Abstract
Human primordial germ cells (PGCs) have proven to be a source of pluripotent stem cells called embryonic germ cells (EGCs). Unlike embryonic stem cells, virtually little is known regarding the factors that regulate EGC survival and maintenance. In mice, the growth factor bone morphogenetic protein 4 (BMP4) has been shown to be required for maintaining mouse embryonic stem cells, and disruptions in this gene lead to defects in mouse PGC specification. Here, we sought to determine whether recombinant human BMP4 could influence EGC derivation and/or human PGC survival. We found that the addition of recombinant BMP4 increased the number of human PGCs after 1 week of culture in a dose-responsive manner. The efficiency of EGC derivation and maintenance in culture was also enhanced by the presence of recombinant BMP4 based on alkaline phosphatase and OCT4 staining. In addition, an antagonist of the BMP4 pathway, Noggin, decreased PGC proliferation and led to an increase in cystic embryoid body formation. Quantitative real-time (qRT)–polymerase chain reaction analyses and immunostaining confirmed that the constituents of the BMP4 pathway were upregulated in EGCs versus PGCs. Downstream activators of the BMP4 pathway such as ID1 and phosphorylated SMADs 1 and 5 were also expressed, suggesting a role of this growth factor in EGC pluripotency.
Introduction
Pluripotent stem cells with the ability to generate virtually all cell types of the body provide hope for treating a variety of human afflictions and present powerful models to study development (reviewed in refs. [1,2]). Compared with the plethora of laboratories that have been working with embryonic stem cells (ESCs) derived from the inner cell mass of preimplantation blastocysts [3–6] and induced pluripotent stem cells from genetically engineered adult tissue, much fewer laboratories currently work with stem cells derived from germ cells. Germline stem cells are derived either in late embryonic development from primordial germ cells (PGCs) known as embryonic germ cells (EGCs) [1,7–12] or from spermatogonial stem cells referred to as adult germline stem cells (GSCs) [13–18]. Germline stem cells provide a unique system to study developmental reprogramming as they are the only example in which a more differentiated progenitor cell dedifferentiates into the pluripotent state without the use of genetic engineering. Moreover, it is not known whether germline reprogramming shares mechanisms that are universal to facilitate pluripotency in other cell lines. However, similarities in the molecular signature among different pluripotent stem cell lines lend support to common mechanisms regulating this process. Therefore, studying germline reversion into EGCs or GSCs could provide critical insights into reprogramming mechanisms, for which little is known. Two main reasons that curtail the study of human germline stem lines such as EGCs include the difficulties in deriving new lines and, in some cases, the maintenance of the lines after they are generated.
Derivation of EGCs can benefit from obtaining pure populations of PGCs and utilizing the factors involved in PGC survival and proliferation and EGC self-renewal. Pure populations of PGCs have now been obtained from both mouse and human tissue via their expression of stage specific embryonic antigen (SSEA1) antigen [19,20]. However, very little is known regarding the factors required for EGC derivation or maintenance in culture. Here the primary difficulty with maintaining EGC cultures is the propensity of EGC colonies to undergo spontaneous differentiation into embryoid bodies (EBs). Factors that have been previously utilized for EGC derivation include stem cell factor (Kit Ligand), leukemia inhibitory factor (LIF), and fibroblast growth factor-2 (FGF2) [21,22]. The role of these factors in EGC derivation is contributed in part by increasing PGC survival in culture. Nonetheless, EGC derivation remains poorly inefficient, and compared with ESC derivation, EGCs more frequently undergo spontaneous differentiation into EBs. Thus, defining factors that are involved in the establishment of EGCs and their long-term maintenance are critical for optimizing their utilization in future studies.
One factor that could potentially promote EGC derivation is bone morphogenetic protein 4 (BMP4). A member of the BMP family, this growth factor has putative roles in germ cell and stem cell maintenance [23,24]. Specifically, BMP4 along with other family members, BMP2 and BMP8b, have been shown to be essential in the formation and possibly proliferation of PGCs in the mouse [25–27]. In this report, we demonstrated that BMP4 enhances human PGC propagation in vitro. The addition of BMP4 also increased the efficiency of EGC derivation and improved EGC maintenance by reducing their spontaneous differentiation into cystic EBs. BMP4 function was assessed using a BMP4 antagonist, Noggin, which decreased PGC survival and EGC derivation and promoted formation of cystic EBs. BMP4 activation during EGC formation was also consistent with elevated expression of downstream activators ID1 and phosphorylated SMAD1 and SMAD5 in EGCs compared with ESCs and PGCs.
Materials and Methods
Collection of tissue
Gonadal tissues were obtained using a protocol approved by the Joint Committee on Clinical Investigation of the Johns Hopkins University School of Medicine from human fetuses at 8–11 weeks postfertilization as a result of termination of pregnancy. Gestational age was estimated through a comparison of anatomical markers including crown heel and crown rump measurements, limb and digit formation, and also the first day of the last maternal menstrual cycle. Ages are discussed in terms of fetal development and not the age from the last menstrual period. Sex of the tissue was determined by gross morphological examination of the gonad and by fluorescent in situ hybridization of tissue connected to the gonads.
PGC acquisition and EGC derivation
PGCs were isolated using magnetic cell sorting technology and an indirect labeling of cells with magnetically tagged goat anti-mouse IgM antibodies toward a mouse-anti-SSEA1 antibody (Miltenyi Biotech). Briefly, gonads were minced in 1 mg/mL collagenase, incubated at 37°C for 20 min, rinsed, and incubated with SSEA1 antibody (1:5 dilution) for 15 min on ice. Afterward, secondary antibody was applied at 1:100 dilution for another 30 min on ice and sorted on magnetic columns as previously described [28]. SSEA + PGCs from a single gonad were sorted and ∼50 cells were seeded in each of 12 wells of a 96-well plate with irradiated mouse embryonic feeder cells, SIM 6-thioguanine resistant ouabain (STO) (∼125,000 cells/well; ATCC). To determine the concentration of SSEA1+ cells, a small portion of sample was sacrificed for counting using a hemocytometer. Stage specific embryonic antigen (SSEA1) cells (stromal cells: STR) were collected from the column flow through. Purity of SSEA1− subfractions were characterized by the absence of alkaline phosphatase (AP) staining, which demonstrated that the majority, >99%, of these cells were AP negative and thus not PGCs. EGC derivation was performed on gamma-irradiated STO as described previously [8]. Media for PGCs and EGCs consisted of Dulbecco's modified Eagle's medium-199 (Invitrogen) supplemented with 20% Knockout serum (Invitrogen), 2 ng/mL FGF2 (R&D Systems), 1000 U LIF (Millipore), 10 mM Forskolin, and where noted, various concentrations of BMP4 (R&D Systems) and/or Noggin (R&D Systems).
