Abstract
The osteochondral microenvironment involves a complex milieu of cues that facilitate proper tissue development, homeostasis, and repair. This environment is disrupted in disease states such as osteoarthritis. Mesenchymal stem cells (MSCs) are under clinical investigation for the treatment of osteoarthritis given their capacity to differentiate into chondrocytes as well as to secrete a wide array of biologically active factors that support cell proliferation and tissue formation. In fact, the therapeutic action of these cells in many clinical applications is now thought to be at least partially dependent on their secretory capacity. Previous work demonstrated that MSCs were capable of stimulating chondrocyte growth and tissue production, whereas tissue-derived osteoblasts were not stimulatory. This study investigated the stimulatory capacity of MSCs during osteogenesis and the impact of MSC phenotype on cartilage stimulation. Cell interactions were examined in 3 coculture systems to confirm that trends were not dependent on material: traditional cell culture insert coculture, bilayered poly(ethylene glycol) gels, and a scaffold comprised of a layer of poly(ethylene glycol) polymerized onto a poly(lactic-co-glycolic) acid-based scaffold. Results demonstrated that MSCs predifferentiated toward an osteogenic phenotype for 3 days exhibited enhanced stimulation of chondrocyte extracellular matrix production, whereas longer periods of predifferentiation decreased the magnitude of observed stimulation. Further, tissue formation by the MSCs themselves showed greater dependence on the coculture system than the presence of other cells or length of predifferentiation.
Background
Stem cells isolated from numerous sources are under investigation for the repair and regeneration of tissues given their proliferative and differentiation capacity. However, the actual therapeutic mechanism of stem cells may not be related to their differentiation despite its use in their initial characterization. Mesenchymal stem cells (MSCs) are one class of stem cells that have been extensively studied for use as a cellular therapy. In addition to their differentiation capacity [1], MSCs possess the capacity to home to sites of injury [2,3] and modulate the immune response [4,5]. This makes them enticing candidates as delivery vehicles of therapeutic agents, in addition to their potential as a cell source for tissue regeneration. Ongoing research into the modification of MSCs for targeted delivery of agents [6,7] is one capacity in which MSCs may serve as a therapeutic delivery vehicle. However, MSCs have also been shown to secrete a wide array of bioactive factors that have the potential to impact tissue remodeling [8,9].
Many of the bioactive factors that MSCs secrete play an important role in matrix production and turnover as well as cellular proliferation for a variety of tissues, including bone and cartilage. For example, MSCs have been shown to secrete matrix metalloproteinase (MMPs), tissue inhibitor of metalloproteinases (TIMPs), transforming growth factor B-1, and fibroblast growth factor-2 [9–12]. These molecules have a significant impact on the musculoskeletal system, making the cells ideal candidates for treatment of osteochondral ailments, a major source of permanent disability with nearly 40 million Americans suffering from either osteoporosis or osteoarthritis [13,14]. Recent studies on the application of MSCs for treatment of bone defects [15] and cartilage degeneration [16,17] have confirmed the potential of MSCs as a cellular therapy. In fact, clinical use of injectable MSCs is being pursued for cartilage repair in ongoing trials of products such as Chondrogen™ [18].
Despite these advances and clinical testing, there remains controversy as to whether the primary function of MSCs in tissue repair is as a cell source for new tissue formation or a stimulator of host tissue remodeling. In vivo, mesodermal cells are known to serve as a cell source in the development of cartilage and bone in the developing embryo; however, their potential role as a stimulator has not been fully elucidated. In the adolescent, the relationship between bone and cartilage remains intricately linked in the growth plate [19]. This relationship remains a vital aspect of tissue homeostasis in the adult as illustrated by the fact that diseases such as osteoarthritis demonstrate deleterious effects on both cartilage and bone [20–23]. To enhance the therapeutic potential of MSCs for degenerative joint diseases, we must understand the function of these cells in tissue development and remodeling.
The bioactive factors mesenchymal cells secrete can be manipulated even without genetic modification of the cells. Pretreatment of MSCs with differentiation cues has previously been shown to increase the therapeutic potential in a model of myocardial infarction [24]. Additionally, the profile of bioactive factors, such as MMP, TIMP, and aggrecanase, which MSCs secrete, is altered during progressive stages of osteogenesis [25,26]. It is therefore important to consider that the therapeutic modality and efficacy of the MSCs for secreting therapeutic molecules may be altered during the differentiation process. Biomaterials and other enabling technologies can be leveraged to deliver stem cells in the optimal differentiation state to promote tissue development.
The overall goal of this study is to examine the effects of MSC stimulation on chondrocytes and cartilage tissue growth and to determine the relationship between the MSC differentiation state and stimulation of adjacent cartilage tissue formation.
Materials and Methods
Cell isolation and medium conditions
Goat MSCs were isolated from caprine bone marrow aspirates from adult animals (Thomas D. Morris Inc.) as previously described [27]. Briefly, marrow samples with 6,000 U of heparin were washed twice with Dulbecco’s modified Eagle medium (DMEM) and plated at a density of 120,000 mononuclear cells/cm2. MSCs were maintained in MSC medium [DMEM supplemented with 1% l-glutamine, 1% penicillin/streptomycin (Pen/Strep), and 10% fetal bovine serum (FBS)], and used by passage 5, and tested for differentiation capacity. Predifferentiation toward an osteogenic phenotype was achieved through culture in osteogenic medium (OM) comprised of DMEM supplemented with 10% FBS, 10 mM β-glycerophosphate, 0.1 mg/mL ascorbic acid-2-phosphate, 100 nM dexamethasone, and 1% Pen/Strep [28].
