Abstract
Tonic smooth muscles play pivotal roles in the pathophysiology of debilitating diseases of the gastrointestinal and cardiovascular systems. Tonic smooth muscles differ from phasic smooth muscles in the ability to spontaneously develop myogenic tone. This ability has been primarily attributed to the local production of specific neurohumoral substances that may work in conjunction with calcium sensitization via signal transduction events associated with the Ras homolog gene family, member A (RhoA)/Rho-associated, coiled-coil containing protein kinase 2 (ROCK II) pathways. In this article, we discuss the molecular pathways involved in the myogenic properties of tonic smooth muscles, particularly the contribution of protein kinase C versus the RhoA/ROCK II pathway in the genesis of basal tone, pathophysiology, and novel therapeutic approaches for certain gastrointestinal and cardiovascular diseases. Emerging evidence suggests that manipulation of RhoA/ROCK II activity through inhibitors or silencing of RNA interface techniques could represent a new therapeutic approach for various gastrointestinal and cardiovascular diseases.
Myogenic properties of tonic smooth muscles
Smooth muscles can be broadly classified according to the contractile patterns: phasic and tonic. Phasic smooth muscles contract transiently in response to neural stimulation and neurohumoral substances such as angiotensins and prostanoids. By contrast, tonic smooth muscles develop and sustain myogenic basal tone in the absence of an external stimulation. The term ‘myogenic’ implies that the stimulus for tone development and maintenance originates in the muscle itself, and its response is carried out via specialized properties of the smooth muscle cells (SMC) [1–4]. Classic examples of tonic smooth muscles in gastrointestinal (GI) tract include the internal anal sphincter (IAS) and the lower esophageal sphincter (LES). Such smooth muscles maintain tone and relax in response to nonadrenergic noncholinergic inhibitory neurotransmission, allowing the passage of food or waste products [5–7]. In the cardiovascular system, small arteries are the most well-established examples of tonic smooth muscles, and they play crucial roles in a number of physiologically important functions such as the establishment of basal vascular tone and autoregulation of blood flow [8,9]. The purpose of this article is to synthesize information on the cellular mechanisms underlying the myogenic properties in tonic smooth muscles and their role in the pathophysiology of debilitating diseases such as rectoanal incontinence, esophageal reflux, and achalasia, Hirschsprung’s disease (HPD), recurrent anal fissures, and hemorrhoids (examples of hypo- and hypertensive sphincteric smooth muscles, respectively), and cardiovascular hypertension. We will also discuss potential novel therapeutic approaches towards those diseases based on the molecular mechanisms underlying myogenic tone.
Role of the Ca2+/calmodulin pathway in the smooth muscle motor response
The motor response in SMCs is controlled by the sliding of myosin and actin filaments over each other. This process requires chemical energy, which is provided by the hydrolysis of ATP (Figure 1). Myosin utilizes ATP for molecular conformational changes in its own structure to facilitate its attachment and interaction with actin filaments, leading to the formation of crossbridges. Myosin attachment happens through protruding globular heads in the myosin filaments that interact with actin filaments. These heads tilt and drag along the actin filament to produce movement. The crossbridges then release the actin filament and adopt their original conformation, resulting in relaxation. This process is known as crossbridge cycling and is considered to be common to all smooth muscles [10].
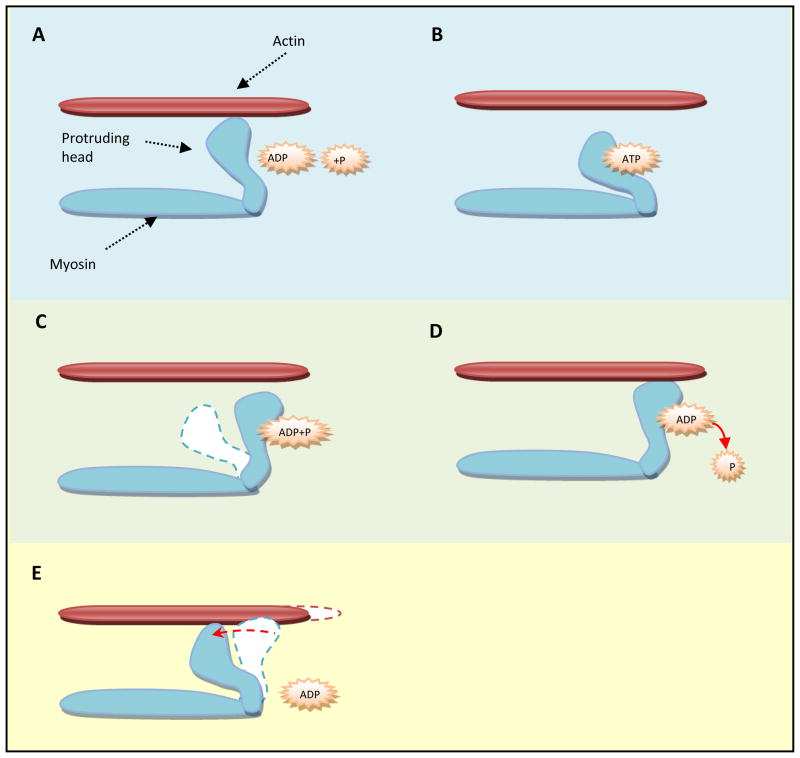
Figure 1.
Cross-bridge cycling in smooth muscle contraction. A. An initial rise in [Ca2+]i accompanied with increase in p-MLC20 via calmodulin-dependent MLCK activation causes activation of myosin ATPase and the formation of the ATP-myosin complex. B. ATP binding to protruding head in myosin structure causes a conformation change. C. As the ATP is hydrolyzed to ADP + P, the protruding head assumes an “erectile” position. D. When the P leaves myosin, the protruding head interacts with actin. E. These heads tilt and drag along the actin filament to produce movement. As ADP leaves the protruding head, the head tilts back towards the original conformation and drags along the actin filament to produce movement or smooth muscle contraction. Whether this phosphorylated cross-bridges cycling and contraction is very brief or sustained is determined by the balance between the forces that initiates the contraction (Ca2+/calmodulin/MLCK/p-MLC20) and dephosphorylation of p-MLC20 by MLCP. For the sustained contraction, it is important that dephosphorylation is inhibited by MLCP inhibition (via p-MYPT1) which is primarily mediated via RhoA/ROCK II or PKC activation. This has been explained further in the following figures.
Myosin activation is prerequisite for the crossbridge cycling. The protruding heads in the myosin filaments contain heavy chains and light chains. Phosphorylation of the 20 kDa myosin light chains (MLC20) is primarily responsible for the activation of myosin to initiate the smooth muscle contraction. The enzyme responsible for the initiation of MLC20 phosphorylation is called myosin light-chain kinase (MLCK), the activity of which depends largely on free intracellular Ca2+ ([Ca2+]i). Once SMCs are stimulated to contract via activation of G-protein-coupled transmembrane receptors (GPCRs) by specific agonists or otherwise as described below, there is an increase in [Ca2+]i via Ca2+ influx, release from the membrane or the intracellular storage organelles such as endoplasmic reticula and mitochondria. Following the increase in [Ca2+]i, Ca2+ binds to calmodulin (CaM) to form a Ca2+-CaM complex. This complex phosphorylates MLCK to induce the phosphorylation of myosin light chain (p-MLC20) followed by the onset of the smooth muscle contraction [10–12]. p-MLC20 is immediately dephosphorylated by myosin light chain phosphatase (MLCP), thus terminating the contraction, and the smooth muscle comes back to its original position, as is the case in the phasic contraction (Figure 2).
