Abstract
An idiopathic capillary leak syndrome (“engraftment syndrome”) often occurs in recipients of hematopoietic cells, manifested clinically by transient azotemia and sometimes fever and fluid retention. Here we report the renal pathology in 10 recipients of combined bone marrow and kidney allografts. Nine developed graft dysfunction on day 10–16 and renal biopsies showed marked acute tubular injury, with interstitial edema, hemorrhage and capillary congestion, with little or no interstitial infiltrate (≤10%) and marked glomerular and peritubular capillary (PTC) endothelial injury and loss by electron microscopy. Two had transient arterial endothelial inflammation; and 2 had C4d deposition. The cells in capillaries were primarily CD68+MPO+ mononuclear cells and CD3+CD8+ T cells, the latter with a high proliferative index (Ki67+). B cells (CD20+) and CD4+ T cells were not detectable, and NK cells were rare. XY FISH showed that CD45+ cells in PTCs were of recipient origin. Optimal treatment remains to be defined; two recovered without additional therapy, six were treated with anti-rejection regimens. Except for one patient, who later developed thrombotic microangiopathy and one with acute humoral rejection, all fully recovered within 2–4 weeks. Graft endothelium is the primary target of this process, attributable to as yet obscure mechanisms, arising during leukocyte recovery.
Keywords: renal transplant, bone marrow transplant, engraftment syndrome
Introduction
HLA-mismatched kidney transplantation without maintenance immunosuppression has recently been described in a cohort of 5 patients from our institution (1). This clinical protocol was based on non-human primate studies demonstrating that allograft kidneys can be accepted following induction of transient mixed chimerism in which donor and recipient hematopoiesis coexist (2–9). During these studies, and in follow-up studies in five additional patients, transient acute kidney injury (AKI) accompanied by a capillary leak syndrome (CLS) beginning about the 10th post-transplant day was observed in nine patients, a phenomenon not observed in preclinical trials.
CLS or engraftment syndrome (ES) has been reported in patients receiving autologous stem cell or allogeneic bone marrow transplants (BMTs), a condition occurring during neutrophil recovery, as described by Spitzer and colleagues (10, 11). Varied manifestations include fever, rash, pulmonary infiltrates, fluid retention, diarrhea, and hepatic injury and AKI (10–16). ES may be independent of graft versus host disease and a host-versus-graft reaction (10, 11, 17, 18), but its pathogenesis is unknown. AKI in the first 3 months after allogeneic or autologous hematopoietic cell transplant has been attributed to drug toxicity, infection or ischemia. However, the pathologic changes in kidney biopsies have not been extensively studied in hematopoietic cell transplantation recipients (19, 20). In particular, we were unable to find reports of autologous renal pathology in association with ES.
Since the previous report of patients from our institution (1), five additional patients have been enrolled into a similar kidney/BMT regimen. All experienced a condition resembling ES. Here, we report comprehensive studies characterizing the pathologic processes in kidney biopsies obtained during this syndrome
Materials and Methods
Patients
Ten allograft recipients in two sequential Immune Tolerance Network (ITN) sponsored, Institutional Review Board (IRB) approved trials underwent simultaneous kidney/BMTs from parent or sibling donors mismatched for one human leukocyte antigen (HLA) haplotype. The five patients in the first trial (1) received a conditioning regimen with cyclophosphamide (60 mg/kg on day -5 and -4), anti-CD2 monoclonal antibody (MEDI 507, MedImmune, Gaithersburg, MD), rituximab (patients 4–5) (Biogen Idec, Cambridge, MA, and Genentech, South San Francisco, CA), thymic irradiation and post transplant cyclosporine [previously described (1)]. The subsequent 5 recipients received more intensive rituximab treatment and tacrolimus instead of cyclosporine. Donor/recipient chimerism was monitored with flow cytometry.(1, 21)
Histologic assessment
Kidney allograft tissue obtained by percutaneous core or open wedge biopsy was processed for light (LM), immunofluorescence (IF), and electron microscopy (EM) by standard techniques. C4d deposition was evaluated using a monoclonal antibody to C4d (clone 10–11, Biogenesis, Sandown, NH) (22, 23). The results were compared with kidney allografts from recipients on conventional regimens at the same time post-transplant (10–12 days), with normal transplant function (n=6) or acute tubular injury (ATI) (n=5), and biopsies with acute cellular rejection, Banff type 2 (ACR2) (n=5), obtained from our biorepository. Immunohistochemistry (IHC) was performed using Ki-67 (MIB-1, DAKO, Carpinteria, CA) CD68 (KP1, DAKO), CD3 (A0452, DAKO), CD4 (1F6, Biocare Medical, Concord, CA), CD8 (SP16, Biocare), CD20 (L26, DAKO), NKG2D (3.1.1.1, Millipore, Billerica, MA), CD34 (My10, Becton-Dickinson, San Jose, California), CD31 (JC70A, DAKO), myeloperoxidase (MPO, ab15484, Abcam, Cambridge, MA) and C4d (12–500, American Research Products, Belmont, MA). XY fluorescence in situ hybridization (FISH) was performed on gender mismatched cases to determine leukocyte and endothelial cell origin (detailed methods in supplemental material).
Statistical analysis
Statistical analysis was performed in Microsoft Excel and SAS JMP version 8.0 (SAS Institute, Cary, NC). A p value of <0.05 was accepted as a significant using two-tailed t-tests.
Results
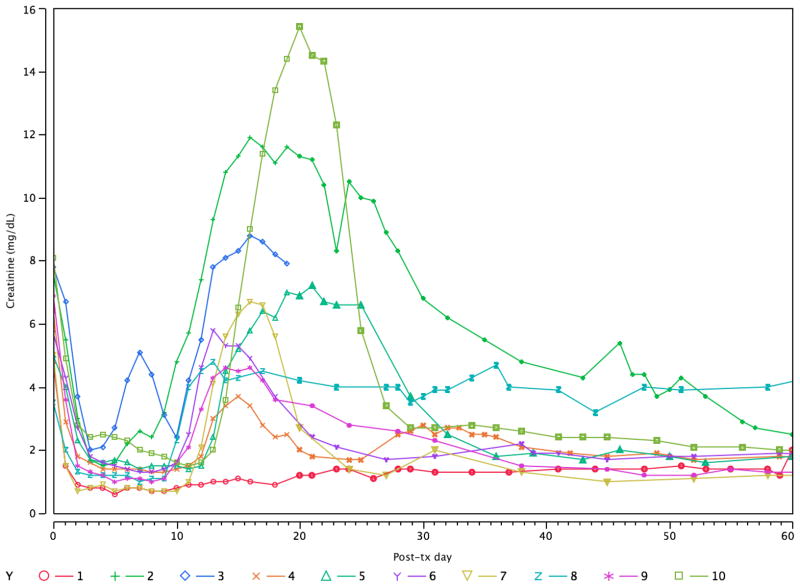
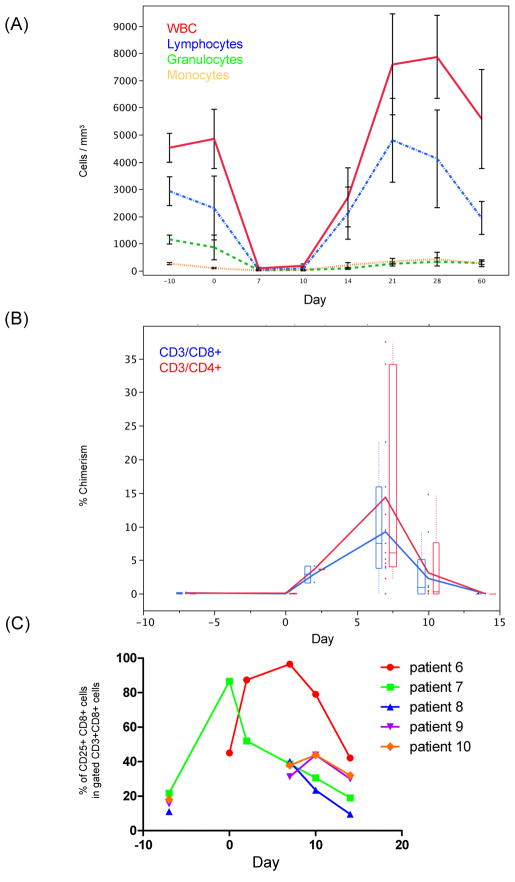
Nine of 10 patients developed AKI in the second week after kidney/BMT, manifested by a creatinine (Cr) rise and sometimes fever and fluid retention, features compatible with ES (Table 1, Figure 1). This occurred in association with a circulating leukocyte count nadir, when donor hematopoietic cells were disappearing and recipient cells were recovering (Figure 2). Donor cell chimerism in the blood peaked at day 7 and was undetectable after 14 days by flow cytometry(24). Flow cytometry showed a transient upregulation of CD25+ among CD8+ T cells during the peri-transplant period (Figure 2).
