Abstract
Heat shock protein 70 (HSP70) is a molecular chaperone involved in protein folding and resistance to the deleterious effects of stress. Here we show that HSP70 suppresses transcription of c-fos, an early response gene that is a key component of the ubiquitous AP-1 transcription factor complex. HSP70 repressed Ras-induced c-fos transcription only in the presence of functional heat shock factor1 (HSF1). This suggests that HSP70 functions as a corepressor with HSF1 to inhibit c-fos gene transcription. Therefore, besides its known function in the stress response, HSP70 also has the property of a corepressor and combines with HSF1 to antagonize Fos expression and may thus impact multiple aspects of cell regulation.
INTRODUCTION
HSP70 is a member of the heat shock protein (HSP) family that enables the organism respond to stress. Under physiological conditions, HSP70 and its cognates play essential roles in modulating protein-protein interaction, participating in the folding, assembling, and translocation of intracellular proteins (Martin 1997; Nover and Scharf 1997; Pilon and Schekman 1999). HSP70 binds to unfolded proteins as a consequence of metabolic stress in vivo (Beckmann et al 1992). Recently, HSP70 has been found to bind its own transcriptional regulator–HSF1 and functions as an autoregulatory transcriptional repressor of HSF1 (Shi et al 1998). In addition, a number of other critical proteins, including p53 and NF-kB (Fourie et al 1997; Guzhova et al 1997), have been found to bind or interact with HSP70. This suggests that HSP70, in addition to its function as a molecular chaperone, may also be involved in cellular regulatory processes.
c-fos, an early response gene as well as a key component of the ubiquitous AP-1 transcription factor complex, is considered a master switch for both cell division and differentiation (Piechaczyk and Blanchard 1994). The Ras protein, which serves as a signal transducer in a manner similar to other signal GTP-binding proteins, transmits signals from the plasma membrane to nuclear targets such as transcription Fos. Previously, based on the finding that the expression of HSP70 and c-fos mRNAs was reciprocally affected by heat shock, we found that HSF1 is directly involved in a process that leads to antagonism of c-fos transcription and thus could influence multiple aspects of cell regulation (Chen et al 1997).
In this study, we demonstrate that HSP70 represses Ras-induced c-fos gene transcription in vitro under normal condition and after heat shock. However, this repression not only was dependent on HSP70 expression but also required active cooperation with HSF1. Our results therefore suggest that the molecular chaperone HSP70 functions with HSF1 as a corepressor of Ras-mediated c-fos gene transcription.
MATERIALS AND METHODS
Cells and growth conditions
Chinese hamster ovary K1 (CHO K1) cell line was purchased from the American Type Tissue Culture Collection (Rockville, MD, USA). Cells were was maintained in F-12 nutrient mixture (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, 100 units/mL penicillin G, 100 μg/mL streptomycin, and 2 mMl-glutamine and 2% sodium bicarbonate. Cells were cultured at 37°C in 5% CO2/95% air-humidified incubator. At approximately 70% to 90% confluence, cells were harvested using trypsin-EDTA, washed, and counted using a hemocytometer.
Constructs
Ha-Ras expression plasmid pH06T1 was a gift from Dr. M.C. Ostrowski (Ohio State University, USA). Human HPS70 plasmid pH2.3 was a gift from Dr. Richard Morimoto (Northwestern University, USA). pGL.reporter–fos396D219-81 was prepared as described before (Chen et al 1997). HSF1 expression plasmid was made as previously described (Chen et al 1997). Human HSP70 was cut with BamHI and EcoRI from pH 2.3 and then fitted in pcDNA 3.1/His B (Invitrogen Corp.). HSF1 deletion mutants were made by PCR with Xho I on 5′ primers and Afl II on 3′ primers and fitted in pcDNA 3.1(−) and then were subjected to sequencing for confirmation.
Oligonucleotides
HSP70 antisense oligo sequence was: cgc ggc ttt ggc cat. HSP70 sense oligo sequence was: gcg ccg aaa ccg gta.