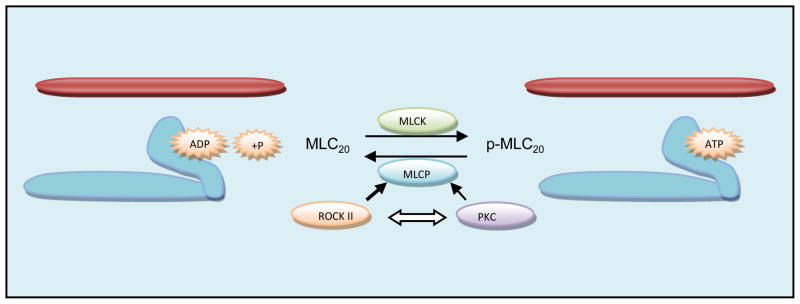
Figure 2.
Maintenance of initiated cross bridge-cycling (by Ca2+/MLCK/phosphorylated-MLC20 or p-MLC20) in the sustained smooth muscle contraction or the basal myogenic tone depends on the mechanisms that inhibit MLCP. (Reaction that represents lack or low level of p-MLC20 points towards the left, and the one for the higher levels of p-MLC20 points towards right). MLCK drives the reaction towards right, and MLCP will change the direction to the left. Likewise, MLCP inhibition either by ROCK II or PKC will drive the reaction to the right. In this regard, as discussed in the text and in the Figure 4 legend below, RhoA/ROCK pathway is predominant as compared with the PKC in the basal myogenic tone. In addition, as indicated, there appears to be a cross-talk between these pathways. In the phasic smooth muscles such as that of the esophageal body and the ASM in the basal state, MLCP may be unleashed (because of the subdued inhibitory RhoA/ROCK or PKC pathways), thus keeping these smooth muscles totally relaxed in the absence of any agonist or stimulus. In such phasic smooth muscles however, in response to an appropriate agonist or stimulus, the tissues respond to a phasic or transient contraction following an increase in p-MLC20 which is dephosphorylated immediately (in a matter of a few sec., depending upon the stimulus), by the leftward reaction by MLCP, returning it to its original relaxed state.
Although p-MLC20 via MLCK is the main signaling event in initiating SMC contractions, MLCP phosphorylation, certain endogenous kinases as further described below, inhibit MLCP, thus prolonging the SMC contraction as is the case in the sustained contraction or in the basal tone. MLCP is composed of three subunits: i). A regulatory 110 to 130 kDa subunit anchors MLCP to phosphorylated MLC20, termed as myosin-targeting subunit of MLCP (MYPT1); ii) a 37 to 38 kDa catalytic subunit (type 1 serine/threonine phosphatase, PP1c); and iii) a 20 kDa subunit (with unknown function). The targeting subunit is called the myosin phosphatase target subunit (MYPT). There are several MYPT isoforms but MYPT1 is the major subunit expressed in smooth muscles that regulates the enzymatic activity of MLCP by targeting and phosphorylating the catalytic subunit PP1c. Therefore, the kinases that modulate the PP1c and MYPT1 activities will regulate MLCP activity (and thus the smooth muscle contraction). In this regard, protein kinase C (PKC) and Rho-associated, coiled-coil containing protein kinase 2 (ROCK II) have been reported to be the primary regulators of MLCP activity [10–13]. Different mechanisms could contribute to the inhibition of MLCP activity such as: the alteration of the heterotrimeric structure of MLCP, the phosphorylation of MYPT1 at a specific site, and by the inhibitory protein CPI-17 (17 kDa PKC-potentiated inhibitory protein of PP1c). Other kinases that might also phosphorylate MYPT1 at the inhibitory site and lead to increase in p-MLC20 include Zip-like kinase, Zip kinase, myotonic dystrophy protein kinase [14].
The RhoA/ROCK pathway
RhoA belongs to a family of small GTPases that include RhoA, RhoB, and RhoC isoforms, of which RhoA is the best understood because of its important roles in the smooth muscle motility. RhoA might have high-affinity binding to guanosine trisphosphate (GTP) as well as guanosine diphosphate (GDP). However, the active form of RhoA is bound to GTP located in the cell membrane, whereas the GDP-bound form is inactive and located in the cytoplasm. Switching between the GDP/GTP-bound forms is controlled by specific regulators called guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs). GEFs facilitate RhoA-GTP binding, whereas GAPs facilitate RhoA-GDP binding. GDIs control RhoA diffusion between the membrane and the cytosol by forming a large complex with RhoA and allowing for specific geographic control of RhoA activation [15,16]. Therefore, RhoA activation and inactivation may be initiated by the activation of RhoA-specific GEFs and RhoA-specific GAPs, respectively [17–19]. Therefore, one of the logical mechanisms for the sustained RhoA/Rho-kinase activation will be inhibition of RhoA-specific GAP activity via phosphorylation of p190A RhoGAP (shown to be at Ser1150) in vitro and in vivo [17].
Additional studies have shown that the activation and inactivation of RhoA-specific GEFs and RhoA-specific GAPs may be dependent on tyrosine phosphatase SHP2 [18]. These molecular processes responsible for RhoA/ROCK activation may be linked to activation of GPCRs (via G12/13 family of heterotrimeric G-proteins) by Ang II and prostanoids [18,20,21]. They might also be GPCR-independent [22] or by triggered by depolarization [23]. It has been established that RhoA/ROCK is key molecule in G-protein-mediated Ca2+ sensitization for smooth muscle contraction [19,24]. (It has been recognized that the sphincteric SMC may be distinctly characterized by the presence of biosynthetic machinery for and high levels of Ang II [25–27] and prostanoids [28–32] with the high levels ongoing, spontaneous, spike-like action potentials with more positive resting membrane potential [33]). Additional studies in the colon and IAS have shown that phosphorylation of heat shock protein (HSP27, a member of small HSP family) [34,35], and RhoA prenylation [36,37] may also play important roles in RhoA activation.
ROCK is a cytoplasmic serine/threonine-specific kinase that serves as an effector of RhoA. Upon RhoA activation, ROCK migrates to the cell membrane and interacts with RhoA-GTP, resulting in autophosphorylation and activation [15,19,38–40]. ROCK I and ROCK II are the two ROCK isoforms expressed in SMCs; ROCK II, nevertheless, is most implicated in contraction [12,41–43]. The mechanism of ROCK II-mediated smooth muscle contraction is primarily based on the inhibition of MLC20 dephosphorylation (causing increase in p-MLC20) via phosphorylation of pThr696, or Thr803-MYPT1 subunit of MLCP; pThr38-CPI-17 and consequent inhibition of PP1c of MLCP; and MLCK-like activity (Figure 3) [16,24,41,44–51].
Figure 3.
Molecular pathways involved in smooth muscle contraction. Activation of GPCRs (or a mechanism independent of GPCR activation) increases the intracellular concentrations of Ca2+ ([Ca2+]i) (either via Ca2+ influx, or release from the membrane or the intracellular storage organelles such as endoplasmic reticulum (ER) or mitochondria) leading to formation of Ca2+/CaM complex and activation of MLCK. MLCK phosphorylates MLC20, resulting in SMC contraction. Conversely, MLCP dephosphorylates MLC20 resulting in SMC relaxation. GPCR activation might also induce RhoA to bind to GTP, a reaction catalyzed by RhoGEF and reversed by RhoGAP. RhoAGTP activates ROCK II, which inhibits MLCP either directly or via phosphorylating CPI-17. In addition, ROCK II, as indicated, may also cause increase in p-MLC20 via MLCK-like action. In the tonic smooth muscles, constitutively active RhoA/ROCK appears to be responsible for the sustained inhibition of MLCP that maintains higher levels of p-MLC20.
