Abstract
Human bone marrow mesenchymal stromal cells (MSCs) with self-renewal and multiple differentiation potentials are considered a possible cell source for tissue engineering and regenerative medicine. However, the limited amount of MSCs in bone marrow and the loss of differentiation capacity following in vitro expansion restrict their practical application. Effective improvement of MSC proliferation is necessary for the clinical application of MSC-based tissue engineering. The effects of estrogen supplements on proliferation and characterizations of human MSCs were investigated at the present study. Supplements of 17-β estradiol (E2) significantly increase the proliferation of human MSCs in vitro. The dose range of E2 to significantly increase MSC proliferation differs in the gender of MSC donor. E2 supplementation in cell proliferation maintains characterizations of MSCs, including cell surface markers, and osteogenic and adipogenic differentiation capacities. These data indicate that estrogen treatment can play an important role in improving human MSCs' expansion in vitro, which will effectively facilitate MSCs' function in the practical application of tissue engineering and regeneration.
Introduction
Autologous adult stem cell-based tissue engineering and regenerative medicine has been considered a promising substitute for current clinical treatments that restore tissue and organ deficiencies [1–3]. Successful tissue regeneration requires a sufficient cell population with high differentiation potential. Self-renewal and multipotent bone marrow mesenchymal stromal cells or mesenchymal stem cells (MSCs) have been identified as a potential cell source for this approach [2,4–7]. However, feasibly isolated cell number of MSCs is limited. Many factors reduce MSC population, proliferation rate, and differentiation potential, including the advanced age and degenerative diseases of donors [8,9]. The bone-forming capacity of MSC-based tissue-engineered constructs declined with the age of donors [10–11]. Although it was considered as an effective means to increase cell population, current in vitro cell expansion system was found to reduce MSC proliferation and differentiation capacity. It has been demonstrated that in vitro proliferation of MSCs decreases telomerase, shortens telomere length, and accelerates MSC senescence [9,12]. Decreased telomerase impairs the multiple differentiation potential of MSCs, and senescent MSCs are always associated with low differentiation potential [8]. To generate a sufficiently large population of MSCs with the differentiation capacity for successful tissue regeneration, the improvement of MSC proliferation and inhibition of senescence is necessary.
Estrogen has multifunctional roles that influence growth, differentiation, and metabolism in many tissues. Estrogen exerts regulatory functions via estrogen receptor (ER)-α and ER-β, which exist on multiple types of cells, including MSCs [13–18]. Recent studies demonstrate that 17-β estradiol (E2) can effectively improve bone marrow stromal cell proliferation in mice and rats. Estrogen has been confirmed to enhance differentiation potentials of human MSCs, whereas their effects on proliferation of human MSCs are controversial [19–22]. In addition, estrogen has a proven potential to upregulate the telomerase activity of endothelial progenitor, myometrial cells, and MSCs, and to prevent the shortening of the telomere of hepatic cells and MSCs via ER-α [23–28], which suggests that estrogen might have the potential to inhibit the senescence of MSCs. We investigated the effect of E2 supplementation on proliferation of MSCs in both steroid-free and conventional cell culture media. Also, we estimated the characterizations of MSC after in vitro cell expansion with E2 supplementation.
Materials and Methods
Human MSC preparation
Fresh human bone marrow donated by 12 healthy donors (6 males and 6 females, average age = 27.4 ± 6.1) were purchased (Allcells, Emeryville, CA). Mononuclear cells were isolated using density gradient centrifugation (Histopaque-1.077; Sigma, St. Louis, MO). Then, cells were cultured with Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA), 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA), and 1% antibiotic–antimycotic (Sigma). On day 5, nonadherent cells were removed after medium exchange. The cells were continued to culture and were collected when cells reach 70%–80% confluence.