Bovine chondrocytes were isolated from 4- to 8-week-old calves (Research 87) as previously described [29]. Briefly, cartilage pieces were dissected from the femoral patellar groove and minced to 1 mm3. Cartilage pieces were washed 3 times with phosphate-buffered saline (PBS) with 1% Pen/Strep and digested in 120 U/mL type II collagenase for 16.5 h. Chondrocyte medium was made with DMEM supplemented with 10% FBS, 1% nonessential amino acids, 1% HEPES, 10 mg/mL ascorbic acid, 0.1 M proline, 1% sodium pyruvate, and 1% Pen/Strep [29]. Coculture studies were carried out in each model system utilizing a combination medium (COMBO medium), previously shown to support both chondrocyte growth and osteogenesis [30]. COMBO medium was made by supplementing DMEM with 2% FBS, 50 μg/mL ascorbic acid, 40 μg/mL L-proline, 10 mM β-glycerophosphate, 100 nm dexamethasone, 100 μg/mL sodium pyruvate, and 1% ITS-Plus Premix. Additional coculture conditions were examined in OM or basic growth medium (BGM) comprised of DMEM supplemented with 10% FBS and 1% Pen/Strep. Goat MSCs and bovine chondrocytes were used in all studies unless otherwise noted.
For human studies, human MSCs (hMSCs) were obtained from the Caplan Lab, Case Western Reserve University, hMSCs were maintained in MSC medium with FBS from a lot defined by the Caplan Lab as supporting MSC growth. Human cartilage pieces from cadaveric sources were obtained from National Disease Research Interchange. Human chondrocytes were isolated by washing the cartilage pieces 3 times with PBS with 1% Pen/Strep, mincing to 1 mm3, and digesting overnight in 120 U/mL type II collagenase.
Cell culture insert coculture
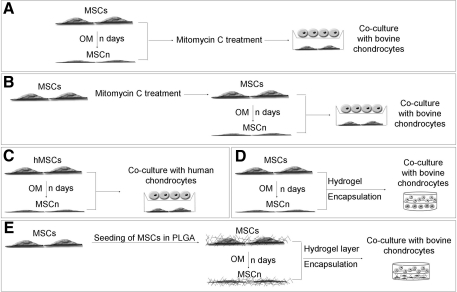
Cell culture insert coculture of cells was carried out in 12-well plates using polyethylene terephthalate membranes with a 1-μm pore size (BD Biosciences 353103). MSCs were plated in monolayer in the wells of 12-well plates and maintained in MSC medium. For predifferentiation of MSCs, n days before coculture was initiated, MSCs were switched into OM (MSC-n, n = 0, 2, 4, 8, 10, 14). To avoid overconfluence of MSCs in monolayer culture, cell proliferation was arrested with mitomycin C treatment. MSCs were treated with a solution of 0.01 mg mitomycin C per 1 mL of DMEM for 2.5 h, and then washed 3 times with PBS and returned to culture media either before initiation of coculture (Fig. 1A), or after 1 week of culture in MSC medium before the start of predifferentiation (Fig. 1B), as indicated. The day before coculture was initiated, chondrocytes were seeded into cell culture inserts and fed with chondrocyte medium. Upon initiation coculture, cell culture inserts were transferred to wells preseeded with MSC-n and wells and cell culture inserts were fed with COMBO medium or BGM as indicated. After 1 week, cells in the cell culture inserts were fixed with 10% formalin and analyzed for evidence of glycosaminoglycan (GAG) accumulation using standard histological techniques for alizarin red and Safranin-O staining.
FIG. 1.
Experimental design. (A) System A: MSCs were plated in monolayer and switched to OM-n (n = 0, 4, 8) days before mitomycin C treatment and subsequent coculture in cell culture inserts. (B) System B: MSCs were plated in monolayer, treated with mitomycin C, and then switched to OM-n (n = 0, 2, 4, 8, 10, 14) days before coculture in transwell inserts. (C) System C: Human MSCs were plated and switched into OM-n (n = 0, 3, 8, 14) days before cell culture insert coculture with human chondrocytes. (D) System D: MSCs were plated in monolayer and predifferentiated in OM-n (n = 0, 3, 7) days before subsequent encapsulation in hydrogels for coculture. (E) MSCs were seeded into PLGA scaffolds and then predifferentiated for n (n = 0, 3, 10) days in OM. A hydrogel layer was then encapsulated onto the PLGA to create a bilayered coculture system. MSC, mesenchymal stem cell; OM, osteogenic medium; PLGA, poly(lactic-co-glycolic) acid.
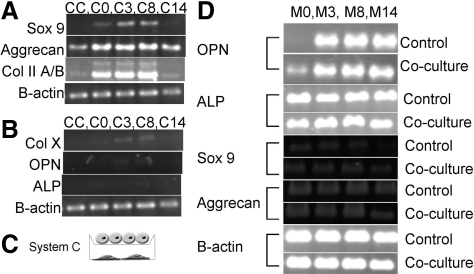
Human coculture studies were also carried out in cell culture inserts. Plating and predifferentiation of hMSCs (M-n, n = 0, 3, 8, 14) was carried out in the same manner as goat MSCs with the following modifications (Fig. 1C): mitomycin C treatment was not performed and all media were made using FBS from defined lots. Human chondrocytes were plated in monolayer and maintained in COMBO media as a control (CC) or in coculture with MSCs predifferentiated for n days (C-n, n = 0, 3, 8, 14) for a period of a week. As histological analysis did not demonstrate significant GAG accumulation, PCR analysis of mRNA production by the chondrocytes and MSCs was performed. Briefly, mRNA was isolated using Trizol, cDNA was made, and PCR was run. Analysis of cartilage markers was done for chondrocytes, including Sox 9 (F: ACG TCA TCT CCA ACA TCG AGA CC; R: CTG TAG TGT GGG AGG TTG AAG GG) [31], collagen type II HA/HB (Col II A/B) (F: GTG AGC CAT GAT TCG CCT CGG; R: CAC CAG GTT CAC CAG GAT TGC C) [32], and aggrecan (F: GCC TTG AGC AGT TCA CCT TC; R: CTC TTC TAC GGG GAC AGC AG) [29]. PCR was also done for the marker of hypertrophic chondrocytes, collagen type X (F: CCC TTT TTG CTG CTA GTA TCC; R: CTG TTG TCC AGG TTT TCC TGG CAC) [32], and the bone markers osteopontin (OPN) (F: CTA GGC ATC ACC TGT GCC ATA CC; R: AGT GAC CAG TTC ATC AGA TTC ATC) [1], and alkaline phosphatase (ALP) (F: ACG TGG CTA AGA ATG TCA TC; R: CTG GTA GGC GAT GTC CTT A) [33]. B-actin was used as a control (F: TGG CAC CAC ACC TTC TAC AAT GAG C; R: GCA CAG CTT CTC CTT AAT GTC ACG C).