Transient transfection and luciferase assay
CHO K1 cells were dispensed into 24-well plates at 3 × 104 cells/well and incubated for 20 to 24 h prior to liposome from Boehringer/Mannheim-mediated transfection (DOTAP) with reporter plasmids, expression plasmids, and control (to β-galactosidase plasmids) as described elsewhere (Chen et al 1997). Luciferase activity was normalized to β-galactosidase activity, which was used as a standard control. Results were expressed as fold increase in activity relative to control: vector transfected only (vector) or non–heat shock (NH).
Statistical analysis
The data were analyzed using a 2-tailed t-test after applying analysis of variance (ANOVA) to the data. Differences were considered significant when P ≤ 0.01.
RESULTS
HSP70 represses transcription of c-fos in vitro
To follow up on our previous finding that the expression of HSP70 and c-fos mRNAs was reciprocally affected by heat shock (Chen et al 1997), we first examined whether HSP70 is involved in the transcriptional repression of c-fos. By using CHO K1 cells in 24-well plates and cotransfection of human HSP70 (50 to 100 ng/well) and c-fos promoter (reporter plasmid pfos396D219–81, 250 ng/well) with or without cotransfection of Ha-Ras (100 ng/well), the c-fos promoter was repressed by HSP70 expression under both basal and Ras-induced conditions (Fig 1A). Since we previously reported that HSF1 represses Ras-induced c-fos, HSF1 was used as a positive control in this experiment (Chen et al 1997) (Fig 1A). In each experiment, HSF1 was more effective in repressing c-fos than HSP70. HSP70 repressed Ras (100 ng/well)-dependent c-fos activity in a dose-dependent manner (Fig 1B). To confirm that c-fos repression by HSP70 was due to the transcription/translation of transfected HSP70, different doses of an HSP70 antisense oligonucleotide were cotransfected with constant amounts of HSP70 (50 ng/well) and Ras (100 ng/well) expression plasmids. As shown in Figure 2, HSP70-mediated repression was totally blocked by increasing doses of antisense oligonucleotide, while sense oligonucleotide had no significant inhibition. These experiments indicate that the overexpressed HSP70 is indeed involved in transcriptional repression of Ras-activated c-fos gene in vitro.
Fig 1.
HSP70 overexpression represses activity of the c-fos promoter under basal conditions as well as under Ras-activation in vitro. (A) CHO-K1 cells transfected with c-fos promoter reporter plasmid pfos396D219-81 were cotransfected with either 50 ng/well human-HSP70 (HSP70), 50 ng/well human-HSF1 (HSF1) expression plasmids, or empty vector (pcDNA3.1(−)) with or without cotransfection of Ras (100 ng/ml) expression plasmid or vector (100 ng/ml). Cells were harvested 16 to 20 hours after transfection, and whole-cell extracts were subjected to luciferase assay. Bars represent mean relative luciferase activity ± standard deviation and show results from 3 independently performed experiments. (B) A constant amount of Ras (100 ng/well) and c-fos reporter (250 ng/well) were cotransfected with increasing doses of HSP70 expression plasmid, and whole-cell extracts were then subjected to Luciferase assay. Cells were harvested 16 to 20 hours after transfection, and whole-cell extracts were subjected to luciferase assay. Bars represent mean relative luciferase activity ± standard deviation and show results from 3 independently performed experiments
Fig 2.