In vitro proliferation of MSCs with E2 supplement
Passage-1 MSCs were used to test for proliferation effect of E2 supplementation. To test E2's effect on proliferation of human MSCs in steroid-free conditions, cells were cultured with a steroid-free medium 24 h before the test. The steroid-free medium consisted of phenol red-free DMEM (Invitrogen), 10% charcoal-stripped FBS (Gemini Bio-product, West Sacramento CA), and 1% antibiotic–antimycotic (Sigma). To investigate E2's effect on MSCs' proliferation at the conventional cell culture medium, MSCs were cultured with DMEM, 10% FBS, and 1% antibiotic–antimycotic. To investigate the effect of E2 supplements on cell proliferation, 1,000 MSCs were placed into each well of 96-well plates and cultured by either conventional medium or steroid-free medium supplemented with E2 (Sigma). The E2 concentrations ranged from 10−6 to 10−12 M. The MSC proliferation without E2 supplement was controlled. The cell proliferation was measured using an MTS-based colorimetric method according to the manufacturer's manual (CellTiter 96®; Promega, Madison, WI). The cell proliferations with E2 supplements were normalized to the controls.
Characterization of MSCs after proliferation with E2 supplement
To investigate the characterization of MSCs after proliferation with E2 supplementation, the MSCs were purified by cell sorting using antihuman antibodies positive to CD105, CD166, CD29, and CD44 and negative to CD14, CD34, and CD45. MSCs were cultured by the conventional medium supplemented with E2 at 10−8M. Corresponding MSCs without E2 supplementation were used as controls. Upon reaching 80%–90% confluence, the isolated MSCs were trypsinized and divided in a ratio of 1:6 to subculture. The capacity of MSC proliferation was assessed after 3 and 8 passages. We also analyzed differentiation capacities of osteogenesis and adipogenesis, cell surface markers, ER, and telomerase activity of MSCs after proliferation with E2 supplement.
Measurement of differentiation capacities of MSCs
For investigating the osteogenic differentiation potentials of MSCs, the cells proliferated with E2 supplements were placed in 12-well plates at a density of 2–2.5 × 104 cells per well. The cells were exposed to the osteogenic differentiation medium consisted of the basic medium supplemented with 100 nM dexamethasone, 10 mM β-glycerophosphate, and 0.05 mM ascorbic acid-2-phosphate. After 1 week the osteogenic differentiated MSCs were collected by washing twice with phosphate-buffered saline (PBS) and lysing with 1% Triton-X100 solution. The mixture was subsequently homogenized by sonication. The amount of DNA, alkaline phosphatase (ALP), calcium concentration, and protein was measured as described previously [20,21]. For testing adipogenic differentiation potential, the cells were seeded into 12-well plates at a density of 4–5 × 104 per well, and then exposed to adipogenic differentiation medium consisted of basic medium supplemented with 500 nM of dexamethasone, 10 μM of insulin, and 0.5 mM of isobutyl-methylxanthine. After 2 weeks of culture, cells were fixed and stained with Oil-Red-O staining. The Oil-Red-O-stained lipid accumulation was eluted using isopropanol and quantitatively assayed by absorbance values at 510 nm according to our previous studies [20,21].
Analysis of cell surface markers, ERs, and telomerase activity
MSCs after proliferation with E2 supplement were collected and washed twice with PBS containing 0.5% bovine serum albumin and 2 mM ethylenediaminetetraacetic acid. After centrifugation the cells were stained with fluorescein isothiocyanate-conjugated antihuman CD105 and CD166 for 30 min (Abcam, Inc., Cambridge, MA). Then, the cells were suspended in 300 μl PBS containing 0.5% bovine serum albumin and 2 mM ethylenediaminetetraacetic acid for flow cytometry assay (Coulter EPICS Elite ESP flow cytometer; Beckman-Coulter, Inc., Hialeah, FL). Fluorescence intensities for the whole cell population and fluorescent-positive cell numbers were analyzed. To investigate ERs of MSCs after proliferation with E2 supplement, RNA of cells were isolated using the Aqua Pure RNA Isolation Kit (Bio-Rad, Hercules, CA) as recommended by the manufacturer's manual. Real-time polymerase chain reaction (PCR) amplification was performed on the iCycler iQ detection system, and the data were be collected and analyzed using iCycler iQ version 3.0 software (Bio-Rad Laboratories Inc). The Syber Green real-time PCR primers and probe for human glyceraldehyde 3-phophate dehydrogenase, ER-α, and -β were purchased (SuperArray, Frederick, MD). The telomerase activity of MSCs was measured by a quantitative telomerase detection kit (US Biomax, Inc. Rockville, MD) as recommended by the manufacturer's manual. The thresholds of E2-treated MSCs were normalized to that of the controls.
