Abstract
Immunization with heat shock proteins has protective effects in models of induced arthritis. Analysis has shown a reduced synovial inflammation in such protected animals. Adoptive transfer and immunization with selected T cell epitopes (synthetic peptides) have indicated the protection to be mediated by T cells directed to conserved hsp epitopes. This was shown first for mycobacterial hsp60 and later for mycobacterial hsp70. Fine specificity analysis showed that such T cells were cross-reactive with the homologous self hsp. Therefore protection by microbial hsp reactive T cells can be by cross-recognition of self hsp overexpressed in the inflamed tissue. Preimmunization with hsp leads to a relative expansion of such self hsp cross-responsive T cells. The regulatory nature of such T cells may originate from mucosal tolerance maintained by commensal flora derived hsp or from partial activation through recognition of self hsp as a partial agonist (Altered Peptide Ligand) or in the absence of proper costimulation. Recently, we reported the selective upregulation of B7.2 on microbial hsp60 specific T cells in response to self hsp60. Through a preferred interaction with CTLA-4 on proinflammatory T cells this may constitute an effector mechanism of regulation. Also, regulatory T cells produced IL10.
INTRODUCTION
Besides their significance as intracellular molecular chaperones and their possible role in triggering innate forms of immunity (signaling “danger”) (Chen et al 1999; Gallucci et al 1999), microbial heat shock proteins (Hsps) are also regular protein immunogens that easily induce secondary adaptive immune responses at the level of both B cells and T cells (Kaufmann et al 1987). In fact, immunization with complex microbial organisms leads to production of antibodies and T-cell responses to Hsp in the first place. In the past, microbial Hsp60 (GroEL) was known as the common antigen of gram negatives, documenting the immunodominance of this microbial antigen.
Given their strong sequence conservation and in accordance with the predictions as formulated by Burnet (elimination of self-reactive cells from the immune repertoire), one would expect reactivity to Hsp to be confined to nonconserved, microbe-specific areas of these molecules. However, contrary to this expectation, many studies have shown reactivity to Hsp to include reactivity to conserved Hsp sequences and to include reactivity to mammalian Hsp (self-Hsp) (Brett et al 1989; Cohen 1992; Quayle et al 1992; Anderton et al 1993; de Graeff-Meeder et al 1995; Konen-Waisman et al 1995; Birk et al 1996; Prakken et al 1996; Abulafia-Lapid et al 1999). Currently, in contrast to the original proposal by Burnet, self-reactivity is appreciated as a normal component of a healthy immune repertoire, such self-reactivity being controlled by mechanisms of T-cell regulation (peripheral tolerance). In our studies, mainly in experimentally induced arthritis, self-Hsp reactivity at the level of T cells was induced by immunization with mycobacterial Hsp, and it was documented that such self-Hsp cross-reactive T cells were the possible mediators of anti-inflammatory regulatory activity themselves.
SUPPRESSION OF ARTHRITIS BY SELF-Hsp CROSS-REACTIVE T CELLS
Evidence for Hsp as a critical antigen in arthritis was obtained when arthritis-associated T cells derived from arthritic rats were found to recognize mycobacterial Hsp60 (van Eden et al 1988). Extensive follow-up experiments in various laboratories subsequently revealed the capacity of microbial Hsp60 to produce resistance against experimental arthritis on immunization (various models in rats and mice). In addition, also for members of other Hsp families, protective qualities in arthritis models were reported. The following are some of the main reports.