Human ESC culture and differentiation
Human ESCs from the H1 line (WiCell, Fed ID# 0043) were cultured on matrigel (BD Biosciences) in 10-cm cell culture dishes with Dulbecco's modified Eagle's medium/F12-Knockout serum-based media conditioned by mouse embryonic fibroblast cells (MEFs) and supplemented with 4 ng/mL FGF2 (R&D Systems) as described previously [29]. Trophoblastic differentiation was performed as previously described by adding 20 ng/mL BMP4 to culture media for 10 days [30]. These experiments included 4 females and 4 males at 8, 9, 10, and 11 weeks postfertilization plated onto 12 wells of a 96-well dish. For every specimen, each of 2 wells was treated with either 0, 5, 10, or 20 ng/mL BMP4, Noggin plus BMP4, or Noggin alone.
Immunostaining
EBs were rinsed in Dulbecco's phosphate-buffered saline (DPBS), frozen in OCT freezing compound (TissueTek), and stored at 80°C. For immunohistochemistry, tissue were cut into 5-μm sections, placed on slides (ProbeOn Plus, Fisher Scientific), and immediately prepared for indirect immunofluorescent staining. Sections were fixed either in 4°C acetone for 10 min to detect cell surface markers or in 4% paraformaldehyde for 10 min to detect nuclear markers. For immunocytochemistry, cells were fixed in 4% paraformaldehyde (Sigma). For markers localized internally in the cell, 0.3% Triton X-100 (Sigma) was used for cell permilization prior to antibody staining.
Antibodies and the concentrations used are summarized in Supplementary Table S1 (available online at www.liebertonline.com/scd). Briefly, antibodies were diluted in 15% goat serum in DPBS and incubated with tissue for 1 h at room temperature. All antibodies were detected by using fluorescently labeled secondary antibodies (1:200 dilution; Millipore) in 15% goat serum in DPBS for 1 h at room temperature. Cells were counterstained with DAPI (Sigma) and mounted using ProLong antifade mounting medium (Millipore) for immunohistochemistry. Negative controls were also performed using secondary antibodies only and with mouse ascites fluid.
Microscopic imaging
Fluorescent images were captured using a Nikon Eclipse E800 microscope. Alexa Fluor 594 fluorescence was detected using a G2ERHOD 541–551 nm excitation filter, a 575 nm dichroic mirror, and a barrier filter with a band width of 590 nm. Alexa Fluor 488 was detected using an fluorescein isothiocyanate (FITC) excitation filter, a 505 nm dichroic mirror, and a barrier filter with a band width of 515–555 nm. DAPI was detected using a standard DAPI/Hoechst filter set, UV 2E/C 340380 nm excitation filter, 400 nm dichroic mirror, and a barrier filter with a band width of 435–485 nm. Barrier filters were manufactured by Chroma, Inc. Images were captured with a Photometrics 20 MHz cooled interlined CCD camera and imported into Metamorph software, v.6.2 (Universal Imaging Corp.).
AP activity
To detect AP activity, EGC colonies were fixed on plates in 66% acetone–3% Paraformaldehyde (PFA) for 5 min and then stained with naphthol/FRV–alkaline AP substrate (Sigma) for 20 min, following the manufacturer's instructions [19].
Quantitative real-time reverse transcriptase–polymerase chain reaction analysis
EGCs and PGCs isolated for quantitative real-time (qRT)–polymerase chain reaction (PCR) were cultured without BMP4 to compare the baseline expression of BMP pathway constituents in these cells. Total RNA from SSEA1+ (PGCs), SSEA− (STR), picked EGCs, and ESCs was obtained using MiniRNeasy kits (Qiagen 74124) with the RNA clean-up protocol and optional on-column DNase treatment, and complementary DNA was generated with SuperScript® III First-Strand Synthesis System RT Kits, following the manufacturer's instructions (INV18080-051). Real-time qRT-PCR analyses were performed using ABi7900HT in 96-well plates (Abi N801-0560) in which each real-time amplification had a template equivalent to 5 ng of total RNA. Each primer set was tested in at least triplicate across 6 biological replicates. Negative controls consisted of a reverse transcription–negative blank per sample plus a no-template blank. Taqman Assay-on-Demand designed oligonucleotides for detection of the specific genes (Supplementary Table S2, available online at www.liebertonline.com/scd). Using the ΔΔCt method, quantification within the log-linear phase of the amplification curve acquired for each probe/primer set was normalized to β-actin.
Fluorescent in situ hybridization
Fluorescent in situ hybridization was performed using CEP X (Spectrum-Orange)/CEP Y (SpectrumGreen) DNA probes (Vysis; www.vysis.com) from extragonadal tissue. Slides were prepared as described for immunostaining, fixed in Carnoy's fixative for 45 s, pretreated in 1 M sodium thiocyanate for 5 min at 75°C, and postfixed in 100% methanol for 1 min. Sections were then denatured in 60% formamide in standard saline citrate (SSC) (sodium chloride and citric acid) buffer, pH 5.3, at 75°C for 3 min, followed by 1 min in cold 70%, 95%, and 100% ethanol, and then incubated with CEP X/Y DNA probe overnight at 37°C. The following day, sections underwent 3 posthybridization washes in 60% formamide (Sigma-Aldrich) and 0.3% Nonidet P40 (Igepal; Sigma-Aldrich) in SSC (pH 5.3). Sections were then counterstained with DAPI and mounted using ProLong antifade mounting medium (Molecular Probes). Supplementary Fig. S1 (available online at www.liebertonline.com/scd) depicts staining of the extragonadal tissue of a male and female specimen used in the following experiment.
Karyotype analysis
EGC cultures were incubated with 0.02 g/mL Colcemid for 1 h, washed in PBS, trypsinized, and spun down. The pellet was resuspended carefully in hypotonic solution (0.56% KCl), to obtain a single-cell suspension, and left at room temperature for 6 min. After spinning and removing hypotonic solution, cells were fixed with 5 mL of ice-cold fixative (methanol:acetic acid in 3:1 ratio) added dropwise to the suspension, left at room temperature for 5 min, and then spun down. The cells were then dropped onto slides and stained with Giemsa. For each cell line, at least 20–30 metaphases were examined.