Quantification of staining with ImageJ
Matrix production was quantified using the ImageJ measure function. Following application of the RGD split function to obtain the “Red” portion of the staining, the measure function was used to quantify the relative amount of staining in representative images. The measure function gives a value of 255 for no staining (white image) and 0 for the darkest staining, that is, black. This number was converted to a more traditional scale wherein a score of 0 represented no staining and 255 represented the darkest staining. The relative staining was then determined by scaling this number to that obtained for the cells in the control.
Formation of poly(ethylene glycol) gels
Formation of bilayered poly(ethylene glycol) (PEG) gels was completed as described previously [29]. Briefly, a 10% w/v solution of PEG, molecular weight 3,400 (Sunbio), was formed in PBS with 1% Pen/Strep. Irgacure 2959 (Ciba Specialty Chemicals) was added at a final concentration of 0.05% to act as a photoinitiator. MSCs predifferentiated for n days in monolayer (MSC-n) were generating by replacing the MSC culture, plated at a density of 5,000 cells/cm2, into OM-n (n = 0, 3, 7) days before encapsulation (Fig. 1D). MSCs were then trypsinized before encapsulation. Alternatively, chondrocytes were used immediately after isolation. Uniform gels were formed by pelleting cells, followed by addition and mixing into the polymer solution at a concentration of 20 million cells/mL, and polymerizing in sterile molds. Polymerization was carried out under 365 nm UV light at an intensity of 3 mW/cm2 for 6–9 min. For bilayered gels, a first layer of cell-laden polymer was first polymerized for 3 min after which a second layer of cell-laden gel was placed atop the first layer, and polymerization was allowed to proceed for an additional 6 min. Constructs were harvested after 3 weeks of culture in COMBO or OM. For biochemical analysis, bilayered constructs were dissected into 3 pieces with the layer in the center discarded to avoid contamination between the layers. Each of the 2 individual cell types was then analyzed individually for chondroitin sulfate production using the DMMB dye assay and DNA content using the Hoechst Dye 33258 Assay. Other constructs were fixed in 4% paraformaldehyde for histological analysis via Safranin-O and alizarin red staining.
PEG/poly(lactic-co-glycolic) acid construct formation
Poly(lactic-co-glycolic) acid (PLGA) scaffolds doped with hydroxyapatite (HA) were formed through dissolution of 0.1 g of PLGA, 0.1 g of poly(lactic) acid, and 0.2 g of HA into 4 mL of chloroform. Two hundred fifty microliters of this solution was added to 400 mg of NaCl for each construct. The chloroform was then evaporated and the salt was leached out to create porous scaffolds. After sterilization with 70% EtOH, scaffolds were coated with fibronectin, via incubation in a 50 μg/mL solution of fibronectin (Sigma F1141) for 3 h, rinsed with PBS, and seeded with 2 million MSCs each. Scaffolds were cultured in MSC medium and replenished with OM-n days before a layer of 10% PEG was encapsulated onto the PLGA scaffolds (Fig. 1E). On the day of coculture initiation, control scaffolds had an acellular layer of PEG attached, whereas experimental conditions received PEG-laden hydrogel with 20 million chondrocytes/mL. Bilayered scaffolds were formed by prepolymerizing the PEG gel for ∼20–30 s before addition of the PLGA layer and continuation of polymerization for 6 min. The medium was replenished 3 times weekly with COMBO medium. After 3 weeks of culture, constructs were dissected by peeling apart the PEG and the PLGA layers and removal of the intermediate layer with a scalpel. Constructs were then harvested for either biochemical analysis or histology as performed on the PEG gels.
Statistics
Statistical analysis was performed on measured samples using t-tests. P values <0.05 were considered statistically significant. Results are presented as averages ± standard deviations.
Results
The MSC stimulation of the chondrocytes and the cartilage tissue production was observed in all 3 coculture systems and was dependent on the differentiation state of the stem cell. However, the MSC behavior depended on the properties of the culture and biomaterial system in which they were studied.
Monolayer coculture
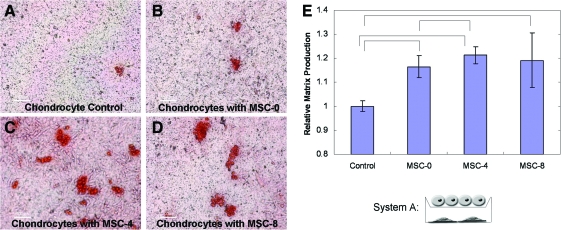
The influence of the MSC phenotype (differentiation state) on the chondrocyte behavior was first evaluated in monolayer cell culture inserts. The results in System A (Fig. 1A), mitomycin C treatment after osteogenic differentiation, demonstrated the potential of the MSCs to stimulate GAG accumulation by the chondrocytes. Safranin-O (Safo) staining of GAG accumulation by the chondrocytes demonstrated a small increase with the MSC coculture compared to the chondrocytes cultured alone (Fig. 2A, B). The chondrocyte GAG accumulation increased further when the MSCs were predifferentiated for 4 days before coculture was initiated (Fig. 2C). However, when the MSCs were predifferentiated for 8 days before coculture, the chondrocytes decreased GAG accumulation relative to 4 days (Fig. 2D). Similar trends in the chondrocyte stimulation were observed when the coculture was carried out in BGM and quantified using ImageJ (Fig. 2E) with a control of the chondrocytes grown in both the cell culture insert and well below.
FIG. 2.