Statistics
All quantitative data were expressed as means ± standard deviation. The proliferation and differentiation of each group with different treatments were compared to the controls using paired Students' t-test with the use of commercially available statistical software (SPSS, Inc., Chicago, IL). One-way ANOVA was used to analyze the differences among treatment of E2 with different concentration. P values <0.05 are considered significant.
Results
E2 significantly enhances human MSC proliferation in vitro
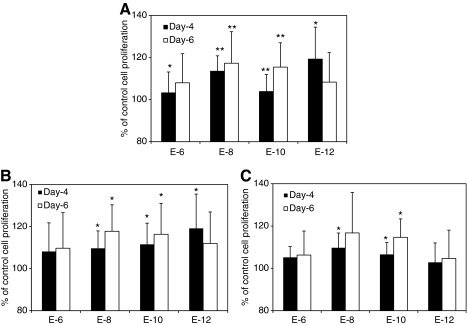
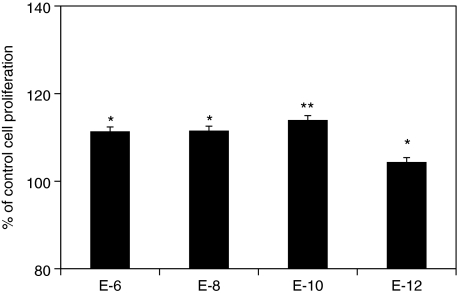
Figure 1 summarizes the effects of E2 supplementation on proliferation of human MSCs at the steroid-free culture medium. E2 at the concentrations of 10−8 and 10−10 M significantly increase cell proliferation after 4 and 6 days of treatment (Fig. 1A, n = 12, P < 0.01), whereas the E2 at concentrations of 10−6 and 10−12 M show no statistical significance compared to the control after 6 days. No statistical difference between the groups with E2 was observed. A wider range of E2 concentrations was observed to significantly increase cell proliferation in male MSCs (10−8–10−12M) than that in female MSCs (10−8–10−10 M) (Fig. 1B, C; n = 6). However, there is no significant difference of improvement among the treatment groups. E2 supplements also significantly increased MSC proliferation in the conventional cell expansion medium at the concentrations of 10−6–10−10 M (Fig. 2, n = 12). However, no statistical difference was observed among the groups with different concentrations of E2.
FIG. 1.
Human MSCs' proliferation at the steroid-free medium with E2 supplement at different concentrations. (A) The percentages of cell proliferation of human MSCs after the treatment of E2 for 4 and 6 days (n = 12). (B, C) The percentages of cell proliferation of human MSCs from male (B, n = 6) and female (C, n = 6) donors after 4 and 6 days of E2 supplements with various concentrations. *P < 0.05, **P < 0.01. MSC, mesenchymal stromal cell; E2, 17-β estradiol.
FIG. 2.
The percentages of cell proliferation of human MSCs after 6 days of E2 supplements in the conventional medium (n = 12). *P < 0.05, **P < 0.01.
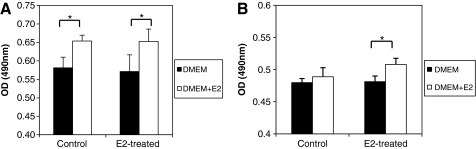
MSCs maintain proliferation rate after expansion with E2 supplement
After 3 passages of MSC proliferation with E2 supplementation, MSCs exhibit proliferation capacity similar to control MSCs cultured with conventional DMEM. Supplementation with E2 effectively enhances the growth rate of both E2-proliferated and control MSCs (Fig. 3A), indicating that MSCs possess the same responsiveness to E2, whether or not they are modulated by E2 before treatment. After 8 passages of proliferation, MSCs of the control group decrease proliferation and lose their responsiveness to E2. However, MSCs proliferated with E2 maintain significantly higher growth rates (Fig. 3B).