Role of PKC vs. RhoA/ROCK in the maintenance of basal tone
Both PKC and ROCK have been implicated in the inhibition of MLCP activity via phosphorylation of CPI-17 at threonine-38 (Thr38) residue (pThr38-CPI-17 or simply p-CPI-17). CPI-17 is an endogenous inhibitory protein of the catalytic subunit of MLCP [15,49,52–54]. p-CPI-17 is ~7,000-fold more potent than nonphosphorylated CPI-17. Phosphorylation of CPI-17 by ROCK has also been suggested by in vivo studies [15,55,56]. In these studies, increase in p-CPI-17 was specifically inhibited by the ROCK inhibitors. In some of these studies, however, the possibility of cross-talk between PKC and RhoA/ROCK pathways may not be completely ruled out.
Earlier studies testing the effects of selective inhibition of PKC in the LES and IAS suggested PKC to be the primary molecular mechanism in the basal tone of the gastrointestinal smooth muscles [2,57,58]. Recent studies however, aimed at assessing the relative contribution of ROCK vs. PKC show that ROCK-mediated inhibition of MLCP is primarily responsible for the basal tone in the LES of humans and animals, and for the IAS of various animals [2,11,13,59,60]. The role of these pathways however, in the intact human IAS remains to be determined.
To examine the molecular bases for the myogenic tone in the IAS, recent multipronged studies using force measurements and molecular biology were focused on the role of RhoA/ROCK in the rat IAS vs. the adjoining smooth muscles of rectum (RSM; that has a mixture of tonic and phasic activities) and the anococcygeus (ASM; a purely phasic smooth muscle) [11,61–63]. The studies also compared cellular distribution of RhoA/ROCK, levels of RhoA-GTP (the active form of RhoA), RhoA-Rho guanine nucleotide dissociation inhibitor (GDI) complex formation, levels of phosphorylated MYPT1 (at threonine 696; pThr696-MYPT1), under different experimental conditions, including inhibition of RhoA/ROCK. Levels of RhoA/ROCK were found to be higher at the cell membrane in the IAS SMCs compared with those from the RSM and ASM. C3 exoenzyme (RhoA inhibitor) and Y27632 (ROCK inhibitor) caused concentration-dependent relaxations in the IAS SMCs. In addition, active ROCK-II (primary isoform of ROCK in the SMC contraction) caused further shortening in the IAS SMCs. C3 exoenzyme increased RhoA-RhoGDI binding and reduced the levels of RhoA-GTP and pThr696-MYPT1. Y27632 attenuated PKC-induced contractions in IAS SMC. Conversely, a PKC inhibitor (Gö 6850, which only causes a partial relaxation of the SMC) had no significant effect on ROCK-II-induced contractions. Further experiments showed the highest levels of RhoA, RhoA-GTP, ROCK-II, MLC20, p-MYPT1, and p-MLC20 in the IAS vs. RSM and ASM SMCs. The trend was the reverse with the levels of inactive RhoA (GDP-RhoA-RhoGDI complex) and MYPT1 [62].
Attenuation of PKC-induced contraction of the IAS SMC by not only the PKC inhibitor but also the selective blockade of active ROCK II by a ROCK inhibitor suggests that PKC pathway is partially mediated via ROCK activation [62]. In agreement with this, it has been shown that vascular smooth muscle contractions caused by PKC are mediated primarily via ROCK activation [15,64]. These conclusions were based on the use of ROCK inhibitors, and also via the use of dominant-negative ROCK and pseudosubstrates peptides as PKC inhibitors [65], and via ezrin, radixin and moesin-binding (ERM) phosphorprotein (EBP50) depletion by siRNA [66]. (It is well known that ERM proteins serve as ROCK substrates). Furthermore, it has been shown recently that ROCK mediates PKC-dependent apoptosis in prostate cancer cells [67]. In addition, recent studies in humans have shown that ROCK inhibitors cause a concentration-dependent decrease in the basal tone of the LES, causing near obliteration of the tone in the maximal effective concentrations. These studies also show that PKC inhibition only had a limited effect [59]. Together, these data suggest that ROCK and PKC pathways may lie in series, PKC being upstream of RhoA/ROCK. In this process (although not fully proven), in the basal tone of the IAS, RhoA/ROCK regulate the higher levels of p-MLC20 and the basal tone via p-MYPT1 and p-CPI-17 while effect of PKC may be limited to p-CPI-17 [15,39,40,58,59,62]. However, direct effect of RhoA/ROCK in the p-MLC20 via MLCK-like effect in intact smooth muscles remains to be determined.
Therapeutic potential of RhoA/ROCK inhibitors in GI diseases
Prototypes of tonic tissues, the LES and IAS play major roles in the pathophysiology of a number of the gastrointestinal motility disorders. The LES maintains tone in the basal state and relaxes during swallowing to allow passage of food. Similarly, the IAS maintains spontaneous tone and relaxes to allow passage of processed food in response to the rectoanal inhibitory reflex that is initiated by rectal distension caused by the stool. Significant changes in the basal tone of the IAS and LES have been directly associated with the pathophysiology of debilitating diseases such as rectoanal incontinence, certain forms of constipation, recurrent anal fissures, hemorrhoids, Hirschsprung’s disease (HPD), achalasia, and gastroesophageal reflux disease[4,6,7,68–76]. Therefore, agents able to restitute the hypo- or hypertonic states of the IAS or LES, particularly those acting on the RhoA/ROCK pathway, have novel and significant therapeutic potentials (Figure 4) [59,60,62].
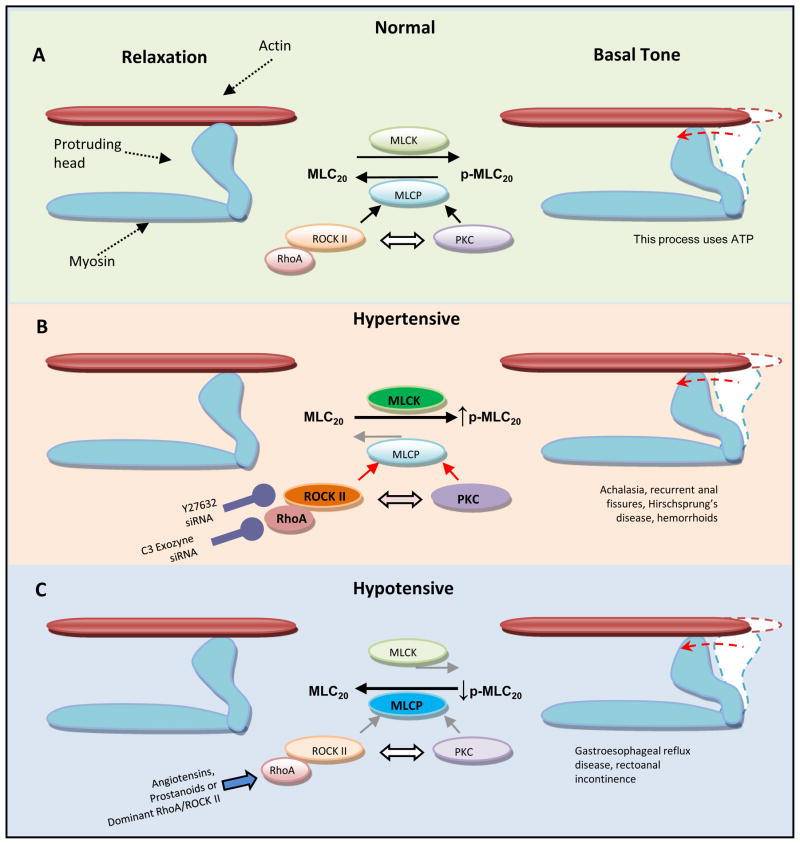
Figure 4.