System A: Safranin-O staining of chondrocytes grown in cell culture insert culture with MSCs predifferentiated in OM and treated with mitomycin C before initiation of coculture. Culture was carried out for 1 week in COMBO medium. Chondrocytes were grown alone (A), or after predifferentiation for 0 (B), 4 (C), or 8 (D) days. Culture was carried out for 1 week in growth medium (E) with other chondrocytes (control) or with MSCs predifferentiated for n days (MSC-n). The relative Safranin-O staining was then determined with ImageJ. Bars indicate significant differences with P values of <0.05. In both culture media chondrocytes produced increased levels of GAG in coculture. Maximum stimulation of GAG accumulation was observed by MSCs predifferentiated for 4 days in both cases. COMBO medium, combination medium; GAG, glycosaminoglycan. Color images available online at www.liebertonline.com/scd.
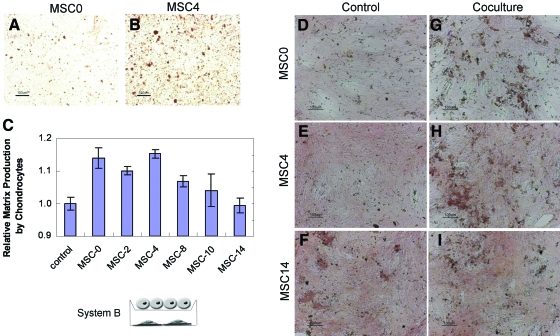
The MSCs were subsequently differentiated toward the osteogenic lineage for longer time periods before coculture with the chondrocytes. Further, the number of MSCs cocultured with the chondrocytes was controlled by treatment with mitomycin C before differentiation (Fig. 1B). The MSCs were still capable of differentiating and producing calcified matrix after mitomycin C treatment in as little as 4 days, as exhibited through alizarin red staining (Fig. 3A, B). Longer times of MSC osteogenic differentiation before coculture decreased the chondrocyte stimulation (Fig. 3C). Although the chondrocytes showed a statistically significant increase in GAG accumulation when cultured with the MSCs predifferentiated for shorter time periods, after 10 days of osteogenic differentiation there was no longer a statistically significant increase in GAG accumulation between the coculture and control cultures. The MSCs also demonstrated increased mineralization with coculture as demonstrated by alizarin red staining (Fig. 3D, G), recapitulating trends previously reported by Gerstenfeld et al. [34]. Increased MSC osteogenesis was also observed with the chondrocyte coculture when the MSCs were predifferentiated for short period (Fig. 3E, H). However, the chondrocyte coculture did not enhance the MSC mineralization when the MSCs were predifferentiated for longer times (Fig. 3F, I). Hence, results in this system once again demonstrated a peak stimulation of GAG accumulation with MSC-4. In addition, longer periods of predifferentiation were shown to result in a lack of stimulatory capacity by the MSCs. Further, the MSCs were shown to increase calcified matrix accumulation in coculture with the chondrocytes.
FIG. 3.
System B: Coculture of chondrocytes with MSCs predifferentiated for n days after treatment with mitomycin C. Alizarin red staining of MSCs harvested before coculture period demonstrates that even after treatment with mitomycin C, MSCs are capable of producing matrix characteristic of osteogenic differentiation in as little as 4 days. MSCs are shown after mitomycin C treatment and 4 days of growth in MSC medium (A) or OM (B). (C) GAG accumulation by chondrocytes grown for 1 week of coculture in COMBO medium with MSCs predifferentiated for n days in OM after inactivation with mitomycin C. GAG accumulation was significantly increased (P < 0.05) when chondrocytes were cocultured with MSCs predifferentiated for up to 8 days; however, an increase was not observed when MSCs were predifferentiated for 10 or more days. (D–I) Alizarin red staining of MSCs treated with mitomycin C and predifferentiated for n days in OM before culture for 1 week in COMBO medium alone (D, E, F) or in cell culture insert coculture with chondrocytes (G, H, I). n = 0 (D, G), 4 (E, H), or 14 days (F, I). Alizarin red staining demonstrates that undifferentiated MSCs produce increased calcified matrix in coculture, but after longer periods of predifferentiation, this increase is no longer observed. Color images available online at www.liebertonline.com/scd.
Three-dimensional hydrogel coculture
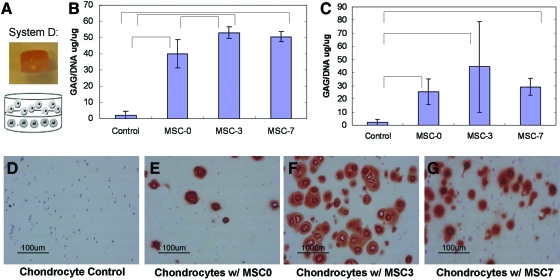
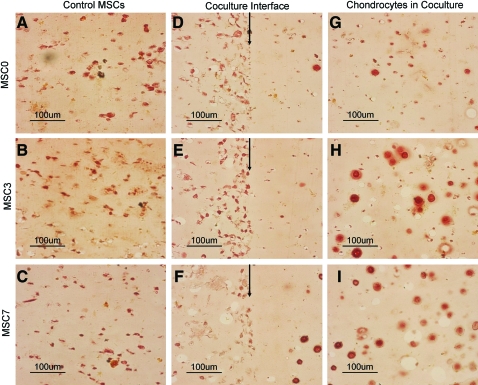
Coculture of the chondrocytes and MSCs in various differentiation states was subsequently evaluated in bilayered hydrogels (Fig. 4A). Multilayered PEG gels have previously been applied to building complex tissue structures [35]. In addition to their ability to produce a 3-dimensional (3D)-layered architecture with distinct cell layers where cells can communicate via soluble factors, these gels have a proven capacity to support chondrocyte tissue formation. This coculture system can also mimic to a degree the in vivo interactions that chondrocytes and an ossifying mesenchyme may experience during development and in the growth plate. Predifferentiation of the MSCs was carried out in monolayer to control for cell number, with a maximum of 1 week of predifferentiation possible before overconfluence (Fig. 1D). Biochemical analysis of GAG accumulation again suggested that shorter times of MSC predifferentiation resulted in peak stem cell stimulation of the chondrocytes and cartilage growth (Fig. 4B, C). This trend was observed regardless of whether coculture was carried out in osteogenic or COMBO medium (Fig. 4B, C). Histological staining of GAGs secreted in the hydrogels also confirmed the MSC stimulation of cartilage growth (Safo, Fig. 4D–G), with the strongest staining occurring when the chondrocytes were cocultured with the MSCs that were predifferentiated for 3 days (Fig. 4F). Further, a higher concentration of DNA per dry scaffold weight was found for the chondrocytes cocultured with the MSCs that were predifferentiated for 3 or 7 days, indicating increased proliferation of the chondrocytes along with the greater GAG accumulation. Alizarin red staining of hydrogel controls containing the MSCs alone demonstrated secretion of a mineralizing matrix (Fig. 5A–I), with the most intense staining occurring when the MSCs were predifferentiated for 7 days before encapsulation (Fig. 5C). However, alizarin red staining of the MSCs decreased with the chondrocyte coculture (Fig. 5A–F). The difference in alizarin staining was most dramatic between bilayered hydrogels containing the undifferentiated MSCs and those predifferentiated for 7 days (Fig. 5C, F). The chondrocytes cocultured with the MSCs that were not predifferentiated produced negligible mineralization (Fig. 5G); however, some mineralization was present in the chondrocytes cocultured with the predifferentiated MSCs (Fig. 5H, I). While PEG hydrogels support cartilage growth, osteogenesis in the PEG hydrogels, without any modifications, is limited [36]. Therefore, bilayered scaffolds that support both bone and cartilage formation were also evaluated.