Table 1.
Clinical and Pathologic Features
| Recipient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bx | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | |||
| Day Post-transplant | 24 | 10 | 10 | 12 | 13 | 11 | 12 | 13 | 10 | 10 | 13 | 16 | |||
| Serum Cr | At Bx | 1.4 | 4.8 | 4.2 | 1.8 | 4.5 | 2.5 | 2.1 | 4.1 | 2.3 | 1.6 | 2.0 | 9.0 | ||
| Peak | 1.4 | 11.9 | 8.3 | 3.7 | 7.2 | 5.8 | 6.7 | 4.8 | 4.6 | 15.4 | |||||
| Last | 1.3 | 2.0 | 2 | 1.8 | 2.0 | 1.6 | 0.7 | 3 | 1.1 | 1.7 | |||||
| Time | 7 y | 6.5 | 2 m | 3 y | 4 y | 1 y | 1 y | 6 mo | 6 mo | 6 mo | |||||
| Symptoms | None | Fever, fluid retention | Fever, fluid retention | Minor fever, fluid retention | Minor fever, fluid retention | Minor fluid retention | Minor fluid retention | No symptoms | Minor fluid retention | Minor fever, minor fluid retention | |||||
| Treatment | No additional treatment | Steroid pulse | ATG, PE, Rituxan, IVIG | Steroid pulse x3 | ATG, PE, Rituxan | No additional treatment | ATG, PE x3 | No additional treatment (ATG later) | No additional treatment | ATG; stopped tacrolimus | |||||
| Banff Acute Rejection Category | None | None | AHR | None | AHR | None | ACR2 | None | None | None | None | ACR2 | |||
| Light Microscopy | PTC Congestion | - | +++ | +++ | + | +++ | ++ | +++ | ++ | ++ | ++ | + | +++ | ||
| Hemorrhage | - | +++ | ++++ | + | ++ | + | +++ | ++ | + | - | - | +++ | |||
| Edema | - | +++ | + | + | + | + | +++ | ++ | + | - | - | ++ | |||
| Tubular Injury | - | +++ | +++ | + | + | + | +++ | + | + | - | + | +++ | |||
| Tubulitis4 | - | - | - | - | - | - | + | - | - | - | - | + | |||
| Interstitial Infiltrate5 | - | - | | - | - | - | - | - | - | - | - | + | |||
| Glomerulitis | - | - | + | + | + | - | ++ | - | + | - | - | ++ | |||
| Endarteritis6 | - | - | - | - | - | - | + | - | - | - | - | ++ | |||
| Immunofluorescence C4d PTC | - | - | >50% | - | >50% | - | - | - | - | 3–5% | - | - | |||
| Electron microscopy | Peritubular Capillaries | Endothelium | Loss | +++ | ++ | - | - | ++ | - | + | ++ | ||||
| Activation | ++ | ++ | + | + | ++ | + | + | ++ | |||||||
| Fibrin and/or platelets | ++ | ++ | - | - | + | - | + | +++ | |||||||
| Cells | RBC | RBC | - | RBC | RBC | - | - | RBC, N | |||||||
| Glomeruli | Endothelium | Loss | - | ++ | ++ | NA | + | + | + | + | |||||
| Activation | + | + | ++ | ++ | + | ++ | ++ | ||||||||
| Foot process effacement | 0 | + | + | +++ | + | + | + | ||||||||
No acute graft dysfunction.
Retransplanted under conventional therapy and stable at 6 yrs.
Developed TMA attributed to calcineurin inhibitor toxicity and currently on dialysis.
Tubulitis: -, none; + up to 2 lymphocytes/tubule;
Interstitial inflammation, -, none, +, up to 10%
Arteritis: +, one artery, ++ more than one artery.
Abbreviations: ATI, acute tubular injury; ACR2, acute cellular rejection, Banff type 2; AHR, acute humoral rejection; Bx: biopsy; PTC: peritubular capillary, TMA, thrombotic microangiopathy; RBC red blood cells, NA, not applicable, ATG, anti-thymocyte globulin; PE, plasmapheresis; N, neutrophils.
Figure 1.
Longitudinal plot of serum Cr in the 10 recipients of combined kidney/bone marrow transplantation. Each one except #1 experienced a transient episode of renal dysfunction in the second to third week, here termed the engraftment syndrome (ES). Patient #3 lost the graft due to acute humoral rejection and #8 developed thrombotic microangiopathy.
Figure 2.
(A) A plot of the mean levels of circulating blood leukocytes (overall white blood cells [WBC], lymphocytes, granulocytes, and monocytes) after combined bone marrow and kidney transplantation shows that a nadir of the circulating cells occurs at 7–10 days, just at the onset the engraftment syndrome. Granulocytes and monocytes recover by day 21, a time when the kidney function is also improving. Plotted are means from patients #2, 4, 6, 7, 8, 9, and 10 and the standard error of the mean. (B) Flow cytometry shows that the mean % donor circulating CD3+CD4+ and CD3+CD8+ cells vary in the post-transplantation period, peaking around day 7. Data is given as an average of patients 1–7 and 9–10, with box plots depicting the distribution of the data. Individual data points on each day are depicted. (C) For patients with available data, flow cytometry shows a transient upregulation of CD25+ among CD8+ T cells during the peri-transplant period. The percentage of CD25+CD8+ cells in gated CD3+CD8+ cells was determined in all patients on days 7, 10 and 14 post-transplant. Additional determinations were available pre-transplant (day -7) in patients 7 to 10, and at day 0 and 2 post-transplant in patients 6 and 7.
Histology and Immunofluorescence (Table 1)
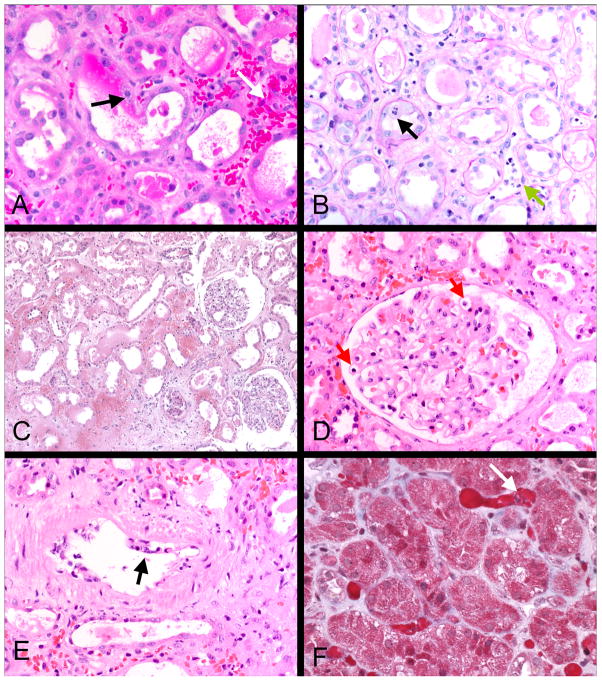
Renal biopsies were obtained 10–16 days post-transplant (11.9±2.0 days) when the Cr was 1.4–9.0 mg/dl (3.4±2.4 mg/dl). Routine LM (Table 1, Figure 3) showed marked ATI with flattened tubular epithelial cells, vacuolization, nuclear and brush border loss, and occasional tubular mitoses in patients diagnosed with ES. ATI was often accompanied by interstitial edema and hemorrhage. Peritubular and glomerular capillary congestion were most prominently seen as a red blood cell “stasis” or “sludging” in PTCs. Occasional mononuclear and polymorphonuclear cells could be appreciated in peritubular and glomerular capillaries. In contrast to typical findings in ACR2 controls, these biopsies had minimal or no interstitial mononuclear infiltrate (0% in all except one case, which had 10%) or tubulitis (none in all except two cases, having ≤2 cells/tubule). Focal endothelialitis was present in one of two biopsies from each of the two recipients 1 and 3 days apart. Patient #7 had arterial endothelial injury and mononuclear inflammation in the subendothelium in one artery on the first biopsy but not in a second biopsy 24 hours later. Patient #10 had similar lesions in two arteries only in the second biopsy. Patient #1 had a protocol biopsy taken on day 24, which was within normal limits, aside from mild donor arteriosclerosis.
