Abstract
The Notch signaling pathway is an important regulation system for the development and self-renewal of different tissues. A specific feature of this signaling cascade is the function of Notch as a surface receptor and regulator of gene expression. Hence, Notch activation and signal transduction requires the proteolytic release of the Notch intracellular domain (NICD), which activates the transcription of cell-specific genes after its transport into the nucleus. To date, little is known about the mechanisms that mediate NICD nuclear import. We here show that transport of NICD into the nucleus is mediated by the canonical importin α/β1 pathway. GST pull-down experiments revealed that NICD binds via one of its four potential nuclear localization signals to importins α3, α4, and α7, but not to α1 and α5. siRNA-mediated knockdown experiments showed that importins α3, α4 (and to a lesser extent, α7) mediate nuclear import of NICD and thus are directly involved in Notch signaling.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0378-7) contains supplementary material, which is available to authorized users.
Keywords: Notch signaling, Nuclear import, Importin alpha, Myoblast
Introduction
The Notch signaling pathway is essential for numerous developmental decisions in all parts of the body and is conserved from invertebrates to vertebrates [1]. In mammals, the Notch family consists of four isoforms, Notch1-4 [2], that function as both transmembrane receptors and transcriptional modulators. Signaling is initiated by ligands of the DSL (Delta/Serrate/LAG-2) family, membrane proteins located on adjacent cells. Binding of the ligand induces a conformational change that exposes a cleavage site to ADAM (a disintegrin and metalloproteinase)17/TACE (tumor necrosis factor-α-converting enzyme) that leads to removal of most of the Notch extracellular domain. The remaining membrane-anchored fragment termed NotchΔE is substrate for γ-secretase, which executes an intramembrane proteolytic cleavage resulting in liberation of a small peptide into the extracellular space [3] and the Notch intracellular domain (NICD) into the cytoplasm [4]. Upon nuclear translocation, NICD cooperates with CSL (CBF1/Su(H)/LAG-1), Mastermind, and coactivators to induce transcription of target genes. Regulation of Notch signaling takes place at various stages and by different means. Posttranslational modifications and trafficking events regulate Notch activity, for example by ubiquitinylation and subsequent endocytosis [5]. Within the nucleus, Notch activity is modulated by chromatin and histone modifications [for review see 6]. In general, the regulation of nuclear transport has been shown to be important for transcriptional regulation in development and disease [7, 8], but whether this applies to Notch signaling as well has yet to be demonstrated. In that respect, the PI3K/Akt pathway has been implicated in nuclear translocation of NICD, suggesting that phosphorylation modulates nuclear import [9, 10]. Typically, transport of large molecules into the nucleus is mediated by a heterodimeric complex of importin α and β1 [11]. The adaptor protein importin α binds to classical nuclear localization signals (NLSs) on cargo molecules, whereas the transport receptor importin β1 mediates translocation through the nuclear pore complex. Five importin α family members have been identified in mouse: importins α1, α3, α4, α5, and α7. In humans, there is an additional importin α6.
In this study, we provide for the first time evidence that importins α3, α4, and α7, adapter proteins in the classical importin α/β1 transport pathway, mediate the nuclear import of NICD in mouse myoblast and human HeLa cells.
Materials and methods
Antibodies
The following antibodies were used. Rabbit anti-cleaved Notch1 (Val1744; Cell Signaling Technology), rat anti-importin α1 (1A6; Sigma), goat anti-importin α3 (Everest Biotech), goat anti-importin α4 (Everest Biotech), rabbit anti-importin α7 [12], mouse anti-importin β1 (3E9; Dianova), mouse anti-myc (9E10; Santa Cruz Biotechnology), rabbit anti-beta Actin (ab8227; Abcam), goat anti-GST (GE Healthcare) and normal goat IgG (Santa Cruz Biotechnology). In Western blotting, secondary horseradish peroxidase-conjugated anti-mouse and anti-rabbit (Promega), anti-goat and anti-rat (Santa Cruz Biotechnology) immunoglobulins were used. Secondary anti-rabbit or anti-goat antibodies conjugated to fluorophore Alexa 488/555 for immunofluorescence were purchased from Invitrogen.
Cell lines and transfections
HeLa Kyoto cells and mouse myoblast C2C12 cells were kindly provided by Rainer Pepperkok (EMBL, Heidelberg) and Rüdiger Rudolf (Forschungszentrum Karlsruhe, Eggenstein-Leopoldshafen), respectively. Cells were grown in standard culture conditions with Dulbecco’s modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. For stable transfections of HeLa Kyoto and HEK293 cells Lipofectamine 2000 (Invitrogen) was used according to the manufacturer’s instruction. Transfections of C2C12 cells were performed with a 2:1 ratio (μl/μg) of Lipofectamine 2000 to plasmid DNA immediately after plating freshly trypsinized cells.