A model to explain the role of the constitutively active RhoA/ROCK II pathway in the basal state of the LES and IAS tone (normal), and in the pathophysiology of the hypertensive and hypotensive states associated with the corresponding motility disorders. A. In the basal state, RhoA/ROCK II displaces the equilibrium between MLCK and MLCP activities towards higher levels of p-MLC20 and maintained cross-bridge interactions with actin. B. In the hypertensive state, upregulation of the RhoA/ROCK II pathway via further displacement of the MLCK/MLCP equilibrium may lead to the still higher levels of p-MLC20 [106–108]. RhoA and ROCK II are targets for potential therapeutic interventions via traditional molecules such as C3 exozyme and Y27632 or via novel therapies such as selective siRNAs. C. In the hypotensive state, downregulation of the RhoA/ROCK II might displace the MLCK/MLCP equilibrium towards lower levels of p-MLC20 resulting in the hypotensive tonic smooth muscles [109–112]. In that case, the tone may be improved by the agonists (e.g. angiotensins and prostanoids) via RhoA/ROCK activation. Although, PKC also has the potential of inhibiting MLCP as explained above, its role in these tonic smooth muscles (as described above based on the humans and animal data in the LES and IAS), may be limited. In addition, as indicated, there appears to be a cross-talk between the RhoA/ROCK and PKC pathways. Exact relative contribution of these pathways and the nature of interaction between them remain to be determined.
Higher levels of RhoA/ROCK II (along with the higher levels of p-MLC20) have been reported at the SMC level in the IAS compared with the adjoining RSM, and the ASM [61,62]. In addition, introduction of active ROCK-II into the IAS SMCs causes a concentration-dependent increase in the spontaneous contractions of the IAS SMCs, which is attenuated by the ROCK inhibitor Y27632 [62]. These results are in line with a transgenic mice study showing that inhibition of RhoA/ROCK II lowers the local levels of p-MLC20, increases the basal length of IAS SMCs and inhibits tone development in the isolated strips of the IAS [77]. The studies also show that inhibition of the RhoA/ROCK II pathway correlates with upregulation of H-ras, which has been implicated in the pathogenesis of certain colorectal cancers, and rectoanal incontinence associated with the lower intraluminal pressures in the IAS [78,79]. However, the role for RhoA/ROCK II upregulation in the pathophysiology of the spontaneously hypertensive IAS via modulation of the signal transduction pathways for the locally-produced angiotensin II (Ang II) and cyclooxygenase (COX) products (thromboxanes and prostaglandins) [25,26,28,29], remains to be determined. In different systems, these agents are known to activate GPCRs and stimulate the RhoA/ROCK signaling pathway (Figure 4) [2,18,20,80].
In the LES, ROCK inhibitors have been shown to attenuate the basal tone and its increase caused by Ang II and prostanoids in various animal species [2,57,81]. Additional studies in the human LES [59] have shown that ROCK inhibitors (Y27632 and HA-1077) produce full relaxation, whereas PKC inhibitors (calphostin C and chelerythrine) only have limited effects. These studies concluded that a mechanism of Ca2+ sensitization mediated by the RhoA/ROCK pathway plays an important role in the basal tone in the human LES. Interestingly, mice lacking lsc/p115-GEF developed hypertension of the LES and showed impaired relaxation to inhibitory stimuli [82]. Native Lsc/p115-GEF has been related to potential reduction in RhoA activity because of its high affinity for RhoA devoid of bound nucleotide. The hypothesis is that GTP would be less able to displace the exchange factor and potentially slow the overall rate of GDP to GTP cycling, thus reducing RhoA activity [83].
Therapeutic potential of RhoA/ROCK inhibitors in cardiovascular diseases
As in gastrointestinal smooth muscles, the contractile state of vascular SMC (VSMC) depends on the levels of p-MLC20 determined by the balance between the activities of Ca2+/CaM/MLCK-dependent (plus Ca2+-independent) and the MLCP pathways [19,38,43]. A number of neurohumoral agonists (e.g. Ang II and prostanoids) are known to modulate these pathways via GPCRs that lead not only to increases in the [Ca2+]i but also stimulate RhoA/ROCK. Thus, as outlined above, the inhibition of MLCP via p-MYPT1 and/or p-CPI-17 by ROCK leads to increased p-MLC20 and enhanced VSMC contractility [38–40,50,84]. In addition, modulation of VSMC contractility via ROCK is of particular importance during tonic contractions in different vascular beds, including the pulmonary artery, mesenteric artery and portal vein. Another important role for ROCK has been shown to be the maintenance of myogenic tone in small arteries [38–40,85,86]. Therefore, the RhoA/ROCK pathway and its interventions have important roles in the pathophysiology and therapy of cardiovascular hypertension resulting from increased peripheral vascular resistance attributed to increased contractility of VSMC. Studies show that the ROCK inhibitors such as Y27632 lower blood pressure in spontaneously hypertensive (SHR) and deoxycorticosterone-acetate (DOCA)/salt-treated and renal hypertensive rats [39]. Interestingly, in the therapeutic doses, Y27632 does not cause a significant decrease in blood pressure in normotensive animals [39,87].
Studies in isolated vascular segments confirm an association of RhoA/ROCK in the pathophysiology of cardiovascular hypertension. In mesenteric and cerebral arteries from SHR, the relaxation induced by treatment with Y27632 is markedly higher as compared with the control Wistar-Kyoto (WKY) rats [88,89]. By comparison, the selective PKC inhibitors calphostin C and Ro 31-8220 had little or no effect on arterial diameter. Vasodilator responses to Y27632 were found to be independent of PKC. In two models of chronic hypertension in SHR and rats treated with N-nitro-L-arginine methyl ester (nitric oxide synthase or NOS inhibitor), Y27632 elicited cerebral vasodilation significantly greater than in normotensive WKY rats. This indicates that the chronically hypertensive state (and not genetic factors) contributes to the increased responses to ROCK inhibition. In the same experimental models, PKC inhibition had no significant effect on arterial diameter in chronically hypertensive rats. These data suggest that ROCK, but not PKC, contributes to chronic hypertension [89]. Similar results have also been shown for mesenteric arteries from DOCA/salt-treated rats [90]. Direct evidence for increased activity of RhoA in hypertensive states has also been found in stroke-prone SHR, DOCA/salt- and renal hypertensive rats [91–93].
Therapeutic potential of RhoA/ROCK silencing with small interfering RNA (siRNA)
The main advantage of an siRNA approach over conventional inhibitors is that the conventional inhibitors such as Y27632 do not exhibit specificity for the ROCK isoforms. In addition, siRNAs are potentially ~1,000-fold more potent than the conventional ROCK inhibitors [13,41,94–100].