Figure 3.
Light microscopy of representative engraftment syndrome cases: Interstitial hemorrhage and congestion are prominent in these cases, as illustrated in A, C and F. Intracapillary mononuclear and polymorphonuclear cells are present in peritubular and glomerular capillaries (B green arrow and D red arrows), a hallmark of this condition. Tubular injury is also characteristically present, as shown by loss of brush border and tubular epithelial mitoses (black arrow B). Two cases had transient arterial endothelial inflammation, as shown in E (arrow); some cells were neutrophils, in contrast to the usual T cell mediated rejection. Little or no interstitial inflammation or tubulitis was evident in contrast to cell mediated rejection. A, C, D, and E, H&E; B, PAS; F, trichrome.
C4d was negative in 5 and rarely detected in 3 (<1% of PTC in two and 3–5% in one) of the 10 recipients by IF and IHC. Two cases (#3,5) had widespread C4d in PTC (>50% by IF) in conjunction with newly detectable DSA in their sera. The C4d pattern often had a web-like or granular quality, in contrast to the usual sharp ring-like staining seen in acute antibody-mediated rejection (AHR). These biopsies both had more prominent neutrophils in PTC than the C4d negative cases and were interpreted as primarily AHR.
Mesangial IgM was present in 4 recipients (1–2+) occasionally with trace C3. Fibrin was noted in the glomeruli, interstitium, and PTCs in 2, 3, and 1 case, respectively (9). Other stains were negative, aside from two cases with trace IgA also detected in the donor biopsy.
Electron microscopy (Tables 1 and 3, Figure 4)
Table 3.
Follow-up Protocol Biopsies
| Recipient | Days Post-Tx | Light Microscopy | C4d | EM |
|---|---|---|---|---|
| 1 | 2716 | Normal1 | − | Normal |
| 2 | 1087 | Normal2 | − | Not done |
| 4 | 1088 | Mild transplant glomerulopathy3 | + | cg1 |
| 5 | 1000 | Normal | − | Normal |
| 6 | 374 | Normal1 | − | Normal |
| 7 | 364 | Normal | − | Normal |
| 9 | 440 | Normal | − | Normal |
| 10 | 177 | Normal4 | − | Normal |
Mild arteriosclerosis (donor disease)
Protocol biopsy at 7 years showed subclinical recurrent MPGN, type I.
Protocol biopsy at 5.4 years showed chronic humoral rejection (cg3)
Indication biopsy at day 311 showed acute cellular rejection (C4d−)
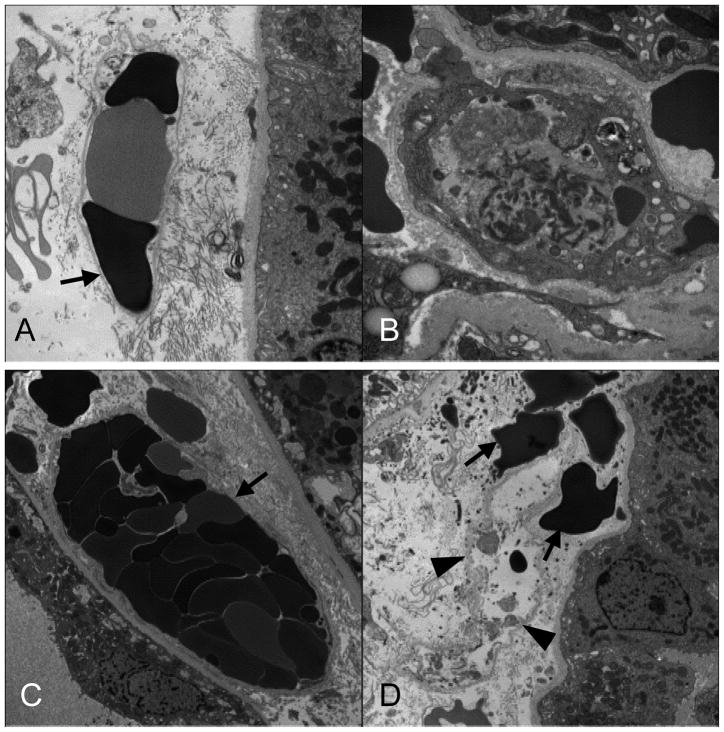
Figure 4.
EM of representative engraftment syndrome cases illustrate the widespread and characteristic peritubular capillary endothelial injury that occurs in this condition. (A and C) Peritubular capillary (PTC) channels no longer lined with endothelium have red blood cell stasis. The bare basement membrane is indicated by black arrows. A capillary with residual, but activated endothelium has fibrin in the lumen (B). A ghost capillary is shown in (D) in which only the basement membrane remains [arrowheads]; extravasated red blood cells are in the renal interstitium [black arrows].
EM (Table 1, Figure 4) showed endothelial activation in PTCs and glomeruli, determined by loss of fenestrations, increased cytoplasmic volume, and increased ribosomes. In 5/8 cases, focal loss of PTC endothelium was accompanied by intravascular accumulation of platelets and fibrin. Erythrocytes were frequently found in PTCs, and extravasated into the interstitium. Glomerular endothelial cells were segmentally lost in 5 of the 7 cases with glomeruli. Subendothelial lucencies were present in glomerular capillary loops without electron dense deposits. Foot process effacement was segmentally present in 5 cases, and extensive in one. The GBM was normal in 6/7 cases. In case #7, the GBM was thinned with concurrent prominent erythrocyte stasis. The pretransplant donor biopsy and later samples had a normal GBM thickness, suggesting that thinning was due to acute distention.
Immunohistochemistry (Figures 5–8)
Figure 5.
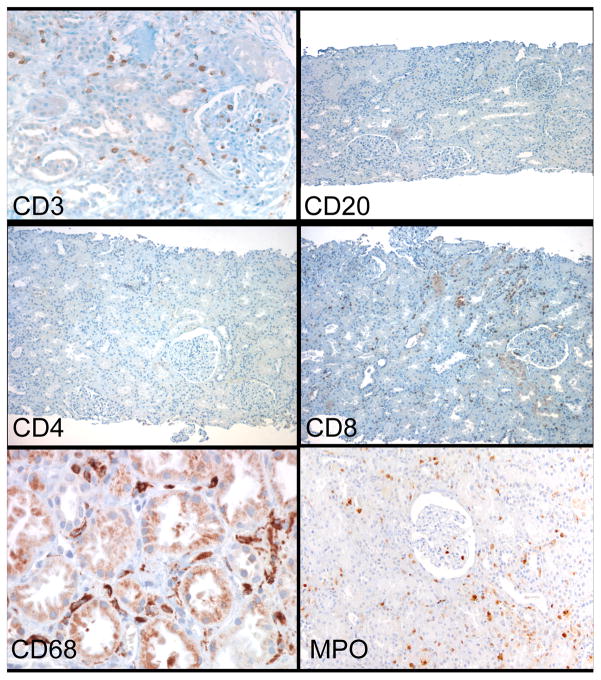
Immunohistochemistry of ES shows the sparse number of T cells, primarily in peritubular and glomerular capillaries. The T cells are almost exclusively CD8+, with rare or no CD4+ cells. The other major cell type is MPO+ and CD68+. B-cells (CD20+) are almost absent.
Figure 8.
