Abstract
Background
Cigarette smoking is common in adolescents with attention-deficit/hyperactivity disorder (ADHD) and substance use disorders (SUD). However, little is known about the relationship between cigarette and cannabis use trajectories in the context of treatment for both ADHD and SUD. To address this research gap, we report collateral analyses from a 16-week randomized, controlled trial (n=303) of osmotic-release methylphenidate (OROS-MPH) in adolescents with ADHD concurrently receiving cognitive behavioral therapy (CBT) targeting non-nicotine SUD.
Methods
Participants completed cigarette and cannabis use self-report at baseline and throughout treatment. Analyses were performed to explore the relationships between cigarette smoking, cannabis use, and other factors, such as medication treatment assignment (OROS-MPH versus placebo).
Results
Baseline (pre-treatment) cigarette smoking was positively correlated with cannabis use. Negligible decline in cigarette smoking during treatment for non-nicotine SUD was observed in both medication groups. Regular cigarette and cannabis users at baseline who reduced their cannabis use by >50% also reduced cigarette smoking (from 10.8±1.1 to 6.2±1.1 cigarettes per day).
Conclusions
Findings highlight the challenging nature of concurrent cannabis and cigarette use in adolescents with ADHD, but demonstrate that changes in use of these substances during treatment may occur in parallel.
Keywords: Smoking, Tobacco, Nicotine, Cigarette, Cannabis, Marijuana, Treatment, Adolescent, ADHD, Methylphenidate
1. Introduction
Cigarette smoking typically begins during adolescence, and is particularly common among adolescents with substance use disorders (SUD) (Backinger et al., 2003; Eckhardt et al., 1994; Myers and Brown, 1994; Vega and Gil, 2005). Youth enrolled in treatment for SUD tend to smoke more heavily and have more negative health consequences, compared to adolescent smokers without other SUD (Arria et al., 1995; McDonald et al., 2000; Myers et al., 1994; Myers and MacPherson, 2004). Most smoke daily, and many become highly dependent, long-term smokers (Chassin et al., 1996; Lindsay and Rainey, 1997).
Despite the common co-occurrence of cigarette smoking in adolescents with SUD, and expert clinical guidelines calling for integrated treatment (Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff, 2008), most adolescent SUD treatment programs do not specifically incorporate interventions to target smoking (Myers and Brown, 2005). In the absence of such interventions, it is not clear what happens to cigarette smoking during treatment, as few prospective studies have examined this issue. Post-treatment smoking outcomes have been discouraging. Adolescents enrolled in non-nicotine SUD treatment are at greater risk for persistence of cigarette smoking and progression to more severe nicotine dependence (Myers and Brown, 1998; Myers and Prochaska, 2008). The trajectory of cigarette smoking during treatment has potentially important implications, particularly among adolescent cannabis users. Cannabis is the most commonly used illicit substance among adolescents, and is typically the primary target among those enrolled in SUD treatment (Substance Abuse and Mental Health Services Administration, 2010). Nicotine and cannabis share the same route of administration, and the use of each fosters initiation, escalation, and prolonged, problematic use of the other (Agrawal et al., 2008; Agrawal et al., 2009; Amos et al., 2004; de Dios et al., 2009; Highet, 2004; Kandel and Yamaguchi, 1993; Okoli et al., 2008; Patton et al., 2005; Swift et al., 2008; Timberlake et al., 2007). In the only large-scale study to date that has tracked cigarette smoking in adolescents enrolled in cannabis treatment, cigarette smokers decreased days of smoking, but heavy smokers only decreased their smoking minimally and temporarily (Shelef et al., 2009).
The comorbidity of ADHD adds another smoking risk factor. Individuals with ADHD are known to initiate smoking at a younger age, possess increased risk for developing nicotine dependence, and have a more difficult time quitting smoking, compared with the general population (Fuemmeler et al., 2007; Humfleet et al., 2005; Lambert and Harsough, 1998; Milberger et al., 1997; Pomerleau et al., 1995; Rodriguez et al., 2008; Tercyak et al., 2002). Additionally, there has been some evidence suggesting that stimulant medication (first-line treatment for ADHD) may acutely increase cigarette smoking (Cousins et al., 2001; Rush et al., 2005; Vansickel et al., 2007), though recent data suggest that it may not increase long-term smoking in individuals with ADHD (Huss et al., 2008; Winhusen et al., 2010). It is unclear whether stimulant treatment might impact cigarette use among adolescents with ADHD who are concurrently enrolled in SUD treatment.
