Abstract
Inflammation of the skin and systemic fever, both of which occur with injury or infection, include a hyperthermic component that many believe constitutes a physiological stress. Such increases in local or systemic body temperature may also have a regulatory effect on immune function. Langerhans cells (LCs), the dendritic cells of the skin, continuously monitor the extracellular matrix of the skin by taking up particles and microbes that they then carry to draining lymph nodes for presentation to T lymphocytes. We hypothesize that the thermal element of inflammation and/or fever may help regulate the activation and migration of LCs out of the epidermis. To test this hypothesis, Balb/c mice were exposed to a mild (39.8°C ± 0.2°C), long-duration (6 hours) whole body hyperthermia (WBH) treatment, which mimics the thermal component of fever. The number of LCs and their morphology were analyzed at various time points up to 7 days after the initiation of WBH. The LCs of the ear epidermis were visualized using a fluorescein isothiocyanate–conjugated antibody specific for the major histocompatibility complex (MHC) class II molecule and confocal microscopy. Although MHC class II staining was diffuse on the surface of the LC body and dendritic extensions of both WBH and control samples, the WBH-treated LCs exhibited a more punctate morphology with fewer dendritic processes compared with control LCs. A significant decrease in the number of LCs was also observed 1 to 5 days after WBH treatment. Furthermore, in vitro heating of Balb/c ear skin cultures at 40°C for 6 to 8 hours enhanced the numbers of viable LCs that migrated into the culture wells. These results suggest that WBH treatment stimulates epidermal LCs in the absence of foreign antigen.
INTRODUCTION
The body is continuously exposed to a variety of antigens, including parasites, fungi, bacteria, viruses, and the compounds that they secrete. The primary physical barrier to these antigens is the skin. If such antigens penetrate the skin, they are taken up by Langerhans cells (LCs) that reside in the epidermis (Maurer and Stingl 1999). LCs are antigen-presenting cells that are continuously sampling the extracellular matrix of the skin for foreign substances. When a foreign antigen is encountered, the LC phagocytoses it and begins a process of maturation in which it simultaneously migrates to the draining lymph node (Maurer and Stingl 1999). This maturation and migration process is accompanied by both physiological and morphological changes in the LC. Once in the lymph node, the mature LC presents the antigen in the context of a major histocompatibility complex (MHC) and with the appropriate costimulatory signals to T cells (Maurer and Stingl 1999).
Infection with foreign antigens is also characterized by other inflammatory processes, which involve vasodilation, increased vascular permeability, neutrophil recruitment and activation, and fever (Rosenberg and Gallin 1999). Although there is obvious benefit to vasodilation, increases in vascular permeability and neutrophil recruitment in controlling an infection, the benefit of fever is poorly understood (Rosenberg and Gallin 1999). The fever response to infection occurs at high metabolic cost in vertebrates (Kluger 1986). Ectotherms also exhibit behavioral fevers that dramatically improve survival following infections (Kluger et al 1975; Covert and Reynolds 1977). These facts support the notion that there may be a beneficial role for fever-range hyperthermia, because it is unlikely that the fever response would have been retained evolutionarily if it has no survival value. Furthermore, if such increases in temperature are considered a physiologically relevant heat stress, it might be suspected that fever-range temperatures have the potential to drive an immunomodulatory stress response. Indeed, this fever-range hyperthermia treatment has been shown to be sufficient to induce heat shock protein expression in lymphocytes and other tissues (Di et al 1997, J.R. Ostberg et al, in preparation). Yet, despite the natural occurrence of increased body temperature in association with an immune challenge, temperature is not a variable in most immunological investigations.
The paucity of knowledge regarding the immunoregulatory role of increased body temperature led us to directly evaluate the effects of the thermal component of fever on epidermal LCs in Balb/c mice. Herein, we report the effects of a fever-range whole body hyperthermia (WBH) protocol (39.8°C ± 0.2°C for 6 hours) on the numbers and morphology of LCs in the mouse ear epidermis.
MATERIALS AND METHODS
Mice
Female Balb/c mice (Taconic Laboratories, Germantown, PA, USA), ranging from 8 to 10 weeks of age, were used in all experiments with age-matched controls. All protocols involving these mice were approved by the Roswell Park Institute Animal Care and Use Committee.
