Abstract
A tumor-selective cell surface localization of heat shock protein 70 (Hsp70), the major heat-inducible member of the Hsp70 group, correlates with an increased sensitivity to lysis mediated by human natural killer (NK) cells and, therefore, might be of clinical relevance. With the exception of mammary carcinomas, an Hsp70 plasma membrane expression was found on freshly isolated human biopsy material of colorectal, lung, neuronal, and pancreas carcinomas, liver metastases, and leukemic blasts of patients with acute myelogenous leukemia. Since normal tissues and bone marrow of healthy human individuals do not express Hsp70 on the cell surface, Hsp70 can be considered as a tumor-selective structure in vivo. Furthermore, we demonstrate that autologous, Hsp70-positive leukemic blasts can be killed by NK cells stimulated with low doses of interleukin 2 plus recombinant Hsp70 protein.
INTRODUCTION
Besides their intracellular chaperoning functions, heat shock proteins (Hsps) have been found to play key roles in cancer immunity. Members of the Hsp70 and Hsp90 family are known to chaperone tumor-derived peptides to major histocompatibility complex (MHC) class I molecules to elicit an anticancer immune response mediated by T cells (Tamura et al 1997). Hsp70, the major heat-inducible member of the Hsp70 group, has been detected on the cell surface of tumor cells but not on normal cells (Ferrarini et al 1992; Multhoff et al 1995a). This unusual Hsp70 plasma membrane expression correlates with an increased sensitivity to allogeneic natural killer (NK) cells (Botzler et al 1996a; Botzler et al 1996b; Multhoff et al 1997). Recently, we demonstrated that the cytolytic activity against Hsp70-expressing tumor cells could be enhanced by an incubation of NK cells with low doses of interleukin 2 (IL-2) plus recombinant Hsp70 (rHsp70) protein (Multhoff et al 1999). With respect to these findings and due to the fact that normal cells fail to express Hsp70 on the plasma membrane, we ask the following question: “Does Hsp70 act as a tumor-selective recognition structure in vivo?” Therefore, we determined Hsp70 membrane expression on freshly isolated primary tumor material, liver metastases, bone marrow of leukemic patients, and normal tissues. Furthermore, the immunostimulatory capacity of rHsp70-stimulated NK cells was analyzed within an autologous tumor model.
RESULTS AND DISCUSSION
Previously, we and others showed an unusual plasma membrane localization of cytoplasmic Hsps with a molecular weight of 70 and 90 kDa (Ferrarini et al 1992; Tamura et al 1993; Multhoff et al 1995a; Piselli et al 1995; Altmeyer et al 1996). Although Hsps are ubiquitously distributed and among the most highly conserved proteins, they have been shown to elicit a specific, cellular anticancer immune response mediated by cytotoxic T cells (Tamura et al 1997). The paradoxical role of Hsps in cancer immunity can be explained by species-specific differences in the sequence of Hsps that might act as classic foreign antigens, by molecular mimicry, or by breaking peripheral tolerance (Srivastava 1994). On the one hand, immunization with Hsp-peptide preparations isolated from tumors has been reported to protect against cancer (Tamura et al 1997; Schild et al 1999). On the other hand, membrane-expressed Hsps might bind peptides and, therefore, elicit an immune response. Beside T cells, IL-2–stimulated NK cells have been shown to play key roles in cancer immunotherapy (Whiteside et al 1998; Rosenberg et al 1998). We were the first who demonstrated that rHsp70-stimulated, transiently plastic adherent NK cells are potent effector cells in the recognition of Hsp70-expressing tumor cell lines (Multhoff et al 1995b). The importance of Hsp70 as a relevant target structure for NK cells was confirmed by different assays: (1) Hsp70-specific antibodies specifically block the lysis of Hsp70-expressing tumor cells (Multhoff et al 1995b); (2) incubation of NK cells using human rHsp70 protein not only increases the cytolytic activity against Hsp70-expressing tumors but also stimulates the proliferation of plastic-adherent NK cells (Multhoff et al 1999); and (3) T cells exhibited no differences in the lytic activity of Hsp70-expressing and nonexpressing tumor cells (Multhoff et al 1995b).
