Abstract
Adipose-derived stem cells (ASCs) possess significant therapeutic potential for tissue engineering and regeneration. This study investigates the endothelial differentiation and functional capacity of ASCs isolated from elderly patients. Isolation of ASCs from 53 patients (50–89 years) revealed that advanced age or comorbidity did not negatively impact stem cell harvest; rather, higher numbers were observed in older donors (>70 years) than in younger. ASCs cultured in endothelial growth medium-2 for up to 3 weeks formed cords upon Matrigel and demonstrated acetylated-low-density lipoprotein and lectin uptake. Further stimulation with vascular endothelial growth factor and shear stress upregulated endothelial cell-specific markers (CD31, von Willebrand factor, endothelial nitric oxide synthase, and VE-cadherin). Inhibition of the PI3K but not mitogen-activated protein kinase pathway blocked the observed endothelial differentiation. Shear stress promoted an anti-thrombogenic phenotype as demonstrated by production of tissue-plasminogen activator and nitric oxide, and inhibition of plasminogen activator inhibitor-1. Shear stress augmented integrin α5β1 expression and subsequently increased attachment of differentiated ASCs to basement membrane components. Finally, ASCs seeded onto a decellularized vein graft resisted detachment despite application of shear force up to 9 dynes. These results suggest that (1) advanced age and comorbidity do not negatively impact isolation of ASCs, and (2) these stem cells retain significant capacity to acquire key endothelial cell traits throughout life. As such, adipose tissue is a practical source of autologous stem cells for vascular tissue engineering.
Introduction
Use of adult stem cells for vascular tissue engineering and regeneration continues to gain momentum as research reveals their improved potency and function. The majority of work involves mesenchymal stem cells (MSCs) derived from bone marrow aspiration and endothelial progenitor cells (EPCs) obtained from blood. Each of these cell types have been used to line vascular scaffolds in the creation of a tissue engineered bypass graft [1–3], as well as in various strategies to promote therapeutic angiogenesis in the coronary and peripheral circulations [4–7]. Although these cells are appropriate for vascular tissue engineering, their availability in patients most likely to benefit from this technology raises practical concerns. The number of stem and progenitor cells derived from bone marrow and blood decrease significantly with age and patient comorbidity [8–10]. Further, it has also been suggested that differentiation potential of bone-marrow-derived MSCs decreases with age [11]. Recent data also indicate that EPC function is diminished in patients with severe vascular disease and multiple coronary risk factors [12,13].
An alternative source for autologous adult stem cells is adipose tissue. Adipose-derived stem cells (ASCs) are multipotent, with the capacity to differentiate into adipocytes, chondrocytes, osteocytes, hepatocytes, and neurons, among other cell types [14–16]. Our group has examined the differentiation of ASCs into vascular cells, including both endothelial cells (ECs) and smooth muscle cells, for the purposes of vascular tissue engineering [17,18]. It is not clear in the literature if these stem cells, which closely resemble MSCs derived from bone marrow, share the limitations noted above for stem cells isolated from bone marrow or blood. ASCs are routinely derived from liposuction specimens, and given the majority of patients undergoing liposuction are relatively younger and healthier compared to those with coronary and peripheral arterial disease, it is possible that the usefulness of ASCs in the latter population may also be limited.
This study evaluates the availability and subsequent endothelial differentiation and function of ASCs isolated from elderly patients with vascular disease. Herein, we isolated ASCs from 53 patients aged 50–87 years undergoing elective vascular surgical procedures. Using both growth factors and shear stress, we describe the acquisition of various specific EC markers and phenotypes by these stem cells over a 3-week period. The role of the PI3 and mitogen-activated protein kinase (MAPK) signaling pathways in stem cell differentiation is also evaluated. Lastly, we evaluate the attachment of the cells on fibronectin in vitro and retention of ASCs onto a decellularized vein exposed to shear force ex vivo. Taken together, these studies demonstrate the usefulness of ASCs isolated from patients most likely to benefit from their use in cardiovascular tissue engineering.
Materials and Methods
Study subjects
Patients scheduled for elective vascular surgery were asked to donate up to 15 g of fat under a protocol approved by the Institutional Review Board of Thomas Jefferson University. After obtaining informed consent, patient demographics were collected, including age, gender, race, height, weight, operation to be performed, known history of diabetes, cardiovascular disease (defined as history of myocardial infarction, angina, or coronary artery revascularization), and smoking history. A total of 53 patients with an average age 66 ± 1 years (range 50–89 years) were enrolled for study.
Isolation and culture of ASCs
Adipose tissue was obtained by liposuction of the periumbilical subcutaneous tissues at the beginning of the vascular procedure. Through a stab incision in the lower umbilicus, 60 mL of tumescence solution (30 mL 1% lidocaine, 30 mL 0.5% bupivicaine, 10 mL 4.2% sodium bicarbonate, and 1 mg epinephrine in 1 L normal saline) was infiltrated. Using a Mercedes cannula, up to 60 mL of lipoaspirate was obtained, put on ice, and transported to the laboratory. The lipoaspirate was washed with phosphate-buffered saline (PBS), filtered through a 250 μm sieve, and incubated for 1 h at 37°C with collagenase I solution [1 mg/mL+4 mg/mL bovine serum albumin (BSA); Worthington], as previously described.17 The slurry was centrifuged for 10 min at 1,500 g followed by a wash with 0.1% BSA (Sigma). The resultant pellet, named the stromal vascular fraction, was suspended in the expansion medium containing M199 (Mediatech), HEPES buffer, heparin sulfate (7.5 U/mL), and 13% fetal bovine serum (Gemini Bio Products), and plated onto a gelatin-coated T-75 flask (Fisher Scientific) at 37°C with 5% CO2 atmosphere. Nonadherent cells were removed after 24 h and the culture medium was subsequently replaced twice weekly.