• Mycobacterial Hsp60 in incomplete Freund's adjuvant (adjuvant arthritis) (van Eden et al 1988)
• Mycobacterial Hsp60 in incomplete Freund's adjuvant (streptococcal cell wall–induced arthritis) (van de Broek et al 1989)
• Mycobacterial Hsp60 in incomplete Freund's adjuvant (pristane arthritis) (Beech et al 1997)
• Mycobacterial Hsp60 in incomplete Freund's adjuvant (adjuvant arthritis and collagen arthritis) (Billingham et al 1990)
• Mycobacterial Hsp60 in vaccinia (adjuvant arthritis) (Hogervorst et al 1991)
• Mycobacterial Hsp60 epitope in dimethyl dioctadecyl ammonium bromide (adjuvant arthritis and avridine arthritis) (Anderton et al 1995)
• Mycobacterial Hsp10 in incomplete Freund's adjuvant (adjuvant arthritis) (Ragno et al 1996)
• Mycobacterial Hsp70 in incomplete Freund's adjuvant (adjuvant arthritis and avridine arthritis) (Kingston et al 1996)
• Mycobacterial Hsp60 oral (adjuvant arthritis) (Haque et al 1996)
• Escherichia coli Hsp70 (OM-89) (adjuvant arthritis) (Bloemendal et al 1997)
• Mycobacterial Hsp60 naked DNA (adjuvant arthritis) (Ragno et al 1997)
• Mycobacterial Hsp70 epitope in incomplete Freund's adjuvant (adjuvant arthritis) (Tanaka 1999)
• Mycobacterial Hsp70 epitope intranasal (adjuvant arthritis) (Wendling et al 2000)
• Mycobacterial Hsp60 oral with soybean trypsin inhibitor as treatment (adjuvant arthritis) (Cobelens et al 2000)
A comprehensive analysis of Hsp60 T-cell epitopes in adjuvant arthritis uncovered the principle of the induction of self-Hsp cross-recognition as the underlying mechanistic principle of the arthritis-suppressive potential of mycobacterial Hsp60 (Anderton et al 1994; Anderton et al 1995). By testing an overlapping set of 15-mer peptides spanning the complete mycobacterial Hsp60 sequence, 9 distinct, dominant T-cell epitopes were detected. Subsequent adoptive transfer studies using T-cell lines generated to all epitopes revealed that only T cells directed to a very conserved 256–265 sequence transferred protection. The same T cells were shown to recognize the self-Hsp60 homologous peptide and also heat-shocked syngeneic cells. Furthermore, active immunization with the conserved peptide protected against the induction of both mycobacteria-induced adjuvant arthritis and avridine arthritis induced with a nonimmunogenic synthetic compound. All other (nonconserved) peptides failed to produce such protection.
Recently, the Hsp60 mode of action in suppressing arthritis has been reproduced very similarly for Hsp70 by others (Tanaka 1999) and ourselves (Wendling et al 2000). Also, in these latter cases, T cells that recognize the very conserved Hsp70 peptides were found to produce protection.
Given the multitude of models involving different triggering substances or antigens, where single molecules such as mycobacterial Hsp60 and Hsp70 were found to be protective, the induction of regulatory self-Hsp cross-reactive T-cell responses may well explain the observations made so far.
HOW SELF-Hsp CROSS-REACTIVE T CELLS BECOME REGULATORS
Recent studies of peripheral immunological tolerance have amply substantiated the potential of self-reactive T cells as regulatory T cells that have the capacity to control autoimmune diseases. These T cells have regulatory activities based on either skewed regulatory cytokine production (Th1 vs Th2, Tr1 [Groux et al 1997; Sakaguchi et al 1995; Wendling et al 2000]) or a diverted functional status such as anergy (Sakaguchi 2000). In the case of anergy, T cells may produce suppressive interleukin (IL) 10 (Buer et al 1998) or modulate antigen-presenting cell (APC) functional activity (Taams et al 1998; Chai et al 1999; Jordan et al 2000).
Given the particular nature of Hsps, self-Hsp60–reactive T cells can be regulatory through grossly 2 additive mechanisms (van Eden et al 1998a; van Eden et al 1998b).
First, presence of constitutive self-Hsp (low levels) in parenchymal cells and microbial homologues in the gut-associated lymphoid tissue maintains tolerance in the peripheral self-Hsp–specific T-cell repertoire through induction of anergy (lack of costimulation or cross-tolerance induction by professional APCs) and induction of regulatory cytokine profiles (mucosal tolerance). Second, self-Hsp60 (due to the uniquely subtle amino acid sequence variations between self- and microbial homologues) does provide natural altered peptide ligands (APLs) or partial agonists for the microbial Hsp60–oriented T-cell repertoire and, therefore, sets the self-Hsp cross-reactive repertoire in the regulatory mode. Thus, the actively tolerated self-Hsp60 repertoire exerts its regulatory activity in the context of stress up-regulated self-Hsp60 at the site of inflammation.