Plasmids and DNA manipulations
NotchΔE without the RAM and PEST domain tagged C-terminally with 6 myc-tags (pCS2+ NΔE(ΔRAM), derived from mouse Notch1) [4] was obtained from Raphael Kopan (Washington University, St. Louis, USA). To clone NotchΔE without tag and with EGFP-tag (NotchΔE-EGFP), standard PCR was performed using the forward primer 5′-GGATCCACTAGTAACGGCCG-3′ (binds to pCS2+ vector sequences upstream of the 5′-UTR and signal sequence of Notch1) and reverse primer either with stop codon (underlined) 5′-TCTAGA TTACTCGAGCTGTCCAACAGGCAG-3′ or without 5′-TCTAGACTCGAGCTGTCCAACAGGCAG-3′. Both products were cloned into BamHI/XbaI sites of pcDNA3.1/Hygro(+) (Invitrogen). EGFP was amplified from pEGFP-N1 (Clontech) and subsequently cloned C-terminally of NotchΔE. To create NLS mutations in NotchΔE we used QuikChange™ site-directed mutagenesis kit (Stratagene). The primers used are 5′-GGAGACGAAGACCTGGAGACCGCCGCATTCGCCTTTGAGGAGCCAGTAGTTCTCC-3′ (mNLS3), 5′-CTCAAGTCTGCCACACAGGGCGCCGCTGCCGCCGCACCCAGCACCAAAGGGCTGGC-3′ (mNLS4a) and 5′-GCAAGGAAGCTAAGGACCTCGCCGCAGCCGCTGCCGCCTCCCAGGATGGCAAGGGCTGCC-3′ (mNLS4b). Mouse NICD as well as importin α1, α3, α4, α5 and α7 genes were amplified from C2C12 cDNA using the following forward and reverse primers: NICD (GenBank™ accession number NM_008714) 5′-GGATCCAGGTGCTGCTGTCCCGCAAGCG-3′ and 5′-GTCGACTTATTTAAATGCCTCTGGAATGTGGGTG-3′; importin α1 (Kpna2, GenBank™ accession number NM_010655) 5′-GGATCCAGATGTCCACGAACGAGAATGCTAA-3′ and 5′-CTCGAGTTAGAAGTTAAAGGTCCCAGGAGCTCC-3′; importin α3 (Kpna4, GenBank™ accession number NM_008467) 5′-GGATCCAGATGGCGGACAACGAGAAATTGGAC-3′ and 5′-CTCGAGCTAAAACTGGAACCCCTCTGTTGGTAC-3′; importin α4 (Kpna3, GenBank™ accession number NM_008466) 5′-GGATCCAGATGGCCGAGAACCCCGGCTTG-3′ and 5′-CTCGAGTTAGAAATTAAATTCTTTTGTTTG-3′; importin α5 (Kpna1, GenBank™ accession number NM_008465) 5′-GGATCCAGATGTCCACACCAGGAAAAGAG-3′ and 5′-CTCGAGTCAAAGCTGGAAACCTTCCATAG-3′; importin α7 (Kpna6, GenBank™ accession number NM_008468) 5′-GGATCCAGATGGAGACCATGGCAAGCCC-3′ and 5′-CTCGAGCTATTATAGCTGGAAGCCCTC-3′. Restriction sites in all primers are in bold. PCR products were subsequently cloned into pGEX-5X-1 (Amersham Biosciences). To create mNLS3 in GST-tagged NICD, the primers described above were used. The luciferase-based Notch reporter (12×CSL-Luz) was constructed by inserting a 12×CSL promoter cassette into pGL4.20 (Promega). The promoter cassette was cut out from 12×CSL-DsRed Express DR construct [13] (kindly provided by Urban Lendahl, Karolinska Institute, Stockholm) with XbaI/HindIII and ligated into pGL4.20 cut with NheI/HindIII. NICD-myc [10] and human Delta1 were kindly provided by Incheol Shin, Hanyang University, Seoul and Falk Fahrenholz, Universität Mainz, respectively.
Recombinant expression and purification of proteins
Glutathione-S-transferase (GST) protein and GST fusion proteins were expressed in Escherichia coli Rosetta cells at 25°C for 2 h with 0.1 mM isopropyl-β-D-thiogalactopyranosid induction. Bacteria were lysed in phosphate buffered saline (PBS) and protease inhibitor mix (Sigma) by sonication and clarified by centrifugation (12,000 rpm, 15 min, 4°C). Bacterial cell extracts containing GST protein or GST fusion proteins were allowed to bind to Glutathione Sepharose™ 4 Fast Flow beads (GE Healthcare) in PBS with rotation at 4°C for 60 min, followed by three times washing with PBS. His-importin α1 (human KPNA2) [14], His-S-importin β1 [15], His-transportin [16] and wild type Ran [17] were expressed and purified as described.
In vitro nuclear import assays
Nuclear import assays of NICD in digitonin-permeabilized HeLa cells were essentially performed as described [18]. Import mixtures contained 200 nM of GST-NICD, 30 μM BSA, 4 μM Ran, an ATP regenerating system (1 mM ATP, 5 mM Kreatinphosphat, 20 U/ml Kreatinphosphatkinase) and 500 nM of His-tagged importins or transportin. After the reaction, cells were washed with transport buffer (20 mM HEPES pH 7.3, 110 mM KOAc, 2 mM Mg(OAc)2, 1 mM EGTA, 2 mM DTT, protease inhibitor mix), fixed with 3.7% formaldehyde and subjected to indirect immunostaining using anti-GST and donkey anti-goat Alexa 488 antibodies as well as DAPI to stain DNA. For quantitation of import efficiencies, a Cell Profiler Pipeline for automated analysis was used [19]. Briefly, DAPI fluorescence was used for single-cell discrimination and creation of image masks of the nuclear area. The nuclear image masks were then used to measure the nuclear EGFP signal that corresponds to the amount of imported protein.
Preparation of lysates
Cells were lysed in STEN lysis buffer (50 mM Tris pH 7.6, 150 mM NaCl, 2 mM EDTA, 1% NP40 and protease inhibitor mix) on ice for 30 min. Cell debris was removed by centrifugation (13,000 rpm, 15 min, 4°C). Cell lysates for GST pull-down and immunoprecipitation experiments were diluted with 1× STE (50 mM Tris pH 7.6, 150 mM NaCl, 2 mM EDTA and protease inhibitor mix) to achieve a final concentration of 0.5% NP40. Homogenization of mouse skeletal muscle was performed with a ratio of 100 mg tissue to 1 ml STEN lysis buffer using a syringe, and debris was removed by centrifugation (13,000 rpm, 15 min, 4°C).