Because the pathophysiology of many diseases is based on the over expression of certain genes, and intense research on gene function has allowed the identification of many putative target genes, approaches based on siRNA are emerging as particularly attractive therapeutic strategies. siRNA is highly versatile since saran molecules can be easily designed to specifically target virtually any gene because of their dependence on complementary base-pair interactions. The siRNA approach that silences a specific gene at the post-transcriptional and post-translation levels comprises the introduction or local generation of double-stranded RNA (dsRNA) molecules specifically designed to match the mRNA sequences coding a protein of interest [94]. Once in the cell, the dsRNA interacts with an RNase III family enzyme named dicer. The dicer fragments RNA into 21–23 bp segments called small-interfering RNA (or siRNA). The siRNA then attaches to a multi-protein complex of endonucleases (RNA-induced silencing complex, RISC), unwinds, pairs with, and fragments the target mRNA and, consequently, inhibits the synthesis of specifically targeted protein.
Regarding siRNA delivery in vivo, there are several studies showing that it can be successfully done. The direct siRNA administration to the eye has been used to target VEGF after laser-induced choroidal neovascularization, and to induce alterations of synaptic function through down-regulation of amyloid precursor protein (APP)/amyloid precursor-like protein 2 after intra-ocular injection of siRNA for APP in rodent models [101,102]. There are also studies describing the downregulation of target genes relevant in depression (serotonin transporter), or hyperanalgesia (pain-related cation channel P2X3) after intrathecal/intraventricular siRNA administration in rodent models [103,104]. Noteworthy, one of the studies showed a significant reduction of P2X3 protein expressed in dorsal root ganglia or translocated into the dorsal horn of the spinal cord, and a blockade of pathophysiological pain response and relief from neuropathic pain [104]. Another study using intranasal application of siRNAs targeting RSV or PIV with or without transfection reagents showed that the siRNA therapy protected mice from respiratory syncytial virus (RSV) and parainfluenza virus (PIV) infections [105].
There are several studies describing the effects of RhoA/ROCK siRNA in vitro. In a study assessing the role of the ROCK isoforms (ROCK I and ROCK II) in aortic VSMC contractility, siRNA specific for the ROCK isoforms was introduced into cultured VSMCs [41]. VSMC contraction induced by lysophosphatidic acid (LPA) was used to measure cellular force production. When LPA was applied, control cells (transfected with scrambled siRNA) underwent a gradual contraction that was abolished by pre-treatment with the ROCK inhibitor Y27632. These results support the notion that VSMC contractility was mediated by ROCK. ROCK II siRNA-silenced cells contracted significantly less than scrambled control and ROCK I siRNA-silenced cells. These results were accompanied by significant reductions in p-MLC20 and suggest prominent role of ROCK II in the pathophysiology and a possible role for ROCK silencing via ROCK II-specific siRNA in the treatment of cardiovascular hypertension [41]. Comparable results have been reported in pulmonary arteries showing that ROCK II siRNA-induced specific and significant decrease in the VSMC motility [95]. However, additional studies using in vivo animal models are necessary to assess the therapeutic potentials of ROCK II siRNA.
The effects of RhoA/ROCK II silencing in the GI tract have also been reported. A study designed to evaluate the effects of COX and RhoA silencing with selective siRNAs in colon carcinoma cells established an association of COX type II and RhoA in decreasing the cellular motility via RhoA/ROCK pathway [96]. Besides, preliminary results from ongoing studies in our laboratory (where topical application of ROCK II siRNA to the anal region restituted the hypertensive IAS and the output of fecal pellets in rats), the effects of such approaches in the functional and dysfunctional GI smooth muscle proper, remain to be determined.
Because of the original reports of RNA interference (RNAi) in cells from a range of species [97–99], there has been increasing interest in harnessing this endogenous mechanism as a novel approach to human therapy. From a drug discovery perspective, siRNAs have distinct advantages over conventional drug therapies; these include higher selectivity, potency, increased number of potential leads, reduced time for lead development, and simpler scalable processes than conventional therapeutics) [100]. Nevertheless, several major obstacles, such as detailed in vivo data, for example, lack of data for the safety and efficacy in humans, and the development of efficacious delivery systems, need to be overcome before the introduction of RNAi therapy i humans.
Concluding remarks
Basal tone in the smooth muscles of the gastrointestinal sphincters (typified by the LES and IAS) and those of certain blood vessels of the cardiovascular system provide true representations of the sustained contraction in the absence of any exogenous stimulus or agonist. The basal tone in such smooth muscles is primarily myogenic. However, the molecular mechanisms underlying the myogenic control in relation to the external triggers (perhaps produced within the cells) and other modulatory neurohumoral stimuli are poorly understood. Such information on the molecular control mechanisms and the associated signal transduction cascade is extremely vital for our advanced understanding of the pathophysiology, and novel and specifically targeted therapy of a number of debilitating disorders. Because of the lack of this knowledge, presently, the therapeutic avenues for such abnormalities are limited to temporary symptomatic approaches that are marred with a number of side effects. In this regard, RhoA/ROCK is emerging as an important pathway that may explain the molecular mechanism underlying the pathophysiology of such disorders, and serve as specific molecular target for the safer and more potent therapy. In addition, because of the factors outlined above, exploration of RhoA/ROCK siRNA approaches with specific distinct advantages in treating such disorders, deserve special consideration.