FIG. 3.
The proliferation capacity in vitro of human MSCs after expansion with E2 supplements (A) The proliferation rates of MSCs 3 passages after culture with E2 supplement. The proliferation is measured 4 days after exposure to the conventional medium supplemented with or without 10−8 M E2. (B) The proliferation rates of MSCs 8 passages after culture with E2 supplement. The growth rate is measured 4 days after exposure to the conventional medium supplemented with or without 10−8 M E2. *P < 0.05. DMEM, Dulbecco's modified Eagle's medium.
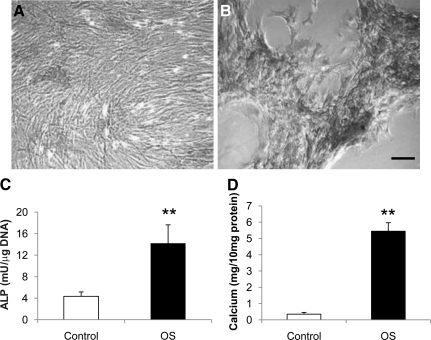
MSCs expanded by E2 supplementation possess osteogenic and adipogenic capacity
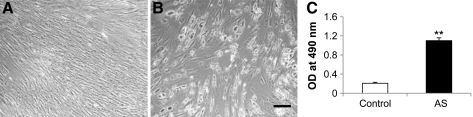
MSCs expanded with E2 supplementation possess strong osteogenic potential. After 1 week of exposure to the osteogenic medium, MSCs condensed and formed high-cell-density nodules positively stained by ALP and von Kossa (Fig. 4A, B). Quantitatively, ALP and calcium content of these osteogenic differentiated human MSCs were significantly increased after exposure to the osteogenic medium (Fig. 4C, D).
FIG. 4.
Osteogenic potentials of human bone marrow MSCs proliferated with E2 supplement. Microphotographs of E2-treated human bone marrow MSCs 1 week after exposure to the basic (A) and osteogenic (B) medium (ALP and von Kossa staining; scale bar = 50 μm). Note that ALP-positive areas are stained dark gray and mineral deposit areas are stained black. Quantitative ALP (C) and calcium content (D) of E2-treated MSCs 1 week after exposure to osteogenic differentiation and control media. **P < 0.01 versus controls. Control, basic medium; OS, osteogenic stimulation medium; ALP, alkaline phosphatase.
MSCs cultured with E2 supplement also exhibit a strong adipogenic potential. After 2 weeks of exposure to the adipogenic medium, E2-modulated MSCs morphologically resembled adipocytes by exhibiting a rounded appearance. This result was similar to that of control MSCs cultured with the basic medium. A positive reaction of Oil-Red-O staining, specifically demonstrating accumulation of lipid droplets, was observed (Fig. 5A, B). Quantitatively, lipid accumulation is significantly increased for adipogenic differentiation of human MSCs proliferated with the E2-supplemented medium (Fig. 5C).
FIG. 5.
Adipogenic potentials of human bone marrow MSCs proliferated with E2 supplement. Microphotographs of E2-treated human bone marrow MSCs 2 weeks after exposure to the basic (A) and adipogenic differentiation (B) medium (Oil-Red-O staining; scale bar = 50 μm). (C) Quantitative lipid accumulations of E2-treated MSCs 2 weeks after exposure to differentiation media. **P < 0.01 versus controls. AS, adipogenic stimulation medium.