Results
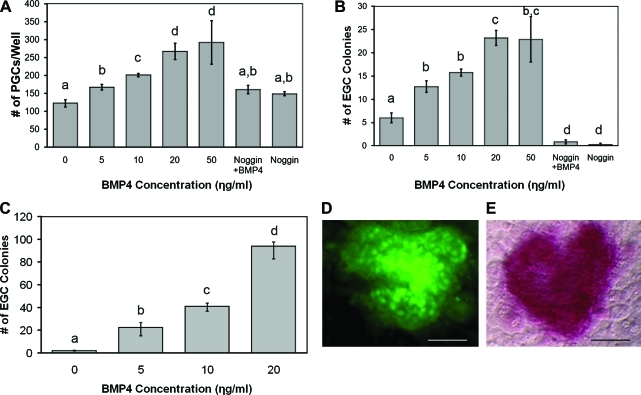
We found that the addition of recombinant BMP4 increased the number of PGCs after the first week in culture in a dose-responsive manner (Fig. 1A). The optimal concentration for PGC proliferation was 20 ng/mL BMP4, which increased PGC number 3-fold compared with controls. Higher concentrations, such as 50 ng/mL BMP4, did not further increase PGC proliferation. Moreover, the effect of BMP4 was negated by the addition of Noggin, an antagonist of the BMP pathway.
FIG. 1.
(A) Dose response in PGC number following 1 week in culture with either increasing doses of BMP4, equimolar concentrations of Noggin (50 ng/mL) plus BMP4 (20 ng/mL), or 50 ng/mL Noggin alone. PGCs were isolated by stage specific embryonic antigen (SSEA1) expression and equal numbers were seeded in a 96-well plate with irradiated SIM 6-thioguanine resistant ouabain (STO) mouse feeder cells. After 7 days, PGCs were counted live using SSEA1 and Alexa 488-secondary antibodies. Dose response in EGCs generated (B) after 1 week from PGCs in A and (C) after 3 weeks in subculture. Lower-case letters denote statistically significant differences among treatments (t-tests, P < 0.05; n = 8). (D) Indirect immunofluorescence demonstrating OCT4 expression (green) and (E) phase-contrast alkaline phosphatase expression colocalized in an undifferentiated human EGC colony. Scale bars: 150 μM. PGC, primordial germ cell; BMP, bone morphogenetic protein; EGC, embryonic germ cell. Color images available online at www.liebertonline.com/scd.
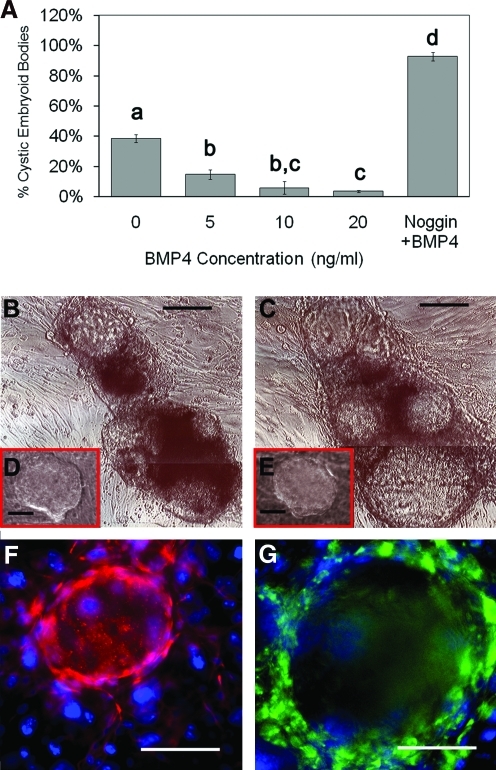
The efficiency of EGC derivation in culture was also enhanced by the presence of recombinant BMP4 (Fig. 1B). After the first week, EGC colonies were identified by morphology and manually counted. The results revealed an increase in EGC derivation of ∼5-fold in 20 ng/mL BMP4. However, after 3 weeks the efficiency was increased by 50-fold in the presence of BMP4 (Fig. 1C). EGC colonies were confirmed by coexpression of pluripotent markers, AP and Oct4 (Fig. 1D, E). During this time period, the number of EGC colonies in the normal derivation culture without BMP4 decreased. These results were primarily due to the spontaneous differentiation into cystic EBs. Additionally, the increase in EGC colonies treated with BMP4 after 3 weeks was concomitant with the reduction in spontaneous differentiation into EBs, whereas Noggin in the absence of BMP4 significantly hampered EGC formation when applied to PGCs in culture. When Noggin was added to EGCs cultured in 20 ng/mL BMP4, cystic EB formation increased to ∼90% (Fig. 2). These results suggest that the majority of BMP inactivation is primarily due to BMP4. This is significant as other BMPs such as BMP8 and BMP7 have demonstrated roles similar to BMP4 in mouse PGCs. Our attempts using BMP8 with human PGCs did not demonstrate any significant additive effects with BMP4, suggesting that it does not play a significant role in the EGC phenotype. Moreover, the effect of BMP4 in these experiments did not appear to be dependent on the sex or age of the tissue studied because similar responses in PGC proliferation and EGC derivation were seen among these groups. However, further study with more specimens may be warranted to address the differences in PGC response to BMP4 during development between both sexes. EGC cultures that were maintained in BMP4 for ∼30 subcultures or 6 months exhibited normal karyotypes (Supplementary Fig. S2, available online at www.liebertonline.com/scd).
FIG. 2.
(A) The incident of cystic EB formation (the number of EBs per total number of colonies) decreases in the presence of BMP4 and significantly increases with the addition of Noggin after 1 week in culture. Dose response in cystic EB formation following 1 week in culture with either increasing doses of BMP4 or equimolar concentrations of Noggin (50 ng/mL) plus BMP4 (20 ng/mL). EGC differentiation is enhanced in the presence of BMP4 inhibitor Noggin. Cystic EB formation is enhanced after (B) 24 h and (C) 7 days following exposure to 50 ng/mL Noggin in culture compared with controls (insets D and E, respectively). Inset figures D and E represent a single colony grown in the presence of 20 ng/mL BMP4 alone after 24 h and 7 days. Lower-case letters denote statistically significant differences among treatments (t-tests, P < 0.05; n = 8). Indirect immunofluorescence of cryosectioned EBs demonstrate a fluid-filled center surrounded by cells labeled by markers of the (F) ectoderm (Nestin: red) and (G) endoderm (AFP: green) lineage but not mesoderm. STO feeder cells surrounding EBs do not express these markers. DAPI (blue) stains the nuclei. Scale bars: 150 μM. EB, embryoid body. Color images available online at www.liebertonline.com/scd.