Repression of the c-fos promoter in HSP70 overexpressing cells is blocked by cotransfection with antisense oligonucleotides directed against HSP70 mRNA. Increasing concentrations of HSP70 antisense oligonucleotide (μM) and sense oligoneucleotide (μM) were cotransfected with constant dosage of Ras (100 ng/well), HSP70 (50 ng/well) expression plasmid, and c-fos reporter in CHO-K1 cells. Cellular luciferase activity was assayed as described in the Materials and Methods section. Anti: HSP70 antisense oligos; Sense: HSP70 sense oligos. Cells were harvested 16 to 20 hours after transfection, and whole-cell extracts were subjected to luciferase assay. Bars represent mean relative luciferase activity ± standard deviation and show results from 3 independently performed experiments
HSP70 requires the cooperation of HSF1 to repress c-fos transcription
The next question addressed was whether transcriptional repression by HSP70 occurs directly or is mediated through the interaction of HSP70 with other protein(s). As HSP70 was shown previously to bind HSF1 in nuclear extracts (Nunes and Calderwood 1995) and to interact with HSF1 under non–heat shock conditions (Baler et al 1996) and HSF1 was found to mediate c-fos repression (Chen et al 1997), we examined whether repression could involve cooperation between HSP70 and HSF1. To investigate this, we first made a series deletion mutants of HSF1 to map the repression domains (Y. Xie and S.K. Calderwood, in preparation). HSF1-(1-229), which contains only the N-terminal DNA-binding domain and a part of a leucine-zipper domain, was used as a dominant negative mutant to compete with the endogenous HSF1. HSF1-(1-229) was shown previously to be null for repression of target promoters such as c-fos and c-fms (Y. Xie and S.K. Calderwood, in preparation). This construct was able to compete with wild-type HSF1 and block repression of c-fos by HSF1 (data not shown). In subsequent experiments, c-fos transcriptional repression by HSP70 was totally abolished by HSF1-(1-229) in a dose-dependent manner (Fig 3A). This suggests that the effect of HSP70 repression on Ras-induced c-fos transcription is dependent on the existence of functional endogenous HSF1. We also attempted to determine whether the converse is true, that is, whether HSF1 dependent repression requires HSP70 expression. To investigate this hypothesis, a dose range of HSP70 antisense oligonucleotides was cotransfected with a constant amount of HSF1. The data show that the repression of Ras-induced c-fos transcription by HSF1 is partially reversed by HSP70 antisense oligonucleotides (Fig 3B), which indicates that HSP70 functions as a corepressor of HSF1 in the transcriptional repression of Ras-induced c-fos.
Fig 3.
HSP70 functions in combination with HSF1 in the repression of the c-fos promoter. (A) HSP70 repression of c-fos promoter activity was abolished in cells transfected with HSF1-dominant negative construct cotransfection. Varying amounts of dominant negative HSF1 (HSF1-(1-229)) were cotransfected with constant dosages of Ras (100 ng/well) and HSP70 (50 ng/well) plasmids in CHO-K1. Cells then were subjected to luciferase assay 20 hours later. HSF1-M: HSF1 (1-229). Bars represent mean relative luciferase activity ± standard deviation and show results from 3 independently performed experiments. (B) HSP70 antisense oligonucleotides (μM) were partially effective in reversing HSF1 repression of c-fos. Different dosages of HSP70 antisense oligos were cotransfected with a constant amount of HSF1 (25 ng/well) as well as Ras (100 ng/well) expression plasmids in CHO-K1. Cells were collected 20 hours later and subjected to luciferase assay. Anti: HSP70 antisense oligos; Sense: HSP70 sense oligos. Bars represent mean relative luciferase activity ± standard deviation and show results from 3 independently performed experiments
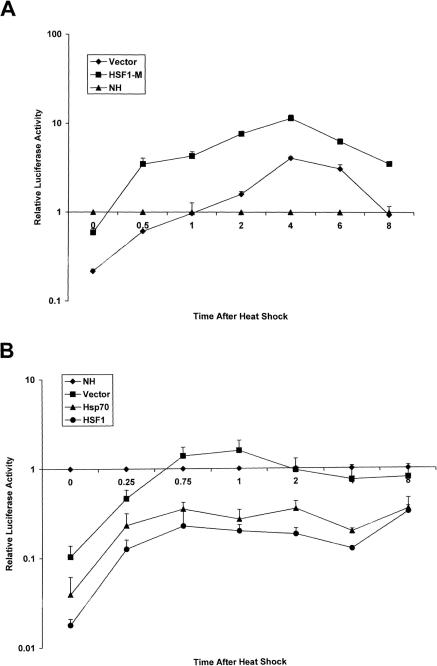
A combination of transient transfection and heat shock was also introduced to check the effect of HSP70 on c-fos transcriptional repression by endogenous HSF1. HSF1-(1-229) was used as a dominant negative construct to antagonize potential heat-induced c-fos transcriptional repression by endogenous HSF1 (Fig 4A). Heat caused initial repression of c-fos activity followed by a delayed increase (Fig 4A). In the presence of the dominant negative inhibitor, luciferase activity was increased by 5- to 6-fold under heat shock compared with the empty vector transfection control (Fig 4A). Endogenous HSF1 thus apparently represses c-fos activity during heat shock. By contrast, increased repression (up to 6-fold) was found in heat shocked cells subjected to HSP70 cotransfection when compared with empty vector controls (Fig 4B). Cotransfection of heat-shocked cells with HSF1 was used as a positive control in this experiment and also caused enhanced c-fos repression (Fig 4B). The significant difference between cells transfected with HSP70 and vector (up to 6-fold) could be observed both during the period when c-fos was repressed by heat shock and when rebound transcription appeared after about 40 minutes recovery from heat shock (Fig 4B).