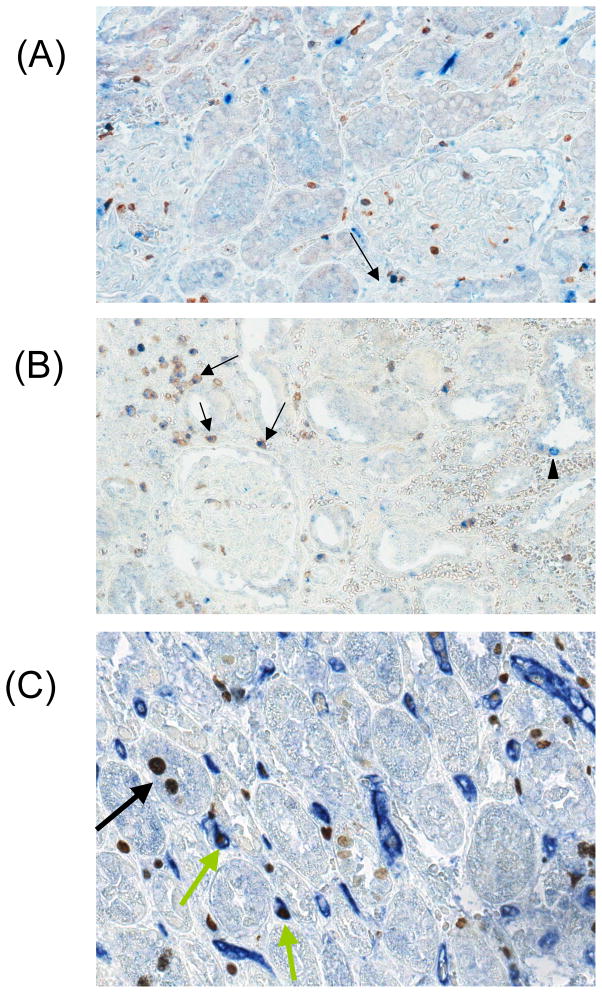
(A) CD3/Ki67 double staining in the engraftment syndrome (ES) showing a CD3+ cell also positive for Ki67 in a glomerulus [arrow], indicating that T-cells are proliferating in the allograft; (B) CD3/Ki67 double staining in the ES showing CD3+Ki67+ double positive cells in the interstitium [arrows], in the location of peritubular capillaries, and a Ki67 single positive cell in the flattened tubular epithelium [arrowhead], indicative of proliferation of the injured tubular epithelium; and (C) CD31+Ki67+ double staining showing Ki67+ cells in PTCs [green arrows] and tubules [black arrow] and areas with loss of PTC endothelial staining.
In contrast to ACR2, the panel of lineage markers revealed a sparse, diffuse accumulation of CD3+ T cells and CD68+ or MPO+ cells in the PTC and glomeruli, with little or no infiltration of the interstitium, not appreciably different from ATI or normal biopsies (Figures 5,6). CD8+ but no CD4+ or CD20+ cells were detected. MPO appeared to be mainly in mononuclear cells, rather than granulocytes. There were rare interstitial NKG2D+ cells in ES biopsies, not appreciably different from ACR2 or ATI (Figure 8).
Figure 6.
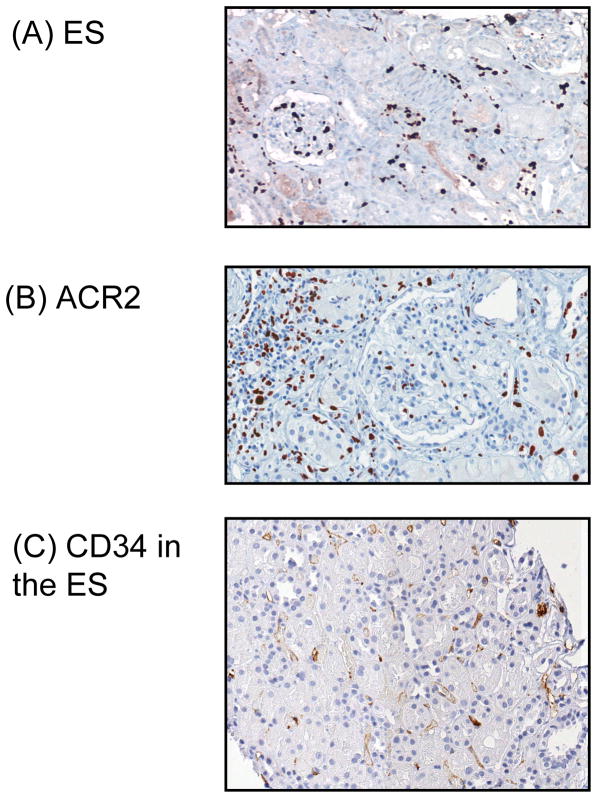
Comparison of Ki67 staining in (A) the engraftment syndrome (ES) with (B) acute cellular rejection, type II (ACR2). Ki67 stains most of the nuclei of intracapillary cells (peritubular capillaries and glomeruli) in ES, but in ACR2, Ki67 is mostly present in the cells infiltrating the interstitium and tubules. (C) Loss of CD34 staining of the peritubular capillary (PTC) endothelium in the ES.
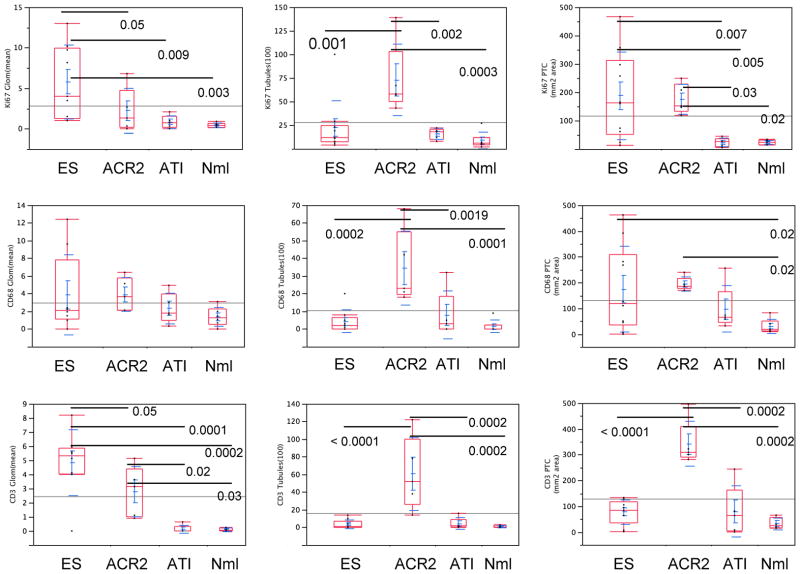
Quantitative studies were performed to compare the pathologic process during ES with acute cellular rejection and ATI (Figure 7). ES biopsies had no more CD3+ T cells in the interstitium/PTC (77.9±47.4/mm2) than ATI (79.5±98.4) or normal transplant biopsies (32.3±22.8) (all p>0.3), and significantly less than in ACR2 (341.9±87.5; p<0.0001). Counts of mononuclear cells in the interstitium included those in PTCs, since it was difficult to distinguish their location in ACR2. Similarly, intratubular CD3+ or CD68+ cells in ES did not differ from ATI or normal biopsies and were significantly less than in ACR2 (p=0.0001 and 0.0002, respectively; Figure 7). In contrast, glomerular CD3+ cells were higher in ES biopsies (4.8±2.3/glomerulus) than in ACR2 (2.8±1.8), ATI (0.1±0.3), or normal controls (0.1±0.1) (p=0.05, p<0.0001, and p<0.0001, respectively) (Figure 7). The density of CD68+ in the interstitium/PTC in ES was highly variable (174.3±166.0/mm2), but overall was greater than in ATI (97.1±90.1) and normal biopsies (30.4±27.7), a difference that was significant compared to normal biopsies (p=0.02) but not ATI (p=0.36). ACR2 had a tighter distribution of CD68+ cell density (145.8±20.1), but overall was not statistically different from ES.
Figure 7.
Cell quantitation statistics for the engraftment syndrome (ES) and the following controls: acute cellular rejection, type II (ACR2), acute tubular injury (ATI), and normal (Nml), with respect to the number of cells positive for Ki67, CD68, and CD3 in the glomeruli, tubules, and interstitium, respectively. Lines and numbers denote statistically significant relationships and the corresponding p value. ES is distinguished from ACR2 by increased CD3+, Ki67+ and CD68+ cells in glomeruli and decreased CD3+, Ki67+ and CD68+ cells in tubules, as well as decreased numbers of CD3 cells in PTC/interstitium. ES is distinguished from ATI by increased CD3+, Ki67+ cells in glomeruli, and from normal kidneys by the same as well as CD68+ cells in the PTC/interstitium.