Importin binding assay, immunoprecipitation, SDS-PAGE, and Western blotting
For GST pull-down experiments, Glutathione Sepharose™ beads alone or loaded with GST protein or GST fusion proteins were mixed with cell lysate and rotated at 4°C for 2 h, followed by three times washing with 1× STEN (50 mM Tris pH 7.6, 150 mM NaCl, 2 mM EDTA, 0.2% NP40 and protease inhibitor mix). Sepharose™ beads were dissolved in 2× Laemmli sample buffer and the proteins were separated on SDS-PAGE. Gels were cut at appropriate positions and either stained with Coomassie Brilliant Blue or transferred onto PVDF membranes and blotted with antibodies as indicated. For immunoprecipitation experiments, Protein G Sepharose™ (GE Healthcare) was incubated with 1 μg of goat anti-importin α4 antibody or normal goat IgG in STEN lysis buffer containing 0.5% NP40 for 2 h. The beads were then mixed with lysate of C2C12 cells transiently transfected with NICD-myc and rotated overnight at 4°C, followed by three times washing with 1× STEN. Proteins were separated on SDS-PAGE and Western blotting was performed using indicated antibodies.
siRNA
The following siRNAs were purchased from Dharmacon. ON-TARGETplus siRNAs for mouse importins: Non-targeting siRNA pool, D-001810-10; importin α1, L-041791-00; importin α3, L-058423-01; importin α4, L-058757-01; importin α7, L-047028-01; importin β1, L-058740-00. siGENOME siRNAs for human importins: Non-Targeting siRNA Pool #2, D-001206-14; importin α1, D-004702-02; importin α3, D-017477-01; importin α4, D-011306-01; importin α7, D-017295-01; importin β1, D-017523-01.
Automated microscopy
HeLa Kyoto cells stably transfected with Notch∆E-EGFP were reverse transfected with appropriate siGENOME siRNAs according to the manufacturer’s instruction. A mixture of siRNA (final concentration 25 nM) and Dharmafect 1 (Dharmacon) (0.1 μl per well) diluted in OPTI-MEM was aliquoted in 384 well microtiter plates. Cells diluted in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin were added to the wells at a concentration of 1.750 cells per well. To inhibit γ-secretase DAPT (ALX-270-416, Alexis Biochemicals) was used at a concentration of 1 μM. After incubation for 60 h the cells were fixed with 4% PFA and stained with DAPI. Images were acquired on an ArrayScan VTi automated imaging platform (Thermo Scientific Cellomics) and analyzed with the “CompartmentalAnalysisV3” BioApplication of the platform. For Fig. 1b, images were taken manually from HeLa Kyoto cells plated on coverslips and transiently transfected with NotchΔE constructs and analyzed as described above.
Fig. 1.
NICD is imported into the nucleus via the canonical importin α/β1 pathway and a classical NLS. a Digitonin-permeabilized HeLa cells were incubated with GST-NICD as well as Ran (ctrl) and with human importin α1, importin β1 or transportin (transp.) as indicated. After indirect immunofluorescence with anti-GST antibodies and DAPI to stain DNA, cells were imaged under identical conditions by fluorescence microscopy. The mean nuclear fluorescence of 250–379 cells per condition is depicted and was set to 0 in the control (ctrl). Error bars indicate the variation from the mean. AU, arbitrary units. The small stimulation of nuclear import by importin α1 alone probably results from residual importin β1 that is not removed from the permeabilized cells [35]. b NotchΔE or NotchΔE with NLS mutations as detailed in Fig. S1A were transfected in HeLa cells, fixed after 24 h and immunostained with NICD-specific antibody. DNA was stained with DAPI. The ratio EGFPnuc/EGFPenuc was determined as depicted in Fig. 3b. Displayed is the log(ratio), error bars indicate SEM of 120–200 cells per condition. Nuc, nuclear; enuc, extra-nuclear. Scale bar, 10 μm
Reporter assays
C2C12 cells (6-cm dishes) were transfected with 200 pmol of appropriate ON-TARGETplus siRNAs and transfected again after 24 h with 4 μg of total DNA containing 2 μg of reporter construct 12×CSL-Luz, 2 μg of Delta1 cDNA, and 40 ng of pGL4.74 (Promega) as an expression control. After a further 24 h, analysis was performed using the Dual-Luciferase® Reporter Assay System (Promega) according to the manual.
Quantitative real-time PCR
Total RNA from C2C12 cells was isolated using NucleoSpin® RNA II kit (Macherey–Nagel, Germany). Then 2 μg RNA was reverse-transcribed using the Transcriptor High Fidelity cDNA Synthesis Kit (Roche, Germany) for first-strand cDNA synthesis with 2.5 μM oligo(dT)18 primer according to the manufacturer’s protocol. All cDNA samples were diluted with RNase-free water before being used as template in quantitative real-time PCR (RT-qPCR) analysis. RT-qPCR was performed using an iCycler (Bio-Rad, USA) and the SYBR Green SensiMix™ dT (Quantace, Germany). Amplification was carried out in 20-μl reaction mixtures containing 5 μl of diluted cDNA template and 2.5 μM of each primer. The following primers were used: Hey1: 5′-TGAGCTGAGAAGGCTGGTAC-3′ and 5′-ACCCCAAACTCCGATAGTCC-3′; cyclophilin D (housekeeping gene for normalization): 5′-GCAAGGATGGCAAGGATTGA-3′ and 5′-AGCAATTCTGCCTGGATAGC-3′. Thermocycling conditions were set as an initial polymerase activation step at 95°C for 10 min, followed by 45 cycles at 95°C for 15 s, at 55°C for 30 s, and at 72°C for 20 s and fluorescence measurement. Samples were analyzed in triplicate and a dilution series was used in each run to determine the PCR efficiency for each primer pair.
Microscopy
Immunofluorescence was performed using standard protocols [20]. Cells were analyzed on a Zeiss Axiovert 200 microscope (Carl Zeiss AG, Jena, Germany) equipped with a 63×/1.25 and a 20×/0.7 objective and standard FITC and TRITC fluorescence filter sets, using an Axiocam Mrm Camera and AxioVision software. Images were assembled and processed using Adobe Photoshop.