After reaching near-confluency (average 7–10 days), the primary isolate underwent negative selection to remove coisolated CD31 (micro-ECs) and CD45 (leukocytes)-positive cells using magnetic-activated cell sorting (Miltenyi Biotec). The cells were sequentially mixed with MicroBeads attached to CD31 and CD45 antibodies for 15 min at 4°C. After washing with buffer (PBS with 2 mM EDTA and 0.5% BSA), the CD31- and CD45-positive cells were removed by an LS separation column. The flow-through cells (those negative for CD31 and CD45), defining the ASC population, were counted using a Coulter counter (Beckman; Coulter), yielding the number of ASCs isolated per gram of fat initially harvested. The ASCs were subsequently cultured in T75 flasks with the expansion medium, fed 3 times/week, and split 1:3 when 80% confluent. The cells were submitted for experimentation at passages 3–5.
Flow cytometry
Cell surface antigens were characterized by flow cytometry. The cells were detached with trypsin (0.25% with 0.1% EDTA in HBSS) and suspended in cold staining buffer. Approximately 5 × 105 cells were incubated with fluorescence-conjugated antibodies for 30 min. The antibodies used were CD13 (APC-conjugated), CD29 (PE-Cy5-conjugated), CD31 (PE-conjugated), CD44 (PE-Cy5-conjugated), CD45 (FITC-conjugated), CD73 (FITC-conjugated), CD90 (FITC-conjugated), CD105 (PE-conjugated), and von Willebrand factor (vWF). All the antibodies were obtained from BD Biosciences. Isotype antibodies served as controls to exclude nonspecific binding. Quantitative analysis was performed using FACScalibur flow cytometer (Beckman) and FlowJo software.
Multipotency of ASCs
To demonstrate that the cultured cells are multipotent, differentiation into both adipocytes and osteocytes was performed. For adipogenic differentiation, ASCs were cultured for 3 weeks in NH AdipoDiff Medium (Miltenyi Biotec) per manufacturer's instructions. Differentiated cells were then incubated with cooled methanol for 5 min and stained with fresh Oil Red O solution for 20 min. Light microscopy was used to identify lipid droplets within the cultures. For osteogenic differentiation, ASCs were cultured for 3 weeks in NH OsteoDiff Medium (Miltenyi Biotec) per manufacturer's instructions. Differentiated cells were then stained for alkaline phosphatase activity with NBT substrate (Sigma). Light microscopy was used to identify a typical ruptured morphology within the cultures.
Endothelial differentiation of ASCs
To stimulate endothelial differentiation, ASCs were cultured for up to 3 weeks in endothelial growth medium-2 (EGM-2; Lonza) with SingleQuots containing vascular endothelial growth factor (VEGF), human basic fibroblast growth factor (hFGF-b), epidermal growth factor, insulin-like growth factor-1, heparin, ascorbic acid, and 5% fetal bovine serum. The medium was exchanged 3 times/week and cultures were split 1:3 when near confluent. In separate experiments, an additional 50 ng/mL VEGF was added to the EGM-2 to evaluate the effect of VEGF supplementation on endothelial differentiation.
To evaluate the roles of the PI3 and MAPK pathways on endothelial differentiation, the PI3K inhibitor LY294002 (Echelon Bioscience) and MAPK inhibitor PD98059 (LC Laboratories) were added to the EGM-2, respectively, for a final concentration 15 μM each. These have been previously shown to specifically inhibit the respective pathways [19,20].
Introduction of shear stress in vitro
Shear stress experiments were performed using an orbital shaker as described by Dardik et al. [21]. ASCs cultured in EGM-2 for 2 weeks were seeded onto 6-well tissue culture plates (Fisher Scientific) at 5 × 104 cells/cm2, seated on an orbital shaker (Bellco Biotechnology) located in an incubator, and maintained at 37°C and 5% CO2. The shaker plate was rotated for 48 h at 210 cycles/min to produce 12 dyn at the periphery of the wells.
Determination of acetylated-low-density lipoprotein uptake
ASCs cultured in EGM-2 for 2 weeks were incubated with DiI-labeled acetylated-low density lipoprotein (ac-LDL; 10 μg/mL; Sigma) at 37°C for 2 h, fixed, and labeled with FITC-conjugated human lectin (10 mg/mL; Sigma) for 1 h. Cultures were viewed with an immunofluorescence microscope to determine uptake of ac-LDL, human lectin, or both.
Matrigel tube formation assay
ASCs cultured for 2 weeks in EGM-2 were plated on top of Matrigel substrate (at a density of 2 × 105/100 μL Matrigel; BD Biosciences) and incubated at 37°C in a 5% CO2 for up to 12 h. Formation of cord-like structures was observed by phase-contrast microscopy.
Evaluation of endothelial molecular markers
Expression of EC-specific molecular markers was determined by reverse transcription (RT)-polymerase chain reaction (PCR) and real-time PCR. Total RNA was extracted through RNeasy mini columns (Qiagen). To detect the specific genes expressed, RT-PCR was performed using the following primers: endothelial nitric oxide synthase (eNOS): 5-primer (5′-TCCCCCAGAACTCTTCCTT-3′) and 3′-primer (5′-CTCATTCTCCAGGTGCTTCA-3′); vWF: 5-primer (5′-TCTTCCAGGACTGCAACAAG-3′) and 3′-primer (5′-TCCGAGATGTCCTCCACATA-3′); CD31: 5-primer (5′-CACAGCAATTCCTCAGGCTA-3′) and 3′-primer (5′-TTCAGCCTTCAGCATGGTAG-3′); VE-cadherin (CD144): 5-primer (5′-ACCCCCACAGGAAAAGAATC-3′) and 3′-primer (5′-GACTTGGCATCCCATTGTCT-3′). Messenger RNA levels were quantified using TaqMan real-time PCR with the 7500 Fast real-time PCR system (Applied Biosystems). PCR primers targeting human eNOS, vWF, CD31, VE-cadherin, and tissue-plasminogen activator (tPA) and TaqMan probes were obtained from Applied Biosystems. PCR conditions were 48°C for 30 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The housekeeping gene GAPDH was amplified in separate tubes to normalize for variance in input RNA. The level of target mRNA in samples was estimated by the relative standard method with a series of dilutions of RNA from human vascular cells.
Western blot analysis
Whole cell lysates (50 μg) of cells were resolved on 4%–12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis acrylamide gel (Invitrogen). Proteins were transferred on polyvinylidene fluoride (PVDF) membranes and imaged with the infrared imaging Odyssey system (LI-COR Biosciences). Monoclonal anti-CD31 and anti-actin were obtained from Santa Cruz Biotechnology.