Fig 4.
The role of HSP70 in repression of the c-fos promoter under heat shock conditions. (A) CHO-K1 cells were cotransfected with the c-fos promoter reporter plasmid (250 ng/well) and either the dominant negative HSF1-M construct (100 ng/well) or the empty (pcDNA 3.1(−)) plasmid (100 ng/well). Sixteen to 20 hours after transfection, cells were treated with or without heat shock (42.5°C for 1 hour). Cells were then assayed for luciferase activity at various intervals. Luciferase activity in heat-shocked cells is normalized to activity in non–heat-shocked cells. Lines represent mean relative luciferase activity ± standard deviation and show results from 3 independently performed experiments. (B) CHO-K1 cells were cotransfected with the c-fos promoter reporter plasmid (250 ng/well) and either the wild-type HSF1 construct (100 ng/well) or HSP70 (100 ng/well) or the empty (pcDNA 3.1(−)) plasmid (100 ng/well). Sixteen to 20 hours after transfection, cells were treated with or without heat shock (42.5°C for 1 hour). Cells were then assayed for luciferase activity at various intervals. Luciferase activity in heat-shocked cells is normalized to activity in non–heat-shocked cells. Lines represent mean relative luciferase activity ± standard deviation and show results from 3 independently performed experiments
DISCUSSION
Our studies suggest that mitogenic stimulation of c-fos transcription is blocked by heat shock at least partially through transcriptional corepression by HSP70 (Figs 1–2) and HSF1 (Chen et al 1997). Interestingly, HSP70-mediated c-fos repression is completely abolished by a dominant negative inhibitor of HSF1 repression with or without heat shock, while the repression of c-fos by HSF1 was partially reversed by HSP70 antisense oligos (Figs 3–4). This indicates that HSF1 plays the dominant role in gene repression, while HSP70 plays a subordinate role as a corepressor. There are a number of known mechanisms involved in transcriptional repression (Gray and Levine 1996; Herschbach and Johnson 1993; Sakai et al 1988; Chen et al 1997). Previous studies argue against a mechanism for c-fos repression by HSF1 involving the displacement of activating factors from the promoter. This was indicated by the finding that HSF1 does not bind to the fragment of the c-fos promoter used in these studies (Chen et al 1997). Similarly, it seems unlikely that HSP70 binds to the c-fos promoter, as no evidence for HSP70 binding to DNA has been obtained (Freeman et al 1995; Fung et al 1996; Henics et al 1999). These findings suggest a role for protein-protein interactions in gene repression by HSP70. Since HSP70 is a versatile molecular chaperone, this function may underlie interactions with either upstream signaling events or a specific coactivator in order to corepress the c-fos promoter with HSF1 (Chen et al 1997).
The domains within HSP70 that mediate c-fos repression are not indicated by this study. However, it has been shown that mammalian HSP70 interacts with HSF1 within the C-terminal activation domain through its carboxyl-terminal substrate binding domain to block transactivation of heat shock promoters (Shi et al 1995, 1998). A similar interaction may activate HSF1 to a transrepression active form. c-fos corepression is also specific for HSF1 and was not observed with other HSF family members, such as HSF2 (Chen et al 1997). HSF1 and HSF2 are highly homologous in the N-terminal DNA-binding domains and trimerization domains while diverging in the C-terminal regulatory and transactivation domains (Voellmy 1994; Wu 1995). This is evidence supporting a role for the C-terminus of HSF1 in interaction with HSP70.