Fever-range WBH
To prevent dehydration, mice were injected with 1 mL of nonpyrogenic saline intraperitoneally (Shen et al 1991) immediately before being placed in microisolator cages preheated to 38.8°C. The cages (≤5 mice per cage) were then placed in an environmental chamber with preheated incoming fresh air (Memmert model BE500, East Troy, WI, USA). Within 20 minutes, the average core body temperature of the mice was raised from 37.5°C (normal core temperature of mice) to 39.8°C ± 0.2°C, and this body temperature was maintained for up to 6 hours by adjusting the temperature of the environmental chamber. Core temperatures were monitored with the Electronic Laboratory Animal Monitoring System from Biomedic Data Systems (Maywood, NJ, USA) using nonexperimental Balb/c mice that had 14 × 2.2-mm microchip transponders subcutaneously implanted into the dorsal thoracic area. Variations in temperature readings between animals are reproducibly within 0.2°C of the mean. Control mice were kept at room temperature and subjected to the same manipulations as the heated mice. All experiments were started at approximately the same time each day (7:30 to 9:30 AM) to avoid the possible influence of diurnal cycling.
Staining of epidermal LCs
Control or WBH-treated mice were killed by cervical dislocation at various times after WBH treatment. Ears were removed, placed in phosphate-buffered saline (PBS), and split into dorsal and ventral halves. Dorsal halves of ears were placed dermal side down in 0.5 M ammonium thiocyanate for 20 minutes at 37°C, and epidermis was separated from dermis using fine forceps. Epidermis was fixed in acetone at room temperature for 5 minutes and then thoroughly washed using PBS. Epidermal sheets were then incubated with 0.05 μg of fluorescein isothiocyanate–conjugated mouse anti-mouse I-Ad monoclonal antibody (PharMingen, San Diego, CA, USA) in 200 μL of PBS for 30 minutes in a dark, humidified box at room temperature. The epidermis was washed thoroughly with PBS, mounted with AquaPolyMount (Polysciences Inc, Warrington, PA, USA), and covered with a coverslip. Fluorescent images were obtained by confocal microscopy.
Statistical analysis
Control values were compared with the experimental values at each time point after hyperthermia treatment using unpaired Student's t-tests. P values less than 0.05 were considered to represent statistically significant differences.
Skin organ culture
Ears were rinsed in 70% ethanol, sterilely trimmed if necessary to ensure similar sizes in heated and control cultures, and split with forceps into dorsal and ventral halves. Ear halves were cultured dermal side down in 2 mL of media (RPMI with 10% fetal bovine serum, 2 mM glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin, 55 mM β-mercaptoethanol) per well of a 24-well tissue culture plate. For the first 6 to 8 hours of culture, plates were divided between 37°C and 40°C incubators, each with 5% CO2. The rest of the culture time was continued at 37°C. At various time points following the hyperthermic exposure, the numbers of viable cells per well were determined by trypan blue dye exclusion using a hemacytometer.
RESULTS
Fever-range WBH alters epidermal LC morphology
To examine the effects of WBH on epidermal LCs in the ears of Balb/c mice, a fluorochrome-conjugated antibody against MHC class II was used to visualize the LCs in epidermal sheets by confocal microscopy. The MHC class II staining was uniformly bright on the surface of the LC body and dendritic extensions of both WBH and control samples, making them easy to identify. However, the WBH-treated LCs exhibited a more punctate morphology and fewer dendritic processes compared with control LCs (Fig 1). It also appeared that the density of LCs in a given field was more sparse in many of the images from WBH-treated mice compared with controls. These observations led us to hypothesize that the WBH treatment was stimulating the resident LCs to migrate out of the ear epidermis.
Fig 1.