Unlike ESCs, EGCs have an increased propensity to undergo spontaneous differentiation into cystic EBs. In our hands, ∼40% of the EGC colonies derived without the addition of BMP4 developed EBs after 1 week in culture. Cystic EBs developed from ESCs have been characterized as resembling blastocysts with a central cavity consisting of an inner ectodermal layer and an outer endodermal layer enclosing the cavity [31]. This is also demonstrated by EBs from human EGCs cultured in the presence of Noggin (Fig. 2).
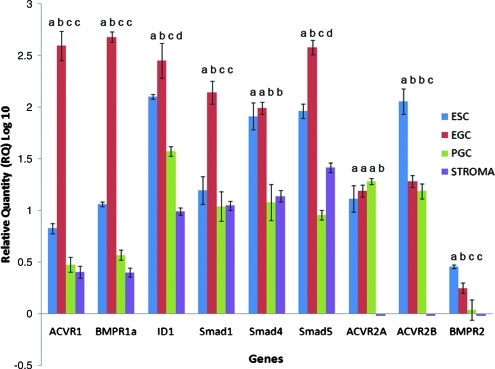
To corroborate these results, BMP4 and members of its pathway were also investigated at the molecular level by measuring mRNA and protein expression. Semiquantitative real-time RT-PCR analysis was performed on PGCs (SSEA1+), gonadal somatic stromal cells (STR, SSEA1−), EGCs, and ESCs in the undifferentiated state without BMP4 (Fig. 3). Data were normalized to the expression of β-actin and presented relative to PGC expression. Results showed that SSEA1+ PGCs express the key constituents of the BMP pathway similar to results shown previously in mice [32]. Moreover, differential expression of key constituents of the BMP pathway between SSEA1+ PGCs and pluripotent stem cells was also observed. EGCs expressed higher levels than PGCs in components involved in BMP regulation, including those that are specific to BMP activation, such as Acvr1, Bmpr1a, Bmpr2, Smad1, and Smad5, as well as those shared by other transforming growth factor-β (TGFβ) family members, such as Smad4 and Id1. In contrast, there was no difference between PGCs and EGCs in the expression of TGFβ family receptors such as Activin A type II receptor, Acvr2a, or the Nodal receptor Acvr2b. Together, these results along with the effects of Noggin and BMP4 in culture define a role for BMP4 activation in promoting PGCs to the pluripotent state. Interestingly, compared with PGCs, ESCs also expressed higher levels of several of these constituents. Significantly higher expression of these markers in ESCs compared with PGCs or gonadal fibroblast cells may support emerging evidence that suggests a common progenitor of ESCs and EGCs [33]. This may also explain the potential of these cells to develop into germ cells under BMP4-inducing conditions [34,35].
FIG. 3.
Quantitative real-time qRT–polymerase chain reaction of BMP signaling components in human EGCs, ESCs, PGCs, and gonadal stromal cells (STR) cultured without BMP4. BMP receptors (Bmpr1A, Acvr1, Bmpr2), Smad1, Smad4, and Smad5 were enriched in EGCs compared with PGCs and stromal cells. In contrast, the activin receptor ACVR2A and the nodal receptor ACR2B were differentially expressed in these cells. Id1, a known BMP downstream activator, was also highly expressed in EGCs and undetectable in stromal cells. Samples were tested from 6 biological samples, 3 male and 3 female age-matched specimens, in triplicate. Data are represented as relative quantity log 10 with standard error of the mean; lower-case letters denote statistically significant differences between cell lines for each gene; P < 0.05. ESC, embryonic stem cell. Color images available online at www.liebertonline.com/scd.
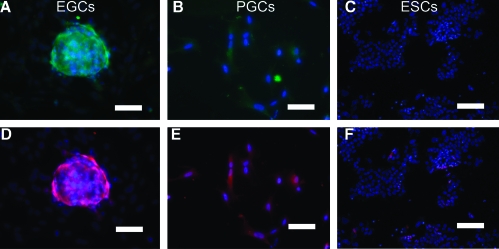
Elevated differences in the BMP constituents of EGCs compared with ESCs demonstrate a unique molecular signature for EGCs. EGCs express more Acvr1, Bmpr1a, Smad1, Smad5, and Id1 than ESCs. SMAD1 and SMAD5 are associated with the BMP pathway that is active in germ cells, whereas Smad4 is a co-SMAD utilized by many other TGFβ family members [36]. In comparison, it is interesting to note that Bmpr2 and other TGFβ family receptors such as Acvr2a and Acvr2b were expressed at significantly lower levels in EGCs.
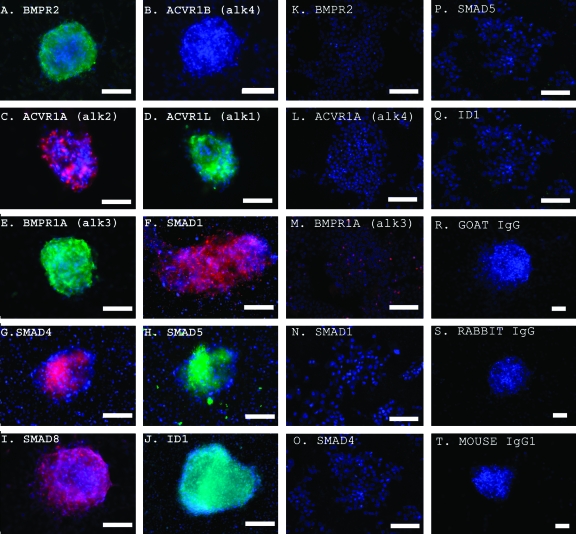
To validate the mRNA analyses, protein expression was measured using indirect immunofluorescence staining. Immunostaining revealed that BMP4 pathway protein levels were also elevated in EGCs compared with ESCs (Fig. 4). Controls using secondary antibody alone did not demonstrate staining of EGCs. BMP4 activation in these cells was also evidenced by the phosphorylation of SMAD1 and SMAD5 proteins and by ID1 expression. To study SMAD activation, immunostaining was performed using antibodies specific to SMAD1 and SMAD5 phosphorylated forms, SMAD1P and SMAD5P, respectively. Immunostaining demonstrated higher expression for SMAD1P and SMAD5P in EGCs compared with PGCs cultured in 20 ng/mL BMP4 (Fig. 5). Importantly, ESCs did not express SMAD1P or SMAD5P in the presence of BMP4, even though they produced significant levels of Smad1 and Smad5 mRNA when grown in the absence of BMP4. Collectively, these results demonstrate that BMP4 plays an active role in the induction of EGC formation and maintenance.