PROTECTION AGAINST ARTHRITIS SEEMS TO BE A UNIQUE QUALITY OF Hsp
Recently, we have tested whether other bacterially derived immunogens of a conserved nature have similar protective qualities in experimental arthritis. For this we selected bacterial antigens such as superoxide dismutase of E coli, glyceraldehyde-3-phosphate dehydrogenase of Bacillus (G3PDH), and aldolase of Staphylococcus. These antigens were found to be immunogenic, because they induced readily proliferative T-cell responses and delayed-type hypersensitivity reactions. All 3 antigens were relatively conserved and have homologous enzymes present in the mammalian hosts. However, none of the antigens were seen to affect arthritis induction on immunization in both adjuvant and avridine arthritis.
Why would Hsp, including minor subfamilies such as Hsp10, protect against arthritis and other inflammatory processes, whereas other conserved and immunogenic bacterial proteins do not?
One major difference between ordinary bacterial proteins and Hsp is the stress protein nature of Hsp. Inflammation leads to the locally up-regulated expression of Hsp, which was documented by various studies to occur in the synovium in the case of arthritis (Boog et al 1992). Therefore, the up-regulated presence of the target for regulatory T-cell activity can focus the regulatory control toward the inflammatory process.
In addition, it is possible that in comparison with other bacterial antigens, Hsps have specialized receptors (such as CD14 [Asea et al 2000] and Toll-like receptors [Ohashi et al 2000]) for their entry into, for instance, macrophages, as suggested by the evidence that Hsps have a unique capacity to signal “danger.” For gut flora–associated Hsp, this could possibly mean that Hsps have a relatively easy entry into the gut-associated lymphoid tissues. In this case, the immune system would have a more continuous and intense relationship with Hsp than with other microbial antigens.
GUT-ASSOCIATED TOLERANCE AND ARTHRITIS PROTECTION
The significance of gut flora for resistance to arthritis has been widely documented. In most cases, presence of gut flora was seen to produce a relative resistance to arthritis induction. Classic experiments by Kohashi et al (1986) have shown in Fisher rats that germ-free animals were susceptible to adjuvant arthritis, whereas conventional animals were not. Reconstitution of the germ-free with the original flora or with E coli was shown to reproduce resistance.
Of interest in this respect seems to be a slow-acting, orally administered antirheumatic drug called Subreum or OM89 (Laboratoires OM, Geneva, Switzerland) (Vischer and van Eden 1994). Subreum consists of a extract of selected E coli strains and contains E coli Hsp, mainly Hsp70. On intragastric administration in Lewis rats, the material was found to inhibit adjuvant arthritis and to induce proliferative T-cell responses specific for Hsp60 and Hsp70 (Bloemendal et al 1997). It is possible that the mode of action of this material is due to the induction of regulatory T-cell activity directed to Hsp. Indeed, recent experiments by Cobelens et al (2000) have provided the evidence that oral Hsp can directly mediate an arthritis therapeutic effect. Mycobacterial Hsp60 was administered from the time adjuvant arthritis was manifest at several days orally in combination with soybean trypsin inhibitor to avoid intragastric disintegration. This led to an immediate reduction of arthritis severity in the treated animals.
More indirect evidence was collected by Nieuwenhuis et al (2000) when they showed that an antibiotic regimen that led to a demonstrable predominance of E coli bacteria in the gut produced a strong resistance to adjuvant arthritis induction. Interestingly, the same regimen produced resistance to induction of experimental autoimmune encephalomyelitis.
SELF-Hsp CROSS-REACTIVE T CELLS PRODUCE IL-10
Immunization with mycobacterial Hsp70 in rats was found to induce production of IL-10. Draining lymph node cells were analyzed by reverse transcriptase–polymerase chain reaction. In samples obtained after Hsp70 immunization, message for IL-10 was detected, whereas in samples obtained after immunization with other control bacterial antigens (as mentioned above), it was not detected (Wendling et al 2000). Follow-up experiments showed, by intracellular staining for cytokines in selected CD4+ T cells, that Hsp70- and Hsp60-specific T cells were producing IL-10 and IL-4 to a lesser extent. Control T cells specific for aldolase were found to produce interferon-γ and tumor necrosis factor α and not IL-10 or IL-4. In the same assay system, a T-cell line raised by immunization with a conserved Hsp70 epitope was also found to produce IL-10. In accordance with that, the same epitope in the form of a synthetic peptide was shown to be arthritis protective on nasal administration.