Surface markers, ERs, and telomerase activity of E2-proliferated MSCs
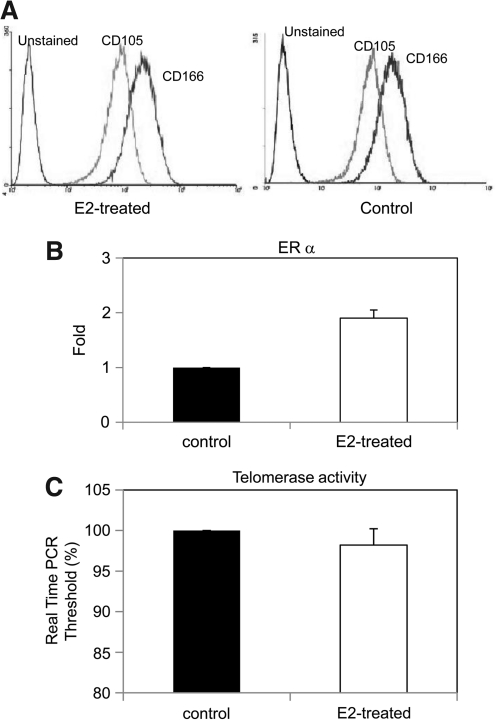
CD105 and CD166 as MSC surface markers were used to detect the variation of MSCs after estrogen modulation. The cells retain and express their cell surface markers of CD105 and CD166. Figure 5 shows the results of flow cytometry using antihuman CD105 and CD166 antibodies. No marked difference of CD105 and CD166 antigens were observed for E2-proliferated MSCs as compared to the controls (Fig. 6A). Real-time PCR revealed that ER-α expression in MSCs with E2 supplementation is 1.9 ± 0.15 folds higher than that of control cells, whereas ER-β has no upregulation (Fig. 6B). The real-time PCR threshold to detect telomerase is lower than that of the controls, indicting a higher telomerase activity of MSCs proliferated with E2 supplementation (Fig. 6C).
FIG. 6.
Characterizations of MSCs after E2 supplementation. (A) Histograms representing fluorescent intensity of cell surface markers of CD105 and CD166 of human MSCs treated with E2 supplementation. (B) ER-α expression of MSCs treated with E2 supplementation. (C) The telomerase activity of MSCs treated with E2 supplementation. ER, estrogen receptor.
Discussion
Although previous studies have demonstrated the effects of estrogen on increasing proliferation of MSCs and osteoblasts in rats, its effects on human MSCs were controversial [19,22]. Holzer et al. reported that estrogen has more growth inhibition on human MSCs although E2 moderately improves the proliferation of human MSCs at concentrations of 10−9–10−8 M [19]. On the contrary, after studying human MSCs from male donors, DiSilvio et al. concluded that E2 increases proliferation of human MSCs [22]. As estrogen may function differently on MSCs depending on the gender of donors [29–31] and endogenous estrogen may influence the outcome of estrogen supplementation; thus, these studies are not convincible to determine the effects of estrogen on human MSC proliferation. At the present study, we investigated effects of E2 on the proliferation of human MSCs from both male and female donors after a wide range of doses of E2 (10−12–10−6 M) were tested in both steroid-free and conventional cell culture media. We verified that E2 increases proliferation of human MSCs in both steroid-free and conventional media. Further, with E2 treatment, MSCs maintain their characterizations, including cell surface markers of CD105 and CD166, and multiple differentiation potentials. Consisting with a previous study, E2 supplements increase telomerase activity of human MSCs during proliferation via the regulation of ER-α, but not ER-β [28]. These results demonstrated that 17-β estradiol can serve as a regulator to improve MSC proliferation in vitro. This will effectively improve MSC amount and differentiation capacity required for stem cell-based tissue engineering.