FIG. 4.
(A–J) Indirect immunofluorescence detection of BMP4 pathway constituents in human EGCs cultured on mouse feeders without BMP4. A and B demonstrate expression of BMP4 type 2 but not the activin/nodal type 1 receptor ACVR1B in the same EGC colony. Colocalization is shown for 2 BMP4 type 1 receptors, (C) ACVR1A (alk2) and (D) ACVRL1 (alk1), which are utilized by BMP4 to maintain mouse ESC pluripotency [34], (E) BMP4 type 1 receptor, BMPR1A (alk3), and (F) SMAD1. Colocalization of (G) SMAD4, (H) SMAD5, (I) SMAD8, and (J) ID1 are demonstrated. (K–Q) Indirect immunofluorescence detection of BMP4 pathway constituents in human ESCs cultured on matrigel without BMP4. (K) Absence of BMP4 type 2 receptor. (L) Absence of ACVR1A (alk2). (M) Absence of BMP4 type 1 receptor, BMPR1A (alk3). Absence of expression of (N) SMAD1, (O) SMAD4, (P) SMAD5, and (Q) ID1 in ESCs is demonstrated. (R–T) Negative controls in EGCs. (R) Goat IgG. (S) Rabbit IgG. (T) Mouse IgG1. DAPI (blue) stains the nuclei. Scale bars: 50 microns or μm. Color images available online at www.liebertonline.com/scd.
FIG. 5.
Indirect immunofluorescence detection of the BMP pathway SMAD activation in human EGCs, SSEA1+ PGCs, and ESCs cultured in the presence of 20 ng/mL BMP4. A, B, and C demonstrate increased SMAD1P expression in EGCs compared with PGCs and undetectable levels in ESCs. D, E, and F demonstrate a similar pattern for SMAD5P. Moreover, A and D demonstrate that SMAD1P and SMAD5P are colocalized in EGCs, and B and E demonstrate the same in isolated SSEA1+ PGCs. The initiation of ESC differentiation is evident by the loss of tight compacted colony formation that is occurring in these cultures. DAPI (blue) stains the nuclei. Scale bars: 150 μM. Color images available online at www.liebertonline.com/scd.
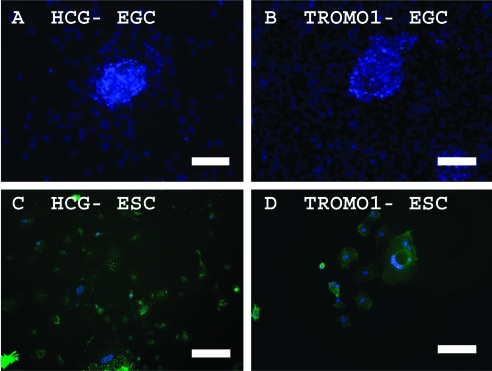
Although BMP4 supports self-renewal in mouse ESCs [37], the addition of BMP4 in human ESC cultures induces trophoblast differentiation [30]. To determine whether BMP4 also induced trophoblast differentiation in human EGCs, markers for trophoblastic differentiation were studied in these cultures. Expression of HCG and Troma-1 was not detected in EGCs compared with human ESCs when cultured in BMP4-containing media (Fig. 6). Microarray analyses also showed that other trophoblastic markers such as CG-B, glial cells missing-1 (GCM1), the nonclassical HLA class I molecule HLA-G1, and CD9 were not enhanced in our human EGCs cultured in BMP4 compared with undifferentiated ESCs (Supplementary Table S3, available online at www.liebertonline.com/scd).
FIG. 6.
Indirect immunofluorescence detection of markers of trophoblastic differentiation in EGCs and ESCs cultured in the presence of 20 ng/mL BMP4. (A, C) HCG and (B, D) TROMO1 protein expression is not detected in EGCs but clearly shown in trophoblastic cells derived from human ESCs. DAPI (blue) stains the nuclei. Scale bars: (A, B) 150 μM; (C, D) 300 μM. Color images available online at www.liebertonline.com/scd.
Discussion
One critical factor to optimize EGC derivation is identifying the key components that regulate the conversion of a unipotent PGC into a pluripotent EGC stem cell. Indeed, PGCs alone do not form EGCs in culture. PGCs are unipotent in that they exhibit complex patterns of imprinted gene expression and methylation, are short lived in culture, and are unable to contribute to chimeras or form EBs in culture [38]. In fact, these cells die within days in cell culture without mitotically inactivated mouse feeder cells or certain growth factors [19].
Previous studies by this laboratory have demonstrated the ability to isolate human PGCs [28]. These PGCs express the germ cell–specific marker VASA along with stem cell markers OCT4 and NANOG [28,39]. Our study demonstrates more efficient EGC derivation and identifies a pathway that contributes to the reprogramming of PGCs to EGCs. The significance of more efficiently deriving human EGCs is that it provides the opportunity to study mechanisms underlying their pluripotency, a relationship that cannot be declared for ESCs and their progenitors, which are comprised of a heterogeneous population of cells of the inner cell mass [40].
BMPs, such as BMP4, are members of the TGFβ superfamily [41]. BMPs exert their influence by binding and forming heterodimeric complexes with type I (ACVRL1, ACVR1, BMPR1A, and BMPR1B) and type II (BMPRII) receptor tyrosine kinases. This binding facilitates the phosphorylation of receptor-regulated (R)-Smads (Smad1, Smad5, and Smad8), which bind to the cooperating (Co)-Smad, Smad4. These Smad complexes then translocate to the nucleus and activate the transcription of BMP target genes [42]. Roles of BMPs in germ cell development and function have been confirmed by targeted and spontaneous mutations in various Bmps, Bmp receptors, and Smads in the mouse. Specifically, BMP2 [43], BMP4 [25], and BMP8 [27] have been demonstrated to determine PGCs' fate and possibly their survival and proliferation. Evidence from multiple groups have also suggested that out of these 3 BMPs, BMP4 plays an essential role in regulating pluripotency in mouse ESCs [37,44]. This was confirmed by demonstrating that BMP4 maintained pluripotency in mouse ESCs by inhibiting neural differentiation [44–47]. Moreover, BMP4 maintained pluripotency only in the presence of LIF. Without LIF, BMP4 promoted mesodermal differentiation. However, no studies in mice or humans have identified a role of BMP4 in promoting the EGC stem cell phenotype.