In a recent study by van Halteren et al (personal communication), the role of Hsp60-specific T cells in mouse non-obese diabetes (NOD) diabetes was studied. From the analysis comparing the diabetes-susceptible NOD with the diabetes-resistant transgenic strain expressing the resistant major histocompatibility complex type I-A g7asp on the NOD background, it appeared that Hsp60-reactive T cells were triggered by insulitis, although Hsp60 was not a primary autoantigen in NOD diabetes. Furthermore, the analysis of cytokines produced suggested that IL-10–producing Hsp60-specific T cells were associated with the control of insulitis. A drop in IL-10–producing Hsp60-reactive T cells was found to precede clinical expression of diabetes.
Other studies have also indicated a propensity of Hsp to induce production of IL-10. For example, in the mouse it was shown that chlamydial Hsp60 primarily induced proinflammatory cytokines (Yi et al 1997). However, in a similar setup, the mouse Hsp60 protein was found to induce primarily IL-10, and in the combination of chlamydial and mouse Hsp60, both proinflammatory cytokines and IL-10 were produced. Therefore, it was postulated that in the natural situation of chlamydial infection both cytokines that drive inflammation and cytokines that control inflammation are produced. A similar situation was reported for experimental Listeria infections in the rat (Kimura et al 1998). In that case, Listeria Hsp70-specific T cells were found to have a regulatory role during listeriosis through production of transforming growth factor β and IL-10. It was suggested that Hsp-reactive T cells were involved to terminate the Th1 cell–mediated excessive inflammation after the “battle against Listeria monocytogenes has been won.”
Other forms of inflammation, apart from those resulting from infection, have also been seen to be associated with production of IL-10. In fact, it appears as if cellular injuries of diverse origin lead to production of IL-10. Therefore, Stordeur and Goldman (1998) have called IL-10 a “stress cytokine.”
Altogether, it is possible that IL-10 production by stressed cells and the propensity of self-Hsp cross-reactive T cells to produce IL-10 are both a reflection of the regulatory arm of the immune system meant to control excessive or nonproductive inflammation.
THE PHENOTYPES OF SELF-Hsp CROSS-REACTIVE T CELLS IN RATS AND HUMANS
Apart from their propensity to produce IL-10, some distinct phenotypic markers were found to become up-regulated on self-Hsp–reactive T cells. Recent experiments in rat adjuvant arthritis have shown the selective up-regulation of B7.2 on Hsp60-reactive T cells when stimulated by the self (rat)–Hsp60 sequence (Paul et al 2000). T-cell lines were raised against the 256–270 conserved mycobacterial Hsp60 peptide. Earlier, such lines had been found to induce arthritis protection (Anderton et al 1995). On restimulation with the mycobacterial peptide (256–265), analysis by fluorescence-activated cell sorter showed that the cell was up-regulating activation markers such as IL-2R, intercellular adhesion molecule 1, and CD134. Furthermore, the T-cell receptor was down-modulated and the costimulatory molecule B7.1 was up-regulated at a higher peptide concentration. Stimulation of the homologous self-peptide (rat 256–265) did lead to up-regulation of the same activation markers. However, B7.1 was not up-regulated, and there was a strong up-regulation of B7.2 already at a low peptide concentration. The same selective B7.2 up-regulation was seen after stimulation of this T-cell line with heat-shocked APCs (spleen). Thus, in this case, the stimulation of the T cells specific for the mycobacterial conserved epitope did not proliferate in the presence of the corresponding rat self-epitope; however, the cell was activated and up-regulated B7.2. By this, the self-epitope acted as a partial agonist or APL. So far, no data were reported on the differential expression of B7.1 and B7.2 on T cells after activation with APLs. However, several reports suggested a role for B7.2, expressed on T cells, in the down-modulation of T-cell responses, including anti–T-cell responses. Greenfield et al (1997) reported that B7.2-transfected T-cell tumors did not provide costimulation to other T cells in vitro and, in fact, inhibit antitumor immunity in vivo. In addition, they showed that the B7.2 expressed on the T-cell tumor preferentially bound CTLA-4, which was also observed for the B7.2 expressed on normal murine T cells, indicating that the B7.2 expressed on T cells differed from that on APCs. Indeed, B7.2 on human T cells, having a different glycosylation form, bound CTLA-4 but not CD28 (Hollsberg et al 1997). In addition, the interaction of B7.2 on murine T cells with CTLA-4 was shown to be responsible for down-modulation of T cell responses in vitro (Stremmel et al 1999). Recently, B7.2 on T-cell tumors was reported to suppress tumor immunity, with IL-4– and/or IL-10–producing CD4+ T cells playing a critical role in the suppression.