A recently completed 16-week multi-site controlled trial of osmotic release methylphenidate (OROS-MPH) in adolescents with ADHD (n=303) concurrently receiving cognitive behavioral therapy (CBT) targeting non-nicotine SUD provided the opportunity to evaluate, via collateral analyses, the relationship between cigarette and cannabis use during SUD treatment.
2. Methods
2.1 Participants
Participants for the parent study were 303 adolescents (ages 13–18) meeting DSM-IV criteria for current ADHD (Orvaschel and Puig-Antich, 1987) and at least one non-nicotine SUD (Cottler et al., 1989), recruited by 11 participating substance abuse treatment programs. Exclusion criteria were current or past psychotic disorder, bipolar disorder, suicide risk, opioid dependence, methamphetamine abuse or dependence, cardiac illness or serious medical illness, pregnancy, past month use of psychotropic medications, or participation in other substance or mental health treatment. All participants (and parents/guardians for those <18) were given a thorough explanation of the study and signed an informed consent form that was approved by the Institutional Review Boards of the participating sites.
2.2 Procedures
See Riggs et al. (2009) for a full description of study procedures. Briefly, the parent study involved randomizing adolescents with ADHD and SUD to OROS-MPH or placebo. For OROS-MPH, the starting dose of 18 mg/day was escalated during the first two study weeks to a maximum of 72 mg/day or to the highest dose tolerated. The study included a 16-week active treatment phase during which participants took OROS-MPH or placebo and were scheduled for weekly one-hour individual cognitive behavioral therapy (CBT) sessions targeting SUD. For the present analyses, the investigative team formulated a list of clinically relevant questions regarding cigarette and cannabis use (see section 2.4).
2.3 Measures
Relevant to the present secondary analysis, participants completed 28-day baseline and weekly Timeline Follow-Back self-report of cigarette (smoking days, cigarettes per day) and cannabis (days using, joints per day) use (Sobell and Sobell, 1992). They additionally completed the DSM-IV ADHD Rating Scale (ADHD-RS) (DuPaul et al., 1998) at baseline and weekly throughout the 16-week trial. Clinician Global Impression of Improvements (CGI-I) in ADHD symptoms was assessed weekly by the study physician or medical clinician (Conners et al., 1985).
2.4 Data Analysis
The analytic approach was designed to address several clinically relevant questions as detailed below.
-
Are baseline cigarette and cannabis use correlated?
Spearman correlations were conducted to evaluate the association between cigarette (smoking days, cigarettes per day, smoking status [+/−]) and cannabis use (days using, joints per day) at baseline among the entire sample (n=303).
-
How does the trajectory of cannabis use compare between those who also regularly smoke cigarettes and those who do not?
Among baseline regular cannabis users (defined as >14/28 days of use), longitudinal analyses compared the trajectories of change in cannabis use (days using, joints per day) in baseline regular cigarette smokers (>14/28 days of use, n=88) and non-cigarette smokers (≤2/28 days of use, n=28). Population-average linear mixed models with AR(1) correlation structure were fitted to estimate change in use in the presence of missing data, assuming those data were missing at random (Brown and Prescott, 1999). Predictors in the models were group (cigarette smoking >14/28 versus ≤2/28 days), time (weeks since baseline), and group by time interaction. The interaction effect is the parameter of interest for differential trajectories of change.
-
Among regular cigarette and cannabis users, what is the impact of significant reduction in cannabis use on cigarette smoking?
Among baseline regular cigarette and cannabis users (>14/28 days of use for both), longitudinal analyses compared the trajectories of change in cigarette smoking (smoking days, cigarettes per day) in participants who decreased days of cannabis use by >50% (n=35) versus ≤10% (n=16) at 16 weeks. Again, population-averaged linear mixed models with AR(1) correlation structure were fitted, where group indicator was cannabis use reduction by >50% versus ≤10%.
-
Does change in ADHD symptom severity or psychostimulant treatment impact change in smoking among regular cigarette smokers?
Population-average linear mixed models with AR(1) correlation structure were used to identify the differences in the trajectories of change in smoking between groups (OROS-MPH versus placebo, using all available participants at each time point; CGI-I ≤2 versus >2, using 16-week completers) among baseline regular cigarette smokers (defined as >14/28 days of use, n=174).