The mechanisms in which BMP4 promotes pluripotency has been attributed to the ability of BMP4 to inhibit extracellular receptor kinase activation caused by LIF, to inhibit p38 mitogen-activated protein kinase pathway, and to induce the expression of X-linked inhibitor of apoptosis (Xiap) [37] and inhibitor of differentiation (Id) genes through SMAD proteins [44,48]. In human ESCs, where LIF-STAT3 activation is not required for pluripotency, the addition of BMP4 alone promotes trophoblastic differentiation [30,49].
Given the potential role of BMP4 in PGC survival, and its demonstrated ability to promote pluripotency in mouse ESCs, which require LIF-STAT3 activation [50], the following study investigated whether BMP4 played a direct role in human PGC survival and EGC maintenance. Our results demonstrate that human PGCs, like mouse PGCs, express BMP receptors and Smads 1, 4, and 5 [32,51,52]. Unlike mouse studies that suggest that BMP4 affects PGC proliferation indirectly via feeder cells, our study investigated the expression of the BMP4 pathway constituents in PGCs in the absence of feeder cells [53]. Our results show that BMP4 directly promotes proliferation of human PGCs in vitro. In addition, freshly isolated PGCs demonstrated that BMP4 signaling components are expressed by mid- to late-stage PGCs (between 8 and 11 weeks of gestation). These included receptors of the BMP family including Bmpr1A (alk3), Acvr1 (alk2), Bmpr2, and ACVRL1 (alk1) as well as their coactivators Smad1, 4, 5, and 8. This is in comparison to 1 study that employed an organ culture system and showed that BMP4 treatment increased PGC numbers, whereas Noggin treatment reduced PGC number and slowed PGC migration in cultured mouse gonads. In this case, the effect of BMP4 on PGC proliferation was primarily contributed to inducing Kitl and Idl expression in the surrounding tissues [32]. This conclusion was based on mRNA expression, which demonstrated higher levels of BMP pathway constituents in the stromal cells of the gonad compared with PGCs. However, our data from sorted PGCs show that both human and mouse PGCs share expression of many BMP constituents, including type 1 and type 2 receptors, SMADs 1, 5, and 8, and other downstream activators such as ID1. This is demonstrated not only at the transcription level but also through protein expression. Importantly, their transcription and protein levels were significantly higher in EGCs, including downstream activation of ID1, SMAD1P, and SMAD5P. Together with the effects of Noggin, these data suggest that BMP4 plays a crucial role in PGC progression to the pluripotent state in a fashion similar to that shown for mouse ESCs. This is the first report defining a role of BMP4 for pluripotency in EGCs.
Although the role of BMPs in mouse EGCs have not been investigated, there are some interesting comparisons that can be made between human EGCs and ESCs. Compared with ESCs, EGCs express higher levels of BMP pathway constituents BMPR1A (alk3), ACVR1 (alk2), and ACVRL1 (alk1) as well as downstream activators ID1, SMAD1P, and SMAD5P. Although others have also shown that human ESCs express Smad1 and Smad5 mRNA, it was not determined whether the pathways were activated [54]. Here, we show that both are phosphorylated in EGCs but not in ESCs, consistent with previous work that suggests that this pathway is not involved in the pluripotent nature of human ESCs. PGCs and EGCs are grown in media supplemented with serum, which has been shown to contain regulatory levels of BMPs [55]. However, when Noggin was added to this media, SMAD1 and SMAD5 phosphorylation was ablated. Similarly, undifferentiated ESCs are maintained in defined culture media without serum or endogenous levels of BMP4, and they did not exhibit phosphorylation of either SMAD1 or SMAD5. In contrast to other members of the TGFβ pathway, ESCs expressed higher levels of the Nodal type II receptor, ACVR2B, and similar concentrations of the Activin A Type II receptor, ACVR2A. This is expected as both pathways have been implicated in regulating pluripotency in human ESCs [56–58].
Here, we demonstrate that ID1 is also enriched in human EGCs and ESCs compared with PGCs and somatic cells. In particular, ID1 is expressed by human PGCs and these levels are elevated after their transition into EGCs. In mouse ESCs, studies have shown that BMP4 activation promoted self-renewal by inhibiting neuroectoderm differentiation through expression of ID proteins including ID1 [44]. ID proteins have demonstrated roles in development, cell cycle, and tumorigenesis [59]. In particular, ID proteins have no DNA binding activity but indirectly inhibit members of the basic helix-loop-helix family of transcription factors known to be involved in many cell fate determinations, including neural differentiation [59]. Although the importance of Id genes in human ESCs has yet to be confirmed, Id1 has been associated with the expression of the pluripotent genes Oct4 and Sox2 in human ESCs using single-cell microarray analyses [60]. Id1 has also been associated with Nanog expression and PGC survival in conditional Nanog-knockdown mice [61]. Like Sox2 and Oct4, Nanog is a regulator of pluripotency and is involved in germline development [62–66]. Thus, the present report demonstrates that increased expression of ID1 may also play a substantial role in maintaining pluripotency of human EGCs and PGC self-renewal.
In human ESC cultures, exogenous BMP4 increases a number of genes associated with trophoblastic differentiation. However, we report here that BMP4 added to EGC culture conditions did not result in trophoblastic differentiation as evidenced by immunostaining of TROMA1 and HCG. Moreover, microarray analysis showed that other markers including CG-B, glial cells missing-1 (GCM1), the nonclassical HLA class I molecule HLA-G1, Troma-1 (KRT8), CDH3, and CD930 [67–69] were not enhanced in our human EGCs compared with undifferentiated ESC controls (Supplementary Table S3).
The effects of BMP4 in EGCs versus ESCs may be explained by differences in their genetic makeup or by the presence of LIF. LIF is required for self-renewal and pluripotency of human EGCs but is not essential for human ESCs. Here, we report a relationship between BMP4 and LIF in human EGC derivation similar to the synergism seen in mouse ESCs. Our results are consistent with previous studies that derived human EGCs without serum or BMP4 but at much lower efficiency [10,12]. The ability to derive EGCs in the absence of serum can be explained by the fact that mouse feeder cells secrete BMP4. In fact, subclones of STO feeder cells that express higher levels of BMP4 are able to generate mouse ESCs more efficiently [37]. In summary, we have shown that BMP4 supports human EGC derivation. Specifically, we have demonstrated RNA and protein expression of the key constituents and downstream activation of the BMP4 pathway in purified human PGCs and their derived EGCs. Activation of downstream BMP pathway constituents provides evidence for a direct role of BMP4 activation in promoting PGC proliferation and the EGC phenotype. Together, these results demonstrate that EGCs and ESCs are different cell types that require alternate signaling pathways for maintenance of self-renewal.