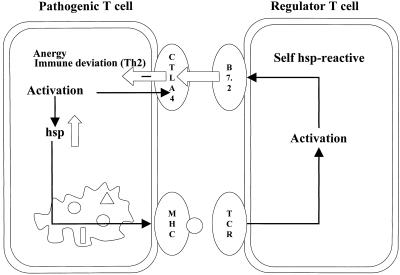
Thus, the up-regulation of B7.2 on T cells, induced on recognition of self-Hsp on APCs, may well provide such T cells with a mechanism to control pathogenic T cells in vivo. Since pathogenic activated rat T cells do express major histocompatibility complex class II antigens (Broeren et al 1995) and enhanced levels of self-Hsp60 (Ferris et al 1988), they may present the self-Hsp60 peptide to T cells that are induced to express B7.2. Also in a more direct manner, this T-T interaction involving B7.2 would lead to a negative signal through CTLA-4 on the activated pathogenic T cells, leading to a suppressive form of regulation. Indeed, a self-Hsp–specific, highly autoreactive T-cell line expressing B7.2 after culture with just APCs and no added antigen was seen to transfer protection in rat adjuvant arthritis. The principle of a possible direct regulatory loop at the level of interacting T cells through recognition of self-Hsp and up-regulated B7.2 is depicted in Figure 1.
Fig 1.
Self-Hsp60–reactive T cells down-modulate Hsp-presenting pathogenic T cells directly through inhibitory T-T cell interactions involving B7.2 and its ligand CTLA-4
Studies in humans have focused on human Hsp60-specific T cells in children with juvenile idiopathic arthritis (JIA) (de Graeff-Meeder et al 1995; Prakken et al 1996). T-cell responses to human Hsp60 were detected both in peripheral blood and synovial fluid samples primarily in patients with JIA who had a remitting course of their disease (mainly oligoarticular JIA), and in time such responses were seen to precede actual remission. Patients with chronic progressive forms of this disease were found to be nonresponsive (polyarticular and systemic forms of JIA). Cytokine analyses of the synovial cells from patients with the remitting forms of the disease revealed the Hsp60 responsiveness to coincide with a relative Th2 skewing with production of IL-4 and IL-10. The analysis of human Hsp60-specific T-cell lines obtained from these patients showed the production of IL-4, IL-10, and transforming growth factor β by these T cells on stimulation with human Hsp60. Interestingly, a remarkable induction of CD30 was similarly noted to occur on stimulation with Hsp60 (Prakken et al 1999). Apart from the fact that CD30 is regarded to be a possible marker for Th2 cells, recently CD30 was documented to be a marker for potentially regulatory T cells in human rheumatoid arthritis. Surface expression of CD30 was found to fully correlate with IL-4 and IL-10 production in synovial T cells. In addition, soluble CD30 was produced in patients with a beneficial response to therapy (Gerli et al 2000).