One of the most striking differences between the ES and the control biopsies was revealed by Ki67 (Figures 6,7). The ES group showed the highest average number of Ki67+ cells in glomeruli (5.8±4.5 cells/glomerulus), which was significantly greater than all other compared groups (ACR2, ATI or normal, p=0.05,0.0088,0.0034, respectively). ES biopsies also had a greater interstitium/PTC Ki67+ cell density (387.6±151.9/mm2) than either ATI or normal groups (23.5±15.6/mm2 and 23.8±8.2/mm2, p=0.0025 and 0.0017, respectively). This finding did not reach statistical significance compared with ACR2 (174.9+52.7/mm2). In contrast, ES had lower numbers of Ki67+ cells in tubules than the ACR2 group (22.4±28.4 vs. 72.8±38.0 cells/100 tubules, p=0.0012). Tubular Ki67 levels in ES were not significantly different from ATI (15.8 nuclei±5.7/100 tubules) or normal (8.8±9.1). The two C4d+ cases had primarily Ki67+ cells in PTCs, similar to the other cases.
Attempts were made to identify the proliferating intravascular cells. CD31 and Ki67 double staining showed that only occasional glomerular and PTC endothelial cells were CD31+Ki67+. However, CD3/Ki67 double staining showed that most of the Ki67+ cells in the interstitium/PTC in either ES or ACR2 were CD3+ (71±18% and 82±10%, respectively) as well as in glomeruli (82±14% and 76±9%, respectively). A higher percentage of CD3+ cells in ES expressed Ki67 (78±13%), compared with ACR2 (43±16%, p < 0.01) (Figure 8). ES CD3+ glomerular cells also had a high level of Ki67 expression (70±14%), although this did not reach statistical significance compared with ACR2 (53±16%). In ATI and normal biopsies, Ki67+ cells expressing either CD3 or CD31 were rare.
Antibodies to antigens expressed by endothelial cells (CD34 and CD31) revealed focal PTC and glomerular endothelial cell loss in both ES and in ACR2 cases, sometimes to a striking degree (Figure 6,8), confirming the EM studies. PTCs in normal biopsies showed a dense ring of staining for CD34 or CD31. In the ES cases, several abnormal patterns were found, ranging from segmental to total loss of CD34, and staining of clumped luminal material believed to be sloughed endothelial cells. CD31 also stained granular aggregates, interpreted as platelets.
Fluorescence In Situ Hybridization (FISH) (Table 2, Figure 9)
Table 2.
Fluorescence In Situ Hybridization (FISH)
| Patient # | Days Post-transplant | Donor/Rec gender | Renal Tubular cells genotype | Endothelial cells (CD34+) genotype | CD45+ cells genotype (Extravesc.) | CD45+ cells Genotype (Intravasc.) |
|---|---|---|---|---|---|---|
| 4 | 731 | Female/Male | XX | XX (>100) | XY (>100) | XY (>100) |
| 4 | 543 | Female/Male | XX | XX (48) | XY (>100) | XY (16) |
| 4 | 1087 | Female/Male | XX | XX (67) | XY (34) | XY (27) |
| 5 | 991 | Female/Male | XX | XX (>100) | XY (>100) | XY (>100) |
| 5 | 667 | Female/Male | XX | XX (58) | XY (>100) | XY (>100) |
| 6 | 12 | Female/Male | XX | XY | ||
| 6 | 183 | Female/Male | XX | XX (76) | XY (23) | XY (few) |
| 7 | 13 | Male/Female | XY | XX | ||
| 7 | 59 | Male/Female | XY | XY (68) | XX (>100) | XX (32) |
Figure 9.
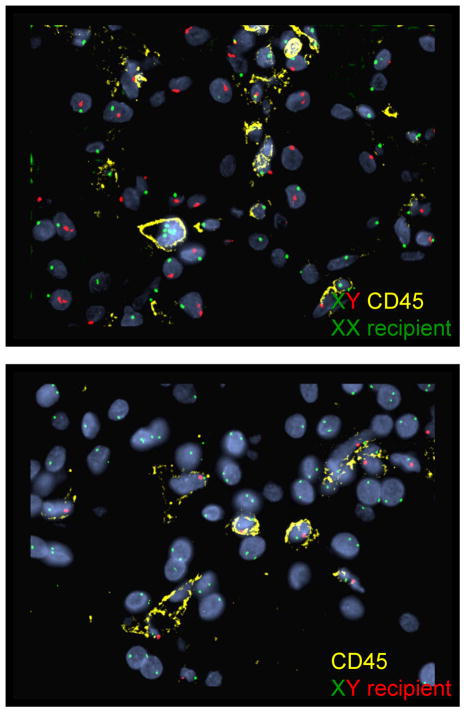
Fluorescence in situ hybridization (FISH) of ES biopsies to determine the origin of the leukocytes in the capillaries. All cells stain for recipient type DNA. In the upper panel the donor was male and recipient female. All of the CD45+ cells stain for XX and none for XY. CD45− cells are largely donor type (tubules, endothelium). In the lower panel, the donor was female and recipient male, and all the CD45+ cells are positive for XY, and none for XX. CD45 (yellow), X chromosome (green), Y chromosome (red) and DAPI nuclear stain (blue).
Two ES samples were studied by XY FISH combined with CD45. The CD45+ cells were all of recipient genotype. In the 7 cases studied in later biopsies, all capillary CD34+ cells were of donor genotype.
Treatment and Outcome
Based on initial biopsy interpretation and severity of the clinical symptoms and signs, during the period of neutrophil recovery, the nine patients with early graft dysfunction were given either no additional treatment (3), steroid pulses (2), or thymoglobulin ± plasma exchange (4) (Table 1). The Cr peaked at 1.4–15.4 mg/dl (mean 7.6±4.4 mg/dl) on the 13th–21st day. As reported previously, one case of C4d+ AHR (#3) was irreversible and the patient underwent successful retransplantion with standard immunosuppression (1). Recipient #8 initially improved over 2 weeks, but then developed severe arterial injury and inflammation, associated with elevated tacrolimus levels which peaked at 21.1 ng/ml 17 days post transplant, which was interpreted at thrombotic microangiopathy (TMA) and/or ACR2. Despite ATG and discontinuance of CNI, allograft failure developed over 6 months requiring reinstitution of dialysis. Recovery was complete in the remaining patients, who currently have a mean Cr of 1.5±0.3 mg/dl at 9 months to 7 years follow-up. Planned withdrawal of maintenance immunosuppressive therapy was completed in all of these 8 patients. To date, only one of these 8 patients (#10) has developed an episode of cellular rejection requiring reinstitution of immunosuppression.
Follow-up Biopsies
Protocol biopsies taken at 2 months-7 years post-transplant showed no residual of the previous ES findings (Table 3). There was no infiltrate, hemorrhage, or tubular injury by LM and no acute endothelial injury by EM. C4d was negative in all follow-up biopsies, except for recipient #4 with late onset Class II donor specific antibodies and transplant glomerulopathy(1). One patient (#10) developed an acute cellular rejection at 10 months post-transplant after an episode of pyelonephritis and is currently on anti-rejection therapy. Two patients had protocol biopsies at 7 years: recipient #2 had subclinical recurrent MPGN, type I, and recipient #1 had a normal biopsy, aside from donor-derived mild arteriosclerosis, which had not progressed.
Discussion
This study describes for the first time the renal pathology associated with post-BMT AKI, which develops as part of what has been termed the “engraftment syndrome.” The most consistent pathological feature was acute injury of glomerular and PTC endothelium. This occurred in the presence of an accumulation of dividing cells in glomerular and PTCs predominately T cells (and exclusively CD8+), accompanied by macrophages and neutrophils. Other changes, considered secondary were ATI and interstitial hemorrhage.