Supplementary Material
Acknowledgments
This work was graciously supported by the Institute for Cellular Engineering, Johns Hopkins University, Baltimore, MD, and by NICHD R21 HD057487-01A1. The authors thank Dr. Ann Burke, Dr. Rameet Singh, and Dr. Roxanne Jamshidi as well as the Birth Defects Laboratory at the University of Washington for their assistance in acquiring tissue.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Donovan PJ. Gearhart J. The end of the beginning for pluripotent stem cells. Nature. 2001;414:92–97. doi: 10.1038/35102154. [DOI] [PubMed] [Google Scholar]
- 2.Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 3.Evans MJ. Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 4.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in media conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson JA. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 6.Amit M. Itskovitz-Eldor J. Derivation and maintenance of human embryonic stem cells. Methods Mol Biol. 2006;331:43–53. doi: 10.1385/1-59745-046-4:43. [DOI] [PubMed] [Google Scholar]
- 7.Turnpenny L. Spalluto CM. Perrett RM, et al. Evaluating human embryonic germ cells: concord and conflict as pluripotent stem cells. Stem Cells. 2005;24:212–220. doi: 10.1634/stemcells.2005-0255. [DOI] [PubMed] [Google Scholar]
- 8.Shamblott MJ. Axelman J. Wang S, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH. Kim SJ. Lee JB, et al. Establishment of a human embryonic germ cell line and comparison with mouse and human embryonic stem cells. Mol Cells. 2004;17:309–315. [PubMed] [Google Scholar]
- 10.Turnpenny L. Brickwood S. Spalluto CM, et al. Derivation of human embryonic germ cells: an alternative source of pluripotent stem cells. Stem Cells. 2003;21:598–609. doi: 10.1634/stemcells.21-5-598. [DOI] [PubMed] [Google Scholar]
- 11.Liu S. Liu H. Pan Y, et al. Human embryonic germ cells isolation from early stages of post-implantation embryos. Cell Tissue Res. 2004;318:525–531. doi: 10.1007/s00441-004-0990-7. [DOI] [PubMed] [Google Scholar]
- 12.Hua J. Yu H. Liu S, et al. Derivation and characterization of human embryonic germ cells: serum-free culture and differentiation potential. Reprod Biomed Online. 2009;19:238–249. doi: 10.1016/s1472-6483(10)60079-x. [DOI] [PubMed] [Google Scholar]
- 13.Guan K. Nayernia K. Maier LS, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 14.Izadyar F. Pau F. Marh J, et al. Generation of multipotent cell lines from a distinct population of male germ line stem cells. Reproduction. 2008;135:771–784. doi: 10.1530/REP-07-0479. [DOI] [PubMed] [Google Scholar]
- 15.Kanatsu-Shinohara M. Inoue K. Lee J, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Conrad S. Renninger M. Hennenlotter J, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 17.Kossack N. Meneses J. Shefi S, et al. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–149. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko K. Tapia N. Wu G, et al. Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell. 2009;5:87–96. doi: 10.1016/j.stem.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Kerr CL. Shamblott MJ. Gearhart JD. Pluripotent stem cells from germ cells. Methods Enzymol. 2006;419:400–426. doi: 10.1016/S0076-6879(06)19016-3. [DOI] [PubMed] [Google Scholar]
- 20.Pesce M. De Felici M. Purification of mouse primordial germ cells by MiniMACS magnetic separation system. Dev Biol. 1995;170:722–725. doi: 10.1006/dbio.1995.1250. [DOI] [PubMed] [Google Scholar]
- 21.Matsui Y. Zsebo K. Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- 22.Resnick JL. Bixler LS. Cheng L. Donovan PJ. Long-term proliferation of mouse primordial germ cells in culture. Nature. 1992;359:550–551. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- 23.Tam PP. Zhou SX. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev Biol. 1996;178:124–132. doi: 10.1006/dbio.1996.0203. [DOI] [PubMed] [Google Scholar]
- 24.Fujiwara T. Dunn NR. Hogan BL. Bone morphogenetic protein 4 in the extraembryonic mesoderm is required for allantois development and the localization and survival of primordial germ cells in the mouse. Proc Natl Acad Sci USA. 2001;98:13739–13744. doi: 10.1073/pnas.241508898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson KA. Dunn NR. Roelen BA, et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ying Y. Qi X. Zhao GQ. Induction of primordial germ cells from murine epiblasts by synergistic action of BMP4 and BMP8B signaling pathways. Proc Natl Acad Sci USA. 2001;98:7858–7862. doi: 10.1073/pnas.151242798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying Y. Liu XM. Marble A. Lawson KA. Zhao GQ. Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol Endocrinol. 2000;14:1053–1063. doi: 10.1210/mend.14.7.0479. [DOI] [PubMed] [Google Scholar]
- 28.Kerr CL. Hill CM. Blumenthal PD. Gearhart JD. Expression of pluripotent stem cell markers in the human fetal testis. Stem Cells. 2008;26:412–421. doi: 10.1634/stemcells.2007-0605. [DOI] [PubMed] [Google Scholar]
- 29.Xu C. Inokuma MS. Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 30.Xu RH. Chen X. Li DS, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 31.Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 32.Dudley BM. Runyan C. Takeuchi Y. Schaible K. Molyneaux K. BMP signaling regulates PGC numbers and motility in organ culture. Mech Dev. 2007;124:68–77. doi: 10.1016/j.mod.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Zwaka TP. Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227–233. doi: 10.1242/dev.01586. [DOI] [PubMed] [Google Scholar]
- 34.Kee K. Gonsalves JM. Clark AT. Pera RA. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006;15:831–837. doi: 10.1089/scd.2006.15.831. [DOI] [PubMed] [Google Scholar]
- 35.West FD. Roche-Rios MI. Abraham S, et al. KIT ligand and bone morphogenetic protein signaling enhances human embryonic stem cell to germ-like cell differentiation. Hum Reprod. 2010;25:168–178. doi: 10.1093/humrep/dep338. [DOI] [PubMed] [Google Scholar]