Our previous study reported that the optimal doses of estrogen and its interaction with glucocorticoid to regulate MSC proliferation differ based on the gender of rats [30]. In the present study we also found that the dose range of E2 to significantly increase the proliferation of human MSCs for male donors (10−8–10−12 M) is wider than that for females (10−8–10−10 M). Although the mechanisms of the gender difference of estrogen regulation are not clear, one of probable explanations is the different characterizations of ERs in bone marrow cells of male and female donors. For example, an in vivo study using a knockout mouse model demonstrated that gender differences exist in ER-α and androgen receptor at regulation of skeletal metabolism [31]. The regulatory roles of ERs in bone remodeling also exhibit a gender difference [32]. Although the concentrations and binding affinity of ERs are unknown in MSCs, these characteristics of ERs exhibit sex differences on bone and brain tissues [33,34]. In addition, ERs have been demonstrated to interact with other steroid hormone receptor, such as glucocorticoid receptors (GRs). GR plays multiple roles in MSC proliferation and differentiation and has synergistic or opposite interaction with ER activity on bone formation [30,35]. ER and GR alter transcription through the activator protein-1 (AP-1) response element, but estrogens and glucocorticoids have opposing effects at this response element [36–38]. As the distribution of GRs vary on genders [39], the gender difference of estrogen regulation may be caused indirectly by GR interaction. Therefore, a future study to investigate the mechanism of gender difference of estrogen effect and understand the characteristics of ERs is needed to maximize estrogen effects on MSCs of donors with different pathophysiological conditions.
Cha et al. demonstrated recently that ER-α mediates the effects of estradiol on improvement of telomerase activity in human MSCs [28]. As antisenescence effectively maintains proliferation and differentiation capacities of MSCs, E2 supplements theoretically will prevent the loss of MSC differentiation potentials and maintain high proliferation capacity of MSCs in current in vitro cell expansion system. At the present study, we discovered that MSCs with E2 treatment exhibit higher proliferation capacity and maintain the characterizations of MSC differentiations and cell surface markers. However, an evaluation in the future is needed to investigate differentiations of MSCs improved by E2 supplements in the cell expansion and its associated mechanism. Nevertheless, the current study demonstrated the function of estradiol on improvement of MSC proliferation. The E2 treatment in MSC proliferation maintains strong differentiation potentials of MSCs. With E2 treatment, it will effectively overcome the limitations of MSCs and make MSCs to become the ideal cell source for the clinical application of tissue engineering.
Acknowledgments
We thank anonymous reviewers for their insightful comments. This research was partially supported by NIH/NIDCR (1R03DE017715) and the start-up funding from the Dows Institute for Dental Research, College of Dentistry, University of Iowa.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Barrilleaux B. Phinney DG. Prockop DJ. O'Connor KC. Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12:3007–3019. doi: 10.1089/ten.2006.12.3007. [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 3.Eberli D. Atala A. Tissue engineering using adult stem cells. Methods Enzymol. 2006;420:287–302. doi: 10.1016/S0076-6879(06)20013-2. [DOI] [PubMed] [Google Scholar]
- 4.Perova MD. Shubich MG. Karpiuk VB. Fomicheva AV. Mel'nik EA. Using stromal stem cells for the periodontal tissue regeneration and their interaction with tissue microenvironment. Morfologiia. 2007;131:7–15. [PubMed] [Google Scholar]