Determination of plasminogen activator inhibitor-1 expression
Concentration of plasminogen activator inhibitor-1 (PAI-1) proteins in the cell culture supernatant was quantified using a quantitative sandwich ELISA kit (R&D Systems) per the manufacturer's instructions. Samples were evaluated on the ELISA plate reader at 450 nm with corrections at 570 nm. A highly linear calibration curve (r2 > 0.99) was obtained.
Measurement of nitric oxide production
Nitric oxide (NO) production by differentiated ASCs was measured within the supernatant of cultured cells before and after stimulation with shear stress for 48 h. The metabolic product NO and its oxidative metabolic products, NO2 and NO3 (collectively known as NOx), were determined using a chemiluminescent NO detector (SIEVER 280i NO Analyzer) as described by Gao et al. [22].
Attachment studies
To determine stem cell attachment to vascular basement membrane components in vitro, ASCs (50,000 cells/well) were plated onto 96-well culture plates coated with 18 μg/mL fibronectin (BD Biosciences). At various time points over a 24 h period, the unattached cells were removed and counted by gently washing with PBS; the remaining adherent cells were stained with crystal violet for 5 min. After eluting the absorbed crystal violet with 0.1 M sodium citrate, absorbance within the eluate was measured on the plate reader at 570 nm. The number of cells attached was then calculated by comparison with a standard curve generated using known numbers of cells.
To determine stem cell attachment to vascular basement membrane ex vivo, intact human saphenous vein specimens procured within 24 h of autopsy (National Disease Research Interchange) were divided into segments 4–5 cm in length. The segments were decellularized by incubation in 0.075% sodium dodecyl sulfate for 15 h, leaving the vascular basement membrane and extracellular matrix intact, as previously described by us.23 Decellularized vein segments were opened longitudinally and pinned lumen side up within a seeding chamber. Cells were seeded onto the surface of the vascular basement membrane at 4 × 105 cells/cm2 and incubated at 37°C for up to 24 h. Cell attachment was quantified indirectly by counting the number of residual cells in the seeding medium after gentle washing with PBS.
The effect of shear force on cell retention after seeding onto a decellularized vein was determined using a bioreactor and flow conditioning system (Lab VIEW; National Instruments) as described by McIlhenny et al. [23]. Briefly, the luminal surface of a decellularized human saphenous vein (5 cm in length) was precoated with fibronectin (18 μg/mL; BD Bioscience) for 1 h at 37°C with rotation. ASCs were placed into the lumen of vessel at a concentration of 2 × 105/cells cm2 luminal surface area and incubated for 2 h with rotation. After 24 h of fluid exchange through the graft (<0.1 dyne), flow within the graft was increased linearly over 5 days whereupon a total of 9 dynes was reached. The graft was removed from the chamber, and splayed open. The morphology of the attached cells was determined after staining the live, seeded cells with CellTracker Green (Molecular Probes) for 20 min and viewing the specimens en face using a laser confocal microscope (Olympus Fluoview).
Integrin studies
Integrin expression was determined using the Alpha/Beta Integrin-Mediated Cell Adhesion Array Combo Kit (Chemicon International) according to manufacturer's instructions. Integrin blockade was accomplished by pretreating differentiated ASCs with anti-α5β1 human monoclonal antibodies (Chemicon International) for 1 h before seeding the cells onto fibronectin-coated plates, as above.
Statistical analyses
Data are expressed as mean ± standard error. A two-tailed Student's t-test was used for 2 group comparisons. The statistical significance regarding multigroup comparisons was determined using one-way analysis of variance. A value of P < 0.05 was considered statistically significant.
Results
Isolation of ASCs from elderly patients
To determine the influence of advancing age on stem cell isolation, ASCs were isolated from a total of 53 patients with an average age of 66 ± 1 years (range 50–89 years). Figure 1A describes the characteristics of the experimental population. The patients had a significant amount of atherosclerosis risk factors, including diabetes (36%) and smoking (25%), with 70% having known cardiovascular disease.
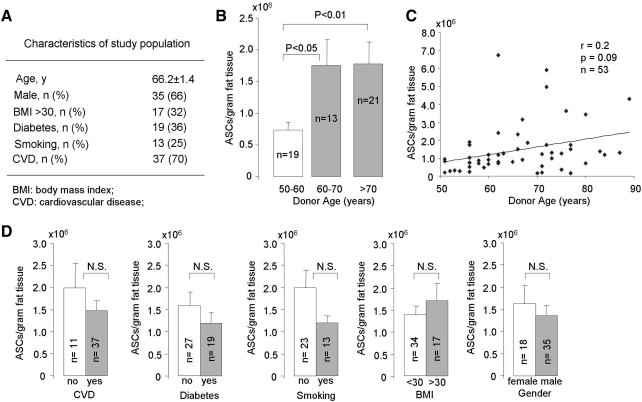
FIG. 1.
Isolation of ASCs from elderly patients with cardiovascular disease. (A) Overall patient characteristics. (B) Number of ASCs isolated per gram of adipose tissue compared with patient age subdivided into decades. (C) Overall correlation between the number of ASCs isolated per gram of adipose tissue and patient age (n = 53). (D) Univariate analysis comparing isolation number and various patient characteristics. ASCs, adipose-derived stem cells; N.S., not significant.
From the population as a whole, we isolated an average of 1.5 million ± 0.5 ASCs per gram of adipose tissue initially harvested. Upon separating the data by age decade (Fig. 1B), we observed that the number of ASCs per gram was significantly higher in the patients over 60 years old compared to the 50–59-year-old group (P < 0.05), resulting in a positive correlation between ASC availability and patient age (Fig. 1C; r = 0.2, P = 0.09). Univariate analysis of the individual risk factors failed to reveal any significant correlation between ASC harvest and the presence of cardiovascular disease, diabetes, smoking, obesity (BMI > 30), or gender (Fig. 1D).