Acknowledgments
This work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grant DK-35385, and an institutional grant from Thomas Jefferson University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruegg JC. Smooth muscle tone. Physiol Rev. 1971;51:201–248. doi: 10.1152/physrev.1971.51.1.201. [DOI] [PubMed] [Google Scholar]
- 2.Harnett KM, et al. Signal-transduction pathways that regulate smooth muscle function I. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005;288:G407–G416. doi: 10.1152/ajpgi.00398.2004. [DOI] [PubMed] [Google Scholar]
- 3.Golenhofen K, Mandrek K. Phasic and tonic contraction processes in the gastrointestinal tract. Dig Dis Sci. 1991;9:341–346. doi: 10.1159/000171321. [DOI] [PubMed] [Google Scholar]
- 4.Farre R, Sifrim D. Regulation of basal tone, relaxation, and contraction of the lower esophageal sphincter. Relevance to drug discovery for esophageal disorders. Br J Pharmacol. 2008;153:858–869. doi: 10.1038/sj.bjp.0707572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culver PJ, Rattan S. Genesis of anal canal pressures in the opossum. Am J Physiol Gastrointest Liver Physiol. 1986;251:G765–G771. doi: 10.1152/ajpgi.1986.251.6.G765. [DOI] [PubMed] [Google Scholar]
- 6.Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008;42:610–619. doi: 10.1097/MCG.0b013e31816b444d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rattan S. The internal anal sphincter: regulation of smooth muscle tone and relaxation. Neurogastroenterol Motil. 2005;17:50–59. doi: 10.1111/j.1365-2982.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PC. Handbook of Physiology. The Cardiovascular System. Vascular Smooth Muscle. 2. II. American Physiological Society; Bethesda, MD: 1980. The myogenic response; pp. 409–442 . [Google Scholar]
- 9.Davis MJ. Myogenic response gradient in an arteriolar network. Am J Physiol Heart Circ Physiol. 1993;264:H2168–H2169. doi: 10.1152/ajpheart.1993.264.6.H2168. [DOI] [PubMed] [Google Scholar]
- 10.Smolock EM, et al. siRNA knock down of casein kinase 2 increases force and cross-bridge cycling rates in vascular smooth muscle. Am J Physiol Cell Physiol. 2007:C876–C885. doi: 10.1152/ajpcell.00343.2006. [DOI] [PubMed] [Google Scholar]
- 11.Rattan S, et al. Rho kinase as a novel molecular therapeutic target for hypertensive internal anal sphincter. Gastroenterology. 2006;131:108–116. doi: 10.1053/j.gastro.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 12.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 13.Rattan S, et al. RhoA/ROCK-kinase: pathophysiologic and therapeutic implications in gastrointestinal smooth muscle tone and relaxation. Gastroenterology. 2010;138:13–18. doi: 10.1053/j.gastro.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano K, et al. Protein kinase network in the regulation of phosphorylation and dephosphorylation of smooth muscle myosin light chain. Mol Cell Biochem. 2003;248:105–114. doi: 10.1023/a:1024180101032. [DOI] [PubMed] [Google Scholar]
- 15.Kandabashi T, et al. Evidence for protein kinase C-mediated activation of Rho-kinase in a porcine model of coronary artery spasm. Arterioscler Thromb Vasc Biol. 2003;23:2209–2214. doi: 10.1161/01.ATV.0000104010.87348.26. [DOI] [PubMed] [Google Scholar]
- 16.Ellenbrock SIJ, Collard JG. Rho GTPases: functions and association with cancer. Clin Exp Metastasis. 2007;24:657–672. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 17.Mori K, et al. Rho-kinase contributes to sustained RhoA activation through phosphorylation of p190A RhoGAP. J Biol Chem. 2009;284:5067–5076. doi: 10.1074/jbc.M806853200. [DOI] [PubMed] [Google Scholar]
- 18.Bregeon J, et al. Angiotensin II induces RhoA activation through SHP2-dependent dephosphorylation of the RhoGAP p190A in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2009;297:C1062–C1070. doi: 10.1152/ajpcell.00174.2009. [DOI] [PubMed] [Google Scholar]
- 19.Puetz S, et al. Regulation of smooth muscle contraction by small GTPases. Physiology. 2009:342–356. doi: 10.1152/physiol.00023.2009. [DOI] [PubMed] [Google Scholar]
- 20.Wilson DP, et al. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochemical Journal. 2005;389:763–774. doi: 10.1042/BJ20050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sward K, et al. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr Hypertens Rep. 2003;5:66–72. doi: 10.1007/s11906-003-0013-1. [DOI] [PubMed] [Google Scholar]
- 22.Ayman S, et al. Receptor-independent activation of Rho-kinase-mediated calcium sensitisation in smooth muscle. Br J Pharmacol. 2003;139:1532–1538. doi: 10.1038/sj.bjp.0705394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodsome TP, et al. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J Cell Sci. 2006;119:1769–1780. doi: 10.1242/jcs.02805. [DOI] [PubMed] [Google Scholar]
- 24.Kureishi Y, et al. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- 25.De Godoy MAF, et al. Evidence for the role of angiotensin II biosynthesis in the rat internal anal sphincter tone. Gastroenterology. 2004;127:127–138. doi: 10.1053/j.gastro.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 26.De Godoy MAF, Rattan S. Autocrine regulation of internal anal sphincter tone by renin-angiotensin system: comparison with phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1164–G1175. doi: 10.1152/ajpgi.00115.2005. [DOI] [PubMed] [Google Scholar]
- 27.Casselbrant C, et al. Actions by angiotensin II on esophageal contractility in humans. Gastroenterology. 2007;132:249–260. doi: 10.1053/j.gastro.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 28.De Godoy MAF, Rattan S. Role of phospholipase A2 (group I secreted) in the genesis of basal tone in the internal anal sphincter smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2007;293:G979–G986. doi: 10.1152/ajpgi.00310.2007. [DOI] [PubMed] [Google Scholar]
- 29.De Godoy MAF, et al. COX-1 vs. COX-2 as a determinant of the basal tone in the internal anal sphincter. Am J Physiol Gastrointest Liver Physiol. 2009;296:G219–G225. doi: 10.1152/ajpgi.90485.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao WB, et al. Group I secreted PLA2 (sPLA2) and arachidonic acid metabolites in the maintenance of cat LES tone. Am J Physiol Gastrointest Liver Physiol. 1999;40:G585–G598. doi: 10.1152/ajpgi.1999.277.3.G585. [DOI] [PubMed] [Google Scholar]
- 31.Cao W, et al. Group I secreted PLA2 in the maintenance of human lower esophageal sphincter tone. Gastroenterology. 2000;119:243–252. doi: 10.1053/gast.2000.19581. [DOI] [PubMed] [Google Scholar]
- 32.Cheng L, et al. Inflammation induced changes in arachidonic acid metabolism in cat LES circular muscle. Am J Physiol Gastrointest Liver Physiol. 2005;288:G787–G797. doi: 10.1152/ajpgi.00327.2004. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, et al. Opposing roles of K+ and Cl− channels in maintenance of opossum lower esophageal sphincter tone. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1226–G1234. doi: 10.1152/ajpgi.2000.279.6.G1226. [DOI] [PubMed] [Google Scholar]
- 34.Patil SB, Bitar KN. RhoA- and PKC-α-mediated phosphorylation of MYPT and its association with HSP27 in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G83–G95. doi: 10.1152/ajpgi.00178.2005. [DOI] [PubMed] [Google Scholar]
- 35.Somara S, et al. Bioengineerd internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology. 2009;137:53–61. doi: 10.1053/j.gastro.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 36.Patel CA, Rattan S. RhoA prenylation inhibitor produces relaxation of tonic smooth muscle of internal anal sphincter. J Pharmacol Exp Ther. 2007;321:501–508. doi: 10.1124/jpet.107.119339. [DOI] [PubMed] [Google Scholar]