Results
The importin α/β1 pathway mediates nuclear import of NICD in vitro
To address the question of how NICD is imported into the nucleus, we first analyzed if transport of NICD could be mediated by the classical importin α/β1 pathway (Fig. 1a). In digitonin-permeabilized cells, a combination of importin α1 and importin β1 promoted efficient import of GST-NICD into the nucleus. Importin α1 or importin β1 alone, by contrast, had only a small or no stimulatory effect on nuclear import of GST-NICD. Likewise, transportin, an alternative nuclear transport receptor [21], did not stimulate nuclear import of GST-NICD. These data suggested that the importin α/β1 dimer is a major import receptor for NICD.
NICD contains one canonical nuclear localization signal
The results described above suggest that NICD contains a classical nuclear localization signal (NLS) that is recognized by the adaptor protein importin α. Notch1 nuclear localization signals have been described and, in some instances, analyzed by mutagenesis [22–27]. However, a single NLS responsible for nuclear import was not identified. Taking these reports into account, and scanning the NICD sequence, revealed four putative NLSs, with 1–3 being putative monopartite, 4 being a putative bipartite NLS (Fig. S1A). To analyze individually the impact of each of these NLSs on nuclear import of NICD, mutations were introduced as depicted in Fig. S1A. NLS1 and 2 were already deleted in the NotchΔE construct we used for our analyses (see experimental procedures). Because the NICD of this construct localized to the nucleus, NLS1 and NLS2 are not essential for nuclear import of NICD. In mNLS3, all three amino acids constituting the consensus motif of a classical NLS (K{K/R}X{K/R}, [28]) were mutated to alanines, in mNLS3KF/LE mutations were introduced as described in [24]. Transfection of NotchΔE with and without NLS mutations in HeLa cells followed by immunofluorescence staining revealed that mutation of NLS4a + b did not inhibit NICD nuclear import (Fig. 1b). In contrast, partial (mNLS3KF/LE) as well as complete mutation of NLS3 (mNLS3) resulted in a strongly reduced nuclear NICD staining, irrespective of an intact or mutated NLS4 (Fig. 1b). Similar observations were made in the myoblast cell line C2C12 (Fig. S1B), demonstrating that the results are not cell type-specific. These data suggested that NLS3, which is conserved in mammalian Notch1 from different species (Fig. S1C), is responsible for importin α/β1-mediated nuclear transport of NICD.
NICD binds to GST-importins α3, α4, and α7
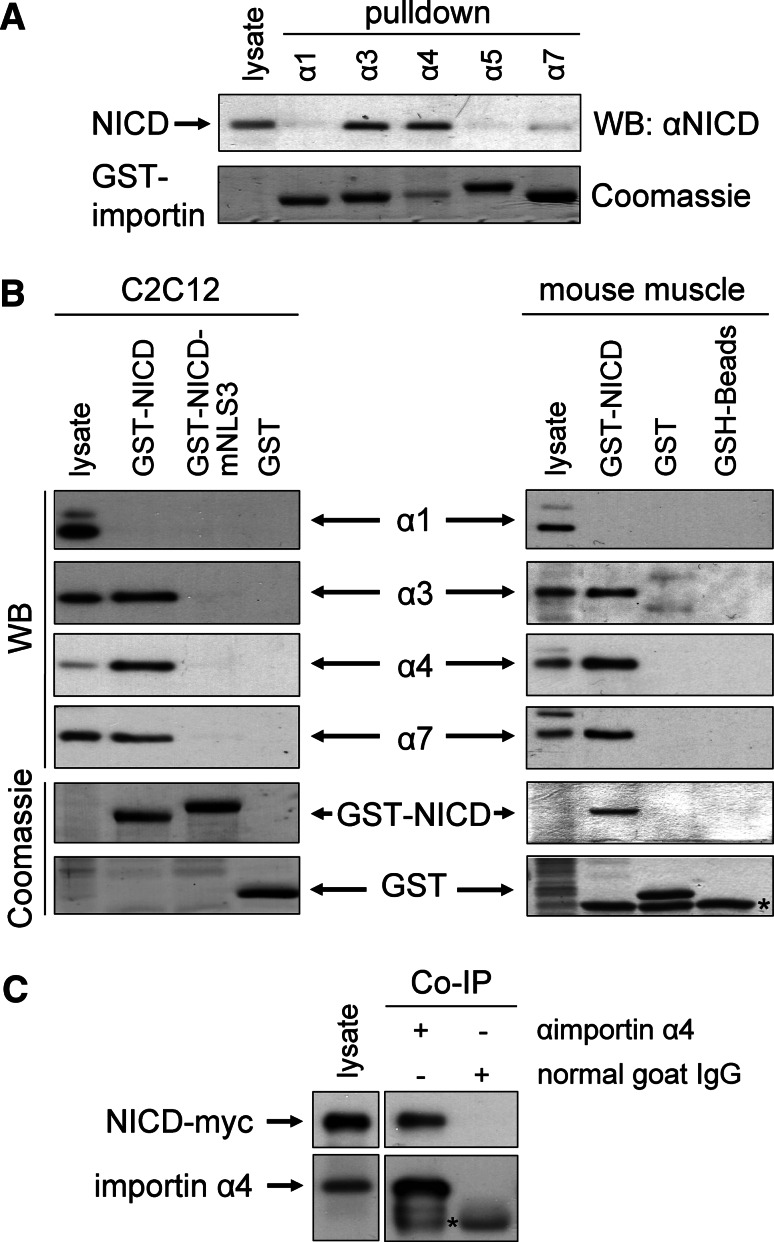
To find out which importin α isoforms are involved in the translocation of NICD, we performed pull-down assays with GST-importins α1, α3, α4, α5, and α7 fusion proteins. As source of NICD HEK293 cells stably expressing NotchΔE were used. Figure 2a demonstrates that GST-importins α3, α4, and α7 precipitated NICD, whereas α1 and α5 did not show any affinity to NICD. Recombinant expression of GST-importin α4 yielded only limited amounts of protein, but nevertheless pulled down robust amounts of NICD, suggesting a high affinity to NICD. GST-importin α7, on the other hand, pulled down NICD less efficiently. These data suggested that importins α3, α4, and α7 are able to bind NICD, α4 having the strongest, α7 the weakest affinity in vitro (Fig. 2a).