Cell surface markers and multipotency of ASC
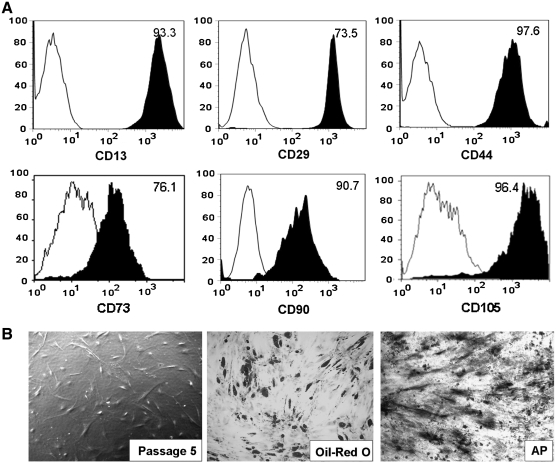
Surface marker expression of the culture-expanded ASCs was analyzed by flow cytometry. ASCs were strongly positive for CD13 (98.4%), CD29 (77.4%), CD44 (97.6%), CD73 (76.1%), CD90 (97.1%), and CD105 (96.4%), markers consistent with those found on MSCs (Fig. 2A). CD31 and CD45 were not found in culture-expanded ASCs after removed the CD31- and CD45-positive cells by magnetic-activated cell sorting (data not shown). The ability of ASCs harvested from elderly patients to differentiate into several cell lines was examined. In addition to endothelial differentiation (see studies below), ASCs were grown in either adipogenic or osteogenic medium for 3 weeks and subsequently stained with Oil Red O or for alkaline phosphatase, respectively. Figure 2B reveals that the stem cells have differentiated into either adipocytes or osteocytes, suggesting that ASCs from elderly patients retain their multipotency.
FIG. 2.
Immunophenotype characterization and multipotency of ASCs. (A) Representative FACS analysis of cultured ASCs (passage 3–5) revealing the majority of ASCs express surface antigens consistent with mesenchymal stem cell markers. Plots show the isotype control (white) versus specific antibody staining (black; n = 12; mean age: 67.4 ± 3). (B) Photomicrograph of ASCs before differentiation (left; phase contrast, 20 ×), and after 3 weeks of culture in adipogenic (middle; Oil Red O stain, 20 ×) and osteogenic (right; AP stain, 20 ×) media (n = 6; mean age: 68 ± 6 years). AP, alkaline phosphatase.
Acquisition of endothelial characteristics by ASCs
We induced endothelial differentiation by culturing ASCs in EGM-2. After 2 weeks of culture, ASCs newly expressed the messenger RNA for the endothelial-specific markers vWF, platelet/endothelial cell adhesion molecule (CD31), eNOS, and VE-cadherin (CD144). Supplementing the EGM-2 with addition VEGF (50 ng/mL) further increased expression of vWF and eNOS demonstrated by quantitative real-time PCR (Fig. 3A, left). Notably, expression of vWF approached the levels noted in endothelial controls. Flow cytometry and western blotting analysis confirmed protein expression of vWF and CD31 in ASCs after 2 weeks of culture in EGM-2 (Fig. 3A, right). Differentiated ASCs stained positive for both DiI-ac-LDL and human lectin, confirming their ability to engulf ac-LDL (Fig. 3B). They also newly formed capillary-like structures when plated onto Matrigel (Fig. 3C) similar to that of control ECs (Fig. 3D).
FIG. 3.
Acquisition of endothelial characteristics by ASCs. (A) After culture for 2 weeks in EGM-2 (“EGM2”), expression of each of these endothelial markers is identified (n = 12; mean age 69 ± 3 years). With the addition of VEGF to the medium (50 ng/mL; “EGM2/VEGF”), further upregulation is noted for vWF and eNOS by quantitative real-time PCR (n = 5; mean age 67 ± 6 years; *P < 0.05 vs. control EGM2). Flow cytometry and western blotting analysis demonstrated protein expression of vWF and CD31 in differentiated ASCs (right panel). (B) Fluorescent photomicrograph (40 ×) of differentiated ASCs revealing concomitant uptake of Dil-labeled acetylated-low-density lipoprotein (red) and FITC-labeled human lectin (green). (C) Photomicrograph (phase contrast, 40 ×) of differentiated ASCs and HUVECs and (D) 24 h after plating onto Matrigel demonstrating their ability to form capillary-like structures, suggesting the ability to participate in angiogenesis. (E) Photomicrograph (phase contrast, 40 ×) of ASCs grown in the differentiating medium supplemented with the PI3K inhibitor LY294002 demonstrating inhibition of capillary growth upon plating on Matrigel. (F) Photomicrograph (phase contrast, 40 ×) of ASCs grown in the differentiating medium supplemented with the mitogen-activated protein kinase inhibitor PD98059 conversely reveals the ability of the ASCs to form capillary-like structures. (G) Reverse transcription (RT)-PCR (left panel) and quantitative PCR analysis (left panel) reveals that expression of both vWF and CD31 in differentiating ASCs appears significantly inhibited by PI3K inhibition (LY) rather than mitogen-activated protein kinase inhibition (PD) (n = 3; mean age 72 ± 6 years; *P < 0.05, **P < 0.01 vs. control). EGM2, endothelial growth medium-2; PCR, polymerase chain reaction; HUVECs, human umbilical vein endothelial cells; VEGF, vascular endothelial growth factor; vWF, von Willebrand factor.
Inhibition of PI3K blocks the endothelial differentiation of ASCs
To investigate possible intracellular pathways involved in the observed endothelial differentiation of ASCs, we evaluated the effects of inhibiting either the PI3K or MAPK pathways, both of which have been shown to be involved with differentiation [9,20,24,25]. To do this, we added to the differentiation medium either specific PI3K (LY294002) or MAPK (PD98059) inhibitors and subsequently evaluated the ability of the cells to form capillary-like structures on Matrigel as well as to express EC molecular markers. Inhibition of the PI3K pathway, but not the MAPK pathway, appeared to inhibit the ability of the ASCs to subsequently form capillary-like structures (Fig. 3E, F, respectively). Similarly, inhibition of the PI3K pathway significantly reduced mRNA expression of CD31 (∼4-fold; P < 0.01) and vWF (∼2-fold; P < 0.05; Fig. 3G). These effects were not seen with inhibition of the MAPK pathway.