- 5.Levicar N. Dimarakis I. Flores C. Tracey J. Gordon MY. Habib NA. Stem cells as a treatment for chronic liver disease and diabetes. Handb Exp Pharmacol. 2007;180:243–262. doi: 10.1007/978-3-540-68976-8_11. [DOI] [PubMed] [Google Scholar]
- 6.Tang Y. Yasuhara T. Hara K. Matsukawa N. Maki M. Yu G, et al. Transplantation of bone marrow-derived stem cells: a promising therapy for stroke. Cell Transplant. 2007;16:159–169. [PubMed] [Google Scholar]
- 7.Marie PJ. Fromigue O. Osteogenic differentiation of human marrow-derived mesenchymal stem cells. Regen Med. 2006;1:539–548. doi: 10.2217/17460751.1.4.539. [DOI] [PubMed] [Google Scholar]
- 8.Sethe S. Scutt A. Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Bonab MM. Alimoghaddam K. Talebian F. Ghaffari SH. Ghavamzadeh A. Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:7–14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenderup K. Justesen J. Clausen C. Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Mendes SC. Tibbe JM. Veenhof M. Bakker K. Both S. Platenburg PP, et al. Bone tissue-engineered implants using human bone marrow stromal cells: effect of culture conditions and donor age. Tissue Eng. 2002;8:911–920. doi: 10.1089/107632702320934010. [DOI] [PubMed] [Google Scholar]
- 12.Baxter MA. Wynn RF. Jowitt SN. Wraith JE. Fairbairn LJ. Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q. Yu JH. Zhai HH. Zhao QT. Chen JW. Shu L. Li DQ, et al. Temporal expression of estrogen receptor alpha in rat bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2006;347:117–123. doi: 10.1016/j.bbrc.2006.06.070. [DOI] [PubMed] [Google Scholar]
- 14.Masuda H. Kalka C. Takahashi T. Yoshida M. Wada M. Kobori M. Itoh R, et al. Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ Res. 2007;101:598–606. doi: 10.1161/CIRCRESAHA.106.144006. [DOI] [PubMed] [Google Scholar]
- 15.Haczynski J. Tarkowski R. Jarzabek K. Slomczynska M. Wolczynski S. Magoffin DA. Jakowicki JA, et al. Human cultured skin fibroblasts express estrogen receptor alpha and beta. Int J Mol Med. 2002;10:149–153. [PubMed] [Google Scholar]
- 16.Hong SH. Nah HY. Lee YJ. Lee JW. Park JH. Kim SJ. Lee JB, et al. Expression of estrogen receptor-alpha and -beta, glucocorticoid receptor, and progesterone receptor genes in human embryonic stem cells and embryoid bodies. Mol Cells. 2004;18:320–325. [PubMed] [Google Scholar]
- 17.Zhou S. Zilberman Y. Wassermann K. Bain SD. Sadovsky Y. Gazit D. Estrogen modulates estrogen receptor alpha and beta expression, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. J Cell Biochem Suppl Suppl. 2001;36:144–155. doi: 10.1002/jcb.1096. [DOI] [PubMed] [Google Scholar]
- 18.Waters KM. Rickard DJ. Riggs BL. Khosla S. Katzenellenbogen JA. Katzenellenbogen BS. Moore J, et al. Estrogen regulation of human osteoblast function is determined by the stage of differentiation and the estrogen receptor isoform. J Cell Biochem. 2001;83:448–462. doi: 10.1002/jcb.1242. [DOI] [PubMed] [Google Scholar]
- 19.Holzer G. Einhorn TA. Majeska RJ. Estrogen regulation of growth and alkaline phosphatase expression by cultured human bone marrow stromal cells. J Orthop Res. 2002;20:281–288. doi: 10.1016/S0736-0266(01)00092-4. [DOI] [PubMed] [Google Scholar]
- 20.Hong L. Colpan A. Peptan IA. Daw J. George A. Evans CA. 17-Beta estradiol enhances osteogenic and adipogenic differentiation of human adipose-derived stromal cells. Tissue Eng. 2007;13:1197–1203. doi: 10.1089/ten.2006.0317. [DOI] [PubMed] [Google Scholar]
- 21.Hong L. Colpan A. Peptan IA. Modulations of 17-beta estradiol on osteogenic and adipogenic differentiations of human mesenchymal stem cells. Tissue Eng. 2006;12:2747–2753. doi: 10.1089/ten.2006.12.2747. [DOI] [PubMed] [Google Scholar]