Fig. 2.
NICD in vitro binds to importins α3, α4, and α7. a GST pull-down assays were performed with lysate of HEK293 cells stably transfected with NotchΔE and purified recombinant GST-importins as indicated. Proteins were separated on SDS-PAGE, blotted and labeled with NICD-specific antibody (top) or stained with Coomassie Brilliant Blue (bottom). b Using purified recombinant GST-NICD, pull-down assays were performed from lysates of C2C12 cells (left) or mouse skeletal muscle (right). Lysates, pull-down, and as controls pull-down with GST protein and GSH-beads were separated on SDS-PAGE, blotted, and labeled with importin-specific antibodies as indicated (top, WB) or gels were stained with Coomassie Brilliant Blue (bottom). Asterisk, unspecific bands; WB, Western blot. c For co-immunoprecipitation experiment (Co-IP) C2C12 cell lysate transiently transfected with NICD-myc was immunoprecipitated with anti-importin α4 antibody or normal goat immunoglobulins. Lysate and Co-IPs were separated on SDS-PAGE, blotted, and labeled with importin α4- and myc-specific antibodies
Importins α3, α4, and α7 bind to GST-NICD
We next tested whether the interaction between NICD and importins could also be demonstrated by the reciprocal approach. We therefore prepared NICD and its NLS3-mutant as GST-fusion proteins and tested which of the importin α family members could be pulled down from a C2C12 myoblast cell lysate. GST-NICD demonstrated robust binding of endogenous importins α3, α4, and α7, whereas α1 was not pulled down (Fig. 2b). As is shown in Fig. 2a, the strongest affinity was found between α4 and NICD, and the least affinity for α7, as is indicated by the ratio input/pull-down. GST-NICD mutated in NLS3 did not pull down significant amounts of any of the importins, confirming the results from Fig. 1b. In a similar approach, we used mouse skeletal muscle lysate (Fig. 2b). GST-NICD precipitated robust amounts of importins α3 and α4 and to a lesser extent α7, but not α1. Due to a lack of specific antibodies, importin α5 was excluded from further analysis. The GST pull-down data were verified by a co-immunoprecipitation approach. Immunoprecipitation using importin α4-specific antibodies, but not control IgG, co-precipitated significant amounts of NICD-myc in transiently transfected C2C12 cells (Fig. 2c). Taken together, our results suggested that importins α3, α4, and α7 bind with descending affinity to NICD and that this binding is dependent on NLS3.
Importins α3, α4, and α7 mediate nuclear import of NICD in vivo
Our in vitro import assay clearly established the importin α/β1 dimer as a major import receptor for NICD. In the absence of competing import substrates, the specific isoform (α1) that was used in these assays promoted transport of NICD. In our binding assays, on the other hand, where many competing substrates were present in the cell lysates, importin α1 did not interact with NICD. To identify the importin α isoform that is responsible for nuclear import of NICD in living cells, we therefore performed siRNA-mediated knockdown (KD) experiments and directly visualized NICD nuclear import using HeLa Kyoto cells stably expressing NotchΔE-EGFP. In untreated cells, NotchΔE-EGFP is processed to NICD-EGFP by γ-secretase and imported into the nucleus, resulting in a nuclear EGFP staining in all cells (Fig. 3a). When γ-secretase was inhibited pharmacologically by DAPT or nuclear import was inhibited by siRNA-mediated downregulation of importin β1, EGFP-labeled NotchΔE or NICD accumulated outside the nucleus at the plasma membrane and in the cytoplasm, respectively (Fig. 3a). HeLa Kyoto NotchΔE-EGFP cells were transfected with importin siRNAs and nuclear versus extra-nuclear EGFP fluorescence was determined using automated microscopy (scheme in Fig. 3b). As control, the reduced ratios of nuclear/extra-nuclear fluorescence indicated that in DAPT treated and importin β1 KD cells nuclear transport of NICD was strongly impaired (Fig. 3c). Likewise, KD of importins α3, α4, and α7 showed a significant inhibition of nuclear import, in line with the in vitro data in Fig. 2a, b. In contrast, KD of importin α1 had no effect on transport of NICD into the nucleus, again confirming the in vitro data in Fig. 2a, b. Specificity and efficiency of the siRNAs was demonstrated in Fig. 3d. KD of importin α1 reproducibly led to a slight upregulation of α3, suggesting a compensatory mechanism. Taken together, these data indicated that nuclear import of NICD in mammalian cells is mediated by importins α3, α4, and α7.
Fig. 3.