Shear stress further stimulates endothelial differentiation of ASCs
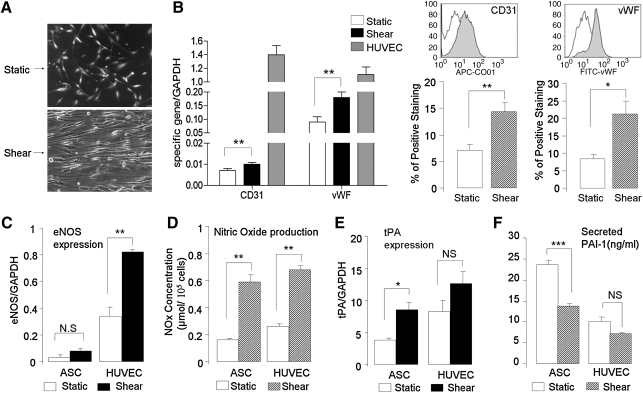
To investigate the effect of shear stress on the endothelial differentiation of ASCs harvested from elderly patients, we exposed ASCs cultured in EGM-2 for 2 weeks to physiological levels of shear stress (12 dynes) in vitro. After 48 h of shear stress, the differentiated ASCs re-aligned in the direction of flow, a morphological characteristic of ECs (Fig. 4A). Additionally, shear force significantly upregulated expression of CD31 (∼1.3-fold) and vWF (∼2-fold) mRNA levels compared with static controls by real-time RT-PCR analysis (Fig. 4B, left). Further, flow cytometry demonstrated significant increases in protein expression of CD31 (∼2-fold) and vWF (∼3-fold) after exposure to shear stress (Fig. 4B, right).
FIG. 4.
Effects of shear stress on ASC differentiation. (A) Photomicrographs (phase contrast, 20 ×) of differentiated ASCs cultured under static conditions or exposed to shear stress (12 dyne × 48 h) revealing re-alignment in the direction of flow. (B) Quantitative PCR (left panels) of differentiated ASCs reveals that exposure to shear stress (12 dyne × 48 h) significantly upregulates expression of the EC markers CD31 and vWF (n = 6; mean age 67 ± 4 years; **P < 0.01 vs. control). Flow cytometry (right panels) confirms that shear stress upregulates protein expression of CD31 and vWF (n = 4; mean age 69 ± 7 years; *P < 0.05, **P < 0.01 vs. control). (C) Quantitative PCR analysis of eNOS expression reveals a small amount of eNOS expression in differentiated ASCs compared to EC controls (n = 4; mean age 65 ± 6 years; **P < 0.01 vs. control). Although the effect did not reach statistical significance, the application of shear stress appeared to upregulate eNOS message. (D) Measurement of total nitric oxide (NOx) production using a chemiluminescence NO detector suggesting significant amounts of NO production by differentiated ASCs, including stimulation by shear stress (n = 4; mean age 68 ± 7 years; **P < 0.01 vs. control). Shear stress increased tPA expression (E, quantitative PCR) and decreased PAI-1 expression (F, ELISA), suggesting the acquisition of an antithrombogenic phenotype by the differentiated ASCs (n = 4; mean age 68 ± 7 years; *P < 0.05, ***P < 0.001 vs. control). EC, endothelial cell; eNOS; endothelial nitric oxide synthase; PAI-1, plasminogen activator inhibitor-1; tPA, tissue-plasminogen activator.
Shear stress upregulates antithrombogenic markers in differentiated ASCs
In ECs, shear stress stimulates the production of eNOS, NO, and tPA, and inhibits the production of PAI-1, thereby producing a nonthrombogenic phenotype. We tested this response to shear stress in ASCs cultured for 2 weeks in EGM-2. Although expression of eNOS mRNA was significantly lower than that in human EC (Fig. 4C), there was a trend (2.5-fold increase) noted in eNOS expression in ASCs in response to shear stress. Notably, total NOx production released from ASCs increased significantly (3.6-fold) upon exposure to shear stress, a result similar to control EC (Fig. 4D).
Similarly, shear stress stimulated production of tPA mRNA significantly in differentiated ASCs (2.2-fold; Fig. 4E). Conversely, the secreted PAI-1 in ASCs significantly reduced (1.7-fold) after exposure to shear stress (Fig. 4F). Together, these results suggest that ASCs differentiated toward EC may acquire a nonthrombogenic phenotype in response to shear stress.
Attachment of differentiated ASCs to vascular basement membrane
To understand the interaction of the differentiated ASCs with blood vessel wall components, and to further understand their potential for use in vascular tissue engineering, we investigated the attachment of these cells in vitro on fibronectin (extracellular membrane component) and ex vivo on decellularized human blood vessels. Regarding the latter experiments, we have previously shown that our decellularization process preserves the vascular basement membrane, and in particular the resident type IV collagen [26].
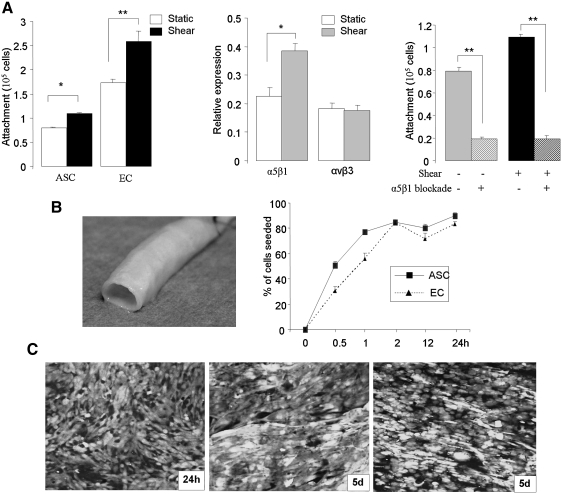
Figure 5A (left) reveals that the differentiated ASCs in vitro do attach to a fibronectin-coated surface, but not as readily as ECs. Of note, however, after exposure to physiological shear stress in vitro (12 dynes × 48 h), there was a significant improvement in attachment (1.4-fold), as was also noted with the EC controls. Given the important role they play in EC attachment to vascular basement membrane, we examined expression of integrins α5β1 and αvβ3 in ASCs (Fig. 5A, middle). We found that while both integrins were present on the ASCs, only the α5β1 integrin was upregulated upon exposure to shear stress (1.7-fold). To further define the importance of the α5β1 integrin on ASC attachment, we added α5β1 blocking antibodies to the medium before attachment (Fig. 5A, right). We observed that α5β1 blockade significantly reduced ASC attachment to fibronectin under both static and shear stress conditions.