FIG. 4.
System D: Chondrocytes encapsulated in bulk PEG gels or in bilayered PEG scaffolds with MSCs predifferentiated for n days in OM before initiation of coculture. Gross image and schematic (A). Biochemical analysis of GAG accumulation (GAG/DNA) by chondrocytes alone (control) or in coculture with MSCs predifferentiated for n days (MSC-n) for 3 weeks in COMBO medium (B) or OM (C). Significant differences (P value <0.05) are indicated with bars. Safranin-O staining of chondrocytes grown alone (D) or with MSCs predifferentiated for 0 (E), 3 (F), or 7 days (G), after 3 weeks of coculture in COMBO medium. Biochemical analysis and staining again demonstrated maximal stimulation of chondrocytes in coculture with MSCs predifferentiated for 3 days. PEG, poly(ethylene glycol). Color images available online at www.liebertonline.com/scd.
FIG. 5.
System D: Alizarin red staining. Control MSCs predifferentiated for 0 (A), 3 (B), or 7 days (C) or in coculture (interface indicated by arrows) of MSCs predifferentiated 0 (D), 3 (E), or 7 (F) days and grown with chondrocytes for 3 weeks in COMBO medium. The left layer of the gels is the MSC layer and the right layer of the gel consists of chondrocytes (D–F). Staining indicates a decrease in calcified matrix by MSCs in coculture. Chondrocytes in coculture with MSCs predifferentiated for 0 (G), 3 (H), or 7 (I) days further from the interface are also depicted. Increased calcified cartilage is observed in chondrocytes cocultured with MSCs predifferentiated for longer periods (H, I) versus control (G). Color images available online at www.liebertonline.com/scd.
3D Hydrogel–sponge coculture
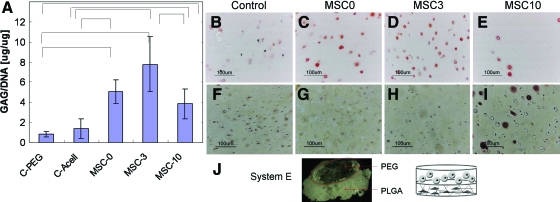
To confirm that the MSC stimulation of cartilage growth was not a biomaterial-specific phenomenon, another bilayered biomaterial was evaluated. Specifically, bilayered scaffolds were created with a PEG hydrogel layer to support chondrocyte growth and a polyester, PLGA layer, to support cell adhesion and osteogenesis. Again, similar trends of the MSC stimulation of the chondrocyte growth and GAG accumulation were found in the PEG-PLGA bilayered constructs, confirming that the observed stimulation was not biomaterial specific (Fig. 6). Biochemical analysis of the chondrocyte GAG accumulation in the hydrogels demonstrated similar results with respect to the MSC differentiation state and stimulation. Short times (3 days) of osteogenic predifferentiation enhanced the MSC stimulation of the chondrocyte GAG accumulation, whereas longer periods of predifferentiation reduced the MSC stimulation (Fig. 6A). This MSC induced increase in cartilage growth and GAG accumulation was also confirmed with histological staining for proteoglycans (Fig. 6B–E). The chondrocytes grown in coculture with the undifferentiated MSCs (Fig. 6C) or the MSCs predifferentiated for 3 days (Fig. 6D) accumulated more GAG than the chondrocytes grown alone (Fig. 6B) or with the MSCs predifferentated for 10 days (Fig. 6E).
FIG. 6.
System E: Chondrocytes grown in coculture with MSCs predifferentiated for n days in PLGA scaffolds for 3 weeks in COMBO medium also demonstrated the ability of MSCs to stimulate chondrocyte GAG accumulation with peak stimulation by MSCs predifferentiated for 3 days. (A) Biochemical analysis of chondrocytes grown in PEG alone (C-PEG), in PEG encapsulated on top of acellular PLGA (C-Acell), or in PEG encapsulated on top of PLGA seeded with MSCs predifferentiated for n days (MSC-n). Statistically significant differences are marked with bars (P value <0.05). MSC-0 versus MSC-3 was nearly significant with a P value of 0.06. A subsequent repeat of this study demonstrated the same trend, but due to technical error in the scaffold fabrication process did not have enough samples for statistical analysis. Safo staining (B–E) and alizarin red (F–I) staining of chondrocytes grown in PEG adjacent to acellular PLGA (B, F), or adjacent to PLGA seeded with MSCs predifferentiated for n days, n = 0 (C, G), 3 (D, H), or 10 days (E, I). (J) Gross image of PEG encapsulated on a PLGA scaffold. Color images available online at www.liebertonline.com/scd.