NICD import in vivo depends on importins α3, α4, and α7. a HeLa Kyoto cells stably expressing NotchΔE-EGFP, a direct substrate for γ-secretase (scheme on the left) show nuclear NICD-EGFP staining (ctrl). Upon KD of importin β1 or incubation with the γ-secretase inhibitor DAPT NICD accumulates outside the nucleus (asterisks) in the cytoplasm (arrows) and at the plasma membrane (arrowheads), respectively. Scale bar, 10 μm. b Scheme of automated image analysis after importin isoform KD. In untreated cells, most EGFP-fluorescence is in the nucleus, resulting in a high nuclear/extra-nuclear (EGFPnuc/EGFPenuc) ratio. Inhibition of nuclear import, as shown for example by importin β1 KD, results in a low EGFPnuc/EGFPenuc ratio. Scale bar, 20 μm. c Result of automated analysis of six independent experiments. The ratio EGFPnuc/EGFPenuc in untreated cells (ctrl) was set to 100%, the ratios of siRNA-transfected cells were related accordingly. In each experiment, more than 250 cells per condition were measured. Shown are means ± SD. Asterisks indicate significance (p < 0.05, Student's t test). d Western-blot analysis of importin KD efficiency and specificity. HeLa Kyoto cell lysates were separated on SDS–PAGE, blotted, and probed for antibodies as indicated
Importins α3, α4, and α7 mediate Notch signaling in myoblasts
Having shown in vitro and with exogenous NICD that importins α3, α4, and α7 are responsible for the nuclear import of NICD, we next tested if also endogenous Notch signaling was mediated by these isoforms. To this end, we used C2C12 cells, an established myoblast cell line with endogenous Notch signaling [29]. Undifferentiated C2C12 cells were first transfected with siRNA against importin isoforms. After 24 h, cells were co-transfected with a luciferase-based Notch reporter construct and, to stimulate signaling, with Delta1 cDNA. After another 24 h, cells were processed for luciferase assay (Fig. 4a). This activation was completely blocked by γ-secretase inhibitor DAPT, demonstrating the suitability of the assay (Fig. 4a). As expected, KD of importin α1, shown above to not interact with NICD, had no effect on Notch activity. KD of importins α3 and α4 showed no decrease in Notch activity, KD of importin α7 even an increase. This suggested that importins α3 and α4 are redundant, and that KD of importin α7 has other effects on Notch signal transduction, for example blocking the nuclear import of an inhibitor or co-repressor. Indeed, the double KD of α3/4 and α3/7 as well as the triple KD of α3, α4 and α7 importin isoforms significantly inhibited endogenous Notch activity, whereas KD of α4/7 showed a moderate reduction of Notch activity (Fig. 4a). Interestingly, the KD of importin β1 lead to a similar increase in Notch signal transduction as the KD of importin α7, suggesting that also in this case the reduced import of an inhibitor or co-repressor has a larger impact than reduction of NICD nuclear transport. All siRNAs specifically and efficiently downregulated their target importin isoform, also when two or three siRNAs were used in combination (Fig. 4b). Further evidence for an involvement of importins α3, α4, and α7 in Notch signaling came from the analysis of a downstream Notch-target. Hey1, also called HERP2, is a transcription factor that is upregulated by Notch signaling [29, 30]. First C2C12 cells were transfected with control siRNA or siRNAs against importins α3, α4, and α7, followed by an induction of Hey1 expression by transfection of Delta1 cDNA. After treatment with DAPT Hey1 expression was downregulated to 20% of the induced level, showing its Notch-dependence. Treatment with siRNAs against importins α3, α4, and α7 led to a downregulation of Hey1 expression, suggesting that Notch could no longer induce Hey1 expression because import of NICD is inhibited (Fig. 4c).
Fig. 4.
Endogenous Notch signaling in myoblasts is mainly mediated by importins α3 and α4. a C2C12 cells transfected with siRNAs against importin isoforms as indicated were transfected with or without Delta1 cDNA and Notch reporter construct. The γ-secretase inhibitor DAPT was used to show γ-secretase dependency of the measured Notch activity. Firefly/renilla activities were determined and the activity in Delta1 transfected cells set to 100%. Means ± SD of five independent experiments are shown. Asterisks indicate significance (p < 0.05, Student's t test). b Western-blot analysis of importin KD efficiency and specificity. C2C12 cell lysates were separated on SDS-PAGE, blotted, and probed with antibodies as indicated. c C2C12 cells were transfected with control (ctrl) siRNA or pooled siRNAs against importins α3, α4, and α7 and subsequently with or without Delta1 as indicated. Where indicated, cells were incubated with DAPT for 24 h. After RNA isolation, quantitative real-time PCR for Hey1 expression was performed. Hey1 expression level after Delta1 induction was set to 100% and the other values related to that. Means ± SD of three independent experiments are shown
Taken together, these data suggested that in myoblasts, NICD is imported into the nucleus mainly by importins α3 and α4, and that reduction of NICD nuclear import affects downstream Notch signaling.
Discussion
Since its discovery 90 years ago [reviewed in 1], the Notch pathway became one of the most extensively studied intracellular signaling pathways. Detailed knowledge is available on the activation, molecular processing, signal transduction and inactivation of Notch with one remarkable exception, the transport of NICD into the nucleus [2]. Analysis of putative NLSs in NICD and their impact on nuclear import were performed before [22–27]. These data are partially inconsistent and the identification of a single NLS responsible for nuclear import of NICD was lacking, as was the elucidation of the import machinery that would use these signals. A role of importin β1 in Notch signaling in oligodendrocytic precursor cells has recently been demonstrated, but on an immunohistochemical level only [31]. We here show that one of the four putative NLSs is responsible for the nuclear targeting of NICD. Mutation of NLS3 prevents nuclear import in transfected cells, demonstrating that this is the relevant monopartite NLS. Aster et al. [24] identified the same NLS as being