FIG. 5.
Attachment of differentiated ASCs to vascular basement membrane. (A) Effect of shear stress and α5β1 integrin expression on ASC attachment. The left panel reveals that cell attachment to fibronectin by ASCs is improved by shear stress (12 dynes × 48 h), but not to the degree that EC attach (n = 3; *P < 0.05, **P < 0.01 vs. control). Integrin-mediated cell adhesion analysis (middle panel) demonstrates that shear stress significantly increased α5β1 expression in differentiated ASCs but not αvβ3 (n = 3; *P < 0.05 vs. control). Specific blockade of the α5β1 integrin (right panel) significantly reduced ASC attachment to fibronectin and eliminated the effect of shear stress (n = 3; **P < 0.01 vs. control). (B) Kinetics of attachment to vascular basement membrane. Photographs of intact human saphenous vein (left) that has subsequently been decellularized for seeding experiments. Attachment of the cells reveals that both differentiated ASCs and EC attach maximally to the scaffolding by 2 h, and remain attached at 24 h after static seeding (right). (C) Cell retention under physiological fluid flow conditioning. Confocal microscopy images of seeded differentiated ASCs and HUVECs on decellularized vein scaffold. Cells were labeled using Cell Tracker Green to allow for observation. Images were taken 24 h and 5 days after seeding and linearly increasing shear force up to 9 dynes.
Finally, we assessed the attachment of differentiated ASCs to vascular basement membrane. A significant amount of differentiated ASCs firmly adhered to the surface in as early as 30 min (50% ± 3% of seeded cells). After 2 h, 84% ± 2% of ASCs attached and remained stable over 24 h (90% ± 3%). Similar findings were noted upon using control ECs (Fig. 5B). The attachment and retention of differentiated ASCs on decellularized vein allograft was examined after seeding within a vascular bioreactor and linearly increasing luminal shear force to physiological levels. As shown in Fig. 5C, a nearly confluent monolayer of differentiated ASCs remains 5 days after seeding and increasing shear force to 9 dynes. These results were similar to the human umbilical vein endothelial cell-seeded control graft (Fig. 5C, right).
Discussion
In this study, we investigated the isolation of adult stem cells derived from the adipose tissue of elderly patients with cardiovascular disease, and the subsequent ability of these cells to differentiate toward an endothelial lineage. The major findings include that (1) ASCs can be isolated in abundance from the patient population most likely to benefit from this technology, unlike other bone marrow- or blood-borne stem and progenitor cells, (2) ASCs from elderly patients acquire several important EC characteristics upon culture in endothelial growth factors, with further upregulation of EC-specific markers when stimulated by shear stress, (3) endothelial differentiation appeared to be dependent upon the PI3K but not MAPK pathway, (4) differentiated ASCs appeared to also acquire an antithrombogenic phenotype upon stimulation with shear stress, and (5) attachment and retention of differentiated ASCs seeded on vascular basement membrane is improved by conditioning with shear force, a finding that appears mediated by upregulation of the α5β1 integrin. Taken together, these results suggest that adipose tissue is a viable and robust source of autologous adult stem cells that can be used as an EC substitute in vascular tissue engineering/regenerative medicine strategies.
This study specifically evaluates the availability of ASCs in an elderly patient population with the usual associated cardiovascular comorbidities. After isolation, attachment to tissue culture plastic, and negative selection for coisolated CD31/45+ cells (an ∼1 week process), we found that an average of 1.8 million ASCs/g of tissue can be obtained from the abdominal wall fat of patients >70 years old. The stem cell harvest from older patients in the study group was higher than that from the younger patients (those 50–59 years old). We did not investigate the reason behind this observation, but suspect this may be due to either a difference in the release of cells from adipose tissue by collagenase between the populations, or a change in water content of the tissue that would alter the weight of the tissue, rather than a true expansion of the population. Because the study did not include far younger (eg, 20–29 years old) control patients, we cannot definitively comment upon isolation differences that may exist with this population; nonetheless, the current study clearly demonstrates that an abundance of cells can be harvested from the elderly, cardiovascular patient. This may prove to be an important advantage for ASCs over stem/progenitor cell populations derived from bone marrow and blood, where isolation number diminishes with advancing age and comorbidities associated with ischemic cardiovascular disease [12,27]. This advantage may become increasingly important as our population age advances; in fact, the U.S. Department of Labor has estimated that there will be 50 million Americans older than age 65 by 2015 [28].
In addition to evaluating stem cell availability with respect to age, this study also examined the impact of gender, obesity, tobacco use, and diabetes—all risk factors for cardiovascular disease—and cardiovascular disease itself on stem cell isolation. The results of this study demonstrate that none of these demographics significantly altered the isolation. Of note, we did not evaluate the impact of various medications, such as statins, which may have an effect on stem cells harvest. Evaluation of the effects of patient comorbidities in vascular tissue engineering/regenerative medicine is critical, given that cardiovascular diseases remain the leading cause of death worldwide [29].
In addition to confirming the availability of these stem cells in an elderly population, this study provides evidence for the first time that ASCs from elderly patients with cardiovascular disease can acquire several important EC characteristics. A number of studies have shown that embryonic and adult stem cells differentiate into ECs in the presence of soluble growth factors [17,30–33]. Using EGM-2, we demonstrate that ASCs newly express the EC markers CD31, vWF, eNOS, and VE-cadherin by 2 weeks. EGM-2 contains up to 10 ng/mL VEGF (per manufacturer), a known stimulus of the endothelial differentiation of endothelial progenitor and MSCs in vitro [34–37]. We found that supplementing the EGM-2 with additional VEGF (50 ng/mL) significantly increases expression of vWF and eNOS within the stem cells. Although we did observe new expression of all of the molecular markers tested in the differentiated stem cells at 2 weeks, we do note that expression of eNOS and VE-cadherin message was significantly less compared with endothelial controls. Interestingly, and despite this observation regarding eNOS, NO production appeared similar to EC controls. Further work will be necessary delineate the timeline of expression for these important EC markers.