- 22.DiSilvio L. Jameson J. Gamie Z. Giannoudis PV. Tsiridis E. In vitro evaluation of the direct effect of estradiol on human osteoblasts (HOB) and human mesenchymal stem cells (h-MSCs) Injury. 2006;37:S33–S42. doi: 10.1016/j.injury.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Sato R. Maesawa C. Fujisawa K. Wada K. Oikawa K. Takikawa Y, et al. Prevention of critical telomere shortening by oestradiol in human normal hepatic cultured cells and carbon tetrachloride induced rat liver fibrosis. Gut. 2004;53:1001–1009. doi: 10.1136/gut.2003.027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DC. Im JA. Kim JH. Lee HR. Shim JY. Effect of long-term hormone therapy on telomere length in postmenopausal women. Yonsei Med J. 2005;46:471–479. doi: 10.3349/ymj.2005.46.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imanishi T. T Hano T. Nishio I. Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity. J Hypertens. 2005;23:1699–1706. doi: 10.1097/01.hjh.0000176788.12376.20. [DOI] [PubMed] [Google Scholar]
- 26.Ling S. Zhou L. Li H. Dai A. Liu JP, et al. Effects of 17beta-estradiol on growth and apoptosis in human vascular endothelial cells: influence of mechanical strain and tumor necrosis factor-alpha. Steroids. 2006;71:799–808. doi: 10.1016/j.steroids.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Imanishi T. Hano T. Nishio I. Estrogen reduces angiotensin II-induced acceleration of senescence in endothelial progenitor cells. Hypertens Res. 2005;28:263–271. doi: 10.1291/hypres.28.263. [DOI] [PubMed] [Google Scholar]
- 28.Cha Y. Kwon SJ. Seol W. Park KS. Estrogen receptor-alpha mediates the effects of estradiol on telomerase activity in human mesenchymal stem cells. Mol Cells. 2008;26:454–458. [PubMed] [Google Scholar]
- 29.von Stechow D. Zurakowski D. Pettit AR. Muller R. Gronowicz G. Chorev M, et al. Differential transcriptional effects of PTH and estrogen during anabolic bone formation. J Cell Biochem. 2004;93:476–490. doi: 10.1002/jcb.20174. [DOI] [PubMed] [Google Scholar]
- 30.Hong L. Sultana H. Paulius K. Zhang G. Steroid regulation of proliferation and osteogenic differentiation of bone marrow stromal cells: a gender difference. J Steroid Biochem Mol Biol. 2009;114:180–185. doi: 10.1016/j.jsbmb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tosi LL. Boyan BD. Boskey AL. Does sex matter in musculoskeletal health? The influence of sex and gender on musculoskeletal health. J Bone Joint Surg Am. 2005;87:1631–1647. doi: 10.2106/JBJS.E.00218. [DOI] [PubMed] [Google Scholar]
- 32.Oz OK. Zerwekh JE. Fisher C. Graves K. Nanu L. Millsaps R. Simpson ER. Bone has a sexually dimorphic response to aromatase deficiency. J Bone Miner Res. 2000;15:507–514. doi: 10.1359/jbmr.2000.15.3.507. [DOI] [PubMed] [Google Scholar]
- 33.Dennison E. Syddall H. Fall C. Brandi ML. Cooper C Hertfordshire Cohort Study Group. Evidence of sexual dimorphism in relationships between estrogen receptor polymorphisms and bone mass: the Hertfordshire study. J Rheumatol. 2005;32:2400–2404. [PubMed] [Google Scholar]
- 34.Weiland NG. Orikasa C. Hayashi S. McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol. 1997;388:603–612. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Sukhu B. Rotenberg B. Binkert C. Kohno H. Zohar R. McCulloch CA. Tenenbaum HC. Tamoxifen attenuates glucocorticoid actions on bone formation in vitro. Endocrinology. 1997;138:3269–3275. doi: 10.1210/endo.138.8.5340. [DOI] [PubMed] [Google Scholar]
- 36.Gaub MP. Bellard M. Scheuer I. Chambon P. Sassone-Corsi P. Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell. 1990;63:1267–1276. doi: 10.1016/0092-8674(90)90422-b. [DOI] [PubMed] [Google Scholar]
- 37.Webb P. Lopez GN. Uht RM. Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- 38.Ponta H. Cato AC. Herrlich P. Interference of pathway specific transcription factors. Biochim Biophys Acta. 1992;1129:255–261. doi: 10.1016/0167-4781(92)90501-p. [DOI] [PubMed] [Google Scholar]
- 39.Mattsson C. Olsson T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr Med Chem. 2007;14:2918–2924. doi: 10.2174/092986707782359972. [DOI] [PubMed] [Google Scholar]