These studies suggest that HSP70 plays a role in cell regulation over and above its role in protein folding and thermotolerance. Besides its role in heat shock and molecular chaperoning (Lund 1995; Martin 1997; Nover and Scharf 1997; Pfanner and Meijer 1997; Pilon and Schekman 1999), HSP70 plays a specialized role as a negative regulator of heat shock transcription factors, such as σ32 in E coli (McCarty et al 1996) and an autonegative regulator of HSF1 in eukaryotes by interacting with HSF1 (Shi et al 1995, 1998). HSP70 may also play some specific roles in other important cellular processes. HSP70 functions during embryogenesis (Luft and Dix 1999), in the immune response (Multhoff and Botzler, 1998), and in antigen processing (Srivastava 1997; Suzue and Young 1996). HSP70 also inhibits apoptosis in prostate carcinoma cell lines (E. Jones and S.K. Calderwood, in preparation) and T-cell hybridomas DO11.10 (Ahn et al 1999). A regulatory function for HSP70 at the interface between protein folding and protein degradation is indicated by some recent finding (Luders et al 2000). In addition, a number of transcription factors have been reported to interact with HSP70, such as p53 (Fourie et al 1997), RAP46 (Zeiner et al 1999), and NF-kB (Guzhova et al 1997), and now we show that HSP70 is involved in c-fos repression by interacting with HSF1.
In summary, HSP70 functions as a corepressor of the c-fos promoter acting in cooperation with HSF1. Thus, HSP70 may function as a transcription factor chaperone and may modulate the activity of its own transcription factor, altering both the activation and the repression functions of HSF1.
REFERENCES
- Ahn JH, Ko YG, Park WY, Kang YS, Chung HY, Seo JS. Suppression of ceramide-mediated apoptosis by HSP70. Mol Cell. 1999;9:200–206. [PubMed] [Google Scholar]
- Baler R, Zou J, Voellmy R. Evidence for a role of HSP70 in the regulation of the heat shock response in mammalian cells. Cell Stress Chaperones. 1996;1:33–39. doi: 10.1379/1466-1268(1996)001<0033:efaroh>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann RP, Lovett M, Welch WJ. Examining the function and regulation of hsp 70 in cells subjected to metabolic stress. J Cell Biol. 1992;117:1137–1150. doi: 10.1083/jcb.117.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Xie Y, Stevenson MA, Auron PE, Calderwood SK. Heat shock factor 1 represses Ras-induced transcriptional activation of the c-fos gene. J Biol Chem. 1997;272:26803–26806. doi: 10.1074/jbc.272.43.26803. [DOI] [PubMed] [Google Scholar]
- Fourie AM, Hupp TR, Lane DP, Sang BC, Barbosa MS, Sambrook JF, Gething MJ. HSP70 binding sites in the tumor suppressor protein p53. J Biol Chem. 1997;272:19471–19479. doi: 10.1074/jbc.272.31.19471. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Myers MP, Schumacher R, Morimoto RI. Identification of a regulatory motif in HSP70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung KL, Hilgenberg L, Wang NM, Chirico WJ. Conformations of the nucleotide and polypeptide binding domains of a cytosolic HSP70 molecular chaperone are coupled. J Biol Chem. 1996;271:21559–21565. doi: 10.1074/jbc.271.35.21559. [DOI] [PubMed] [Google Scholar]
- Gray S, Levine M. Transcriptional repression in development. Curr Opin Cell Biol. 1996;8:358–364. doi: 10.1016/s0955-0674(96)80010-x. [DOI] [PubMed] [Google Scholar]
- Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein HSP70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones. 1997;2:132–139. doi: 10.1379/1466-1268(1997)002<0132:msphiw>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henics T, Nagy E, Oh HJ, Csermely P, von Gabain A, Subjeck JR. Mammalian HSP70 and Hsp110 proteins bind to RNA motifs involved in mRNA stability. J Biol Chem. 1999;274:17318–17324. doi: 10.1074/jbc.274.24.17318. [DOI] [PubMed] [Google Scholar]