REFERENCES
- Abulafia-Lapid R, Elias D, Raz I, Keren-Zur Y, Atlan H, Cohen IR. T cell proliferative responses of type 1 diabetes patients and healthy individuals to human hsp60 and its peptides. J Autoimmun. 1999;12:121–129. doi: 10.1006/jaut.1998.0262. [DOI] [PubMed] [Google Scholar]
- Anderton SM, van der Zee R, Goodacre JA. Inflammation activates self hsp60-specific T cells. Eur J Immunol. 1993;23:33–38. doi: 10.1002/eji.1830230107. [DOI] [PubMed] [Google Scholar]
- Anderton SM, van der Zee R, Noordzij A, van Eden W. Differential mycobacterial 65-kDa heat shock protein T cell epitope recognition after adjuvant arthritis-inducing or protective immunization protocols. J Immunol. 1994;152:3656–3664. [PubMed] [Google Scholar]
- Anderton SM, van der Zee R, Prakken B, Noordzij A, van Eden W. Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis. J Exp Med. 1995;181:943–952. doi: 10.1084/jem.181.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Beech JT, Siew LK, Ghoraishian M, Stasiuk LM, Elson CJ, Thompson SJ. CD4+ Th2 cells specific for mycobacterial 65-kilodalton heat shock protein protect against pristane-induced arthritis. J Immunol. 1997;159:3692–3697. [PubMed] [Google Scholar]
- Billingham MEJ, Carney S, Butler R, Colston MJ. A mycobacterial 65-kDa heat shock protein induces antigen-specific suppression of adjuvant arthritis, but is not itself arthritogenic. J Exp Med. 1990;171:339–344. doi: 10.1084/jem.171.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk OS, Douek DC, Elias D, et al. A role of Hsp60 in autoimmune diabetes: analysis in a transgenic model. Proc Natl Acad Sci USA. 1996;93:1032–1037. doi: 10.1073/pnas.93.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal A, Van der Zee R, Rutten VP, van Kooten PJ, Farine JC, van Eden W. Experimental immunization with anti-rheumatic bacterial extract OM-89 induces T cell responses to heat shock protein (hsp)60 and hsp70; modulation of peripheral immunological tolerance as its possible mode of action in the treatment of rheumatoid arthritis (RA) Clin Exp Immunol. 1997;110:72–78. doi: 10.1046/j.1365-2249.1997.4841378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boog CJ, de Graeff-Meeder ER, Lucassen MA, van der Zee R, Voorhorst-Ogink MM, van Kooten PJ, Geuze HJ, van Eden W. Two monoclonal antibodies generated against human hsp60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J Exp Med. 1992;175:1805–1810. doi: 10.1084/jem.175.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett SJ, Lamb JR, Cox JH, Rothbard JB, Mehlert A, Ivanyi J. Differential pattern of T cell recognition of the 65-kDa mycobacterial antigen following immunization with the whole protein or peptides. Eur J Immunol. 1989;19:1303–1310. doi: 10.1002/eji.1830190723. [DOI] [PubMed] [Google Scholar]
- Broeren CP, Wauben MH, Lucassen MA, Van Meurs M, Van Kooten PJ, Boog CJ, Claassen E, van Eden W. Activated rat T cells synthesize and express functional major histocompatibility class II antigens. Immunology. 1995;84:193–201. [PMC free article] [PubMed] [Google Scholar]
- Buer J, Lanoue A, Franzke A, Garcia C, von Boehmer H, Sarukhan A. Interleukin 10 secretion and impaired effector function of major histocompatibility complex class II-restricted T cells anergized in vivo. J Exp Med. 1998;187:177–183. doi: 10.1084/jem.187.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai JG, Bartok I, Chandler P, Vendetti S, Antoniou A, Dyson J, Lechler R. Anergic T cells act as suppressor cells in vitro and in vivo. Eur J Immunol. 1999;29:686–692. doi: 10.1002/(SICI)1521-4141(199902)29:02<686::AID-IMMU686>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–3219. [PubMed] [Google Scholar]
- Cobelens PM, Heijnen CJ, Nieuwenhuis EES, Kramer PPG, Van der Zee R, van Eden W, and Kavelaars A 2000 Treatment of adjuvant arthritis by oral administration of mycobacterial HSP65 during disease. Arthritis Rheum. in press. [DOI] [PubMed] [Google Scholar]
- Cohen IR. Autoimmunity to hsp65 and the immunologic paradigm. Adv Intern Med. 1992;37:295–311. [PubMed] [Google Scholar]