Evaluation of the role of both the PI3K and MAPK pathways in endothelial differentiation revealed that the acquisition of the endothelial characteristics of capillary formation and CD31 and vWF expression appeared dependent on the PI3K pathway. Several studies report that PI3K signaling also mediates VEGF expression in ECs and inhibition of PI3K signaling interferes with angiogenesis [38,39]. These findings are consistent with those reported by Cao et al., who also reported the importance of the PI3K pathway in the endothelial differentiation of ASCs [40].
Shear force proved to be an important stimulus to upregulate various endothelial markers in the ASCs. A previous study by us revealed that shear alone does not appear to induce differentiation; rather, it requires the presence of endothelial growth factors before its effects were observed.17 Shear force may regulate expression of growth factors, and has been implicated as playing a critical role in the physiological response of blood vessels through ECs signaling [41,42]. Other studies have demonstrated that shear stress can stimulate differentiation of stem cells toward EC phenotypes [37,43,44]. In this study, expression of CD31 and vWF at both the message and protein levels in ASCs were upregulated by exposure to physiological levels of shear stress (12 dynes).
In addition to stimulating various molecular markers upon differentiation of ASCs, shear force also appeared to foster an anti-thrombogenic phenotype. Prior studies involving shear force demonstrate this observation in peripheral blood EPCs [45,46]. Here we have similarly demonstrated that shear force stimulates expression of tPA and inhibits PAI-1. Further, NO production released from ASCs was significantly increased by exposure to shear stress. Given the critical role ECs play in the regulation of thrombosis within blood vessels, these results provide evidence for the ability of ASCs to commit to an EC lineage.
Other functional characteristics of ECs were observed in ASCs after culture in differentiating medium. The ability to form new capillary-like structures upon plating on Matrigel suggests that differentiated ASCs have the potential to participate in angiogenesis. This in vitro observation is consistent with previous work by others that demonstrates the ability of ASCs to participate in postnatal neovascularization in vivo [47]. We also observed the uptake of ac-LDL by these cells, a known characteristic of ECs and their circulating progenitor cells.
In addition to evaluating endothelial differentiation capacity of ASCs isolated from elderly patients, we preliminarily evaluated their multipotency. Several investigators have demonstrated the multipotent potential of stem cells derived from adipose tissue [15,48–50]. As noted, however, many of these studies were performed using adipose tissue from healthy, young patients after cosmetic liposuction. In this study, we preliminarily confirmed that ASCs derived from elderly patients retained the ability to differentiate toward adipocyte and osteocytes lineages, in addition to ECs.
We further investigated the attachment of differentiated ASCs to vascular basement membrane components (specifically, fibronectin), not only to demonstrate similarity to ECs, but also to evaluate their potential for use as EC substitutes in the creation of tissue-engineered vascular grafts. In vivo, ECs reside in a shear stress environment; an important mechanism that allows the cells to remain firmly attached and to align with blood flow is an increase in the number of integrins as well as their affinity for specific ligands in response to shear [51]. Flow conditioning of ECs (a gradual introduction of shear force) has also been used to improve attachment of the cells to vascular grafts [52]. Urbich et al. have demonstrated that upregulation of mRNA and protein expression of integrin subunits α5 and β1 in response to shear leads to a functional increase of endothelial attachment strength [53]. In the current study, we observed that differentiated ASCs attached to fibronectin, but not as readily as endothelial controls. The application of shear force before seeding significantly improved attachment, an observation that correlated with upregulation of the α5β1 integrin (but not the αvβ3 integrin). This was further confirmed by the observation that both cell attachment and the effect of shear were essentially eliminated with monoclonal antibody blockade of the α5β1 integrin.
Finally, these studies evaluated the ability of differentiated ASCs to form a confluent monolayer that resists detachment at physiological levels of shear force. This was accomplished using the luminal surface of decellularized human saphenous vein. We have previously shown that our decellularization method leaves the extracellular matrix of the vessel intact, including the basement membrane composed primarily of collagen IV [26]. Our results suggest that the differentiated ASCs attach to the vessel with similar efficacy and kinetics as do EC. Additionally, differentiated ASCs formed a confluent monolayer within the vascular graft that remained intact over 5 days as luminal flow was linearly increased up to 9 dyn. These preliminary tissue engineering experiments provide further evidence for the similarity between the differentiated stem cells and EC, and suggests the utility of these cells for use as EC substitutes for the creation of vascular grafts. Further work will be necessary to evaluate the retention and function of the ASCs in vivo.
These studies demonstrate the acquisition of a multitude of EC characteristics upon differentiation by ASCs isolated from elderly patients with cardiovascular disease. However, unlike the isolation study (where data could be gathered from all 53 patients), examination of differentiation using low passage number cells significantly limited the number of assays we could perform on each cell line. In total, 42 cell lines were submitted for examination of differentiation; for each individual test, the number of cell lines examined ranged between 3 and 12. Given this study design, we can conclude that ASCs isolated from elderly patients can commit toward an EC lineage; however, we cannot definitively state that comorbid conditions present within these patients do not specifically alter the potency of the isolated ASCs.
In summary, this study provides new and practical information regarding the use of an adult stem cell lineage derived from adipose tissue, namely, (1) unlike stem and progenitor cells derived from bone marrow and blood, the availability of these cells for use does not appear to be adversely affected by age (in fact, harvest was better in the elderly population); (2) ASCs from elderly patients acquire many of the key molecular and functional markers associated with mature ECs, including the acquisition of an antithrombogenic phenotype, in response to chemical and physical signals; and (3) these cells readily attach to vascular basement membrane and form a confluent monolayer of cells within a vascular graft that resists detachment at physiologic levels of shear force. Overall, adipose tissue appears to be a suitable and viable source of autologous stem cells that could be used as EC substitutes in the patient population most like to benefit from this technology.