Reversible AKI (doubling of serum Cr) has been reported as a feature of the ES in about 20% of recipients of either autologous stem cells or allogeneic marrow (22–24). To our knowledge no renal biopsies have been reported on these patients. Although the exact pathophysiology of this syndrome remains to be defined, some patients have been reported to exhibit signs and symptoms consistent with CLS, hypothesized to be mediated by various cytokines (10, 25, 26), including TNFα (27, 28), IL-1, IL-2 (25), IL-8 (29), and interferonγ (30) and associated with elevated blood levels of soluble vascular cell adhesion molecule-1 (31). This condition has been termed “engraftment syndrome”, since it occurs around the nadir of the leukocyte count, when the donor marrow is beginning to recover function. However, in the present situation, in which an allogeneic stem cell transplant has been conducted, the marrow that is beginning to function includes the recipient marrow, so that a more general term might be “marrow recovery syndrome (MRS).”
Our patients differ in from the published ES series, in that they also received an allograft kidney and nephrotoxic drugs. The allograft kidney necessarily suffers some degree of ischemic injury during transplantation, and alloantigens are presented by the kidney. Any of these factors may contribute to the more severe AKI observed in the present series. These considerations make it difficult to extrapolate to the pathology in autologous kidneys in isolated hematopoietic transplantation, but it is likely that fundamental similarities exist, which are augmented in our patients.
Drugs with potential nephrotoxicity were given to these patients before (cyclophosphamide) (32, 33) or after transplantation (cyclosporine or tacrolimus). CNIs cause renal failure by vascular toxicity, leading to vasoconstriction and tubular injury. We did not see isometric vacuolization, a characteristic feature of acute CNI tubular toxicity, in these patients. TMA is a rare but well documented complication of CNIs, as well as chemotherapeutic or immunosuppressive regimens that include cyclophosphamide (34–36). One patient (#8) developed severe TMA after the ES episode, attributed to increased tacrolimus dosages. However, the biopsies in other patients lacked diagnostic features of TMA, such as arterial or glomerular thrombi. Furthermore, features were present which are not typical of TMA, such as interstitial hemorrhage and PTC endothelial injury. Thus, while an element of drug-related endothelial toxicity may contribute to ES, that cannot explain all the features observed.
In autologous stem cell recipients, immune dysregulation and “autoaggression” have been suggested as the basis for the ES.(37, 38) Autoimmunity has been documented as a late complication of autologous stem cell transplantation (39, 40). Usually the autoimmunity is manifested only by detection of autoantibodies, but occasionally autoimmune disease has been reported, including thyroiditis, autoimmune thrombocytopenia and myasthenia gravis (41). The mechanism is not clear, but protracted deficiency of putative Treg cells (CD4+CD25+) cells has been described(42). In the present series, a striking paucity of CD4 cells (which would include CD4+Foxp3+ Treg) was evident in the tissue. Few CD4 cells were present in the peripheral blood at this time (<50/mm2)(24), although the Treg% was enriched (T. Morokata, B. Sprangers and M. Sykes, manuscript in preparation). Similar transient acute graft dysfunction with a hypocellular infiltrate were observed 2 weeks after autologous bone marrow-derived mesenchymal stem cells transplants in recipient of allogeneic kidneys (G. Remuzzi, personal communication, 2010).
We demonstrated that proliferating T cells in the graft are a prominent feature of ES. Most Ki67+ cells in the glomeruli and PTCs/interstitium are CD3+ cells, and the Ki67+ T cell concentration is higher than that detected in ACR. This is probably a reflection of the intense expansion occurring as the lymphocytes recover. CD8 cells recover more quickly after lymphoid depletion by radiation or anti-T cell antibody,(43) and in these patients (1). The disparate recovery can be appreciated in the renal biopsies, in that no CD4+ cells were present in the day 10–16 samples. It is of interest that peripheral blood CD8+ cells showed an activated (CD25+) phenotype on Day 0, suggesting that the conditioning regimen may promote CD8+ cell activation and contribute to the observed pathology. Lymphoid proliferation occurs in immunodeficient animals given mature T cells, a process termed homeostatic proliferation, a normal physiological process triggered by lymphopenia.(44) Adoptively transferred T cells that undergo homeostatic proliferation in syngeneic immunodeficient recipients trigger acute graft injury of MHC class II or class I mismatched kidney allografts in 9–11 days, which survive for 7–10 weeks in wild type recipients (44). The graft pathology after transfer of homeostatically expanded T cells resembled ACR with CD4+ cells in the infiltrate. While the pathology differs in the present patients, we documented a higher level of T cell proliferation and profound lymphopenia, consistent with homeostatic proliferation. We suspect that, although the mechanism remains unclear, the ability to cause transient graft injury may be an additional property of T cells that undergo homeostatic proliferation in this protocol.
Allograft rejection must be included among possible causes of renal allograft dysfunction in these patients. AKI occurs just when the donor marrow-derived elements are disappearing from the circulation and the host leukocytes are returning. Thus the host may be reacting to donor antigen shared by the marrow and kidney. In support of this possibility is the presence in 2 patients of acute arterial inflammation meeting the criteria of Banff for ACR2. However, other features of ACR were absent: little or no interstitial infiltrate or tubulitis was evident and there were no more T cells in the interstitium/PTC and tubules than in ATI or normal transplant biopsies. Since both of these patients were treated with ATG (and recovered), we are unable to determine whether this was indeed a manifestation of ACR or an extension of the ES endothelial injury to arteries. The strongest argument against ACR is the spontaneous recovery without additional immunosuppressive therapy in 2 of the patients diagnosed with ES, which would be exceedingly rare in ACR and more compatible with a resolution of a transient state of hyperreactivity.
Monocytes/macrophages were present in the interstitium/PTC at levels similar to that in patients on conventional therapy with ACR2, and may be part of the mechanism of injury. Macrophages release a variety of cytotoxic molecules in the course of activation, such as TNFα and reactive oxygen species, which could contribute to endothelial injury. In immunodeficient mice, monocytes can recognize non-MHC antigens and promote an inflammatory response (45). Transient increased cytotoxicity of monocyte/macrophages after cyclophosphamide treatment has also been documented in experimental animals and patients. For example, an increased level of cytotoxic activity (presumably TNFα) by blood monocytes has been described in patients treated with cyclophosphamide (46). In mice, cyclophosphamide increases macrophage production of IL-6 and reactive oxygen intermediates and decreases TGFβ production (47). Increased numbers of cytocidal immature macrophages accumulate in the spleen after cyclophosphamide treatment (48) and increased MPO positive immature macrophages with enhanced oxidant generative capacity can be detected by alveolar lavage(49).
Two cases had strongly positive and widespread C4d in the PTC but were otherwise similar to the C4d− cases, so are probably part of the spectrum of the marrow ES. However, both patients had the new appearance of anti-donor HLA antibodies, which were not detected in the other cases, and we have to conclude that these have AHR (with or without an ES component). The regimen in the last five patients includes rituximab and tacrolimus and no AHR or C4d+ biopsies have occurred.
Because of the severe endothelial injury, we postulated that recipient replacement of graft endothelium might occur, as documented in renal allografts after ACR2 (50). However, FISH studies argue that no donor replacement occurs, or is so minimal as to be undetectable. Thus the long-term graft survival off all immunosuppression cannot be attributed to endothelial repopulation.
The optimal therapeutic strategy for ES remains to be defined. Five patients lacked features suggestive of acute rejection (no interstitial infiltrate, endarteritis or C4d). Two recovered without any change in therapy (#6,9) and two recovered after pulse steroids (#2,4). The fifth (#8) started to improve but then developed vascular lesions variously interpreted as ACR or TMA due to high levels of tacrolimus; despite later ATG therapy, the graft was lost. Two in the first cohort had positive C4d (#3,5) and were treated for AHR, with plasmapheresis, rituxan, and ATG); one recovered and one did not. Two had endarteritis at the time of presentation with ES with negative C4d (#7,10), both were treated for ACR with ATG, one with plasmapheresis (#7) and one without (#10), the latter had the tacrolimus discontinued; both recovered fully. Based on this limited experience, we believe that pulse steroids or no therapy is usually sufficient for those without endarteritis or C4d+. Those with endarteritis may benefit from ATG and minimization or discontinuance of CNI, to avoid compounding the vascular injury.