- de Graeff-Meeder ER, van Eden W, Rijkers GT, et al. Juvenile chronic arthritis: T cell reactivity to human HSP60 in patients with a favorable course of arthritis. J Clin Invest. 1995;95:934–940. doi: 10.1172/JCI117801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris DK, Harel-Bellan A, Morimoto RI, Welch WJ, Farrar WL. Mitogen and lymphokine stimulation of heat shock proteins in T lymphocytes. Proc Natl Acad Sci USA. 1988;85:3850–3854. doi: 10.1073/pnas.85.11.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- Gerli R, Pitzalis C, Bistoni O, Falini B, Costantini V, Russano A, Lunardi C. CD30+ T cells in rheumatoid synovitis: mechanisms of recruitment and functional role. J Immunol. 2000;164:4399–4407. doi: 10.4049/jimmunol.164.8.4399. [DOI] [PubMed] [Google Scholar]
- Greenfield EA, Howard E, Paradis T, et al. B7.2 expressed by T cells does not induce CD28-mediated costimulatory activity but retains CTLA4 binding: implications for induction of antitumor immunity to T cell tumors. J Immunol. 1997;158:2025–2034. [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Haque MA, Yoshino S, Inada S, Nomaguchi H, Tokunaga O, Kohashi O. Suppression of adjuvant arthritis in rats by induction of oral tolerance to mycobacterial 65-kDa heat shock protein. Eur J Immunol. 1996;26:2650–2656. doi: 10.1002/eji.1830261116. [DOI] [PubMed] [Google Scholar]
- Hogervorst EJ, Schouls L, Wagenaar JP, Boog CJ, Spaan WJ, van Embden JD, van Eden W. Modulation of experimental autoimmunity: treatment of adjuvant arthritis by immunization with a recombinant vaccinia virus. Infect Immun. 1991;59:2029–2035. doi: 10.1128/iai.59.6.2029-2035.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollsberg P, Scholz C, Anderson DE, Greenfield EA, Kuchroo VK, Freeman GJ, Hafler DA. Expression of a hypoglycosylated form of CD86 (B7–2) on human T cells with altered binding properties to CD28 and CTLA-4. J Immunol. 1997;159:4799–4805. [PubMed] [Google Scholar]
- Jordan MS, Riley MP, von Boehmer H, Caton AJ. Anergy and suppression regulate CD4(+) T cell responses to a self peptide. Eur J Immunol. 2000;30:136–144. doi: 10.1002/1521-4141(200001)30:1<136::AID-IMMU136>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Vath U, Thole JE, Van Embden JD, Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987;17:351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yamada K, Sakai T, Mishima K, Nishimura H, Matsumoto Y, Singh M, Yoshikai Y. The regulatory role of heat shock protein 70-reactive CD4+ T cells during rat listeriosis. Int Immunol. 1998;10:117–130. doi: 10.1093/intimm/10.2.117. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Hicks CA, Colston MJ, Billingham ME. A 71-kD heat shock protein (hsp) from Mycobacterium tuberculosis has modulatory effects on experimental rat arthritis. Clin Exp Immunol. 1996;103:77–82. doi: 10.1046/j.1365-2249.1996.929628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi O, Kohashi Y, Ozawa A, Shigematsu N. Suppressive effect of E. coli on adjuvant-induced arthritis in germ-free rats. Arthritis Rheum. 1986;29:547–552. doi: 10.1002/art.1780290413. [DOI] [PubMed] [Google Scholar]
- Konen-Waisman S, Fridkin M, Cohen IR. Self and foreign 60-kilodalton heat shock protein T cell epitope peptides serve as immunogenic carriers for a T cell-independent sugar antigen. J Immunol. 1995;154:5977–5985. [PubMed] [Google Scholar]
- Nieuwenhuis EES, Visser MR, Kavelaars A, and et al. 2000 Oral antibiotics as a novel therapy for arthritis: evidence for a beneficial effect of intestinal Escherichia coli. Arthritis Rheum. in press. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Paul AGA, van der Zee R, Taams LS, van Eden W. A self-hsp60 peptide acts as a partial agonist inducing expression of B7-2 on mycobacterial hsp60-specific T cells: a possible mechanism for inhibitory T cell regulation of adjuvant arthritis? Intern Immunol. 2000;12:1041–1050. doi: 10.1093/intimm/12.7.1041. [DOI] [PubMed] [Google Scholar]
- Prakken AB, van Eden W, Rijkers GT, Kuis W, Toebes EA, de Graeff-Meeder ER, van der Zee R, Zegers BJ. Autoreactivity to human heat-shock protein 60 predicts disease remission in oligoarticular juvenile rheumatoid arthritis. Arthritis Rheum. 1996;39:1826–1832. doi: 10.1002/art.1780391108. [DOI] [PubMed] [Google Scholar]