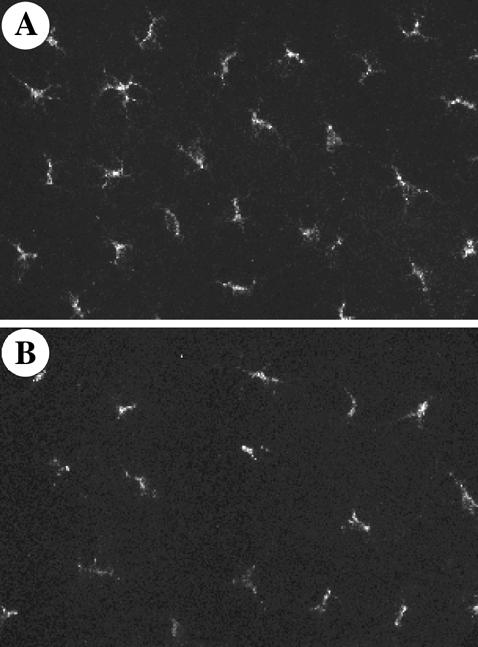
Confocal images of MHC class II–stained epidermal LCs from ears of control and WBH-treated mice. Representative 60× fields of control epidermis (A) and epidermis collected 3 days after WBH treatment (B) are depicted
Fever-range WBH results in decreased numbers of LCs in the ear epidermis
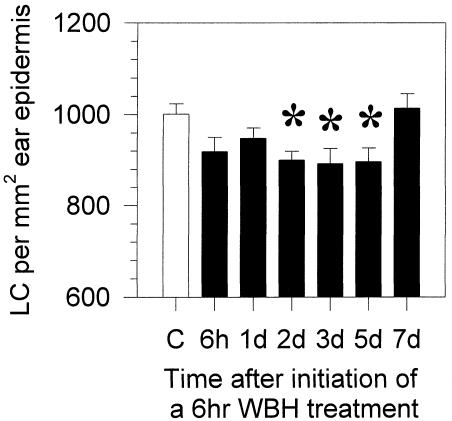
To precisely determine the effects of WBH on epidermal LC numbers, we first determined that LCs are evenly spaced throughout the epidermis of the whole ear (data not shown). Cell counts were then determined from 3 random low-power fields (20×) per ear obtained by confocal microscopy. The average numbers of LCs per square millimeter of ear epidermis collected at various time points after WBH treatment are depicted in Figure 2. Statistically significant decreases in the numbers of LCs per square millimeter were seen between 2 and 5 days after WBH treatment when compared with the control mice. Other experiments also revealed statistically significant decreases in LC numbers as early as 1 day after WBH treatment (data not shown). The numbers of LCs then appeared to return to control levels by 7 days after WBH treatment. These data suggest that WBH treatment enhances the migration of resident LCs out of the epidermis.
Fig 2.
Decreased numbers of LCs in the epidermis of Balb/c mice are observed after physiological WBH treatment. Ear epidermis of Balb/c mice was stained for MHC class II at various time points after a 6-hour WBH treatment. Images were collected using a confocal microscope, and the average number of LCs per square millimeter (± SE) was determined. White column, control mice; black columns, WBH-treated mice; *, P < 0.05 when WBH-treated mice were compared with controls using the unpaired Student's t-test. n = 3–6 mice per group. Data are representative of 3 different experiments
In vitro fever-range hyperthermia treatment results in increased numbers of LCs in the wells of ear skin organ cultures
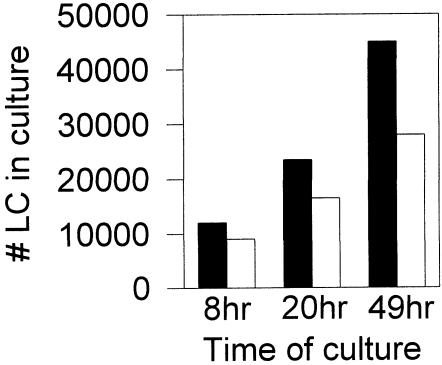
To ensure that the decreased numbers of LCs in the ear epidermis of WBH-treated mice were due to increased migration out of the skin and not to the damage and/or death of these cells, we also performed in vitro hyperthermia treatments of ear skin organ cultures. It has previously been shown that epidermal LCs migrate out of cultured skin explants into the culture medium at the rate of about 5 to 8 × 103 per ear per day (Larsen et al 1990). To determine the effects of fever-range hyperthermia on this LC migration out of explants, ear skin cultures were incubated for the first 6 to 8 hours at either 40°C or 37°C, and then all cultures were placed at 37°C. Numbers of viable cells in the culture wells were then determined at various time points using the trypan blue dye exclusion assay. As shown in Figure 3, increased numbers of viable cells with dendritic morphology were reproducibly found in the cultures of hyperthermia-treated explants compared with controls. Thus, it appears that fever-range temperatures do indeed enhance the migratory capacity of epidermal LCs.
Fig 3.
Increased numbers of dendritic cells are observed in culture after in vitro hyperthermia treatment of mouse ear skin. Dorsal halves of Balb/c ears were cultured dermis side down in 24-well plates with RPMI + 10% fetal bovine serum at either 40°C (black bars) or 37°C (white bars) for the first 8 hours, and then all cultures were incubated at 37°C. Numbers of cells that left the skin were determined by trypan blue dye exclusion of culture supernatant. Visualization of the cultures revealed that these had dendritic morphology by the 20-hour time point. Data are representative of 3 different experiments
DISCUSSION
WBH applied at the temperature range and duration similar to that experienced during a fever appears to regulate epidermal LC activation and migration. Although we had difficulty determining any enhancement of MHC class II intensity that is usually associated with LC activation (Larsen et al 1990), morphological changes that are suggestive of the cells retracting their dendritic processes to facilitate emigration were observed. Further analysis of the effects of fever-range hyperthermia on different LC activation markers are currently under way.
Also indicative of increased LC migration out of the skin, a significant reduction in the numbers of epidermal LCs was observed after WBH treatment. This was despite the fact that our experimental system does not purposely block the ability of new cells to migrate into the epidermis. Furthermore, our in vitro hyperthermia experiments support the notion that the decreased numbers of epidermal LCs after WBH are due to increased migration of viable cells out of the skin, and not to LC death or destruction. This may reflect the ability of WBH to specifically skew the kinetics of LC surveillance toward activation and exiting the epidermis without equally enhancing their replacement by incoming cells. In general, LCs are a mobile cell population with a relatively slow turnover (Maurer and Stingl 1999). They originate from bone marrow precursors that circulate in the peripheral blood en route to populating the skin (Maurer and Stingl 1999). Thus, although fever-range WBH can stimulate resident epidermal LC emigration and maturation, it may be limited in its stimulatory capacity on LC precursor formation or localization to the epidermis. Interestingly, however, the stabilization of LC density between 5 and 7 days after WBH treatment is similar to that seen with mouse skin grafts (Larsen et al 1990).
In conclusion, although this study suggests that body temperatures similar to those experienced during a fever are capable of modulating LC-mediated immune responses, it is important to realize that WBH treatment cannot be directly compared with a naturally occurring fever. For example, this externally applied hyperthermia treatment bypasses the biochemical, immunological, and neurological events that normally lead to fever (Kluger 1991). Yet on consideration of the various observations made using ectotherms (Kluger et al 1975; Covert and Reynolds 1977) and mice (Jiang et al 2000), the relevance of exogenous heating mechanisms in survival against infections cannot be denied. Furthermore, studies such as ours highlight a largely underappreciated immunoregulator: fever range or physiological heat stress. Indeed, one may speculate that the effects of elevated body temperatures are beneficial and may be clinically used for the treatment of various diseases.
Acknowledgments
This work was supported in part by National Institutes of Health grant CA71599, Roswell Park Cancer Institute core grant CA16056-21, and a postdoctoral training fellowship to J.R.O. from the Cancer Research Institute. We also wish to thank Christian P. Larsen for his technical advice regarding the skin organ cultures.
REFERENCES
- Covert JB, Reynolds WW. Survival value of fever in fish. Nature. 1977;267:43–45. doi: 10.1038/267043a0. [DOI] [PubMed] [Google Scholar]
- Di YP, Repasky EA, Subjeck JR. Distribution of HSP70, protein kinase C, and spectrin is altered in lymphocytes during a fever-like hyperthermia exposure. J Cell Physiol. 1997;172:44–54. doi: 10.1002/(SICI)1097-4652(199707)172:1<44::AID-JCP5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Cross AS, Singh IS, Chen TT, Viscardi RM, Hasday JD. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun. 2000;68:1265–1270. doi: 10.1128/iai.68.3.1265-1270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ. Is fever beneficial? Yale J Biol Med. 1986;59:89–95. [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. 1975;188:166–168. [PubMed] [Google Scholar]
- Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Stingl G 1999 Dendritic cells in the context of skin immunity. In: Dendritic Cells, ed Lotze MT, Thomson AW. Academic Press, New York, 111–122. [Google Scholar]
- Rosenberg HF, Gallin JI 1999 Inflammation. In:Fundamental Immunology, ed Paul WE. Lippincott-Raven Publishers, Philadelphia, 1051–1066. [Google Scholar]
- Shen RN, Hornback NB, Shidnia H, Wu B, Lu L, Broxmeyer HE. Whole body hyperthermia: a potent radioprotector in vivo. Intl J Radiat Oncol Biol Phys. 1991;20:525–530. doi: 10.1016/0360-3016(91)90065-c. [DOI] [PubMed] [Google Scholar]