responsible for nuclear import of NICD, but only in conjunction with NLS4. Our data partially confirm Aster et al. although in our experiments the NLS3 is clearly independent of NLS4. Support for NLS3 being the relevant NLS also comes from our pull-down data that demonstrate complete absence of interaction between NICD-mNLS3 and all importins (Fig. 3b). Using an in vitro import assay we could show that the nuclear transport of NICD is mediated by the canonical importin α/β1 transport pathway. Importin α or β1 alone or transportin are not able to transport NICD into the nucleus. The in vitro import assay is well suited to determine the general importin α/β1 dependence of nuclear import, but does not allow to reliably define which α-isoform is involved in import of NICD [see discussion in 32]. However, using additional techniques, several lines of evidence demonstrate a role for the adaptor proteins importins α3, α4, and α7 in nuclear import of NICD and hence Notch signaling. In vitro NICD binds to GST-importins α3, α4, and α7, but not to α1 and α5, at least not under our experimental conditions (i.e., in the presence of competing substrates). GST-NICD binds endogenous importins α3, α4, and α7, but not α1 from C2C12 cells and skeletal muscle. Due to a lack of specific antibodies, importin α5 could not be analyzed in detail. However, GST-importin α5 did not precipitate NICD, suggesting that it does not play a major role in nuclear import of NICD. Direct visualization of NICD-EGFP nuclear transport demonstrated its dependence on importins α3, α4, and α7 in living cells. Finally, evidence that also endogenous Notch signaling is affected by KD of importins α3, α4, α7, but not α1, came from reporter assays measuring activity of endogenous Notch and expression of a downstream Notch target, Hey1. In contrast to the direct visualization experiments, single KD of importin α3 or α4 had little or no effect on endogenous Notch signaling. A likely explanation is the difference in expression levels in the two systems. NotchΔE-EGFP is stably overexpressed, and the EGFP tag stabilizes NICD. In contrast, endogenous NICD levels are extremely low. Therefore, in case of NotchΔE-EGFP overexpression, a lot of NICD will encounter a certain amount of importins, maybe in a ratio close to saturation, and a reduction in either importin α3, α4, or α7 will have direct effects. In the case of endogenous NICD, the ratio is shifted towards the importins, therefore reduction in one isoform is compensated by the availability of others. Indeed, when importins α3/α4 or α3/α7 or α3, α4, and α7 were downregulated together, a significant reduction in endogenous Notch signaling was observed. This demonstrated the redundancy of the importin system, perhaps reflecting the importance of Notch signaling in many developmental and differentiation events, where it would be too risky to rely on a single importin isoform. Single KD of importin α7 increased endogenous Notch activity despite the observation that NICD weakly binds this isoform and NICD nuclear import was reduced (Figs. 2a/b, 3c). Perhaps nuclear import of an unknown Notch inhibitor more strongly depends on importin α7 than that of NICD. Alternatively, an off-target effect might be responsible for this observation. The former explanation would be in line with the lower affinity of NICD to importin α7 compared to that to importin α3 or importin α4. Likewise, the observed increase in Notch activity after downregulation of importin β1, which should affect nuclear import via all importin α isoforms and which has a clear effect on NICD nuclear import (Fig. 3c), seems counterintuitive. However, since the KD is not complete, an explanation could be that NICD import via importins α3 and α4 is not fully suppressed, allowing Notch signaling to occur. A partial reduction of nuclear import of the unknown importin α7-dependent inhibitor could then lead to a net increase in signaling, like the importin α7 KD does. Others have made similar observations and found that inhibition of nuclear import of an inhibitor actually increased Notch activity [33, 34]. Alternatively, importin-independent nuclear import mechanisms could explain why NICD nuclear import is not completely abolished after KD of importins.
Having identified the relevant importins for transport of NICD into the nucleus, it will be interesting to investigate whether Notch signaling is regulated on the level of nuclear import, as has been suggested for other signaling pathways [7, 8].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (KA 1751/1-1 to CK). We thank Raphael Kopan, Incheol Shin, Falk Fahrenholz, Rainer Pepperkok, Urban Lendahl, and Rüdiger Rudolf for providing cell lines or cDNAs and Anna Hassel-Adwan for help in siRNA KD experiments. We are also grateful to Sigrun Horn for help with RT-qPCR.
Abbreviations
- ADAM
A disintegrin and metalloproteinase
- GST
Glutathione-S-transferase
- KD
Knockdown
- NICD
Notch intracellular domain
- NLS
Nuclear localization signal
- TACE
Tumor necrosis factor-α-converting enzyme
References
- 1.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okochi M, Fukumori A, Jiang J, Itoh N, Kimura R, Steiner H, Haass C, Tagami S, Takeda M. Secretion of the Notch-1 Abeta-like peptide during Notch signaling. J Biol Chem. 2006;281:7890–7898. doi: 10.1074/jbc.M513250200. [DOI] [PubMed] [Google Scholar]
- 4.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 5.Le Borgne R, Bardin A, Schweisguth F. The roles of receptor and ligand endocytosis in regulating Notch signaling. Development. 2005;132:1751–1762. doi: 10.1242/dev.01789. [DOI] [PubMed] [Google Scholar]
- 6.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 7.Poon IK, Jans DA. Regulation of nuclear transport: central role in development and transformation? Traffic. 2005;6:173–186. doi: 10.1111/j.1600-0854.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 8.Yasuhara N, Shibazaki N, Tanaka S, Nagai M, Kamikawa Y, Oe S, Asally M, Kamachi Y, Kondoh H, Yoneda Y. Triggering neural differentiation of ES cells by subtype switching of importin-alpha. Nat Cell Biol. 2007;9:72–79. doi: 10.1038/ncb1521. [DOI] [PubMed] [Google Scholar]