3. Results
Participant demographics and baseline characteristics, as a function of analysis (see section 2.4) are presented in Table 1.
Table 1.
Demographics and key baseline characteristics (mean±SD or %), corresponding to section 2.4 analyses a., b., c., and d.
| Analysis | a. | b. | c. | d. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Total sample | Baseline regular cannabis users (>14/28 days) | Baseline regular cigarette and cannabis users (>14/28 days) | Baseline regular cigarette smokers (>14/28 days) | |||||||||||||||
| All participants (n = 303) | Completers (n = 227) |
Drop-outs (n = 76) |
p-value | All (n = 122) |
Regular baseline cigarette smokers (>14/28 days) (n = 88) |
Baseline non- cigarette smokers (≤2/28 days) (n = 28) |
p-value | All (n = 88) |
SUD treatment responders (>50% cannabis reduction) (n = 35) |
SUD treatment non-responders (≤10% cannabis reduction) (n = 16) |
p-value | All (n = 174) |
OROS-MPH (n = 87) |
Placebo (n = 87) |
p-value | CGI-I responder*** (n = 30) |
CGI-I non- responder (n = 96) |
p-value | |
| Age (years) | 16.49 ± 1.27 | 16.52 ± 1.22 | 16.40 ± 1.41 | 0.47 | 16.78 ± 1.19 | 16.91 ± 1.07 | 16.49 ± 1.36 | 0.10 | 16.91 ± 1.07 | 16.77 ± 1.23 | 16.64 ± 1.01 | 0.71 | 16.71 ± 1.21 | 16.63 ± 1.21 | 16.80 ± 1.21 | 0.35 | 16.54 ± 1.10 | 16.68 ± 1.26 | 0.59 |
| Gender (% female) | 21.1% | 22.9% | 15.8% | 0.19 | 19.7% | 22.7% | 14.3% | 0.34 | 22.7% | 25.7% | 31.3% | 0.74** | 22.4% | 20.7% | 24.1% | 0.59 | 23.3% | 22.9% | 0.96 |
| Cigarette smoking days (out of possible 28) | 16.26 ± 12.49 | 15.79 ± 12.57 | 17.66 ± 12.20 | 0.26 | 19.69 ± 11.77 | 26.63 ± 3.21 | 0.32 ± 0.61 | <.0001* | 26.63 ± 3.21 | 26.97 ± 2.73 | 26.19 ± 3.67 | 0.40 | 26.48 ± 3.21 | 26.45 ± 3.27 | 26.51 ± 3.16 | 0.91 | 27.00 ± 2.26 | 26.34 ± 3.35 | 0.22* |
| Cigarettes per day | 5.92 ± 7.61 | 5.80 ± 7.71 | 6.30 ± 7.34 | 0.62 | 7.84 ± 8.25 | 10.75 ± 7.90 | 0.03 ± 0.07 | <.0001* | 10.75 ± 7.90 | 10.70 ± 6.56 | 10.57 ± 6.76 | 0.95 | 10.08 ± 7.71 | 9.31 ± 7.33 | 10.85 ± 8.04 | 0.19 | 11.05 ± 7.17 | 9.91 ± 8.16 | 0.50 |
| Cigarette smoking status (% with any cigarette smoking) | 72.8% | 70.8% | 78.9% | 0.17 | 82.6% | 100% | 25.0% | <.0001 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |||
| Cannabis using days (out of possible 28) | 13.10 ± 10.01 | 12.58 ± 9.76 | 14.67 ± 10.65 | 0.12 | 23.93 ± 4.32 | 24.01 ± 4.19 | 23.79 ± 4.86 | 0.81 | 24.01 ± 4.19 | 23.77 ± 4.24 | 23.19 ± 4.55 | 0.66 | 14.91 ± 10.28 | 13.62 ± 10.11 | 16.18 ± 10.34 | 0.10 | 12.87 ± 9.71 | 15.22 ± 10.30 | 0.27 |
| Joints per day | 1.89 ± 2.20 | 1.77 ± 2.09 | 2.23 ± 2.48 | 0.12 | 3.56 ± 2.48 | 3.36 ± 2.14 | 4.02 ± 3.34 | 0.33* | 3.36 ± 2.14 | 3.21 ± 1.85 | 3.36 ± 2.21 | 0.81 | 2.09 ± 2.09 | 1.77 ± 1.77 | 2.40 ± 2.34 | 0.049* | 1.64 ± 1.86 | 2.05 ± 1.89 | 0.30 |
| ADHD-RS score | 38.73 ± 8.88 | 38.26 ± 8.64 | 40.14 ± 9.50 | 0.11 | 38.38 ± 8.31 | 38.63 ± 8.23 | 37.21 ± 8.12 | 0.43 | 38.63 ± 8.23 | 41.11 ± 8.52 | 37.56 ± 6.85 | 0.15 | 38.79 ± 8.76 | 37.57 ± 8.92 | 40.00 ± 8.46 | 0.07 | 38.23 ± 8.53 | 38.04 ± 8.56 | 0.91 |