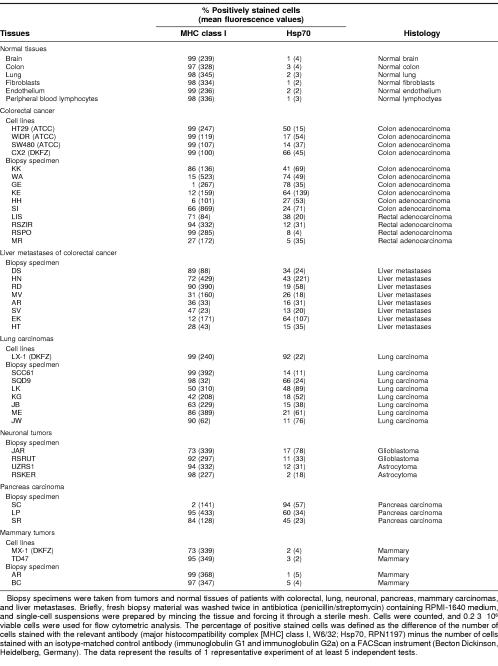
To evaluate the clinical relevance of Hsp70 as a recognition structure for autologous NK cells, the MHC class I and Hsp70 membrane expression was determined on viable, single-cell suspensions generated from tumor biopsy material of patients with carcinomas and bone marrow aspirates of leukemic patients. As controls, tumor cell lines, normal tissues, and bone marrow of healthy human individuals were also analyzed. The percentage of positively stained cells was defined as the difference of the number of cells stained with the relevant antibody minus the number of cells stained with an isotype-matched control antibody. The mean fluorescence, as a marker for the antigen density per cell, is given in parentheses. As shown in Table 1, normal tissues, including, brain, colon, lung, fibroblasts, and umbilical vein–derived endothelial cells and peripheral blood lymphocytes (PBLs), express MHC class I molecules on the cell surface but were negative for Hsp70 membrane expression. However, studies on freshly isolated human tumor biopsy material revealed that, with the exception of mammary carcinomas, Hsp70 molecules could be detected on primary tumors, including colon, lung, neuronal, and pancreas carcinomas. All tested liver metastases of patients with colorectal cancer were also positive for Hsp70; in 5 of 8 liver metastases, the MHC class I expression was down-regulated. With respect to killer cell inhibitory receptors on NK cells that are known to inhibit NK cell activity after interaction with MHC class I molecules (Lanier 1998), we hypothesize that Hsp70-positive metastases with a decreased MHC class I expression provide ideal targets for NK cell therapy.
Table 1.
Hsp70 membrane expression on normal tissues and human tumors
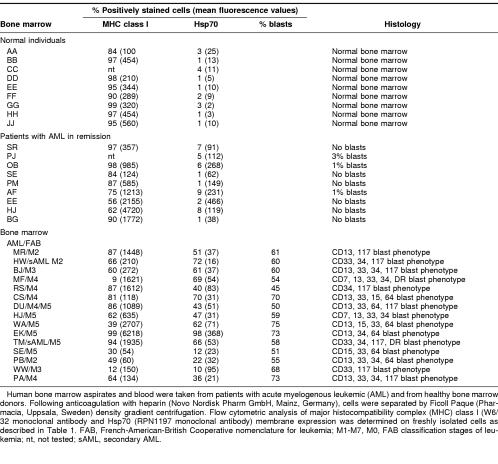
In total, 75 bone marrow aspirates of patients with acute myelogenous leukemia (AML) and different French-American-British Cooperative group types were analyzed; 56 (75%) of 75 bone marrow aspirates exhibit an Hsp70-positive phenotype. The amount and the immunophenotype of the blasts were determined in 15 of 75 bone marrow aspirates of patients with AML by double and triple staining technique using the following fluorescence-labeled antibodies (Becton Dickinson, Heidelberg, Germany) in different combinations: HLA-DR, CD7, CD13, CD14, CD15, CD16, CD18, CD33, CD34, CD64, and CD117. As shown in Table 2, in 11 of 15 tested bone marrow aspirates, a correlation of the percentage of leukemic blasts with the number of Hsp70 positively stained cells was observed. Bone marrow of healthy human individuals (n = 9) or bone marrow of patients with AML in remission (n = 9) did not express Hsp70 on the plasma membrane. Taken together, these findings suggest that Hsp70 acts as a tumor-selective recognition structure in vivo. Furthermore, one might speculate about Hsp70 membrane expression as a prognostic marker to characterize leukemic blasts.
