervical and anal dysplasia and carcinoma.
Consistent with the in vivo results, HspE7 immunization induces cytolytic cells that recognized TC-1 tumor cells in vitro, and splenocytes derived from HspE7-immunized mice secreted the Th1 cytokine IFN-γ following restimulation with E7 protein. This is in contrast to the immune response profile following (h)E7 immunization, which does not induce cytolytic activity against TC-1 cells and is associated with IL-5 production (data not shown). Therefore, immunization of mice with the E7 antigen fused to Hsp65 markedly alters the E7 recall response from that of IL-5 to IFN-γ production, suggesting a shift toward a type 1 response.
It is noteworthy that in the absence of CD4+ T cells (or MHC class II–restricted responses), tumor rejection is observed following treatment with HspE7. At present, it is unknown whether in an immunocompetent animal immunized with HspE7, CD4+ T cells provide help for induction of CD8+ CTL. Under conditions of CD4 depletion or genetic deficiency in MHC class II presentation (as in the present study), possible alternate mechanisms exist for CD8 activation, such as those mediated by CD40 (Bennett et al 1998), or the synergistic action of IL-12 and IL-18 (Okamura et al 1998), via direct stimulation of antigen presenting cells (APC).
Other reports have described the ability of mycobacterial Hsp70 and Hsp65 fusion proteins to induce CTL activity in the absence of CD4+ T cells (Cho et al 2000; Huang et al 2000). The mechanism(s) by which Hsp fusion proteins elicit MHC class I–restricted CTL likely involves direct stimulation of professional APC, bypassing the requirement for CD4 help. For example, it has previously been demonstrated that exposure of macrophages in vitro to bacterial and mammalian Hsp results in the release of proinflammatory cytokines and upregulation of cell adhesion molecules (Verdegaal et al 1996). Most significant is the recent identification of toll-like receptor-4 and CD14 as candidate Hsp60 receptors on murine and human macrophages, respectively (Kol et al 2000; Ohashi et al 2000). Engagement of such “pattern recognition” receptors on APC is reminiscent of Hsp activation of innate immune response effectors such as NK cells (Multhoff et al 1997) and is consistent with the proposal that Hsp represent “danger signals” to the immune system used in the surveillance of abnormal situations such as infection, cell death, and transformation. It is intriguing that in contrast to tumor cell–derived peptides noncovalently associated with Hsp chaperones (Udono et al 1994; Tamura et al 1997), the antitumor immunity induced by Hsp fusion proteins (this study and Yamakazi et al 1999) is CD4 independent. One interpretation of these data is that the fusion construct exposes structural motif(s) within the Hsp that is recognized by the innate immune system, resulting in direct activation of macrophages or dendritic cells (Cho et al 2000).
In summary, HspE7 immunotherapy does not require adjuvant, and because the entire E7 protein has been incorporated, there is no need to select the “appropriate” HLA-binding, antigenic peptides, as for other immunologic approaches. It should be added that because E7 is an oncoprotein, a protein-based approach to HPV immunotherapy is favored over the use of viral or plasmid vectors containing potentially oncogenic DNA sequences. Moreover, the activation of antitumor CTL by HspE7 in the absence of CD4+ T cells suggests that this mode of immunotherapy may have distinct advantages in immunocompromised individuals, such as those with invasive cancer and/or HIV infection.
REFERENCES
- Anthony LSD, Wu H, Sweet H, Turnnir C, Boux LJ, Mizzen LA. Priming of CD8+ CTL effector cells in mice by immunization with a stress protein-influenza virus nucleoprotein fusion molecule. Vaccine. 1998;17:373–383. doi: 10.1016/s0264-410x(98)00199-6. [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Carbone FR, Karamalis F, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- Cho BK, Palliser D, Guillen E, Wisniewski J, Young RA, Chen J, Eisen HN. A proposed mechanism for the induction of cytotoxic T lymphocyte production by heat shock fusion proteins. Immunity. 2000;12:263–272. doi: 10.1016/s1074-7613(00)80179-x. [DOI] [PubMed] [Google Scholar]
- Chu NR, Wu HB, Wu TC, Boux LJ, Siegel MI, Mizzen LA. Immunotherapy of a human papillomavirus (HPV) type 16 E7-expressing tumour by administration of fusion protein comprising Mycobacterium bovis bacille Calmette-Guerin (BCG) hsp65 and HPV16 E7. Clin Exp Immunol. 2000;121:216–225. doi: 10.1046/j.1365-2249.2000.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch M, Fenger C, van den Brule AJC, et al. Variants of squamous cell carcinoma of the anal canal and perianal skin and their relation to human papillomaviruses. Cancer Res. 1999;59:753–757. [PubMed] [Google Scholar]
- Huang Q, Richmond JFL, Suzue K, Eisen HN, Young RA. In vivo cytotoxic T lymphocyte elicitation by mycobacterial heat shock protein 70 fusion proteins maps to a discrete domain and is CD4(+) T cell independent. J Exp Med. 2000;191:403–408. doi: 10.1084/jem.191.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- Lin KY, Guarnieri G, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- Mizzen L. Immune responses to stress proteins: applications to infectious disease and cancer. Biotherapy. 1998;10:173–189. doi: 10.1007/BF02678295. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- Murakami M, Gurski KJ, Steller MA. Human papillomavirus vaccines for cervical cancer. J Immunother. 1999;22:212–218. doi: 10.1097/00002371-199905000-00003. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:+ 259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- Olsen AO, Gjoen K, Sauer T, Orstavik I, Naess O, Kierulf K, Sponland G, Magnus P. Human papillomavirus and cervical intraepithelial neoplasia grade II-III: a population-based case-control study. Int J Cancer. 1995;61:312–315. doi: 10.1002/ijc.2910610306. [DOI] [PubMed] [Google Scholar]
- Suzue K, Zhou XZ, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146–13151. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- Tsukui T, Hildesheim A, Schiffman MH, et al. Interleukin 2 production in vitro by peripheral lymphocytes in response to human papillomavirus-derived peptides: correlation with cervical pathology. Cancer Res. 1996;56:3967–3974. [PubMed] [Google Scholar]
- Udono H, Levey L, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc Natl Acad Sci USA. 1994;91:3077–3081. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdegaal EME, Zegveld ST, van Furth R. Heat shock protein 65 induces CD62e, CD106, and CD54 on cultured human endothelial cells and increases their adhesiveness for monocytes and granulocytes. J Immunol. 1996;157:369–376. [PubMed] [Google Scholar]
- Yamazaki K, Nguyen T, Podack ER. Cutting edge: tumor secreted heat shock-fusion protein elicits CD8 cells for rejection. J Immunol. 1999;163:5178–5182. [PubMed] [Google Scholar]