t-test for unequal variances
Fisher’s exact test
n=48 with no CGI-I
ADHD-RS = DSM-IV ADHD Rating Scale
CGI-I = Clinician Global Impression of Improvements
OROS-MPH = Osmotic Release Methylphenidate
SUD = Substance Use Disorder
3.1 Baseline relationship between cigarette and cannabis use (section 2.4 analysis a.)
Significant positive correlations were found between cigarette and cannabis use for all measures evaluated, including cigarette smoking days and days of cannabis use (ρ [Spearman’s rho]=0.22, p=0.0001); cigarette smoking days and joints per day (ρ=0.20, p=0.0005); cigarettes per day and days of cannabis use (ρ=0.20, p=0.0004); cigarettes per day and joints per day (ρ=0.20, p=0.0004); cigarette smoking status (+/−) and days of cannabis use (ρ=0.13, p=0.03); cigarette smoking status (+/−) and joints per day (ρ=0.14, p=0.01).
3.2 Change in cannabis use as a function of baseline cigarette smoking status (section 2.4 analysis b.)
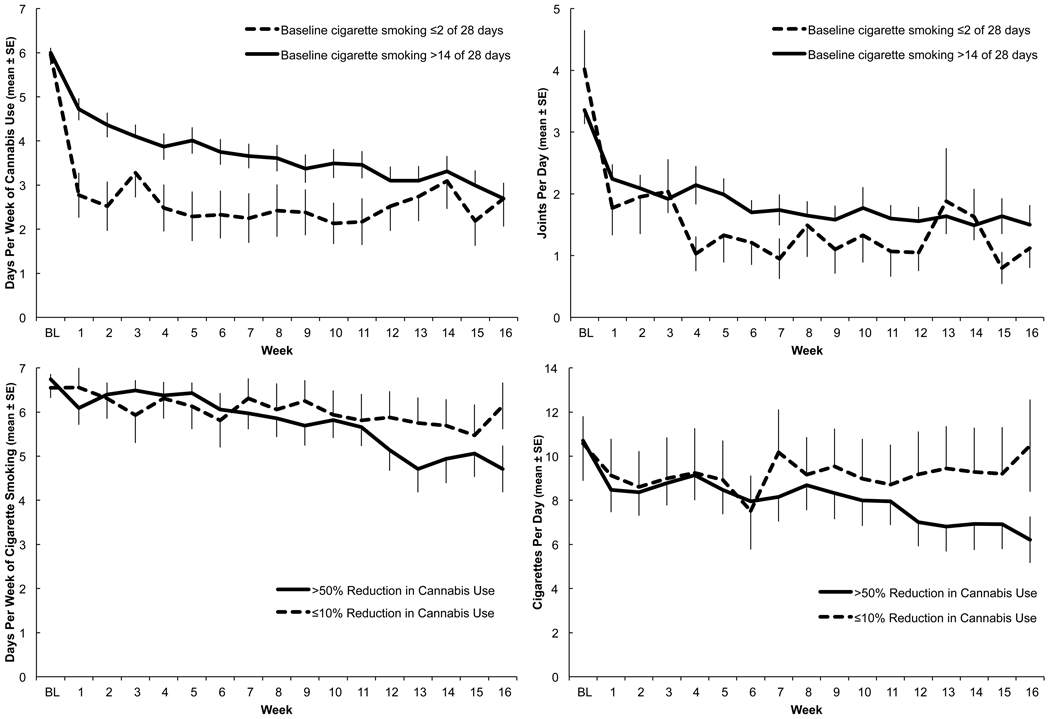
While there was not a significant difference in the linear rate of cannabis reduction, there was a significant quadratic time effect for cannabis use. Significant differences in initial decline, with non-regular cigarette smokers more rapidly reducing cannabis use than regular cigarette smokers (β [slope between baseline and Week 1] = −3.17 vs. −1.33, p<0.0001 for days of cannabis use; β=−2.34 vs. −1.15, p<0.0001 for joints per day), were estimated in the models with a spline term for time at Week 1 (Figure 1 top row).