Table 2.
Hsp70 membrane expression on bone marrow cells of healthy individuals and leukemic patients
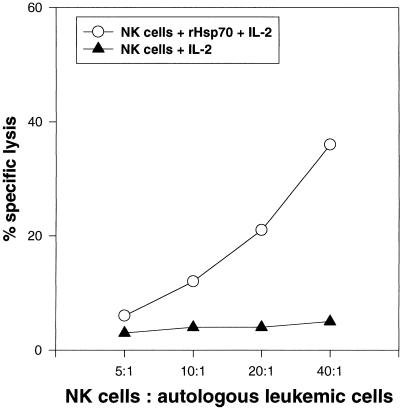
The role of Hsp70 as a recognition structure for allogeneic NK cells was demonstrated for several tumor cell lines, including the colon carcinoma sublines CX+ and CX−, which differ profoundly with respect to their capacity to express Hsp70 on the plasma membrane but exhibit an identical MHC and adhesion molecule expression (Multhoff et al 1995b; Multhoff et al 1997). NK cells, stimulated with low-dose IL-2 plus rHsp70 protein (SPP-755, StressGen, British Columbia, Canada), have the capacity to kill Hsp70-expressing, allogeneic tumor cells significantly better compared with NK cells stimulated with low-dose IL-2 only (Multhoff et al 1999). To exclude an immunostimulatory effect induced by lipopolysaccharide, it is important to note that rHsp70 used for the stimulation of NK cells is endotoxin free as determined by the Limulus ambeocyte lysate kit. In the present study, the immunostimulatory capacity of rHsp70-activated NK cells was analyzed against autologous, Hsp70-positive leukemic blasts. Figure 1 shows the results of 1 of 6 representative experiments. NK-enriched effector cells derived from the peripheral blood of a patient with AML stimulated with low-dose IL-2 plus rHsp70 exhibit a significant cytolytic response against autologous, Hsp70-positive leukemic blasts. In contrast, IL-2 stimulation alone was insufficient to stimulate the cytotoxic response against autologous leukemic blasts. As an internal control, the lytic activity of both NK cell populations was tested against allogeneic CX+ and CX− tumor cells. As expected, the lysis of CX+ cells was enhanced if the NK cells of the patient were stimulated with low-dose IL-2 plus rHsp70; the lysis of CX− tumor cells remained unaffected (data not shown). In summary, we can state that the cytotoxic response against autologous, Hsp70-expressing leukemic blasts is inducible by incubation of NK cells with low-dose IL-2 plus rHsp70. Studies are ongoing that investigate the feasibility and in vitro efficacy of rHsp70-stimulated NK cells against autologous tumor cells in a clinical phase 1 trial.
Fig 1.
Comparison of the cytolytic activity of NK-enriched effector cells (Vujanovic et al 1993) stimulated either with low-dose IL-2 (100 IU/mL) plus rHsp70 (10 μg/mL; SPP-755, StressGen, Victoria, British Columbia, Canada) or with IL-2, against autologous, Hsp70-expressing leukemic blasts. NK cells and leukemic blasts were derived from PBLs and bone marrow of a patient with AML (bone marrow contains 98% Hsp70-positive cells). The cytolytic activity was measured in a standard 51Cr assay (MacDonald et al 1974) on day 4 after stimulation. Different dilutions of the effector cells (40:1, 20:1, 10:1, 5:1) were incubated with 51Cr-labeled (100 μCi of NaCr51O4, NEN-Dupont, Boston, MA, USA) target cells (3 × 103 cells per well) in duplicates at a final volume of 200 μL of RPMI-1640 medium supplemented with 10% fetal calf serum at 37°C for 4 hours in 96-well U-bottom plates (Greiner, Nuertingen, Germany). The radioactivity of the supernatants was counted in a γ-counter (Packard Instruments). The percentage of specific lysis was determined according to the following equation: (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. Percentage of spontaneous release of primary leukemic cells was always less than 30%
Acknowledgments
This work was supported by grant number SFB455.