- 37.Rattan S. 3-hydroxymethyl coenzyme A reductase inhibition attenuates spontaneous smooth muscle tone via RhoA/ROCK pathway regulated by RhoA prenylation. Am J Physiol Gastrointest Liver Physiol. 2010;298:G962–G969. doi: 10.1152/ajpgi.00034.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barman SA, et al. RhoA/Rho-kinase signaling: a therapeutic target in pulmonary hypertension. Vascular Health & Risk Management. 2009;5:663–671. doi: 10.2147/vhrm.s4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wirth A. Rho kinase and hypertension. Biochim Biophys Acta. 2010;1802:1276–1284. doi: 10.1016/j.bbadis.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Dong M, et al. Rho-kinase inhibition: a novel therapeutic target for the treatment of cardiovascular diseases. Drug Discov Today. 2010;15:622–629. doi: 10.1016/j.drudis.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, et al. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104:531–540. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunes KP, et al. RhoA/Rho-kinase and vascular diseases: what is the link? Cellular & Molecular Life Sciences. 2010;67:3823–3836. doi: 10.1007/s00018-010-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Somlyo AP, Somlyo AV. Signal transduction through the RhoA/Rho-kinase pathway in smooth muscle. J Muscle Res Cell Motil. 2004;25(8):613–615. doi: 10.1007/s10974-004-3146-1. [DOI] [PubMed] [Google Scholar]
- 44.Leung T, et al. The p160 RhoA-binding kinase ROK is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 2007;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murthy KS, et al. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 (20 kDa regulatory light chain myosin II) phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit and protein kinase C/CPI-17 pathway. Biochem J. 2003;374:145–155. doi: 10.1042/BJ20021274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen XQ, et al. Characterization of RhoA-binding kinase ROK implication of the plecktsrin homology domain in ROK function using region-specific antibodies. J Biol Chem. 2002;277:12680–12688. doi: 10.1074/jbc.M109839200. [DOI] [PubMed] [Google Scholar]
- 47.Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20(Suppl 1):39–53. doi: 10.1111/j.1365-2982.2008.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartshorne DJ, et al. Myosin light chain phosphatase: subunit composition, interactions and regulation. J Muscle Res Cell Motil. 1998;19:325–341. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- 49.Koyama M, et al. Phosphorylation of CPI-17, and inhibitory phosphoprotein of smooth muscle myosin phosphatase, by Rho-kinase. FEBS Lett. 2000;475:197–200. doi: 10.1016/s0014-5793(00)01654-9. [DOI] [PubMed] [Google Scholar]
- 50.Kitazawa T, et al. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000;275:9897–9900. doi: 10.1074/jbc.275.14.9897. [DOI] [PubMed] [Google Scholar]
- 51.Amano M, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 52.Eto M, et al. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: Its specific localization in smooth muscle. FEBS Lett. 1997;410:356–360. doi: 10.1016/s0014-5793(97)00657-1. [DOI] [PubMed] [Google Scholar]
- 53.Kitazawa T, et al. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol (Lond) 2003;546:879–889. doi: 10.1113/jphysiol.2002.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem. 2009;284:35273–35277. doi: 10.1074/jbc.R109.059972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang T, et al. Carbachol-induced rabbit bladder smooth muscle contraction: roles of protein kinase C and Rho kinase. Am J Physiol Renal Physiol. 2009;297:F1534–F1542. doi: 10.1152/ajprenal.00095.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiie Z, et al. Role of calcium-independent phospholipase A2 in high glucose-induced activation of RhoA, Rho kinase, and CPI-17 in cultured vascular smooth muscle cells and vascular smooth muscle hypercontractility in diabetic animals. J Biol Chem. 2010;285:8628–8638. doi: 10.1074/jbc.M109.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim N, et al. Distinct kinases are involved in contraction of cat esophageal and lower esophageal sphincter smooth muscles. Am J Physiol Cell Physiol. 2004;287:C384–C394. doi: 10.1152/ajpcell.00390.2003. [DOI] [PubMed] [Google Scholar]
- 58.Bitar KN, et al. Regulation of smooth muscle contraction in rabbit internal anal sphincter by protein kinase C and Ins(1,4,5)P3. Am J Physiol Gastrointest Liver Physiol. 1991;260:G537–G542. doi: 10.1152/ajpgi.1991.260.4.G537. [DOI] [PubMed] [Google Scholar]
- 59.Sims SM, et al. Calcium sensitization in human esophageal muscle: role for RhoA kinase in maintenance of lower esophageal sphincter tone. J Pharmacol Exp Ther. 2008;327:178–186. doi: 10.1124/jpet.108.140806. [DOI] [PubMed] [Google Scholar]
- 60.Park SY, et al. Paticipation of Rho-associated kinase in electrical stimulated and acetylcholine-induced contraction of feline esophageal smooth muscle. Eur J Pharmacol. 2009;607:220–225. doi: 10.1016/j.ejphar.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 61.Patel CA, Rattan S. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2006;291:G830–G837. doi: 10.1152/ajpgi.00130.2006. [DOI] [PubMed] [Google Scholar]
- 62.Patel CA, Rattan S. Cellular regulation of basal tone in internal anal sphincter smooth muscle by RhoA/ROCK. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1747–G1756. doi: 10.1152/ajpgi.00438.2006. [DOI] [PubMed] [Google Scholar]
- 63.Rattan S, Patel CA. Selectivity of Rho Kinase inhibitors in the spontaneously tonic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2008;294:G687–G693. doi: 10.1152/ajpgi.00501.2007. [DOI] [PubMed] [Google Scholar]
- 64.Baek I, et al. A role for Rho-kinase in Ca2+-independent contractions induced by phorbol-12,13-dibutyrate. Clin Exp Pharmacol Physiol. 2009;36:256–261. doi: 10.1111/j.1440-1681.2008.05045.x. [DOI] [PubMed] [Google Scholar]
- 65.Shirao S, et al. Sphingosylphosphorylcholine is a novel messenger for Rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery: unimportant role for protein kinase C. Circ Res. 2002;91:112–119. doi: 10.1161/01.res.0000026057.13161.42. [DOI] [PubMed] [Google Scholar]
- 66.Baeyens N, et al. Identification of functional implication of a rho kinase-dependent moesin-EBP50 interaction in noradrenaline-stimulated artery. Am J Physiol Cell Physiol. 2010;299:C1530–C1540. doi: 10.1152/ajpcell.00175.2010. [DOI] [PubMed] [Google Scholar]
- 67.Xiao L, et al. ROCK Mediates Phorbol Ester-induced Apoptosis in Prostate Cancer Cells via p21Cip1 Up-regulation and JNK. J Biol Chem. 2010;284:29365–29375. doi: 10.1074/jbc.M109.007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hashish M, et al. Surgical implantation of a bioengineered internal anal sphincter. J Ped Surg. 2010;45:52–58. doi: 10.1016/j.jpedsurg.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wald A. Clinical practice. Fecal incontinence in adults. N Engl J Med. 2007;356:1648–1655. doi: 10.1056/NEJMcp067041. [DOI] [PubMed] [Google Scholar]
- 70.Rao SS, Singh S. Clinical utility of colonic and anorectal manometry in chronic constipation. [Review] J Clin Gastroenterol. 2010;44:597–609. doi: 10.1097/MCG.0b013e3181e88532. [DOI] [PubMed] [Google Scholar]
- 71.Gibbons CP, et al. Role of constipation and anal hypertonia in the pathogenesis of haemorrhoids. Br J Surg. 1988;75:656–660. doi: 10.1002/bjs.1800750712. [DOI] [PubMed] [Google Scholar]
- 72.Farouk R, et al. Sustained internal sphincter hypertonia with chronic anal fissure. Dis Colon Rectum. 1994;37:424–429. doi: 10.1007/BF02076185. [DOI] [PubMed] [Google Scholar]
- 73.Madalinski M, Kalinowski L. Novel options for the pharmacological treatment of chronic anal fissure-Role of botulinum toxin. Curr Clin Pharmacol. 2009;4:47–52. doi: 10.2174/157488409787236083. [DOI] [PubMed] [Google Scholar]