Figure 1.
Cigarette and cannabis use trajectories from baseline through 16-week active treatment. Overlapping error bars are omitted for visual clarity.
Top Left: Days per week of cannabis use among participants who, at baseline (BL), smoked cigarettes ≤2 of 28 days versus >14 of 28 days (analysis b. from section 2.4).
Top Right: Joints per day among participants who, at baseline, smoked cigarettes ≤2 of 28 days versus >14 of 28 days (analysis b. from section 2.4).
Bottom Left: Days per week of cigarette use among baseline regular cigarette and cannabis users (>14 of 28 days using each) who achieved >50% versus ≤10% reduction in days per week of cannabis use during study participation (analysis c. from section 2.4).
Bottom Right: Cigarettes per day among baseline regular cigarette and cannabis users (>14 of 28 days using each) who achieved >50% versus ≤10% reduction in days per week of cannabis use during study participation (analysis c. from section 2.4).
3.3 Change in cigarette smoking as a function of change in cannabis use (section 2.4 analysis c.)
Significant rates of reduction in days per week of cigarette smoking (β[slope during 16 weeks]= −0.13, p=0.0002) and cigarettes per day (β=−0.27, p=0.004) were noted among those who achieved >50% reduction in cannabis use. Additional paired t-tests confirmed reduced cigarette use from baseline to Week 16 (smoking days per week p=0.0008, cigarettes per day p=0.0007).
There was not a significant difference in cigarette smoking trajectory between those who did versus those who did not significantly reduce cannabis use (50% versus ≤10% reduction). However, post hoc t-tests revealed borderline differences for changes from baseline to Week 16 cigarette smoking. Participants with >50% reduction in days per week of cannabis use went from 6.7±0.1 (mean±SE) to 4.7±0.5 days per week of smoking and from 10.7±1.1 to 6.2±1.1 cigarettes per day, while those with ≤10% reduction in days per week of cannabis use went from 6.6±0.2 to 6.1±0.5 smoking days per week and from 10.6±1.7 to 10.5±2.1 cigarettes per day. (p=0.08 for both smoking days per week and cigarettes per day) (Figure 1 bottom row).
3.4 Change in cigarette smoking as a function of change in ADHD symptom severity or psychostimulant treatment (section 2.4 analysis d)
Change in ADHD symptom level (ADHD-RS scores, CGI responders versus non-responders) and medication assignment (OROS-MPH versus placebo) did not show significant differences in the trajectories of change in smoking among regular cigarette smokers. Longitudinal plots confirmed these findings.
4. Discussion
Results indicate that baseline cigarette and cannabis use were positively correlated. Although cigarette smoking was not targeted during treatment, reduction in cannabis use was associated with modest but statistically significant reduction in cigarette smoking, consistent with previous findings (Shelef et al., 2009), suggesting that adolescents do not increase cigarette smoking to compensate for reduction in marijuana smoking (same route of administration) during outpatient substance treatment. It is also noteworthy that cigarette smoking did not increase in participants treated with OROS-MPH. This contrasts with findings from a laboratory methylphenidate administration study (Rush et al., 2005), but is consistent with the findings of a double-blind placebo-controlled trial of OROS-MPH in adult smokers with ADHD (Winhusen et al., 2010).
Although both non-cigarette smokers (smoking ≤2/28 days at baseline) and regular smokers (>14/28 days) had similar overall reduction in cannabis use, non-smokers reduced cannabis use significantly earlier (often within the first week of treatment), whereas regular cigarette smokers showed more gradual reduction. This suggests that significant reduction in cannabis use may be more difficult in adolescents that also regularly smoke cigarettes, potentially reflecting priming effects of cigarette smoking and shared route of administration (de Dios et al., 2009).