Staining for aberrant chondrocyte mineralization or MSC chondrogenesis was also performed to evaluate the specificity of differentiation in the 2 layers. Alizarin red staining of the chondrocytes demonstrated limited mineralization when the cells were cultured alone (Fig. 6F). The chondrocyte mineralization decreased in coculture with the MSCs predifferentiated for 0 or 3 days (Fig. 6G, H). However, the chondrocytes cocultured with the MSCs predifferentiated for 10 days produced some cells with pericellular staining for mineralization, though the majority of the cells were not stained (Fig. 6I). The MSCs accumulated little GAG in any of the conditions (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/scd). For the PLGA constructs, proteoglycan production (Safo staining) was most evident in the MSCs predifferentiatied for 3 days and cultured alone (Supplementary Fig. S1A–C). With coculture, no proteoglycan staining was present in the PLGA layer when the MSCs were predifferentiated for 3 or 10 days. However, the MSCs that were not predifferentiated exhibited increased Safo staining with coculture (Supplementary Fig. S1D–F). Differences in mineralization of the MSCs in the PLGA layer of the constructs were not discernable, likely due to the background staining of HA in the scaffolds (Supplementary Fig. S2A–F). Differences in matrix production by the MSCs were not evident based on hematoxylin and eosin staining (Supplementary Fig. S2G–L).
Human cell culture insert coculture
Human coculture studies were performed to ensure that the observed MSC stimulation were not species related. The human cells did not accumulate significant matrix within the week of transwell coculture. Gene expression was further characterized to analyze the effects of coculture on the chondrocytes (Fig. 7). The human chondrocytes increased their expression of cartilage markers, including Sox 9, aggrecan, and collagen type II, in coculture with MSCs (C0) versus control levels (CC) (Fig. 7A). mRNA expression of the chondrocytes in coculture with the MSCs predifferentiated for 3 (C3) or 8 (C8) days was also increased versus control for all 3 cartilage markers, and Sox 9 was increased compared to C0 (Fig. 7A). The MSCs predifferentiated for the longer period of 14 days were unable to stimulate the chondrocyte (C14) mRNA expression of Sox 9 or collagen type II (Fig. 7A). The human chondrocytes expressed no collagen type X alone (CC) or in coculture with the MSCs predifferentiated for 0 or 14 days (C0, C14) (Fig. 7B). Negligible expression of collagen type X was observed for the chondrocytes in coculture with the MSCs predifferentiated for 3 and 8 days (C3, C8) (Fig. 7B). The chondrocytes did not express mRNA for the osteogenic markers OPN and ALP under any conditions (Fig. 7B). The response of the MSCs to coculture with the chondrocytes was also evaluated via mRNA expression. OPN expression was increased for the undifferentiated MSCs (M0) grown in coculture with the chondrocytes versus those grown alone (Fig. 7D), but no differences were observed at other states of differentiation (Fig. 7D). Further, no differences in mRNA expression of ALP, Sox 9, or aggrecan were observed at any of the differentiation states (Fig. 7D).
FIG. 7.
System C: Human chondrocytes in cell culture insert coculture with human MSCs predifferentiated for n (n = 0, 3, 8, 14) days in monolayer (C). Chondrocyte control (CC) and in coculture with MSCs predifferentiated for n days (C-n) were analyzed via PCR analysis of mRNA expression. This mRNA expression analysis demonstrated a similar trend of MSC stimulation of chondrocyte gene expression with a peak stimulation observed at 3–8 days (A). Chondrocytes expressed little or no evidence of hypertrophy as examined through collagen type X mRNA expression and were negative for the osteogenic markers OPN and ALP (B). MSCs demonstrated increased OPN expression in coculture when undifferentiated (M0), but this increase was not evident when cells were predifferentiated (M-n) (D). No changes in MSC gene expression for ALP or the chondrogenic markers Sox 9 and Aggrecan were observed (D). ALP, alkaline phosphatase; OPN, osteopontin.
Discussion
Recent studies have demonstrated that MSCs stimulate cartilage matrix production, whereas coculture with osteoblasts decreased chondrocyte matrix production [37]. Further, proteomic evaluation of MSCs in OM demonstrated that the MSCs remain at an earlier state of differentiation compared to tissue-derived osteoblasts [38]. The differentiation state of MSCs may therefore have an impact on their capacity to stimulate tissue formation. The present work evaluated the capacity of MSCs to stimulate cell proliferation and GAG accumulation depending on the state of differentiation.
Cell and tissue interactions can be analyzed in a variety of coculture systems. Some studies have utilized mixed populations of cells plated together in monolayer [39], whereas others have employed cell culture insert culture systems [40] or conditioned medium [41] to study the interaction of cells without direct contact. Biomaterials can provide the added dimension of a 3D environment for cell and tissue coculture. For example, we previously applied PEG-based hydrogels to create layered structures to build stratified cartilage [35]. Additional studies have sought to create osteochondral composites through chondrocyte pellet coating of a poly(lactic) acid scaffold preseeded with MSCs [30] or PLGA scaffolds wrapped in periosteum [42].
This study employs a number of culture systems and biomaterials to evaluate the effects of MSC coculture on cartilage tissue development. Traditional monolayer culture, bilayered PEG hydrogels, and a novel coculture system comprised of a PEG hydrogel layer adjacent to a HA-doped PLGA scaffold are employed to evaluate cell interactions. The monolayer cell culture insert system allows coculture screening with the benefit of low cellular requirements. However, this 2D system does not fully recapitulate the native 3D architecture cells are exposed to in vivo. Further, culture time is limited in monolayer culture when cells reach confluence whereas 3D systems support long-term tissue development and accumulation of extracellular matrix. The bilayered PEG hydrogel system provides a means of examining the cellular interactions in a 3D, nonadhesive environment. In the second coculture system, the PEG layer that is supportive of chondrocyte growth [27] is combined with a HA-PLGA layer that promotes cell adhesion and is therefore conducive for osteogenesis [31]. Evaluating coculture in multiple environments ensures that the results observed are not a simply a phenomena of a particular biomaterial.