REFERENCES
- Altmeyer A, Maki RG, Feldweg AM, Heike M, Protopopov VP, Masur SK, Srivastava PK. Tumor-specific cell surface expression of the -KDEL containing, endoplasmic reticular heat shock protein gp96. Int J Cancer. 1996;69:340–349. doi: 10.1002/(SICI)1097-0215(19960822)69:4<340::AID-IJC18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Botzler C, Issels R, Multhoff G. Heat-shock protein 72 cell-surface expression on human lung carcinoma cells in associated with an increased sensitivity to lysis mediated by adherent natural killer cells. Cancer Immunol Immunother. 1996a;43:226–230. doi: 10.1007/s002620050326. [DOI] [PubMed] [Google Scholar]
- Botzler C, Kolb H-J, Issels RD, Multhoff G. Noncytotoxic alkyl-lysophospholipid treatment increases sensitivity of leukemic K562 cells to lysis by natural killer cells (NK) Int J Cancer. 1996b;65:633–638. doi: 10.1002/(SICI)1097-0215(19960301)65:5<633::AID-IJC13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 1992;51:613–619. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–367. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- MacDonald HR, Engers HD, Cerottini JC, Brunner KT. Generation of cytotoxic T lymphocytes in vitro. J Exp Med. 1974;140:718–722. doi: 10.1084/jem.140.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells: a recognition structure for Natural Killer cells. J Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Eissner G, Issels R. CD3− large granular lymphocytes recognize a heat-inducible immunogenic determinant associated with the 72-kD heat shock protein on human sarcoma cells. Blood. 1995b;86:1374–1382. [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Müller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995a;61:272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Mizzen L, Winchester CC, Milner CM, Wenk S, Kampinga HH, Laumbacher B, Johnson J. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of NK cells. Exp Hematol. 1999;27:1627–1636. doi: 10.1016/s0301-472x(99)00104-6. [DOI] [PubMed] [Google Scholar]
- Piselli P, Vendetti S, Poccia F, Cicconi R, Mattei M, Bolognesi A, Stripe F, Colizzi V. In vitro and in vivo efficacy of heat shock protein specific immunotoxins on human tumor cells. J Biol Regul Homeost Agents. 1995;9:55–62. [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer with high-dose interleukin-2: identification of the antigen mediating response. Ann Surg. 1998;228:307–319. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild H-J, Arnold-Schild A, Lammert E, Rammensee H-G. Stress proteins and immunity mediated by cytotoxic T lymphocytes. Curr Opin Immunol. 1999;11:109–114. doi: 10.1016/s0952-7915(99)80019-3. [DOI] [PubMed] [Google Scholar]
- Srivastava PK. Heat shock proteins in immune response to cancer: the fourth paradigm. Experientia. 1994;50:1054/FPAGE>–1060. doi: 10.1007/BF01923461. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Tsuboi N, Sato N, Kikuchi K. 70 kDa heat shock cognate protein is a transformation-associated antigen and a possible target for the host's anti-tumor immunity. J Immunol. 1993;151:5516–5524. [PubMed] [Google Scholar]
- Vujanovic NL, Rabinovich H, Lee YJ, Herbermann RB, Whiteside TL. Distinct phenotypic and functional characteristics of human natural killer cells obtained by rapid IL-2 adherence to plastic. Cell Immunol. 1993;151:133–137. doi: 10.1006/cimm.1993.1227. [DOI] [PubMed] [Google Scholar]
- Whiteside TL, Vujanovic NL, Herbermann RB. Natural killer cells and tumor therapy. Curr Top Microbiol Immunol. 1998;230:221–244. doi: 10.1007/978-3-642-46859-9_13. [DOI] [PubMed] [Google Scholar]