These findings should be interpreted in the context of study limitations. Results were generated by conducting secondary, post-hoc analyses of data from a subsample of participants in a larger randomized controlled trial. Additionally, cigarette and cannabis use data were obtained exclusively by adolescents’ self-reports. While several studies support the validity of youth cigarette and cannabis use self-report (e.g., Buchan et al., 2002; Dolcini et al., 2003; Post et al., 2005; Solbergsdottir et al., 2004; Williams and Nowatzki; Zaldívar Basurto et al., 2009), biological verification was not obtained. Also, groups considered for comparison were determined based on clinical reasoning, since there is no accepted standard or empirically based definition of “clinically significant” reduction in cannabis use. Lastly, in order to simplify this initial exploration of the relationship between changes in cigarette and cannabis use, some longitudinal analyses only included participants who completed 16 weeks. In these cases, comparison groups (e.g., reduction in cannabis use, change in ADHD symptom level) were defined using the completers’ sample, thereby introducing some potential for biased estimates. However, those who completed 16 weeks and those who did not were not significantly different on demographic and baseline characteristics, as shown in Table 1.
Despite these limitations, the present findings extend current research, given the lack of published studies on the relationship between change in cigarette and cannabis use in adolescent substance treatment. Compared to non-substance-involved youth, adolescents with SUD are significantly more likely to smoke cigarettes and those with co-occurring ADHD have even higher rates and earlier onset of smoking. If replicated, these preliminary findings suggest that cigarette smoking does not increase in adolescents with ADHD during treatment for cannabis and other non-nicotine SUD with or without psychostimulant medication, though further work is needed to develop evidence-based interventions targeting cigarette smoking in this especially vulnerable group. Counter to the concerns of many clinicians, adult and preliminary adolescent studies have shown that smoking cessation interventions in the context of SUD treatment not only reduce cigarette use but are also associated with improved SUD outcomes (Myers and Brown, 2005; Myers and Prochaska, 2008; Prochaska et al., 2004). Given the tremendous public health impact and high prevalence of cigarette smoking in adolescents with SUD and even higher rates in youth with co-occurring ADHD (30–50% of adolescents in treatment for SUD) (Horner and Scheibe, 1997), there is a critical need for larger scale efficacy studies of smoking cessation interventions in the context of adolescent substance treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Lynskey MT, Pergadia ML, Bucholz KK, Heath AC, Martin NG, Madden PAF. Early cannabis use and DSM-IV nicotine dependence: a twin study. Addiction. 2008;103:1896–1904. doi: 10.1111/j.1360-0443.2008.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, Madden PAF, Pergadia ML, Bucholz KK, Heath AC. Simultaneous cannabis and tobacco use and cannabis-related outcomes in young women. Drug Alcohol Depend. 2009;101:8–12. doi: 10.1016/j.drugalcdep.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos A, Wiltshire S, Bostock Y, Haw S, McNeill A. ‘You can’t go without a fag … you need it for your hash’ —a qualitative exploration of smoking, cannabis and young people. Addiction. 2004;99:77–81. doi: 10.1111/j.1360-0443.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- Arria AM, Dohey MA, Mezzich AC, Bukstein OG, Van Thiel DH. Self-reported health problems and physical symptomatology in adolescent alcohol abusers. J. Adolesc. Health. 1995;16:226–231. doi: 10.1016/1054-139X(94)00066-N. [DOI] [PubMed] [Google Scholar]
- Backinger CL, Fagan P, Matthews E, Grana R. Adolescent and young adult tobacco prevention and cessation: current status and future directions. Tob. Control. 2003;12:iv46–iv53. doi: 10.1136/tc.12.suppl_4.iv46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied Mixed Models in Medicine. New York: John Wiley and Sons; 1999. [Google Scholar]
- Buchan BJ, Dennis ML, Tims FM, Diamond GS. Cannabis use: consistency and validity of self-report, on-site urine testing and laboratory testing. Addiction. 2002;97:98–108. doi: 10.1046/j.1360-0443.97.s01.1.x. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Rose JS, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood: demographic predictors of continuity and change. Health Psychol. 1996;15:478–484. doi: 10.1037//0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am. J. Prev. Med. 2008;35:158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Russell A, Barkley RA. CGI (Clinical Global Impression Scale) NIMH. Psychopharmacology Bull. 1985;21:839–843. [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: a comprehensive substance abuse interview. Br. J. Addiction. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology (Berl.) 2001;157:243–253. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- de Dios MA, Vaughan EL, Stanton CA, Niaura R. Adolescent tobacco use and substance abuse treatment outcomes. J. Subst. Abuse Treat. 2009;37:17–24. doi: 10.1016/j.jsat.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcini MM, Adler NE, Lee P, Bauman KE. An assessment of the validity of adolescent self-reported smoking using three biological indicators. Nicotine Tob. Res. 2003;5:473–483. [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale—IV: Checklists, Norms, and Clinical Interpretation. Bethlehem, PA: Guilford Publications; 1998. [Google Scholar]
- Eckhardt L, Woodruff SI, Elder JP. A longitudinal analysis of adolescent smoking and its correlates. J. Sch. Health. 1994;64:67–72. doi: 10.1111/j.1746-1561.1994.tb06181.x. [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Kollins SH, McClernon FJ. Attention deficit hyperactivity disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood. J. Pediatr. Psychol. 2007;32:1203–1213. doi: 10.1093/jpepsy/jsm051. [DOI] [PubMed] [Google Scholar]
- Highet G. The role of cannabis in supporting young people’s cigarette smoking: a qualitative exploration. Health Educ. Res. 2004;19:635–643. doi: 10.1093/her/cyg089. [DOI] [PubMed] [Google Scholar]
- Horner BR, Scheibe KE. Prevalence and implications of attention-deficit hyperactivity among adolescents in treatment for substance abuse. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:30–36. doi: 10.1097/00004583-199701000-00014. [DOI] [PubMed] [Google Scholar]
- Humfleet GL, Prochaska JJ, Mengis M, Cullen J, Muñoz R, Hall SM. Preliminary evidence of the association between the history of childhood attention-deficit/hyperactivity disorder and smoking treatment failure. Nicotine Tob. Res. 2005;7:453–460. doi: 10.1080/14622200500125310. [DOI] [PubMed] [Google Scholar]
- Huss M, Poustka F, Lehmkuhl G, Lehmkuhl U. No increase in long-term risk for nicotine use disorders after treatment with methylphenidate in children with attention-deficit/hyperactivity disorder (ADHD): evidence from a non-randomised retrospective study. J. Neural Transm. 2008;115:335–339. doi: 10.1007/s00702-008-0872-3. [DOI] [PubMed] [Google Scholar]
- Kandel D, Yamaguchi K. From beer to crack: developmental patterns of drug involvement. Am. J. Public Health. 1993;83:851–855. doi: 10.2105/ajph.83.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J. Learn. Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Lindsay GB, Rainey J. Psychosocial and pharmacologic explanations of nicotine’s “gateway drug” function. J. Sch. Health. 1997;67:123–126. doi: 10.1111/j.1746-1561.1997.tb03430.x. [DOI] [PubMed] [Google Scholar]
- McDonald CA, Roberts S, Descheemaeker N. Intentions to quit smoking in substance-abusing teens exposed to a tobacco program. J. Subst. Abuse Treat. 2000;18:291–308. doi: 10.1016/s0740-5472(99)00067-7. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone S, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Myers MG, Brown SA. Smoking and health in substance-abusing adolescents: a two-year follow-up. Pediatrics. 1994;93:561–566. [PubMed] [Google Scholar]
- Myers MG, Brown SA. Cigarette smoking four years following treatment for adolescent substance abuse. J. Child Adolesc. Subst. Abuse. 1998;7:1–15. [Google Scholar]
- Myers MG, Brown SA. A controlled study of a cigarette smoking cessation intervention for adolescents in substance abuse treatment. Psychol. Addict. Behav. 2005;19:230–233. doi: 10.1037/0893-164X.19.2.230. [DOI] [PubMed] [Google Scholar]
- Myers MG, MacPherson L. Smoking cessation efforts among substance abusing adolescents. Drug Alcohol Depend. 2004;73:209–213. doi: 10.1016/j.drugalcdep.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Myers MG, Prochaska JJ. Does smoking intervention influence adolescent substance use disorder treatment outcomes? Subst. Abuse. 2008;29:81–88. doi: 10.1080/08897070802093361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoli CT, Richardson CG, Ratner PA, Johnson JL. Adolescents’ self-defined tobacco use status, marijuana use, and tobacco dependence. Addict Behav. 2008;33:1491–1499. doi: 10.1016/j.addbeh.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School-Age Children: Epidemiologic. 4th Version. Ft. Lauderdale, FL: Nova University, Center for Psychological Study; 1987. [Google Scholar]
- Patton CG, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction. 2005;100:1518–1525. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J. Subst. Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Post A, Gilljam H, Rosendahl I, Meurling L, Bremberg S, Galanti MR. Validity of self reports in a cohort of Swedish adolescent smokers and smokeless tobacco (snus) users. Tob. Control. 2005;14:114–117. doi: 10.1136/tc.2004.008789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J. Consult. Clin. Psychol. 2004;72:1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Riggs PD, Winhusen T, Davies R, Leimberger J, Mikulich-Gilbertson SK. Los Angeles, CA: American Academy of Addiction Psychiatry Annual Meeting; 2009. A randomized controlled trial of OROS-MPH for ADHD in adolescents with substance use disorders. [Google Scholar]
- Rodriguez D, Tercyak KP, Audrain-McGovern J. Effects of inattention and hyperactivity/impulsivity symptoms on development of nicotine dependence from mid adolescence to young adulthood. J. Pediatr. Psychol. 2008;33:563–575. doi: 10.1093/jpepsy/jsm100. [DOI] [PubMed] [Google Scholar]
- Rush CR, Higgins ST, Vansickel AR, Stoops WW, Lile JA, Glaser PE. Methylphenidate increases cigarette smoking. Psychopharmacology (Berl.) 2005;181:781–789. doi: 10.1007/s00213-005-0021-8. [DOI] [PubMed] [Google Scholar]
- Shelef K, Diamond GS, Diamond GM, Myers MG. Changes in tobacco use among adolescent smokers in substance abuse treatment. Psychol. Addict. Behav. 2009;23:355–361. doi: 10.1037/a0014517. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-Back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. Totowa, NJ: The Humana Press, Inc.; 1992. pp. 41–72. [Google Scholar]
- Solbergsdottir E, Bjornsson G, Gudmondsson LS, Tyrfingsson T, Kristinsson J. Validity of self-reports and drug use among young people seeking treatment for substance abuse or dependence. J. Addict. Dis. 2004;23:29–38. doi: 10.1300/J069v23n01_03. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. NSDUH Series H-38A, HHS Publication No. SMA 10-4586Findings. Rockville, MD: Office of Applied Studies; 2010. [Google Scholar]
- Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychol. 2006;25:549–557. doi: 10.1037/0278-6133.25.5.549. [DOI] [PubMed] [Google Scholar]
- Swift W, Coffey C, Carlin JB, Degenhardt L, Patton GC. Adolescent cannabis users at 24 years: trajectories to regular weekly use and dependence in young adulthood. Addiction. 2008;103:1361–1370. doi: 10.1111/j.1360-0443.2008.02246.x. [DOI] [PubMed] [Google Scholar]
- Tercyak KP, Lerman C, Audrain J. Association of attention-deficit/hyperactivity disorder symptoms with levels of cigarette smoking in a community sample of adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:799–805. doi: 10.1097/00004583-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Timberlake DS, Haberstick BC, Hopfer CJ, Bricker J, Sakai JT, Lessem JM, Hewitt JK. Progression from marijuana use to daily smoking and nicotine dependence in a national sample of U.S. adolescents. Drug Alcohol Depend. 2007;88:272–281. doi: 10.1016/j.drugalcdep.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Glaser PE, Rush CR. A pharmacological analysis of stimulant-induced increases in smoking. Psychopharmacology (Berl.) 2007;193:305–313. doi: 10.1007/s00213-007-0786-z. [DOI] [PubMed] [Google Scholar]
- Vega WA, Gil AG. Revisiting drug progression: long-range effects of early tobacco use. Addiction. 2005;100:1358–1369. doi: 10.1111/j.1360-0443.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Nowatzki N. Validity of adolescent self-report of substance use. Subst. Use Misuse. 2005;40:299–311. doi: 10.1081/ja-200049327. [DOI] [PubMed] [Google Scholar]
- Winhusen TM, Somoza EC, Brigham GS, Liu DS, Green CA, Covey LS, Croghan IT, Adler LA, Weiss RD, Leimberger JD, Lewis DF, Dorer EM. Impact of attention-deficit/hyperactivity disorder (ADHD) treatment on smoking cessation intervention in ADHD smokers: a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry. 2010;71:1680–1688. doi: 10.4088/JCP.09m05089gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaldívar Basurto F, García Montes JM, Flores Cubos P, Sánchez Santed F, López Ríos F, Molina Moreno A. Validity of the self-report on drug use by university students: correspondence between self-reported use and use detected in urine. Psicothemia. 2009;21:213–219. [PubMed] [Google Scholar]