The relationship between MSC differentiation and chondrocyte stimulation was examined under numerous coculture conditions. The differing stimulatory capacity of MSCs at varying stages of differentiation was expected based on previous reports that expression of MMPs, TIMPs, and aggrecanases are altered during osteogenesis [25,26]. However, the repeatedly observed trend of an increase in chondrocyte GAG accumulation at short periods of MSC osteogenic predifferentiation was not expected. The reproducibility of the relationship between period of MSC predifferentiation and the relative stimulation in the varying coculture systems suggests that it is not an artifact of the medium composition, culture system, or differences in cell number. This indicates that predifferentiation of MSCs before their delivery may serve to enhance their therapeutic efficacy.
Tissue formation by MSCs recapitulated previous reports that chondrocytes stimulated osteogenesis in 2D coculture [34], but inhibited in 3D coculture models [37]. Interestingly, hematoxylin and eosin staining did not indicate a decrease in MSC matrix production with coculture in the PEG PLGA/HA scaffolds system. Further, chondrocytes demonstrated the least calcification in the PEG PLGA/HA scaffolds, highlighting the importance of scaffold choice for tissue engineering applications.
The repeatedly observed peak at 3 days of predifferentiation brings forth the idea that this phenomenon is related to a fundamental biologic process. The peak in stimulation at 3 days may have ties to the process of natural fracture healing. Previously published work by Cho et al. demonstrated that inflammatory, chondrogenic, and osteogenic markers are expressed temporally during murine fracture healing in vivo [43]. This study found that an initial small peak in osteogenesis was found 1 to 3 days following the fracture, which was directly followed by a large peak in chondrogenic markers [43]. Alternatively, the stimulation may be related to the relationship between cartilage and mesenchymal precursors in growth plate architecture.
Regardless of the fundamental principle responsible for the 3-day MSC stimulatory peak, this study highlights the importance MSCs as a living and responsive therapeutic. This research illustrates the propensity for MSCs to undergo changes in stimulatory capacity over the differentiation process that impacts their therapeutic potential. This knowledge will allow design of biomaterial delivery systems to specifically maintain stem cells in a desired differentiation state for maximal therapeutic impact. Further, this research suggests that 1 or more soluble factors may mediate the stimulation by MSCs. Identification of these secretory factors could thereby lead to development of a cell-free therapeutic.
Supplementary Material
Acknowledgments
This research was supported in part by a National Institutes of Health R01 GM 074048 and a Maryland Stem Cell award ID 90034364.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science (N Y) 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Granero-Molto F. Weis JA. Longobardi L. Spagnoli A. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther. 2008;8:255–268. doi: 10.1517/14712598.8.3.255. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF. Mesenchymal stem cells from adult bone marrow. Methods Mol Biol (Clifton, N.J.) 2008;449:27–44. doi: 10.1007/978-1-60327-169-1_2. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S. Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 5.Fibbe WE. Nauta AJ. Roelofs H. Modulation of immune responses by mesenchymal stem cells. Ann N Y Acad Sci. 2007;1106:272–278. doi: 10.1196/annals.1392.025. [DOI] [PubMed] [Google Scholar]
- 6.Xiang J. Tang J. Song C. Yang Z. Hirst DG. Zheng QJ. Li G. Mesenchymal stem cells as a gene therapy carrier for treatment of fibrosarcoma. Cytotherapy. 2009;11:516–526. doi: 10.1080/14653240902960429. [DOI] [PubMed] [Google Scholar]
- 7.Yang B. Wu X. Mao Y. Bao W. Gao L. Zhou P. Xie R. Zhou L. Zhu J. Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells. Neurosurgery. 2009;65:610–624. doi: 10.1227/01.NEU.0000350227.61132.A7. [DOI] [PubMed] [Google Scholar]
- 8.Caplan AI. Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 9.Liu CH. Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine. 2005;32:270–279. doi: 10.1016/j.cyto.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Cawston TE. Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther. 1996;70:163–182. doi: 10.1016/0163-7258(96)00015-0. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto E. Ochi M. Kato Y. Mochizuki Y. Sumen Y. Ikuta Y. Beneficial effect of basic fibroblast growth factor on the repair of full-thickness defects in rabbit articular cartilage. Arch Orthop Trauma Surg. 1999;119:139–145. doi: 10.1007/s004020050377. [DOI] [PubMed] [Google Scholar]
- 12.Schinkothe T. Bloch W. Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev. 2008;17:199–206. doi: 10.1089/scd.2007.0175. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence RC. Felson DT. Helmick CG. Arnold LM. Choi H. Deyo RA. Gabriel S. Hirsch R. Hochberg MC. Hunder GG. Jordan JM. Katz JN. Kremers HM. Wolfe F National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomsen JS. Nielsen PT. Christensen PH. Simonsen O. Hoeck HC. Laursen MB. Moller-Madsen B. Ovesen J. Differences in zinc status, bone turnover and femoral head bone density and biomechanical properties between patients with osteoarthritis and osteoporosis. J Musculoskelet Neuronal Interact. 2008;8:22. [PubMed] [Google Scholar]
- 15.Quarto R. Mastrogiacomo M. Cancedda R. Kutepov SM. Mukhachev V. Lavroukov A. Kon E. Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 16.Centeno CJ. Busse D. Kisiday J. Keohan C. Freeman M. Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–353. [PubMed] [Google Scholar]