We conclude that a clinical “engraftment syndrome” occurs commonly in the combined bone marrow/kidney transplant protocol, manifested by acute renal dysfunction and sometimes fever and fluid retention that developed characteristically at the time of recipient marrow recovery. The common pathology in all of the allograft biopsies at the time of ES was acute allograft endothelial injury with little or no cellular infiltrate. Some patients had additional manifestations that suggested acute cellular (endarteritis) or humoral (C4d+) rejection. We believe that ES is caused by an attack on the graft endothelium related to immune dysregulation or the innate immune system during recovery from the profound leukocyte depletion, which leads to transient auto- or allo-reactivity by CD8+ T cells (and in some cases B cells) and accumulation of macrophages. CNI toxicity may also contribute to the endothelial injury. While the molecular mediators and optimal therapy are not established, 80% of patients have had a full recovery from the acute vascular injury as judged by renal function and protocol biopsies at 6–12 months.
Supplementary Material
Acknowledgments
The authors are grateful for the excellent technical assistance of A Ackerman, C Adams, N Brousaides, AB Collins, P Della Pelle, D Sebastian, and M Selig.
The authors are grateful for the review of the pathology of this study and manuscript by Charles E. Alpers, MD, Anthony J. Demetris, MD, and Lorraine C. Racusen, MD, done as a part of the NIH/ITN sponsored clinical trial.
Funding Sources:
Supported by a grants from the Immune Tolerance Network (N01-AI-15416), a collaborative clinical research project supported by the National Institute of Allergy and Infectious Diseases, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Juvenile Diabetes Research Foundation, and the National Institutes of Health (RO1-AI-084074)
Abbreviations
- ACR2
Acute cellular rejection, type 2
- AHR
Acute humoral rejection
- AKI
Acute kidney injury
- ATI
Acute tubular injury
- BMT
Bone marrow transplant
- CLS
Capillary leak syndrome
- CNI
Calcineurin inhibitor
- Cr
Creatinine
- ES
Engraftment syndrome
- FISH
Fluorescence in situ hybridization
- GBM
Glomerular basement membrane
- GVHD
Graft-versus-host-disease
- H&E
Hematoxylin and eosin
- HLA
Human leukocyte antigen
- IHC
Immunohistochemistry
- LM
Light microscopy
- ITN
Immune Tolerance Network
- MPO
Myeloperoxidase
- MRS
Marrow recovery syndrome
- PAS
Periodic acid-Schiff
- PTC
Peritubular capillary
- TGF
Transforming growth factor
- TMA
Thrombotic microangiopathy
- TNF
Tumor necrosis factor
Footnotes
ABF and DT contributed equally as first authors of this paper during both data collection, analysis and manuscript preparation. TK, WW, N T-R, TRS, ABC, MS, DHS devised and supervised the clinical protocols and care of the patients in this study. LF and AJI performed the fluorescence in situ hybridization (FISH) studies. FIP, SLC, BS, SS, and RNS provided laboratory services to the patients in this study and made invaluable contributions to the assays performed. RBC supervised data collection, pathology interpretation and extensively edited the manuscript.
Disclosures: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchimoto Y, Huang CA, Yamada K, Shimizu A, Kitamura H, Colvin RB, et al. Mixed chimerism and tolerance without whole body irradiation in a large animal model. J Clin Invest. 2000;105(12):1779–1789. doi: 10.1172/JCI8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169(2):493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59(2):256–262. [PubMed] [Google Scholar]
- 5.Kimikawa M, Sachs DH, Colvin RB, Bartholomew A, Kawai T, Cosimi AB. Modifications of the conditioning regimen for achieving mixed chimerism and donor-specific tolerance in cynomolgus monkeys. Transplantation. 1997;64(5):709–716. doi: 10.1097/00007890-199709150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Kimikawa M, Kawai T, Sachs DH, Colvin RB, Bartholomew A, Cosimi AB. Mixed chimerism and transplantation tolerance induced by a nonlethal preparative regimen in cynomolgus monkeys. Transplant Proc. 1997;29(1–2):1218. doi: 10.1016/s0041-1345(96)00642-2. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Poncelet A, Sachs DH, Mauiyyedi S, Boskovic S, Wee SL, et al. Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation. 1999;68(11):1767–1775. doi: 10.1097/00007890-199912150-00022. [DOI] [PubMed] [Google Scholar]
- 8.Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4(9):1391–1398. doi: 10.1111/j.1600-6143.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- 9.Pilat N, Wekerle T. Transplantation tolerance through mixed chimerism. Nat Rev Nephrol. 2010;6(10):594–605. doi: 10.1038/nrneph.2010.110. [DOI] [PubMed] [Google Scholar]
- 10.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone marrow transplantation. 2001;27(9):893–898. doi: 10.1038/sj.bmt.1703015. [DOI] [PubMed] [Google Scholar]
- 11.Cahill RA, Spitzer TR, Mazumder A. Marrow engraftment and clinical manifestations of capillary leak syndrome. Bone Marrow Transplant. 1996;18(1):177–184. [PubMed] [Google Scholar]
- 12.Sreedharan A, Bowyer S, Wallace CA, Robertson MJ, Schmidt K, Woolfrey AE, et al. Macrophage activation syndrome and other systemic inflammatory conditions after BMT. Bone Marrow Transplant. 2006;37(7):629–634. doi: 10.1038/sj.bmt.1705305. [DOI] [PubMed] [Google Scholar]
- 13.Marin D, Berrade J, Ferra C, Mateu A, Berlanga J, Salar A, et al. Engraftment syndrome and survival after respiratory failure post-bone marrow transplantation. Intensive Care Med. 1998;24(7):732–735. doi: 10.1007/s001340050653. [DOI] [PubMed] [Google Scholar]
- 14.Marin D, Gonzalez-Barca E, Domingo E, Berlanga J, Granena A. Noninvasive mechanical ventilation in a patient with respiratory failure after hematopoietic progenitor transplantation. Bone Marrow Transplant. 1998;22(11):1123–1124. doi: 10.1038/sj.bmt.1701487. [DOI] [PubMed] [Google Scholar]
- 15.Nellen RG, van Marion AM, Frank J, Poblete-Gutierrez P, Steijlen PM. Eruption of lymphocyte recovery or autologous graft-versus-host disease? Int J Dermatol. 2008;47 (Suppl 1):32–34. doi: 10.1111/j.1365-4632.2008.03956.x. [DOI] [PubMed] [Google Scholar]
- 16.Inaba H, Hale G, Leung W, Woodard P, Burnette K, Handgretinger R, et al. Diagnostic challenge in recurrent skin rash after autologous bone marrow transplantation. J Pediatr Hematol Oncol. 2006;28(8):525–528. doi: 10.1097/01.mph.0000212966.60383.74. [DOI] [PubMed] [Google Scholar]
- 17.Tichelli A, Gratwohl A. Vascular endothelium as ‘novel’ target of graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21(2):139–148. doi: 10.1016/j.beha.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Miano M, Faraci M, Dini G, Bordigoni P. Early complications following haematopoietic SCT in children. Bone Marrow Transplant. 2008;41 (Suppl 2):S39–42. doi: 10.1038/bmt.2008.53. [DOI] [PubMed] [Google Scholar]
- 19.Troxell ML, Pilapil M, Miklos DB, Higgins JP, Kambham N. Renal pathology in hematopoietic cell transplantation recipients. Mod Pathol. 2008;21(4):396–406. doi: 10.1038/modpathol.3801011. [DOI] [PubMed] [Google Scholar]
- 20.Chang A, Hingorani S, Kowalewska J, Flowers ME, Aneja T, Smith KD, et al. Spectrum of renal pathology in hematopoietic cell transplantation: a series of 20 patients and review of the literature. Clin J Am Soc Nephrol. 2007;2(5):1014–1023. doi: 10.2215/CJN.01700407. [DOI] [PubMed] [Google Scholar]