- 9.Baek SH, Kim MY, Mo JS, Ann EJ, Lee KS, Park JH, Kim JY, Seo MS, Choi EJ, Park HS. Zinc-induced downregulation of Notch signaling is associated with cytoplasmic retention of Notch1-IC and RBP-Jk via PI3 k-Akt signaling pathway. Cancer Lett. 2007;255:117–126. doi: 10.1016/j.canlet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Song J, Park S, Kim M, Shin I. Down-regulation of Notch-dependent transcription by Akt in vitro. FEBS Lett. 2008;582:1693–1699. doi: 10.1016/j.febslet.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA. Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol. 2004;14:505–514. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Kohler M, Speck C, Christiansen M, Bischoff FR, Prehn S, Haller H, Gorlich D, Hartmann E. Evidence for distinct substrate specificities of importin alpha family members in nuclear protein import. Mol Cell Biol. 1999;19:7782–7791. doi: 10.1128/mcb.19.11.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson EM, Teixeira AI, Gustafsson MV, Dohda T, Chapman G, Meletis K, Muhr J, Lendahl U. Recording Notch signaling in real time. Dev Neurosci. 2006;28:118–127. doi: 10.1159/000090758. [DOI] [PubMed] [Google Scholar]
- 14.Hu T, Guan T, Gerace L. Molecular and functional characterization of the p62 complex, an assembly of nuclear pore complex glycoproteins. J Cell Biol. 1996;134:589–601. doi: 10.1083/jcb.134.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi NC, Adam SA. Functional domains in nuclear import factor p97 for binding the nuclear localization sequence receptor and the nuclear pore. Mol Biol Cell. 1997;8:945–956. doi: 10.1091/mbc.8.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baake M, Bauerle M, Doenecke D, Albig W. Core histones and linker histones are imported into the nucleus by different pathways. Eur J Cell Biol. 2001;80:669–677. doi: 10.1078/0171-9335-00208. [DOI] [PubMed] [Google Scholar]
- 17.Melchior F, Sweet DJ, Gerace L. Analysis of Ran/TC4 function in nuclear protein import. Methods Enzymol. 1995;257:279–291. doi: 10.1016/S0076-6879(95)57032-2. [DOI] [PubMed] [Google Scholar]
- 18.Waldmann I, Walde S, Kehlenbach RH. Nuclear import of c-Jun is mediated by multiple transport receptors. J Biol Chem. 2007;282:27685–27692. doi: 10.1074/jbc.M703301200. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wacker I, Kaether C, Kromer A, Migala A, Almers W, Gerdes HH. Microtubule-dependent transport of secretory vesicles visualized in real time with a GFP-tagged secretory protein. J Cell Sci. 1997;110:1453–1463. doi: 10.1242/jcs.110.13.1453. [DOI] [PubMed] [Google Scholar]
- 21.Pollard VW, Michael WM, Nakielny S, Siomi MC, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/S0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 22.Stifani S, Blaumueller CM, Redhead NJ, Hill RE, Artavanis-Tsakonas S. Human homologs of a Drosophila enhancer of split gene product define a novel family of nuclear proteins. Nat Genet. 1992;2:343. doi: 10.1038/ng1092-119. [DOI] [PubMed] [Google Scholar]
- 23.Kopan R, Nye JS, Weintraub H. The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development. 1994;120:2385–2396. doi: 10.1242/dev.120.9.2385. [DOI] [PubMed] [Google Scholar]
- 24.Aster JC, Robertson ES, Hasserjian RP, Turner JR, Kieff E, Sklar J. Oncogenic forms of NOTCH1 lacking either the primary binding site for RBP-Jkappa or nuclear localization sequences retain the ability to associate with RBP-Jkappa and activate transcription. J Biol Chem. 1997;272:11336–11343. doi: 10.1074/jbc.272.17.11336. [DOI] [PubMed] [Google Scholar]
- 25.Jeffries S, Capobianco AJ. Neoplastic transformation by Notch requires nuclear localization. Mol Cell Biol. 2000;20:3928–3941. doi: 10.1128/MCB.20.11.3928-3941.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Shelly L, Miele L, Boykins R, Norcross MA, Guan E. Human Notch-1 inhibits NF-kappa B activity in the nucleus through a direct interaction involving a novel domain. J Immunol. 2001;167:289–295. doi: 10.4049/jimmunol.167.1.289. [DOI] [PubMed] [Google Scholar]
- 27.LaVoie MJ, Selkoe DJ. The Notch ligands, Jagged and Delta, are sequentially processed by alpha-secretase and presenilin/gamma-secretase and release signaling fragments. J Biol Chem. 2003;278:34427–34437. doi: 10.1074/jbc.M302659200. [DOI] [PubMed] [Google Scholar]
- 28.Lange A, Mills RE, Lange CJ, Stewart M, Devine SE, Corbett AH. Classical nuclear localization signals: definition, function, and interaction with importin alpha. J Biol Chem. 2007;282:5101–5105. doi: 10.1074/jbc.R600026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buas MF, Kabak S, Kadesch T. Inhibition of myogenesis by Notch: evidence for multiple pathways. J Cell Physiol. 2009;218:84–93. doi: 10.1002/jcp.21571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iso T, Sartorelli V, Chung G, Shichinohe T, Kedes L, Hamamori Y. HERP, a new primary target of Notch regulated by ligand binding. Mol Cell Biol. 2001;21:6071–6079. doi: 10.1128/MCB.21.17.6071-6079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakahara J, Kanekura K, Nawa M, Aiso S, Suzuki N. Abnormal expression of TIP30 and arrested nucleocytoplasmic transport within oligodendrocyte precursor cells in multiple sclerosis. J Clin Invest. 2009;119:169–181. doi: 10.1172/JCI35440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quensel C, Friedrich B, Sommer T, Hartmann E, Kohler M. In vivo analysis of importin alpha proteins reveals cellular proliferation inhibition and substrate specificity. Mol Cell Biol. 2004;24:10246–10255. doi: 10.1128/MCB.24.23.10246-10255.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mummery-Widmer JL, Yamazaki M, Stoeger T, Novatchkova M, Bhalerao S, Chen D, Dietzl G, Dickson BJ, Knoblich JA. Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature. 2009;458:987–992. doi: 10.1038/nature07936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tekotte H, Berdnik D, Torok T, Buszczak M, Jones LM, Cooley L, Knoblich JA, Davis I. Dcas is required for importin-alpha3 nuclear export and mechano-sensory organ cell fate specification in Drosophila. Dev Biol. 2002;244:396–406. doi: 10.1006/dbio.2002.0612. [DOI] [PubMed] [Google Scholar]
- 35.Hutten S, Flotho A, Melchior F, Kehlenbach RH. The Nup358-RanGAP complex is required for efficient importin alpha/beta-dependent nuclear import. Mol Biol Cell. 2008;19:2300–2310. doi: 10.1091/mbc.E07-12-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.