Acknowledgments
This study was supported by the National Institutes of Health grant K08 HL076300-02 (P.J.D.), the American Heart Association Beginning Grant-in-Aid 0565454U (P.J.D.), and the American Vascular Association Lifeline Foundation (P.J.D.). The authors also greatly appreciate the careful review of the article by Sharon Molotsky.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adams B. Xiao Q. Xu Q. Stem cell therapy for vascular disease. Trends Cardiovasc Med. 2007;17:246–251. doi: 10.1016/j.tcm.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 2.de Mel A. Bolvin C. Edirisinghe M. Hamilton G. Seifalian AM. Development of cardiovascular bypass grafts: endothelialization and applications of nanotechnology. Expert Rev Cardiovas Ther. 2008;6:1259–1277. doi: 10.1586/14779072.6.9.1259. [DOI] [PubMed] [Google Scholar]
- 3.Sarkar S. Schmitz-Rixen T. Hamilton G. Seifalian AM. Achieving the ideal properties for vascular bypass grafts using a tissue engineered approach: a review. Med Bio Eng. 2007;45:327–336. doi: 10.1007/s11517-007-0176-z. [DOI] [PubMed] [Google Scholar]
- 4.Isenberg BC. Williams C. Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 5.Griese DP. Ehsan A. Melo LG. Kong D. Zhang L. Mann MJ. Pratt RE. Mulligan RC. Dzau VJ. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108:2710–2715. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 6.Levenberg S. Engineering blood vessels from stem cells: recent advances and application. Curr Opin Biotechnol. 2005;16:516–523. doi: 10.1016/j.copbio.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Sales KM. Salacinski HJ. Alobaid N. Mikhail M. Balakrishnan V. Seifalian AM. Advancing vascular tissue engineering: the role of stem cell technology. Trends Biotechnol. 2005;23:461–467. doi: 10.1016/j.tibtech.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 8.D'Ippolito G. Schiller PC. Ricordi C. Roos BA. Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 9.Heeschen C. Lehmann R. Honold J. Assmus B. Aicher A. Walter DH. Martin H. Zeiher AM. Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 10.Barrilleaux B. Phinney DG. Prockop D. O'Connor KC. Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12:1–13. doi: 10.1089/ten.2006.12.3007. [DOI] [PubMed] [Google Scholar]
- 11.Xin Y. Wang Y. Zhang H. Li J. Wang W. Wei Y. Hu S. Aging adversely impacts biological properties of human bone marrow-derived mesenchymal stem cells: implication for tissue engineering heart valve construction. Artif Organs. 2010;34:215–222. doi: 10.1111/j.1525-1594.2009.00824.x. [DOI] [PubMed] [Google Scholar]
- 12.Oliveras A. Soler MJ. Martinez-Esrada OM. Vazquez S. Marco-Feliu D. Vila JS. Vilaro S. Lloveras J. Endothelial progenitor cells are reduced in refractory hypertension. J Hum Hypertens. 2008;22:183–190. doi: 10.1038/sj.jhh.1002304. [DOI] [PubMed] [Google Scholar]
- 13.Dzau VJ. Gnecchi M. Pachori AS. Morello F. Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 14.Nakagami H. Morishita R. Maeda K. Kikuchi Y. Ogihara T. Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 15.Zuk PA. Zhu M. Ashjian P. De Ugarte DA. Huang JI. Mizuno H. Alfonso ZC. Fraser JK. Benhaim P. Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Bio Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang JI. Beanes SR. Zhu M. Lorenz HP. Hedrick MH. Benhaim P. Rat extramedullary adipose tissue as a source of osteochondrogenic progenitor cells. Plast Reconstr Surg. 2002;109:1042–1043. doi: 10.1097/00006534-200203000-00037. [DOI] [PubMed] [Google Scholar]
- 17.Fischer LJ. McIihenny S. Tulenko T. Golesorkhi N. Zhang P. Larson R. Lombardi J. Shapiro I. Dimuzio PJ. Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force. J Surg Res. 2009;152:157–166. doi: 10.1016/j.jss.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris LJ. Abdollahi H. Zhang P. McIlhenny S. Tulenko T. DiMuzio P. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J Surg Res. 2009. Sep 4, pp. 1–9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 19.Xu J. Liu X. Jiang Y. Chu L. Hao H. Liua Z. Verfaillie C. Zweier J. Gupta K. Liu Z. MAPK/ERK signalling mediates VEGF-induced bone marrow stem cell differentiation into endothelial cell. J Cell Mol Med. 2008;12:2395–2406. doi: 10.1111/j.1582-4934.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang BH. Zheng JZ. Aoki M. Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. PNAS. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dardik A. Chen L. Frattini J. Asada H. Aziz F. Kudo FA. Sumpio BE. Differential effects of orbital and laminar shear stress on endothelial cells. J Vasc Surg. 2005;41:869–880. doi: 10.1016/j.jvs.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Gao F. Gao E. Yue TL. Ohlstein EH. Lopez BL. Christopher TA. Ma XL. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002;105:1497–1502. doi: 10.1161/01.cir.0000012529.00367.0f. [DOI] [PubMed] [Google Scholar]
- 23.McIlhenny SE. Hager ES. Grabo DJ. DiMatteo C. Shapiro IM. Tulenko TN. DiMuzio PJ. Linear shear conditioning improves vascular graft retention of adipose-derived stem cells by up-regulation of the α5β1 integrin. Tissue Eng. 2010;16:245–255. doi: 10.1089/ten.tea.2009.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu W. Chen Z. Zhang J. Zhang L. Ke H. Huang L. Peng Y. Zhang X. Li S. Lahn BT. Xiang AP. Critical role of phosphoinositide 3-kinase cascade in adipogenesis of human mesenchymal stem cells. Mol Cell Biochem. 2008;310:11–18. doi: 10.1007/s11010-007-9661-9. [DOI] [PubMed] [Google Scholar]
- 25.Tamama K. Sen CK. Wells A. Differentiation of bone marrow mesenchymal stem cells into the smooth muscle lineage by blocking ERK/MAPK signaling pathway. Stem Cells Dev. 2008;17:897–908. doi: 10.1089/scd.2007.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaner PJ. Martin ND. Tulenko TN. Shapiro IM. Tarola NA. Leichter RF. Carabasi RA. Dimuzio PJ. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146–153. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Heeschen C. Lehmann R. Honold J. Assmus B. Aicher A. Walter DH. Martin H. Zeiher AM. Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 28.Cohn L. Narayanasamy N. Aortic valve replacement in elderly patients: what are the limits? Curr Opin Cardiol. 2007;22:92–95. doi: 10.1097/HCO.0b013e32802086bc. [DOI] [PubMed] [Google Scholar]
- 29.Ounpuu S. Anand S. Yusuf S. The impending global epidemic of cardiovascular diseases. