Abstract
In the early mammalian embryo, lineage separation of and subsequent crosstalk between the trophectoderm (TE) and inner cell mass (ICM) are required to support further development. Previous studies have shown that the homeobox transcription factor Cdx2 is required for TE differentiation and that lack of Cdx2 expression causes death of embryos at the peri-implantation stage. In this study, we effectively eliminated Cdx2 transcripts by microinjection of siRNA into embryos and evaluated the effect on efficiency of deriving embryonic stem cells (ESCs). By this approach, we successfully created nonviable embryos similar to reported knockout embryos. Accordingly, the efficiency of ESC derivation dropped from 19.1% in control blastocysts to 2% in Cdx2-deficient blastocysts, indicating loss of pluripotency in the ICM. Strikingly, when 8-cell stage embryos were cultured under ESC culture conditions before lineage separation, fully functional pluripotent stem cell lines were obtained, with efficiency even greater than that for control embryos. These results demonstrate that Cdx2 plays an essential role within the microenvironment created by the TE to support ICM pluripotency but that the ESC culture system, with mouse embryonic fibroblasts, could rescue the pluripotent cell population for efficient ESC derivation.
Introduction
At the fourth cell division during development of the mouse embryo, cells on the outer part adopt an epithelial fate, whereas those on the inner part remain pluripotent. The outer epithelium, called trophectoderm (TE), will subsequently differentiate into extraembryonic tissue; whereas the inner cells, called inner cell mass (ICM), will eventually give rise to the embryo proper. Embryonic stem cells (ESCs), derived from the ICM, can be indefinitely propagated in culture and can differentiate into nearly all cell types of the adult body [1,2]. TE is vital for creating a niche providing the appropriate microenvironment to support the pluripotent state and self-renewal capacity of the ICM as well as to regulate its differentiation program. The roles played by this niche have been well demonstrated by experiments monitoring the fate of ESCs in different environments. When subcutaneously injected into immunodeficient nude mice, ESCs can differentiate into multicellular tumor masses, known as teratomas, as they lack the appropriate microenvironment supportive of specific intercellular interactions and cellular organization. However, when amorphous pluripotent ESCs are aggregated with a tetraploid embryo, they can differentiate into a highly organized and morphologically distinct organism, all cells of which originate from the ESCs [3].
Cdx2, a class I homeobox transcription factor, plays an essential role in maintaining TE differentiation in the mouse [4,5]. Cdx2-null embryos generated by heterozygous mating are capable of initiating formation of a blastocyst, but they fail to maintain a blastocoel or to form a functional TE [5]. Recent studies have suggested that, although the positive feedback loop between Cdx2 expression and cell polarity could also affect cell lineage allocation to TE [6], Cdx2 is not required for the formation of the TE lineage [7], and it acts downstream of cell polarization [8] and the Hippo signaling pathway modulated Tead4 activation [9–11]. However, controversy exists regarding the effect of Cdx2 deficiency on the viability of the pluripotent founder cell population. Although it has been reported that ESC lines can be established from Cdx2-deficient embryos and that aggregation of such embryos with control tetraploid embryos helps them recover and assume postimplantation development [12], there have also been accounts of failure of the ICM of Cdx2-deficient embryos to proliferate [13].
In the present study, direct injection of siRNA duplex into zygotes and metaphase II (MII) oocytes was performed to robustly abolish both maternal and zygotic expression of Cdx2. These experiments demonstrated the significance of interactions between the TE and ICM and the effect of Cdx2 depletion at the preimplantation stage on the pluripotent founder cell population, as showed by efficiency of ESC derivation and full pluripotency of the derived ESCs.
Materials and Methods
Embryo culture and microinjection of siCdx2 duplex
Fertilized oocytes were collected in M2 medium 18 h post- human chorionic gonadotrophin (hCG) from oviducts of primed B6C3F1 female mice after mating with nontransgenic CD1 or ROSA26+/+ (LacZ) and OG2+/− (Oct4-GFP) double transgenic male mice, depending on experiments. Oocytes were cultured in KSOMAA medium (potassium simplex optimized medium plus 19 natural amino acids) (37°C, 5% CO2 in air) until microinjection. About half of the embryos derived from B6C3F1 × ROSA26+/+/OG2+/− matings have both the LacZ and the Oct4-GFP transgenes, which enabled us to track the contribution of both somatic and germ cells in chimeras after ESC injection into blastocysts. For MII oocyte microinjection, mature oocytes were collected in M2 medium 14 h post-hCG from oviducts of primed B6C3F1 female mice and fertilized in vitro in modified KSOM [14] with epididymal spermatozoa from adult OG2 male mice after siCdx2 microinjection.
The online tool BLOCK-iT™ RNAi Designer was used to select target sequences for siRNA (https://rnaidesigner.invitrogen.com/rnaiexpress/), which automatically filters sequences for specificity. Lyophilized siRNA duplexes (Invitrogen) were resuspended in 1 mL of diethylpyrocarbonate (DEPC)-treated water according to the manufacturer's instructions and stored in single-use aliquots at −20°C. A highly effective duplex was selected from 3 regular oligonucleotides and 3 Stealth™ RNAi oligonucleotides containing the coding region of Cdx2 gene (sense: GCAGUCCCUAGGAAGCCAAdTdT; antisense: UUGGCUUCCUAGGGACUGCdTd). Unless otherwise specified, a scrambled siRNA duplex was used as control (sense: GCACCCGAUAAGCGGUCAAdTdT; antisense: UUGACCGCUUAUCGGGUGCdTdT).
siRNA microinjections were carried out with an Eppendorf FemtoJet microinjector and Narishige micromanipulators in M2 medium drops covered with mineral oil. Microinjection pipettes were pulled with a Sutter P-97 pipette puller. Five microliters of siRNA (8 μM) were loaded into the pipette, and about 2 pl of siRNA solution was injected into the cytoplasm of oocytes.
After the injection, oocytes were washed and cultured in KSOMAA (37°C, 5% CO2 in air) and evaluated for cleavage twice daily. Implantation capability of early stage embryos was evaluated by transfer of embryos at 3.5 days postcoitum (dpc) into the uteri of pseudopregnant CD1 2.5-dpc female mice.
The animals' care was in accordance with MPI institutional guidelines.
RNA extraction, cDNA synthesis, and real-time reverse transcription polymerase chain reaction
For real-time analysis of gene expression, embryos were harvested in RNA lysis buffer at different stages and processed as previously described [15]. Briefly, total RNA was extracted from single blastocysts using the MicroRNeasy Kit (Qiagen GmbH) according to the manufacturer's instructions. cDNA synthesis was performed with the High Capacity cDNA Archive Kit (Applied BioSystems GmbH) following the manufacturer's instructions. Transcript levels were determined using the ABI PRISM Sequence Detection System 7900 (Applied BioSystems) and the ready-to-use 5′-nuclease Assays-on-Demand.
Oligonucleotides for real-time detection were designed by the TaqMan® Assays-on-Demand™. Three biological replicates were used, and each sample was run with 3 technical replications; an reverse transcription (RT)-blank and a no-template blank served as negative controls. Quantification was normalized to the endogenous Hprt gene, which was found to be more stable than others previously tested [16].
Immunofluorescence staining of embryos
Immunocytochemical staining was performed as previously described with minor modifications [5]. Briefly, samples were fixed in 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton X-100 (Sigma-Aldrich Inc.) for 1 h, and blocked with 3% bovine serum albumin in phosphate-buffered saline (PBS) for 1 h. Control and Cdx2-depleted embryos were processed and examined in parallel. Samples were then incubated overnight with monoclonal mouse anti-Cdx2 IgG (1:100; BioGenex) and rabbit anti-Oct4 IgG (1:2,500; generated in our laboratory [17]) at 4°C overnight. Finally, samples were washed thrice with PBS and stained with Alexa 488–conjugated goat anti-rabbit IgG (1:400; Invitrogen) and Cy3-conjugated goat anti-mouse IgG (1:200; Jackson Laboratory) for 1 h at room temperature. Samples were examined under a Laser Scanning Confocal Microscope (UltraVIEW; PerkinElmer Life Sciences, Inc.) with 488- and 568-nm lasers.
TUNEL measurement of DNA fragmentation
Degree of apoptosis in embryo samples was determined using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-FITC nick-end labeling (TUNEL) with an in-situ cell death detection kit (DeadEnd™ Fluorimetric TUNEL System; Promega Corporation) according to the manufacturer's recommendation. Cells were then viewed and counted under confocal microscopy.
Derivation of ESC lines
The siControl- and siCdx2-injected embryos were cultured to obtain E2.5 8-cell stage embryos and E3.5 blastocysts, which were used to establish cell lines with a standard procedure [18] after removal of the zonae pellucidae by acidic Tyrode's solution (Sigma-Aldrich). The complete ESC medium composition is 4 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 100 μM β-mercaptoethanol, 100 μM nonessential amino acids, 1,000 U/mL leukemia inhibitory factor (LIF) (Chemicon), and 15% fetal bovine serum (Invitrogen) in Dulbecco's modified Eagle's medium (31885-023; Invitrogen). A more recently published protocol was also used with knockout Dulbecco's modified Eagle's medium and 20% serum replacement or 15% fetal calf serum plus 5% serum replacement [19].
Generation and analysis of chimeric mice
To test the pluripotency of stem cell lines, Oct4-GFP+/LacZ+ ESCs were injected into E3.5 blastocysts of C57BL/6 mice using a Piezo-driven micromanipulator (Primetech). The transgenes allowed us to clearly follow the germ and somatic cell contribution of the injected ESCs in the generated chimeras. Each C57BL/6 blastocyst was injected with 15–20 Oct4-GFP+/LacZ+ ESCs, and 11–18 injected blastocysts were transferred into the uterus of each CD1 pseudopregnant recipient 2 h after injection. All 4 foster mothers became pregnant. Nine implanted embryos were harvested on E9.5 from 1 foster mother, and 5 implanted embryos were recovered on E13.5. E9.5 embryos and gonads from E13.5 embryos were examined for germ cell contribution by GFP fluorescence. All embryos were then evaluated for LacZ staining. The remaining foster mothers gave birth to a total of 9 pups. All pups survived, and 4 showed chimeric coat color.
For whole-mount LacZ staining, extraembryonic tissues were removed from E9.5 and E13.5 embryos, fixed for 1 h in 4% paraformaldehyde at 4°C, and stained overnight at 37°C in 1.0 mg/mL β-galactosidase, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, 0.02% NP-40, and 0.01% sodium deoxycholate in 0.1 M PBS (pH 7.3). Embryos were extensively washed in PBS, postfixed in 4% paraformaldehyde for 1 h at 4°C, and embedded in Tissue-Tek O.C.T compound (Sakura Finetechnical) on dry ice. Sagittal sections (10 μm) were cut at −18°C with a LEICA cryostat model CM3050S (Leica Instrument), washed in PBS, and counterstained with Fast Red. Sections were examined through a stereoscope and under a microscope to visualize the distribution of LacZ-positive cells, stained in blue, in different tissues.
Teratoma formation
Teratomas were induced by injecting 2–5 × 106 cells into the subcutaneous tissue at the side of the chest of 6-week-old Nude Swiss (athymic, immunocompromised) mice. Eight to 12 weeks postinjection, teratomas were harvested and fixed overnight in 4% paraformaldehyde at 4°C. Samples were immersed in 30% sucrose overnight before embedding the tissue in the Tissue-Tek O.C.T compound. Cryosections of 10 μm thickness were obtained and stained with 1% Fast Red and hematoxylin.
Aggregation of ESCs with tetraploid embryos
Two-cell stage embryos were flushed 20 h post-hCG from oviducts of B6C3F1 mice and fused with a Cellfusion CF-150/B apparatus in a 250-μm gap electrode chamber (BLS Ltd.) containing 0.3 M Mannitol with 0.3% bovine serum albumin (Sigma-Aldrich). An initial electrical field of 2 V was applied to the embryos, followed by one peak pulse of 50 V for 35 μs. Embryos were washed and immediately transferred back into KSOMAA in a 37°C incubator. After 15–60 min, embryos were examined for fusion. Those embryos that remained unfused after 60 min were discarded. Fused tetraploid embryos were cultured overnight and aggregated with ESCs as reported with a slight modification [20]. Briefly, clumps of loosely connected ESCs (10–20 cells) from short trypsin-treated day-2 cultures were picked out and transferred into microdrops of KSOMAA with 10% fetal calf serum under mineral oil; each clump was placed in a depression in the microdrop. Meanwhile, batches of 30–40 embryos were briefly incubated in acidified Tyrode's solution [21] until dissolution of their zonae pellucidae. Two embryos were placed on top of each clump. All aggregates were assembled in this manner and cultured overnight at 37°C in 5% CO2, then transferred into the uterus of CD1 pseudopregnant recipients. GFP genotyping was carried out by polymerase chain reaction (PCR) with DNA extracted from tails, as described [22], for 35 cycles with primers: ACGTAAACGGCCACAAGTTC (forward) and AAGTCGATGCCCTTCAGCTC (reverse).
Statistical analysis
Real-time PCR results were analyzed using Applied Biosystems SDS v2.0 software and the ΔΔCT method (User Bulletin #2, ABI Prism 7700 Sequence Detection System, 1997). For ESC derivation experiments, statistical analysis was performed using the Pearson's chi-square (χ2) test and Fisher Exact test. A P value of <0.05 was considered significant.
Results
Reduction of Cdx2 RNA by siRNA was robust, and phenotype of resultant embryos was similar to that of Cdx2-knockout embryos
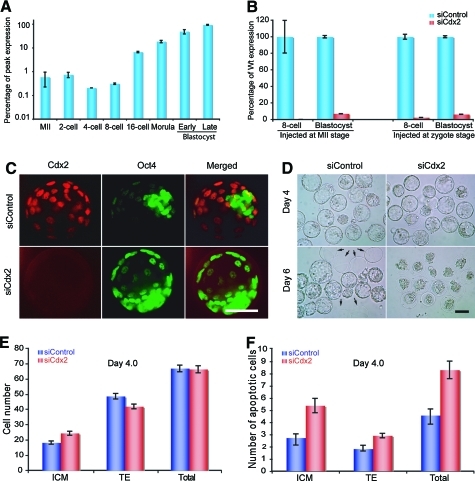
First, we examined the expression of Cdx2 at the mRNA level during early mouse development by quantitative TaqMan Real-Time PCR (qRT-PCR). Maternal Cdx2 mRNA was detectable in oocytes and 2-cell stage embryos and decreased in 4-cell stage embryos and a burst of expression on Cdx2 activation between the 8-cell and 16-cell stages (Fig. 1A). Next, we tested the knockdown efficiency of siRNA on Cdx2 at the mRNA level after siRNA microinjection into single embryos, which was performed in triplicate. Of the 6 Cdx2 siRNA duplexes (siCdx2) tested, siRNA3 was selected for subsequent experiments due to its unique sequence and high efficacy (∼95%) up to the blastocyst stage (Fig. 1B). A scrambled array of siRNA duplexes (siControl) with the same composition was used as control throughout the study. Almost complete elimination of the maternal Cdx2 was evidenced by qRT-PCR with a knockdown efficiency of >99% on treated embryos from 2- to 16-cell stages. Knockdown of Cdx2 mRNA in blastocysts paralleled that of the protein as determined by immunocytochemistry (Fig. 1C). Consequently, the treated embryos showed a complete hatching block (Fig. 1D, 0/86 in siCdx2 vs. 43/71 in siControl at 6.0 dpc) without a reduction in the total cell number (Fig. 1E) and with a mild increase in apoptotic cells in both the ICM and the TE (Fig. 1F). Transfer of 3.5-dpc Cdx2-deficient blastocysts into the uterus of pseudopregnant foster mice did not lead to any implantation (0 implanted/109 transferred in the siCdx2-treated group vs. 60/108 in siControl from 3 separate experiments), which is a phenotype typical of Cdx2-knockout embryos, even though these embryos developed into the 2-cell or blastocyst stage at the same rate as control embryos (Table 1). These results clearly indicated that siCdx2-treated zygotes lost their developmental potential due to the specific effect of Cdx2 depletion.
FIG. 1.
Efficient reduction of Cdx2 mRNA and protein in mouse preimplantation stages by siCdx2 treatment and the resultant phenotypes. (A) Relative Cdx2 mRNA expression levels in logarithmic scale as percentage of peak expression in mouse embryos at different preimplantation stages by quantitative real-time (qRT)–polymerase chain reaction. The maternal mRNA decreased at the 4-cell stage, followed by upregulation at the 8-cell stage due to the zygotic activation of Cdx2. Embryo collection time: 2-cell stage: 40 h post-human chorionic gonadotrophin (hCG); 4-cell stage: 52 h post-hCG; 8-cell stage: 62 h post-hCG; 16-cell stage: 72 h post-hCG; Morula: 85 h post-hCG; Early blastocyst: 96 h post-hCG; Blastocyst: 115 h post-hCG. (B) Comparison of Cdx2 mRNA level in siControl and siCdx2-treated embryos at different preimplantation stages demonstrated robust downregulation of Cdx2 by siCdx2 treatment of oocytes at both metaphase II (MII) and zygote stages. (C) Confocal images of immunohistochemistry of blastocyst-stage embryos with anti-Cdx2 and anti-Oct4 antibodies. The images show that after siCdx2 treatment, Cdx2 was eliminated, but Oct4 was ectopically expressed in the trophectoderm (TE). Scale bar, 100 μm. (D) Differential interference contrast (DIC) images of E4.0 and E6.0 embryos. All siCdx2-treated embryos failed to hatch. Arrows indicate empty zonae pellucidae left by hatched control embryos. Scale bar, 100 μm. (E) Effect of Cdx2 depletion on the TE and inner cell mass (ICM) cell numbers. There was a significant increase in the number of ICM cells (P < 0.01), but the total cell number remained unchanged (P = 0.64). (F) Number of apoptotic cells in a Cdx2-deficient E4.0 blastocyst showing an increase in apoptotic activity. Color images available online at www.liebertonline.com/scd.
Table 1.
Preimplantation Development of siCdx2-Injected Embryos
| Group | 2-Cell stage formation/total (%) | Blastocyst formation (%) | Hatched blastocysts (%) |
|---|---|---|---|
| Not injected | 34/34 (100) | 29/34 (85.3) | 17/26 (65.4) |
| siControl | 259/261 (99.2) | 227/261 (87.0) | 43/71 (60.6) |
| siCdx2 | 248/250 (99.2) | 221/250 (88.4) | 0/86 (0) |
Rates of cleavage, blastocyst formation, and hatching were recorded at 1.5, 3.5, and 6 days postcoitum, respectively. Fewer embryos were used for the hatching observation, as samples at different stages were randomly removed for other experiments. No significant differences were found until the blastocysts began hatching. All siCdx2-treated blastocysts failed to hatch and ultimately collapsed. Data are pooled from 3 independent microinjection experiments.
Efficiency of generating ESC lines from Cdx2-deficient embryos is enhanced at an early stage (8-cell), but compromised at a later stage (blastocyst)
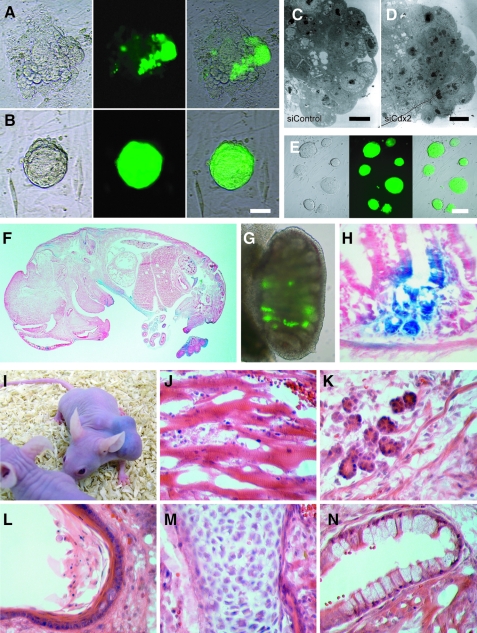
To determine whether siCdx2 has any effect on the pluripotent cell lineage, the ability of 8-cell and blastocyst stage embryos to give rise to pluripotent ESCs was assessed. Stem cell derivation rates at the 8-cell and blastocyst stages of the control group were similar (22.4% vs. 19.1%), indicating that both stages are suitable for ESC derivation under our culture system. Surprisingly though, injection of siCdx2 had opposite effects at different stages (Table 2): Efficiency of deriving stem cell lines from blastocyst stage embryos was reduced by 10-fold (2.0% vs. 19.1% for controls), whereas that from 8-cell stage Cdx2-knockdown embryos was increased (33.4% vs. 22.4% for controls), with the resultant ESCs exhibiting normal colony morphology (Fig. 2E). Improvement in derivation efficiency of 8-cell stage Cdx2-knockdown embryos compared with same stage controls was confirmed by using another ESC derivation protocol [19] after performing triplicate experiments (77/123 vs. 69/133), with pooled data showing statistical significance (97/182 vs. 82/192, P < 0.05). In addition, the morphology of blastocyst outgrowths was different compared with that of controls—almost all colonies obtained from Cdx2-deficient embryos exhibited a markedly adherent spherical morphology without obvious spreading of surrounding TE cells (Fig. 2A, B). Moreover, the transgenic pluripotency marker Oct4-GFP was expressed throughout the spherical outgrowths (Fig. 2B), whereas it was expressed by only a few cells of control outgrowths (Fig. 2A). Electron microscopy of sections revealed the presence of a far larger population of ESC-like cells inside the spherical outgrowths from Cdx2-deficient embryos (Fig. 2D) compared with control embryos (Fig. 2C). These results suggest that feeder cells, in conjunction with appropriate ESC culture conditions, were capable of compensating for the lack of functional TE cells in Cdx2-deficient embryos in maintaining the viability of pluripotent cells. Also, the increased efficiency of ESC derivation may be due to the presence of more GFP-positive, ESC-like cells in the spherical outgrowths.
Table 2.
Stem Cell Derivation Efficiency from Cdx2-Deficient Embryos at Different Stages
| Embryonic stage | Group | ESC lines derived/total number (%) |
|---|---|---|
| Blastocyst | siControl | 9/47 (19.1) |
| Blastocyst | siCdx2 | 1/49 (2.0) |
| 8-Cell | siControl | 13/58 (22.4) |
| 8-Cell | siCdx2 | 20/59 (33.9) |
Derivation rate of ESCs from Cdx2-deficient embryos was significantly higher at the 8-cell stage compared with the blastocyst stage. No significant difference in derivation rate was observed in siControl embryos.
ESC, embryonic stem cell.
FIG. 2.
Embryonic stem cell (ESC) derivation from Cdx2-deficient embryos and test for pluripotency. (A, B) DIC and fluorescent images of typical outgrowths, 4 days after 8-cell stage embryos had been seeded onto a mouse embryonic fibroblast feeder layer. Cdx2-deficient embryo (B) formed an Oct4-GFP–positive cyst-like structure without the normally forming trophoblast outgrowth in Control (A). Scale bar, 50 μm. (C, D) Ultrastructure of outgrowths on mouse embryonic fibroblasts from 8-cell stage embryos revealed that Cdx2 depletion resulted in an increased number of ESC-like cells (relatively small amount of cytoplasm) inside the outgrowth (D) relative to normal outgrowth (C). Scale bars, 6.5 μm. (E) DIC and fluorescent images showed GFP-positive ESC colonies derived from Cdx2-deficient embryos. Scale bar, 100 μm. (F, H) Chimeras: ESC contribution to somatic tissues of different embryonic germ layers (X-Gal stain-positive in F), germ cells (Oct4-GFP positive in G) in an E13.5 chimera fetus and ESC contribution to columnar intestinal epithelium (X-Gal stain-positive) in an adult chimeric mouse (H). (I–N) Teratoma: cell lines derived from Cdx2-deficient embryos are pluripotent, as evidenced by their ability to form a teratoma (I) with derivatives of all three germ layers, including muscle (mesodermal) (J), gland-like tissue (endodermal) (K), keratinized stratified squamous epithelial cells (ectodermal) (L), osteoid islands showing bony differentiation (mesodermal) (M), and ciliated columnar epithelial cells (endodermal) (N). Color images available online at www.liebertonline.com/scd.
ESCs derived from the Cdx2-deficient 8-cell stage embryos were pluripotent—they contributed to germ and somatic cell lineages
The next step was to assess the ability of ESCs derived from Cdx2-deficient 8-cell stage embryos to contribute to germ and somatic cell lineages—and hence their pluripotency—using germline-specific Oct4-GFP [23] and ubiquitously expressed LacZ [24], respectively. Results showed that Cdx2-deficient 8-cell stage embryo−derived ESCs injected into blastocysts were capable of contributing to the development of both somatic (Fig. 2F) and germ cell lineages (Fig. 2G) as well as to that of mature adult columnar intestinal epithelium in the chimera mouse (Fig. 2H), which requires Cdx2 expression [25]. Teratoma experiments with nude mice further confirmed that these ESCs were truly pluripotent, as they differentiated into cell types of all three germ layers (Fig. 2I–N).
ESCs could also be derived from embryos with siCdx2 microinjection at MII-oocyte stage
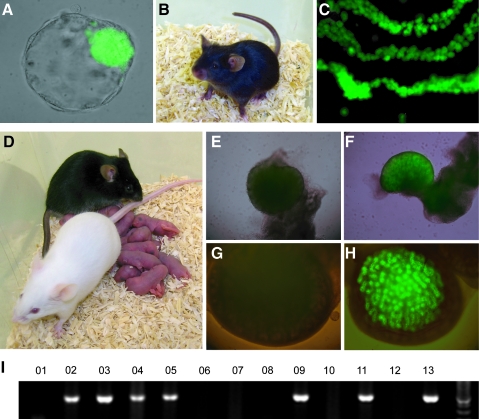
Since oocytes are not considered to represent living organisms, alteration of MII oocytes is not fraught with ethical concerns associated with manipulation of embryos [26]. In addition, therapeutic cloning requires the use of MII oocytes. For these reasons, we pushed our microinjection procedure 1 step further and tested whether treatment of MII oocytes with siCdx2 can eliminate Cdx2 expression as efficiently as that at the zygote stage and can give rise to pluripotent ESCs. Our results clearly demonstrate that Cdx2 mRNA was efficiently reduced in blastocysts obtained from treated MII oocytes (Fig. 1B). When Cdx2-deficient 8-cell stage embryos were cultured on mouse embryonic fibroblast (MEF) cells, spherical structures expressing GFP were also observed. Eleven independent ESC lines were established from such embryos. Two male lines were assessed by the tetraploid embryo/diploid ESC aggregation procedure, the most stringent test for pluripotency. After 24 h of culture, the majority of aggregates had formed blastocysts (Fig. 3A). Eleven to 14 aggregates were transferred into a uterine horn of each 2.5-dpc pseudopregnant recipient. From a total of 145 transferred ESC (male)/tetraploid aggregates, 12 living pups were recovered after cesarean section at E19. All pups were male, corresponding to the sex of the original ESC lines, and genotyped positive for the Oct4-GFP transgene by PCR. Three of the 12 pups failed to breathe after birth and died. One was sacrificed due to a midline closure defect. When testicles from these dead pups were examined under ultraviolet light, all their spermatogonia cells were positive for GFP (Fig. 3C), confirming that the origin of the pups was indeed the ESCs. Three pups were rejected and killed by their foster mothers overnight. The remaining 5 pups all survived to adulthood (Fig. 3B). These male mice were mated with wild-type CD1 female mice, and 29 of the 61 offspring delivered (Fig. 3D) were positive for Oct4-GFP by genotyping (Fig. 3I) and gonad GFP examination (Fig. 3F, H). This ratio was expected according to Mendel's segregation law, as the ESC lines were heterozygous for the Oct4-GFP transgene. These results further strengthened our conclusions that the siRNA-mediated Cdx2 knockdown effect is transient and produces fully functional stem cell lines without the mice incurring any permanent epigenetic or genetic modifications.
FIG. 3.
The tetraploid-derived mice were obtained from ESCs of Cdx2-deficient–embryo origin and they were fully fertile. (A–C) Blastocyst (A) and offspring (B) generated after siCdx2-ESC/tetraploid embryo complementation. (C) Expression of the Oct4-GFP transgene in testis of a full-term pup that died after delivery confirmed its ESC origin. (D) A CD1 female mouse gave birth to pups of normal litter size after mating with a male mouse generated from siCdx2-ESC/tetraploid embryo complementation. (E–H) Expression of the Oct4-GFP transgene in ovary (F) and testis (H) of progeny confirmed that the offspring from tetraploid aggregation was fully fertile and had originated from the ESCs, in comparison to GFP-negative samples (E, G). (I) Oct4-GFP transgene in 7 out of 13 F1 offspring in this test was confirmed by polymerase chain reaction using GFP primers, consistent with GFP expression in the gonads. Color images available online at www.liebertonline.com/scd.
Discussion
Previous studies have demonstrated that Cdx2 plays a pivotal role in TE formation and that Cdx2-deficient embryos fail to hatch and implant [5]. In this study, we examined the impact of Cdx2 deficiency on the pluripotent founder population by ESC derivation experiments and obtained very interesting results. The presence of maternal Cdx2 in mouse oocytes was in doubt, as the transcript could not be detected by mRNA-Seq whole-transcriptome analysis, which incidentally does not detect genes expressed at low expression levels [27]. However, we detected low levels of Cdx2 in MII oocytes by qRT-PCR, consistent with the expression of maternal CDX2 in primate oocytes [28]. Based on our experiments, we provided direct evidence for the first time that both maternal and zygotic Cdx2 are not required for the formation of the TE. However, our results confirmed that Cdx2 is required for proper function of the TE. Consequently, it is involved in establishing a “niche” [29] to maintain the viability and potency of the pluripotent founder population. Studies with Drosophila germ stem cells [30] and mouse hematopoietic stem cells [31] revealed that direct physical interactions between stem cells and their nonstem cell neighbors within the niche are critical for maintaining the characteristics of stem cells, with properly tailored extracellular matrix and cell-cell adhesive molecules—for example, cadherins, β-catenin, and integrins. Indeed, CDX2 overexpression in the human colon adenocarcinoma cell line Caco2-TC7 leads to upregulation of molecules involved in cell–cell and cell–substratum interactions and in the transduction process, such as E-cadherin, integrin-beta4 subunit, laminin-gamma2 chain, hemidesmosomal protein, adenomatosis polyposis coli (APC), and alpha-actinin [32] and Cdx2 is essential for intestinal development [33]. We clearly demonstrated the importance of a niche created by a functional TE for the ICM by the dramatic detrimental effect of Cdx2 knockdown on the efficiency of ESC derivation at 4.0 dpc (2% vs. 19.1% for controls). The failure to derive ESCs from Cdx2-deficient blastocysts was not due to compromised viability as a result of manipulation, as control embryos were manipulated in the same way, except for a scrambled siRNA that was used. Moreover, embryos in both groups exhibited the same rate of blastocyst formation, the same total cell number, and only a small increase in the number of apoptotic cells (Table 1 and Fig. 2E, F). The role of a functional trophoblast in supporting the ICM has also been demonstrated by Fgfr2 mutation experiments. Fgfr2 protein was first detected in peripheral blastomeres of compacted morulae and specifically localized to the TE of the early blastocyst [34]. Although Fgf4/Fgfr2 signaling plays a key role only in the growth and survival of trophoblast stem cells [35], Fgfr2 mutants were arrested in development around the time of implantation with collapsed yolk cavity in vivo and lack of ICM growth in vitro [36], indicating that ICM survival depends on a fully functional trophoblast.
To our surprise, when Cdx2-deficient embryos were cultured on MEFs under ESC culture conditions at the 8-cell stage, before the formation of TE, siCdx2 produced the opposite effect on ESC derivation—that is, a positive effect. This result suggested that MEFs and ESC culture conditions can effectively replace part of the communication normally provided by the trophectodermal “niche” and can, therefore, support pluripotent stem cell derivation without repressing cellular proliferation. The distinct outgrowth phenotype of Cdx2-deficient embryos with increased ESC numbers (“pluripotent cell ball”) provided a clue for the improved efficiency. Our results implied that, as observed in the best studied drosophila systems [30], the niche actually acts as a 2-edged sword in mouse preimplantation embryos: On the one hand, it supports the founder population by maintaining its pluripotency/viability; on the other hand, it inhibits its proliferation. It is possible that the improvement in ESC derivation is also related to the increased number of cells allocated to the ICM, as loss of Cdx2 had an effect on the balance of ICM/TE cell allocation in favor of the ICM, as reported [6]. However, the exact molecular mechanism underlying Cdx2-mediated regulation of the niche is unknown at this time. Further study on embryos may help us gain insight into the specific interplay of interactions within the niche during development of mouse preimplantation embryos.
Since ICM/ESCs and cancer cells share similar features, we would like to point out that CDX2 was overexpressed by human colorectal adenocarcinomas [37] and that CDX2 has tumorigenic potential in the human colon cancer cell lines LOVO and SW48 [38], whereas loss of the tumor suppressor activity of Cdx2 is an important feature in other tumors [39]. Investigation into the role of Cdx2 regulation of the niche in early embryo differentiation and cancer development may yield very fruitful results for both areas of research.
In general, the knockdown effect of siRNA in mammal cells is transient, lasting for up to 6.5 days in mouse embryos [40]. However, studies revealed that in many eukaryotes, including the mouse, siRNA/RNAi can induce epigenetic changes in gene expression via modifications of chromatin heterochromatin [41]. In the current experiments, despite the fact that the TE was severely affected by siCdx2 treatment, ESCs derived from Cdx2-deficient embryos were not only capable of contributing to the development of the germ cell lineage and all 3 germ layers in chimeras but also had the potential to sustain full organismal development in the tetraploid complementation assay. Moreover, ESCs derived by this procedure contributed to the development of columnar intestinal epithelium in adult chimeric mice, which would not have been possible in the absence of Cdx2 [33]. These findings clearly and unequivocally demonstrate that injection of siRNA duplexes targeting critical regulation factors into the zygotes did not lead to any heritable epigenetic changes during mouse preimplantation development.
Therefore, we would like to postulate that, by using the procedure described in this article, derivation of patient-specific ESC lines for therapeutic purposes using siRNA could be accomplished in a straightforward and safe manner. Specifically, this procedure allows for the efficient derivation of pluripotent stem cell lines from Cdx2-deficient 8-cell stage embryos, which even at this early stage have no developmental prospects. More specifically, these findings support the feasibility of Altered Nuclear Transfer [42,43], a variation of SCNT, an approach wherein alterations of the adult cell nucleus and/or the egg cytoplasm preclude the formation of a totipotent embryonic organism, yet allow for the production of ESCs. Combined with alternative sources of human oocytes recently discovered, such as the laboratory maturation of immature eggs unused after clinical in vitro fertilization (IVF), this approach may open up new possibilities in countries, such as the United States and Germany, where objections to embryo-destructive research are encoded as laws or as federally mandated constraints on the funding of ESC research.
Acknowledgments
We would like to thank William B. Hurlbut and Markus Grompe for valuable discussions and Jeanine Mueller-Keuker, Margit Preusser, and Susanne Koelsch for assistance with preparation of the manuscript. This research was supported by the Max Planck Society and grant R01HD059946 from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute Of Child Health & Human Development or the National Institutes of Health. This work was also supported in part by the DFG grant “Germ Cell Potential” ovDFG FOR 1041 SCHO 340/7-1.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Evans MJ. Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagy A. Gocza E. Diaz EM. Prideaux VR. Ivanyi E. Markkula M. Rossant J. Embryonic stem cells alone are able to support fetal development in the mouse. Development. 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- 4.Niwa H. Toyooka Y. Shimosato D. Strumpf D. Takahashi K. Yagi R. Rossant J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 5.Strumpf D. Mao CA. Yamanaka Y. Ralston A. Chawengsaksophak K. Beck F. Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- 6.Jedrusik A. Parfitt DE. Guo G. Skamagki M. Grabarek JB. Johnson MH. Robson P. Zernicka-Goetz M. Role of Cdx2 and cell polarity in cell allocation and specification of trophectoderm and inner cell mass in the mouse embryo. Genes Dev. 2008;22:2692–2706. doi: 10.1101/gad.486108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossant J. Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 2009;136:701–713. doi: 10.1242/dev.017178. [DOI] [PubMed] [Google Scholar]
- 8.Ralston A. Rossant J. Cdx2 acts downstream of cell polarization to cell-autonomously promote trophectoderm fate in the early mouse embryo. Dev Biol. 2008;313:614–629. doi: 10.1016/j.ydbio.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 9.Nishioka N. Yamamoto S. Kiyonari H. Sato H. Sawada A. Ota M. Nakao K. Sasaki H. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech Dev. 2008;125:270–283. doi: 10.1016/j.mod.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Yagi R. Kohn MJ. Karavanova I. Kaneko KJ. Vullhorst D. DePamphilis ML. Buonanno A. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development. 2007;134:3827–3836. doi: 10.1242/dev.010223. [DOI] [PubMed] [Google Scholar]
- 11.Nishioka N. Inoue K. Adachi K. Kiyonari H. Ota M. Ralston A. Yabuta N. Hirahara S. Stephenson RO. Ogonuki N. Makita R. Kurihara H. Morin-Kensicki EM. Nojima H. Rossant J. Nakao K. Niwa H. Sasaki H. The hippo signaling pathway components lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Chawengsaksophak K. de Graaff W. Rossant J. Deschamps J. Beck F. Cdx2 is essential for axial elongation in mouse development. Proc Natl Acad Sci U S A. 2004;101:7641–7645. doi: 10.1073/pnas.0401654101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamai Y. Nakajima R. Ishikawa T. Takaku K. Seldin MF. Taketo MM. Colonic hamartoma development by anomalous duplication in Cdx2 knockout mice. Cancer Res. 1999;59:2965–2970. [PubMed] [Google Scholar]
- 14.Summers MC. McGinnis LK. Lawitts JA. Raffin M. Biggers JD. IVF of mouse ova in a simplex optimized medium supplemented with amino acids. Hum Reprod. 2000;15:1791–1801. doi: 10.1093/humrep/15.8.1791. [DOI] [PubMed] [Google Scholar]
- 15.Boiani M. Gentile L. Gambles VV. Cavaleri F. Redi CA. Scholer HR. Variable reprogramming of the pluripotent stem cell marker Oct4 in mouse clones: distinct developmental potentials in different culture environments. Stem Cells. 2005;23:1089–1104. doi: 10.1634/stemcells.2004-0352. [DOI] [PubMed] [Google Scholar]
- 16.Cavaleri FM. Balbach ST. Gentile L. Jauch A. Bohm-Steuer B. Han YM. Scholer HR. Boiani M. Subsets of cloned mouse embryos and their non-random relationship to development and nuclear reprogramming. Mech Dev. 2008;125:153–166. doi: 10.1016/j.mod.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Palmieri SL. Peter W. Hess H. Scholer HR. Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol. 1994;166:259–267. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- 18.Nagy A. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2003. Manipulating the Mouse Embryo: A Laboratory Manual. [Google Scholar]
- 19.Bryja V. Bonilla S. Arenas E. Derivation of mouse embryonic stem cells. Nat Protoc. 2006;1:2082–2087. doi: 10.1038/nprot.2006.355. [DOI] [PubMed] [Google Scholar]
- 20.Nagy A. Rossant J. Nagy R. Abramow-Newerly W. Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogan B. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1994. Manipulating the Mouse Embryo: A Laboratory Manual. [Google Scholar]
- 22.Kim JB. Zaehres H. Wu G. Gentile L. Ko K. Sebastiano V. Arauzo-Bravo MJ. Ruau D. Han DW. Zenke M. Scholer HR. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimizu T. Sugiyama N. De Felice M. Yeom YI. Ohbo K. Masuko K. Obinata M. Abe K. Scholer HR. Matsui Y. Germline-specific expression of the Oct-4/green fluorescent protein (GFP) transgene in mice. Dev Growth Differ. 1999;41:675–684. doi: 10.1046/j.1440-169x.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 24.Zambrowicz BP. Imamoto A. Fiering S. Herzenberg LA. Kerr WG. Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci U S A. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chawengsaksophak K. James R. Hammond VE. Kontgen F. Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84–87. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- 26.Revazova ES. Turovets NA. Kochetkova OD. Kindarova LB. Kuzmichev LN. Janus JD. Pryzhkova MV. Patient-specific stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2007;9:432–449. doi: 10.1089/clo.2007.0033. [DOI] [PubMed] [Google Scholar]
- 27.Tang F. Barbacioru C. Wang Y. Nordman E. Lee C. Xu N. Wang X. Bodeau J. Tuch BB. Siddiqui A. Lao K. Surani MA. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- 28.Sritanaudomchai H. Sparman M. Tachibana M. Clepper L. Woodward J. Gokhale S. Wolf D. Hennebold J. Hurlbut W. Grompe M. Mitalipov S. CDX2 in the formation of the trophectoderm lineage in primate embryos. Dev Biol. 2009;335:179–187. doi: 10.1016/j.ydbio.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watt FM. Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 30.Song X. Zhu CH. Doan C. Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J. Niu C. Ye L. Huang H. He X. Tong WG. Ross J. Haug J. Johnson T. Feng JQ. Harris S. Wiedemann LM. Mishina Y. Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 32.Lorentz O. Duluc I. Arcangelis AD. Simon-Assmann P. Kedinger M. Freund JN. Key role of the Cdx2 homeobox gene in extracellular matrix-mediated intestinal cell differentiation. J Cell Biol. 1997;139:1553–1565. doi: 10.1083/jcb.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck F. Chawengsaksophak K. Luckett J. Giblett S. Tucci J. Brown J. Poulsom R. Jeffery R. Wright NA. A study of regional gut endoderm potency by analysis of Cdx2 null mutant chimaeric mice. Dev Biol. 2003;255:399–406. doi: 10.1016/s0012-1606(02)00096-9. [DOI] [PubMed] [Google Scholar]
- 34.Haffner-Krausz R. Gorivodsky M. Chen Y. Lonai P. Expression of Fgfr2 in the early mouse embryo indicates its involvement in preimplantation development. Mech Dev. 1999;85:167–172. doi: 10.1016/s0925-4773(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 35.Nichols J. Zevnik B. Anastassiadis K. Niwa H. Klewe-Nebenius D. Chambers I. Scholer H. Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 36.Arman E. Haffner-Krausz R. Chen Y. Heath JK. Lonai P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci U S A. 1998;95:5082–5087. doi: 10.1073/pnas.95.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witek ME. Nielsen K. Walters R. Hyslop T. Palazzo J. Schulz S. Waldman SA. The putative tumor suppressor Cdx2 is overexpressed by human colorectal adenocarcinomas. Clin Cancer Res. 2005;11:8549–8556. doi: 10.1158/1078-0432.CCR-05-1624. [DOI] [PubMed] [Google Scholar]
- 38.Dang LH. Chen F. Ying C. Chun SY. Knock SA. Appelman HD. Dang DT. CDX2 has tumorigenic potential in the human colon cancer cell lines LOVO and SW48. Oncogene. 2006;25:2264–2272. doi: 10.1038/sj.onc.1209247. [DOI] [PubMed] [Google Scholar]
- 39.Guo RJ. Suh ER. Lynch JP. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther. 2004;3:593–601. doi: 10.4161/cbt.3.7.913. [DOI] [PubMed] [Google Scholar]
- 40.Wianny F. Zernicka-Goetz M. Specific interference with gene function by double-stranded RNA in early mouse development. Nat Cell Biol. 2000;2:70–75. doi: 10.1038/35000016. [DOI] [PubMed] [Google Scholar]
- 41.Djupedal I. Ekwall K. Epigenetics: heterochromatin meets RNAi. Cell Res. 2009;19:282–295. doi: 10.1038/cr.2009.13. [DOI] [PubMed] [Google Scholar]
- 42.Hurlbut WB. Altered nuclear transfer. N Engl J Med. 2005;352:1153–1154. doi: 10.1056/NEJM200503173521121. author reply 1153–1154. [DOI] [PubMed] [Google Scholar]
- 43.Meissner A. Jaenisch R. Generation of nuclear transfer-derived pluripotent ES cells from cloned Cdx2-deficient blastocysts. Nature. 2006;439:212–215. doi: 10.1038/nature04257. [DOI] [PubMed] [Google Scholar]