- 17.Murphy JM. Fink DJ. Hunziker EB. Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheumat. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 18.Osiris T. FDA Fast-Track Clearance Expedites Stem Cell Therapy. Osiris Therapeutics Inc.; Columbia, MD: Nov, 2008. [Google Scholar]
- 19.Mackie EJ. Ahmed YA. Tatarczuch L. Chen KS. Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Tetlow LC. Adlam DJ. Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheumat. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 21.Sakao K. Takahashi KA. Mazda O. Arai Y. Tonomura H. Inoue A. Saito M. Fujioka M. Takamiya H. Imanishi J. Kubo T. Enhanced expression of interleukin-6, matrix metalloproteinase-13, and receptor activator of NF-kappaB ligand in cells derived from osteoarthritic subchondral bone. J Orthop Sci. 2008;13:202–210. doi: 10.1007/s00776-008-1227-5. [DOI] [PubMed] [Google Scholar]
- 22.Grynpas MD. Alpert B. Katz I. Lieberman I. Pritzker KP. Subchondral bone in osteoarthritis. Calcif Tissue Int. 1991;49:20–26. doi: 10.1007/BF02555898. [DOI] [PubMed] [Google Scholar]
- 23.Hopwood B. Tsykin A. Findlay DM. Fazzalari NL. Microarray gene expression profiling of osteoarthritic bone suggests altered bone remodelling, WNT and transforming growth factor-beta/bone morphogenic protein signalling. Arthritis Res Ther. 2007;9:R100. doi: 10.1186/ar2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn JY. Cho HJ. Kang HJ. Kim TS. Kim MH. Chung JH. Bae JW. Oh BH. Park YB. Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 25.Filanti C. Dickson GR. Di Martino D. Ulivi V. Sanguineti C. Romano P. Palermo C. Manduca P. The expression of metalloproteinase-2, -9, and -14 and of tissue inhibitors-1 and -2 is developmentally modulated during osteogenesis in vitro, the mature osteoblastic phenotype expressing metalloproteinase-14. J Bone Miner Res. 2000;15:2154–2168. doi: 10.1359/jbmr.2000.15.11.2154. [DOI] [PubMed] [Google Scholar]
- 26.Lind T. McKie N. Wendel M. Racey SN. Birch MA. The hyalectan degrading ADAMTS-1 enzyme is expressed by osteoblasts and up-regulated at regions of new bone formation. Bone. 2005;36:408–417. doi: 10.1016/j.bone.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Williams CG. Kim TK. Taboas A. Malik A. Manson P. Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 28.Jaiswal N. Haynesworth SE. Caplan AI. Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 29.Kim TK. Sharma B. Williams CG. Ruffner MA. Malik A. McFarland EG. Elisseeff JH. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage. 2003;11:653–664. doi: 10.1016/s1063-4584(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 30.Tuli R. Nandi S. Li WJ. Tuli S. Huang X. Manner PA. Laquerriere P. Noth U. Hall DJ. Tuan RS. Human mesenchymal progenitor cell-based tissue engineering of a single-unit osteochondral construct. Tissue Eng. 2004;10:1169–1179. doi: 10.1089/ten.2004.10.1169. [DOI] [PubMed] [Google Scholar]
- 31.Hwang NS. Varghese S. Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2008;60:199–214. doi: 10.1016/j.addr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang DA. Williams CG. Yang F. Cher N. Lee H. Elisseeff JH. Bioresponsive phosphoester hydrogels for bone tissue engineering. Tissue Eng. 2005;11:201–213. doi: 10.1089/ten.2005.11.201. [DOI] [PubMed] [Google Scholar]
- 33.Halvorsen YD. Franklin D. Bond AL. Hitt DC. Auchter C. Boskey AL. Paschalis EP. Wilkison WO. Gimble JM. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7:729–741. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- 34.Gerstenfeld LC. Cruceta J. Shea CM. Sampath K. Barnes GL. Einhorn TA. Chondrocytes provide morphogenic signals that selectively induce osteogenic differentiation of mesenchymal stem cells. J Bone Miner Res. 2002;17:221–230. doi: 10.1359/jbmr.2002.17.2.221. [DOI] [PubMed] [Google Scholar]
- 35.Sharma B. Williams CG. Kim TK. Sun D. Malik A. Khan M. Leong K. Elisseeff JH. Designing zonal organization into tissue-engineered cartilage. Tissue Eng. 2007;13:405–414. doi: 10.1089/ten.2006.0068. [DOI] [PubMed] [Google Scholar]
- 36.Yang F. Williams CG. Wang DA. Lee H. Manson PN. Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Jiang J. Nicoll SB. Lu HH. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem Biophys Res Commun. 2005;338:762–770. doi: 10.1016/j.bbrc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 38.Bennett KP. Bergeron C. Acar E. Klees RF. Vandenberg SL. Yener B. Plopper GE. Proteomics reveals multiple routes to the osteogenic phenotype in mesenchymal stem cells. BMC Genomics. 2007;8:380. doi: 10.1186/1471-2164-8-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y. Volloch V. Pindrus MA. Blasioli DJ. Chen J. Kaplan DL. Murine osteoblasts regulate mesenchymal stem cells via WNT and cadherin pathways: mechanism depends on cell-cell contact mode. J Tissue Eng Regen Med. 2007;1:39–50. doi: 10.1002/term.6. [DOI] [PubMed] [Google Scholar]
- 40.Gerstenfeld LC. Barnes GL. Shea CM. Einhorn TA. Osteogenic differentiation is selectively promoted by morphogenetic signals from chondrocytes and synergized by a nutrient rich growth environment. Connective Tissue Res. 2003;44(Suppl 1):85–91. [PubMed] [Google Scholar]
- 41.Hwang NS. Varghese S. Puleo C. Zhang Z. Elisseeff J. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2007;212:281–284. doi: 10.1002/jcp.21052. [DOI] [PubMed] [Google Scholar]
- 42.Sedrakyan S. Zhou ZY. Perin L. Leach K. Mooney D. Kim TH. Tissue engineering of a small hand phalanx with a porously casted polylactic acid-polyglycolic acid copolymer. Tissue Eng. 2006;12:2675–2683. doi: 10.1089/ten.2006.12.2675. [DOI] [PubMed] [Google Scholar]
- 43.Cho TJ. Gerstenfeld LC. Einhorn TA. Differential temporal expression of members of the transforming growth factor B superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.