- Herschbach BM, Johnson AD. Transcriptional repression in eukaryotes. Annu Rev Cell Biol. 1993;9:479–509. doi: 10.1146/annurev.cb.09.110193.002403. [DOI] [PubMed] [Google Scholar]
- Luders J, Demand J, Hohfeld J. The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/HSP70 and the proteasome. J Biol Chem. 2000;275:4613–4617. doi: 10.1074/jbc.275.7.4613. [DOI] [PubMed] [Google Scholar]
- Luft JC, Dix D.J. HSP70 expression and function during embryogenesis. Cell Stress Chaperones. 1999;4:162–170. doi: 10.1379/1466-1268(1999)004<0162:heafde>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund PA. The roles of molecular chaperones in vivo. Essays Biochem. 1995;29:113–123. [PubMed] [Google Scholar]
- Martin J. Molecular chaperones and mitochondrial protein folding. J Bioenerg Biomembr. 1997;29:35–43. doi: 10.1023/a:1022407705182. [DOI] [PubMed] [Google Scholar]
- McCarty JS, Rudiger S, Schonfeld HJ, Schneider-Mergener J, Nakahigashi K, Yura T, Bukau B. Regulatory region C of the E. coli heat shock transcription factor, sigma32, constitutes a DnaK binding site and is conserved among eubacteria. J Mol Biol. 1996;256:829–837. doi: 10.1006/jmbi.1996.0129. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Botzler C. Heat-shock proteins and the immune response. Ann NY Acad Sci. 1998;851:86–93. doi: 10.1111/j.1749-6632.1998.tb08980.x. [DOI] [PubMed] [Google Scholar]
- Nover L, Scharf KD. Heat stress proteins and transcription factors. Cell Mol Life Sci. 1997;53:80–103. doi: 10.1007/PL00000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes SL, Calderwood SK. Heat shock factor-1 and the heat shock cognate 70 protein associate in high molecular weight complexes in the cytoplasm of NIH-3T3 cells. Biochem Biophys Res Commun. 1995;213:1–6. doi: 10.1006/bbrc.1995.2090. [DOI] [PubMed] [Google Scholar]
- Pfanner N, Meijer M. The Tom and Tim machine. Curr Biol. 1997;7:R100–R103. doi: 10.1016/s0960-9822(06)00048-0. [DOI] [PubMed] [Google Scholar]
- Piechaczyk M, Blanchard JM. c-fos proto-oncogene regulation and function. Crit Rev Oncol Hematol. 1994;17:93–131. doi: 10.1016/1040-8428(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Pilon M, Schekman R. Protein translocation: how HSP70 pulls it off. Cell. 1999;97:679–682. doi: 10.1016/s0092-8674(00)80780-1. [DOI] [PubMed] [Google Scholar]
- Sakai DD, Helms S, Carlstedt-Duke J, Gustafsson JA, Rottman FM, Yamamoto KR. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988;2:1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Shi Y, Kroeger PE, Morimoto RI. The carboxyl-terminal transactivation domain of heat shock factor 1 is negatively regulated and stress responsive. Mol Cell Biol. 1995;15:4309–4318. doi: 10.1128/mcb.15.8.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava PK. Purification of heat shock protein-peptide complexes for use in vaccination against cancers and intracellular pathogens. Methods. 1997;12:165–171. doi: 10.1006/meth.1997.0464. [DOI] [PubMed] [Google Scholar]
- Suzue K, Young RA. Heat shock proteins as immunological carriers and vaccines. Exs. 1996;77:451–465. doi: 10.1007/978-3-0348-9088-5_30. [DOI] [PubMed] [Google Scholar]
- Voellmy R. Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein gene expression in higher eukaryotes. Crit Rev Eukaryot Gene Expr. 1994;4:357–401. [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- Zeiner M, Niyaz Y, Gehring U. The HSP70-associating protein Hap46 binds to DNA and stimulates transcription. Proc Natl Acad Sci USA. 1999;96:10194–10199. doi: 10.1073/pnas.96.18.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]