- 21.Preffer F, Dombkowski D. Advances in complex multiparameter flow cytometry technology: Applications in stem cell research. Cytometry B Clin Cytom. 2009;76(5):295–314. doi: 10.1002/cyto.b.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, et al. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10(10):2208–2214. doi: 10.1681/ASN.V10102208. [DOI] [PubMed] [Google Scholar]
- 23.Nadasdy GM, Bott C, Cowden D, Pelletier R, Ferguson R, Nadasdy T. Comparative study for the detection of peritubular capillary C4d deposition in human renal allografts using different methodologies. Hum Pathol. 2005;36(11):1178–1185. doi: 10.1016/j.humpath.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 24.LoCascio SA, Morokata T, Chittenden M, Preffer F, Dombkowski D, Adnreola G, et al. Mixed chimerism, lymphocyte recovery, and evidence for early donor-specific unresponsiveness in patients receiving combined kidney and bone marrow transplantation to induce tolerance. Transplantation. 2010;90(12):1607–15. doi: 10.1097/TP.0b013e3181ffbaff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jadus MR, Wepsic HT. The role of cytokines in graft-versus-host reactions and disease. Bone Marrow Transplant. 1992;10(1):1–14. [PubMed] [Google Scholar]
- 26.Rabinowitz J, Petros WP, Stuart AR, Peters WP. Characterization of endogenous cytokine concentrations after high-dose chemotherapy with autologous bone marrow support. Blood. 1993;81(9):2452–2459. [PubMed] [Google Scholar]
- 27.Holler E, Kolb HJ, Moller A, Kempeni J, Liesenfeld S, Pechumer H, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75(4):1011–1016. [PubMed] [Google Scholar]
- 28.Kitko CL, Paczesny S, Yanik G, Braun T, Jones D, Whitfield J, et al. Plasma elevations of tumor necrosis factor-receptor-1 at day 7 postallogeneic transplant correlate with graft-versus-host disease severity and overall survival in pediatric patients. Biol Blood Marrow Transplant. 2008;14(7):759–765. doi: 10.1016/j.bbmt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber AR, Kunkel SL, Todd RF, 3rd, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254(5028):99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa J, Maeda T, Miyazaki T, Manabe N, Honda S, Nishiura T, et al. Early onset of hemophagocytic syndrome following allogeneic bone marrow transplantation. Int J Hematol. 2000;72(2):243–246. [PubMed] [Google Scholar]
- 31.Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Martinez C, et al. Endothelial Dysfunction After Hematopoietic Stem Cell Transplantation: Role Of The Conditioning Regimen And The Type Of Transplantation. Biol Blood Marrow Transplant. 2010;16(7):985–93. doi: 10.1016/j.bbmt.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Ayhanci A, Gunes S, Sahinturk V, Appak S, Uyar R, Cengiz M, et al. Seleno L-methionine acts on cyclophosphamide-induced kidney toxicity. Biol Trace Elem Res. 2010;136(2):171–179. doi: 10.1007/s12011-009-8535-2. [DOI] [PubMed] [Google Scholar]
- 33.Sayed-Ahmed MM. Progression of cyclophosphamide-induced acute renal metabolic damage in carnitine-depleted rat model. Clinical and experimental nephrology. 2010;14(5):418–26. doi: 10.1007/s10157-010-0321-0. [DOI] [PubMed] [Google Scholar]
- 34.Fisher DC, Sherrill GB, Hussein A, Rubin P, Vredenburgh JJ, Elkordy M, et al. Thrombotic microangiopathy as a complication of high-dose chemotherapy for breast cancer. Bone marrow transplantation. 1996;18(1):193–198. [PubMed] [Google Scholar]
- 35.Vantelon JM, Munck JN, Bourhis JH, Pico JL, Fadel C, Ulusakarya A, et al. Thrombotic microangiopathy: a new dose-limiting toxicity of high-dose sequential chemotherapy. Bone marrow transplantation. 2001;27(5):531–536. doi: 10.1038/sj.bmt.1702812. [DOI] [PubMed] [Google Scholar]
- 36.Miyata R, Shimazu M, Tanabe M, Kawachi S, Hoshino K, Wakabayashi G, et al. Clinical characteristics of thrombotic microangiopathy following ABO incompatible living donor liver transplantation. Liver Transpl. 2007;13(10):1455–1462. doi: 10.1002/lt.21253. [DOI] [PubMed] [Google Scholar]
- 37.Moreb JS, Kubilis PS, Mullins DL, Myers L, Youngblood M, Hutcheson C. Increased frequency of autoaggression syndrome associated with autologous stem cell transplantation in breast cancer patients. Bone marrow transplantation. 1997;19(2):101–106. doi: 10.1038/sj.bmt.1700615. [DOI] [PubMed] [Google Scholar]
- 38.Carreras E, Fernandez-Aviles F, Silva L, Guerrero M, de Larrea CF, Martinez C, et al. Engraftment syndrome after auto-SCT: analysis of diagnostic criteria and risk factors in a large series from a single center. Bone marrow transplantation. 2010;45(9):1417–22. doi: 10.1038/bmt.2009.363. [DOI] [PubMed] [Google Scholar]
- 39.Daikeler T, Tyndall A. Autoimmunity following haematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2007;20(2):349–360. doi: 10.1016/j.beha.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Bohgaki T, Atsumi T, Koike T. Autoimmune disease after autologous hematopoietic stem cell transplantation. Autoimmunity reviews. 2008;7(3):198–203. doi: 10.1016/j.autrev.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Deligny C, Clave E, Sibon D, Daikeler T, Keshmandt H, Carmagnat M, et al. New onset of myasthenia gravis after treatment of systemic sclerosis by autologous hematopoietic stem cell transplantation: sustained autoimmunity or inadequate reset of tolerance? Human immunology. 2010;71(4):363–365. doi: 10.1016/j.humimm.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Bohgaki T, Atsumi T, Koike T. Multiple autoimmune diseases after autologous stem-cell transplantation. The New England journal of medicine. 2007;357(26):2734–2736. doi: 10.1056/NEJMc076383. [DOI] [PubMed] [Google Scholar]
- 43.Haas G, Halperin E, Doseretz D, Linggood R, Russell P, Colvin R, et al. Differential recovery of circulating T-cell subsets after nodal irradiation for Hodgkin’s disease. J Immunol. 1984;132:1026–1030. [PubMed] [Google Scholar]
- 44.Moxham VF, Karegli J, Phillips RE, Brown KL, Tapmeier TT, Hangartner R, et al. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. J Immunol. 2008;180(6):3910–3918. doi: 10.4049/jimmunol.180.6.3910. [DOI] [PubMed] [Google Scholar]
- 45.Zecher D, van Rooijen N, Rothstein DM, Shlomchik WD, Lakkis FG. An innate response to allogeneic nonself mediated by monocytes. J Immunol. 2009;183(12):7810–7816. doi: 10.4049/jimmunol.0902194. [DOI] [PubMed] [Google Scholar]
- 46.McBride WH, Hoon DB, Jung T, Naungayan J, Nizze A, Morton DL. Cyclophosphamide-induced alterations in human monocyte functions. J Leukoc Biol. 1987;42(6):659–666. doi: 10.1002/jlb.42.6.659. [DOI] [PubMed] [Google Scholar]
- 47.Bryniarski K, Szczepanik M, Ptak M, Zemelka M, Ptak W. Influence of cyclophosphamide and its metabolic products on the activity of peritoneal macrophages in mice. Pharmacol Rep. 2009;61(3):550–557. doi: 10.1016/s1734-1140(09)70098-2. [DOI] [PubMed] [Google Scholar]
- 48.Baccarini M, Bistoni F, Lohmann-Matthes ML. Organ-associated macrophage precursor activity: isolation of candidacidal and tumoricidal effectors from the spleens of cyclophosphamide-treated mice. J Immunol. 1986;136(3):837–843. [PubMed] [Google Scholar]
- 49.Cooper JA, Jr, Merrill WW, Reynolds HY. Cyclophosphamide modulation of bronchoalveolar cellular populations and macrophage oxidative metabolism. Possible mechanisms of pulmonary pharmacotoxicity. Am Rev Respir Dis. 1986;134(1):108–114. doi: 10.1164/arrd.1986.134.1.108. [DOI] [PubMed] [Google Scholar]
- 50.Lagaaij EL, Cramer-Knijnenburg GF, van Kemenade FJ, van Es LA, Bruijn JA, van Krieken JH. Endothelial cell chimerism after renal transplantation and vascular rejection. Lancet. 2001;357(9249):33–37. doi: 10.1016/S0140-6736(00)03569-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.