- 74.Kuribayashi S, et al. Mechanism of gastroesophageal reflux in patients with obstructive sleep apnea syndrome. Neurogastroenterol Motil. 2010;22:611–e172. doi: 10.1111/j.1365-2982.2010.01485.x. [DOI] [PubMed] [Google Scholar]
- 75.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med. 1997;336:924–932. doi: 10.1056/NEJM199703273361306. [DOI] [PubMed] [Google Scholar]
- 76.Richter JE. Achalasia- an update. J Neurogastroenterol Motil. 2010;16:232–242. doi: 10.5056/jnm.2010.16.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Godoy MAF, et al. H-ras inhibits RhoA/ROCK leading to a decrease in the basal tone in the internal anal sphincter. Gastroenterology. 2007;132:1401–1409. doi: 10.1053/j.gastro.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 78.Arber N, et al. Activation of c-K-ras mutations in human gastrointestinal tumors. Gastroenterology. 2000;118:1045–1050. doi: 10.1016/s0016-5085(00)70357-x. [DOI] [PubMed] [Google Scholar]
- 79.van Duijvendijk P, et al. A prospective evaluation of anorectal function after total mesorectal excision in patients with a rectal carcinoma. Surgery. 2003;133:56–65. doi: 10.1067/msy.2003.3. [DOI] [PubMed] [Google Scholar]
- 80.Rattan S, et al. Involvement of rho and rho-associated kinase in sphincteric smooth muscle contraction by angiotensin II. Exp Biol Med. 2003;228:972–981. doi: 10.1177/153537020322800814. [DOI] [PubMed] [Google Scholar]
- 81.Rattan S, et al. Comparison of angiotensin II (Ang II) effects in the internal anal sphincter (IAS) and lower esophageal sphincter smooth muscles. Life Sci. 2002;70:2147–2164. doi: 10.1016/s0024-3205(01)01527-2. [DOI] [PubMed] [Google Scholar]
- 82.Zizer E, et al. Loss of Lsc/p115 protein leads to neuronal hypoplasia in the esophagus and an achalasia-like phenotype in mice. Gastroenterology. 2010;139:1344–1354. doi: 10.1053/j.gastro.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 83.Wells CD, et al. Identification of potential mechanisms for regulation of p115 RhoGEF through analysis of endogenous and mutant forms of the exchange factor. J Biol Chem. 2001;276:288897–28905. doi: 10.1074/jbc.M102913200. [DOI] [PubMed] [Google Scholar]
- 84.Ohtsu H, et al. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1831–1836. doi: 10.1161/01.ATV.0000175749.41799.9b. [DOI] [PubMed] [Google Scholar]
- 85.Bolz S-S, et al. Sphingosin kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation. 2003;108:342–347. doi: 10.1161/01.CIR.0000080324.12530.0D. [DOI] [PubMed] [Google Scholar]
- 86.Gokina NI, et al. Effects of Rho kinase inhibition on cerebral artery myogenic tone and reactivity. J Appl Physiol. 2004;98:1940–1948. doi: 10.1152/japplphysiol.01104.2004. [DOI] [PubMed] [Google Scholar]
- 87.Mukai Y, et al. Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J. 2001;15:1062–1064. doi: 10.1096/fj.00-0735fje. [DOI] [PubMed] [Google Scholar]
- 88.Asano M, Nomura Y. Comparison of inhibitory effects of Y-27632, a Rho kinase inhibitor, in strips of small and large mesenteric arteries from spontaneously hypertensive and normotensive Wistar-Kyoto rats. Hypertension Res. 2003;26:97–106. doi: 10.1291/hypres.26.97. [DOI] [PubMed] [Google Scholar]
- 89.Chrissobolis S, Sobey CG. Evidence that Rho-kinase activity contributes to cerebral vascular tone in vivo and is enhanced during chronic hypertension - Comparison with protein kinase C. Circ Res. 2001;88:774–779. doi: 10.1161/hh0801.090441. [DOI] [PubMed] [Google Scholar]
- 90.Weber DS, Webb RC. Enhanced relaxation to the rho-kinase inhibitor Y-27632 in mesenteric arteries from mineralocorticoid hypertensive rats. Pharmacology. 2001;63:129–133. doi: 10.1159/000056123. [DOI] [PubMed] [Google Scholar]
- 91.Seko T, et al. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res. 2003;92:411–418. doi: 10.1161/01.RES.0000059987.90200.44. [DOI] [PubMed] [Google Scholar]
- 92.Seasholtz TM, et al. Increased expression and activity of RhoA are associated with increased DNA synthesis and reduced p27(Kip1) expression in the vasculature of hypertensive rats. Circ Res. 2001;89:488–495. doi: 10.1161/hh1801.096337. [DOI] [PubMed] [Google Scholar]
- 93.Moriki N, et al. RhoA activation in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Hypertension Res. 2004;27:263–270. doi: 10.1291/hypres.27.263. [DOI] [PubMed] [Google Scholar]
- 94.Zamore PD, et al. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 95.Harvey KA, et al. Role of Rho kinase in sphingosine 1-phosphate-mediated endothelial and smooth muscle cell migration and differentiation. Mol Cell Biochem. 2010;342:7–19. doi: 10.1007/s11010-010-0461-2. [DOI] [PubMed] [Google Scholar]
- 96.Chang Y-WE, et al. RhoA mediates cyclooxygenase-2 signaling to disrupt the formation of adherens junctions and increase cell motility. Cancer Res. 2006;66:11700–11708. doi: 10.1158/0008-5472.CAN-06-1818. [DOI] [PubMed] [Google Scholar]
- 97.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in. Caenorhabditis elegans Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 98.Kennerdell JR, Carthew RW. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell. 1998;95:1017–1026. doi: 10.1016/s0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- 99.Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 100.Vaishnaw AK, et al. A status report on RNAi therapeutics. Silence. 2010;1:1–13. doi: 10.1186/1758-907X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reich SJ, et al. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- 102.Herard AS, et al. siRNA targeted against amyloid precursor protein impairs synaptic activity in vivo. Neurobiol Aging. 2005;27:1740–1750. doi: 10.1016/j.neurobiolaging.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 103.Thakker DR, et al. siRNA-mediated knockdown of the serotonin transporter in the adult mouse brain. Mol Psychiatry. 2005;10:782–789. doi: 10.1038/sj.mp.4001687. [DOI] [PubMed] [Google Scholar]
- 104.Dorn G, et al. siRNA relieves chronic neuropathic pain. Nucleic Acids Res. 2004;32:e49. doi: 10.1093/nar/gnh044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bitko V, et al. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 106.Mizuno Y, et al. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am J Physiol Cell Physiol. 2008;295:C358–C364. doi: 10.1152/ajpcell.90645.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han YJ, et al. Regulation of myosin light chain kinase expression by angiotensin II in hypertension. Am J Hypertens. 2008;21:860–865. doi: 10.1038/ajh.2008.199. [DOI] [PubMed] [Google Scholar]
- 108.Hu W-Y, et al. Involvement of ras-regulated myosin light chain phosphorylation in the captopril effects in spontaneously hypertensive rats. Am J Hypertens. 2007;20:53–61. doi: 10.1016/j.amjhyper.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 109.Ogut O, Brozovich F. The potential role of MLC phosphatase and MAPK signaling in the pathogenesis of vascular dysfunction in heart failure. J Cell Mol Med. 2008;12:2158–2164. doi: 10.1111/j.1582-4934.2008.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Payne MC, et al. Dynamic changes in expression of myosin phosphatase in a model of portal hypertension. Am J Physiol Heart Circ Physiol. 2004;286:H1801–H1810. doi: 10.1152/ajpheart.00696.2003. [DOI] [PubMed] [Google Scholar]
- 111.Dakshinamurti S, et al. Regulation of pulmonary arterial myosin phosphatase activity in neonatal circulatory transition and in hypoxic pulmonary hypertension: a role for CPI-17. Ped Pulmonology. 2005;40:398–407. doi: 10.1002/ppul.20290. [DOI] [PubMed] [Google Scholar]
- 112.Belik J, et al. Myosin light chain phosphatase and kinase abnormalities in fetal sheep pulmonary hypertension. Pediatr Res. 1998;43:57–61. doi: 10.1203/00006450-199801000-00009. [DOI] [PubMed] [Google Scholar]