- 36.Watabe T. Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 37.Qi X. Li TG. Hao J, et al. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc Natl Acad Sci USA. 2004;101:6027–6032. doi: 10.1073/pnas.0401367101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donovan PJ. de Miguel MP. Turning germ cells into stem cells. Curr Opin Genet Dev. 2003;13:463–471. doi: 10.1016/j.gde.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Kerr CL. Hill CM. Blumenthal PD. Gearhart JD. Expression of pluripotent stem cell markers in the human fetal ovary. Hum Reprod. 2008;23:589–599. doi: 10.1093/humrep/dem411. [DOI] [PubMed] [Google Scholar]
- 40.Durcova-Hills G. Tang F. Doody G. Tooze R. Surani MA. Reprogramming primordial germ cells into pluripotent stem cells. PLoS ONE. 2008;3:e3531. doi: 10.1371/journal.pone.0003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Y. Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 42.Murakami G. Watabe T. Takaoka K. Miyazono K. Imamura T. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol Biol Cell. 2003;14:2809–2817. doi: 10.1091/mbc.E02-07-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ying Y. Zhao GQ. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol. 2001;232:484–492. doi: 10.1006/dbio.2001.0173. [DOI] [PubMed] [Google Scholar]
- 44.Ying QL. Nichols J. Chambers I. Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 45.Chambers I. Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004;23:7150–7160. doi: 10.1038/sj.onc.1207930. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki A. Raya A. Kawakami Y, et al. Maintenance of embryonic stem cell pluripotency by Nanog-mediated reversal of mesoderm specification. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S114–122. doi: 10.1038/ncpcardio0442. [DOI] [PubMed] [Google Scholar]
- 47.Finley MF. Devata S. Huettner JE. BMP-4 inhibits neural differentiation of murine embryonic stem cells. J Neurobiol. 1999;40:271–287. [PubMed] [Google Scholar]
- 48.Valdimarsdottir G. Goumans MJ. Rosendahl A, et al. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation. 2002;106:2263–2270. doi: 10.1161/01.cir.0000033830.36431.46. [DOI] [PubMed] [Google Scholar]
- 49.Das P. Ezashi T. Schulz LC. Westfall SD. Livingston KA. Roberts RM. Effects of fgf2 and oxygen in the bmp4-driven differentiation of trophoblast from human embryonic stem cells. Stem Cell Res. 2007;1:61–74. doi: 10.1016/j.scr.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Felici M. Farini D. Dolci S. In or out stemness: comparing growth factor signalling in mouse embryonic stem cells and primordial germ cells. Curr Stem Cell Res Ther. 2009;4:87–97. doi: 10.2174/157488809788167391. [DOI] [PubMed] [Google Scholar]
- 51.Molyneaux K. Wylie C. Primordial germ cell migration. Int J Dev Biol. 2004;48:537–544. doi: 10.1387/ijdb.041833km. [DOI] [PubMed] [Google Scholar]
- 52.Pesce M. Gioia Klinger F. De Felici M. Derivation in culture of primordial germ cells from cells of the mouse epiblast: phenotypic induction and growth control by Bmp4 signalling. Mech Dev. 2002;112:15–24. doi: 10.1016/s0925-4773(01)00624-4. [DOI] [PubMed] [Google Scholar]
- 53.Pesce M. Wang X. Wolgemuth DJ. Scholer H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 1998;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 54.Huang Y. Gu B. Wu R. Zhang J. Li Y. Zhang M. Development of a rabbit monoclonal antibody group against Smads and immunocytochemical study of human and mouse embryonic stem cells. Hybridoma. 2007;26:387–391. doi: 10.1089/hyb.2007.0517. [DOI] [PubMed] [Google Scholar]
- 55.Kodaira K. Imada M. Goto M, et al. Purification and identification of a BMP-like factor from bovine serum. Biochem Biophys Res Commun. 2006;345:1224–1231. doi: 10.1016/j.bbrc.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 56.Xu RH. Sampsell-Barron TL. Gu F, et al. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vallier L. Alexander M. Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118(Pt 19):4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 58.Vallier L. Mendjan S. Brown S, et al. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development. 2009;136:1339–1349. doi: 10.1242/dev.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruzinova MB. Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003;13:410–418. doi: 10.1016/s0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 60.Stahlberg A. Bengtsson M. Hemberg M. Semb H. Quantitative transcription factor analysis of undifferentiated single human embryonic stem cells. Clin Chem. 2009;55:2162–2170. doi: 10.1373/clinchem.2009.131433. [DOI] [PubMed] [Google Scholar]
- 61.Yamaguchi S. Kurimoto K. Yabuta Y, et al. Conditional knockdown of Nanog induces apoptotic cell death in mouse migrating primordial germ cells. Development. 2009;136:4011–4020. doi: 10.1242/dev.041160. [DOI] [PubMed] [Google Scholar]
- 62.Chambers I. Colby D. Robertson M, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 63.Mitsui K. Tokuzawa Y. Itoh H, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 64.Hatano SY. Tada M. Kimura H, et al. Pluripotential competence of cells associated with Nanog activity. Mech Dev. 2005;122:67–79. doi: 10.1016/j.mod.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 65.Hart AH. Hartley L. Ibrahim M. Robb L. Identification, cloning and expression analysis of the pluripotency promoting Nanog genes in mouse and human. Dev Dyn. 2004;230:187–198. doi: 10.1002/dvdy.20034. [DOI] [PubMed] [Google Scholar]
- 66.Yamaguchi S. Kimura H. Tada M. Nakatsuji N. Tada T. Nanog expression in mouse germ cell development. Gene Expr Patterns. 2005;5:639–646. doi: 10.1016/j.modgep.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Sasagawa M. Watanabe S. Ohmomo Y. Honma S. Kanazawa K. Takeuchi S. Reactivity of two monoclonal antibodies (Troma 1 and CAM 5.2) on human tissue sections: analysis of their usefulness as a histological trophoblast marker in normal pregnancy and trophoblastic disease. Int J Gynecol Pathol. 1986;5:345–356. doi: 10.1097/00004347-198612000-00006. [DOI] [PubMed] [Google Scholar]
- 68.King A. Thomas L. Bischof P. Cell culture models of trophoblast II: trophoblast cell lines—a workshop report. Placenta. 2000;21(Suppl A):S113–S119. doi: 10.1053/plac.1999.0526. [DOI] [PubMed] [Google Scholar]
- 69.Cross JC. Genetic insights into trophoblast differentiation and placental morphogenesis. Semin Cell Dev Biol. 2000;11:105–113. doi: 10.1006/scdb.2000.0156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.