- Prakken BJ, Kuis W, and van Eden W 1999 T cell responsiveness to heat shock proteins in patients with juvenile chronic arthritis as an example of immunomodulation in vivo. In: Specific Immunotherapy of Chronic Autoimmune Diseases, ed van Eden W, De Boer M, Bijlsma JWJ, De Vries RRP, Melief CJM. KNAW, Amsterdam, The Netherlands, 33–40. [Google Scholar]
- Quayle AJ, Wilson KB, Li SG, et al. Peptide recognition, T cell receptor usage and HLA restriction elements of human heat-shock protein (hsp) 60 and mycobacterial 65-kDa hsp-reactive T cell clones from rheumatoid synovial fluid. Eur J Immunol. 1992;22:1315–1322. doi: 10.1002/eji.1830220529. [DOI] [PubMed] [Google Scholar]
- Ragno S, Colston MJ, Lowrie DB, Winrow VR, Blake DR, Tascon R. Protection of rats from adjuvant arthritis by immunization with naked DNA encoding for mycobacterial heat shock protein 65. Arthritis Rheum. 1997;40:277–283. doi: 10.1002/art.1780400212. [DOI] [PubMed] [Google Scholar]
- Ragno S, Winrow VR, Mascagni P, Lucietto P, Di Pierro F, Morris CJ, Blake DR. A synthetic 10-kD heat shock protein (hsp10) from Mycobacterium tuberculosis modulates adjuvant arthritis. Clin Exp Immunol. 1996;103:384–390. doi: 10.1111/j.1365-2249.1996.tb08291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Stordeur P, Goldman M. Interleukin-10 as a regulatory cytokine induced by cellular stress: molecular aspects. Int Rev Immunol. 1998;16:501–522. doi: 10.3109/08830189809043006. [DOI] [PubMed] [Google Scholar]
- Stremmel C, Greenfield EA, Howard E, Freeman GJ, Kuchroo VK. B7–2 expressed on EL4 lymphoma suppresses antitumor immunity by an interleukin 4-dependent mechanism. J Exp Med. 1999;189:919–930. doi: 10.1084/jem.189.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taams LS, van Rensen AJ, Poelen MC, van Els CA, Besseling AC, Wagenaar JP, van Eden W, Wauben MH. Anergic T cells actively suppress T cell responses via the antigen-presenting cell. Eur J Immunol. 1998;28:2902–2912. doi: 10.1002/(SICI)1521-4141(199809)28:09<2902::AID-IMMU2902>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Tanaka S. Activation of T cells recognizing an epitope of heat-shock protein 70 can protect against rat adjuvant arthritis [record supplied by publisher] J Immunol. 1999;163:5560–5565. [PubMed] [Google Scholar]
- van de Broek MF, Hogervorst EJ, Van Bruggen MC, van Eden W, van der Zee R, van den Berg WB. Protection against streptococcal cell wall-induced arthritis by pretreatment with the 65-kD mycobacterial heat shock protein. J Exp Med. 1989;170:449–466. doi: 10.1084/jem.170.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W, Thole JE, van der Zee R, Noordzij A, van Embden JD, Hensen EJ, Cohen IR. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988;331:171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- van Eden W, van der Zee R, Paul AG, Prakken BJ, Wendling U, Anderton SM, Wauben MH. Do heat shock proteins control the balance of T-cell regulation in inflammatory diseases? Immunol Today. 1998a;19:303–307. doi: 10.1016/s0167-5699(98)01283-3. [DOI] [PubMed] [Google Scholar]
- van Eden W, van der Zee R, Taams LS, Prakken AB, van Roon J, Wauben MH. Heat-shock protein T-cell epitopes trigger a spreading regulatory control in a diversified arthritogenic T-cell response. Immunol Rev. 1998b;164:169–174. doi: 10.1111/j.1600-065x.1998.tb01218.x. [DOI] [PubMed] [Google Scholar]
- Vischer TL, van Eden W. Oral desensitisation in rheumatoid arthritis. Ann Rheum Dis. 1994;53:708–710. doi: 10.1136/ard.53.11.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling U, Paul L, Van der Zee R, Prakken B, Singh M, van Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–2717. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- Yi Y, Yang X, Brunham RC. Autoimmunity to heat shock protein 60 and antigen-specific production of interleukin-10. Infect Immun. 1997;65:1669–1674. doi: 10.1128/iai.65.5.1669-1674.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]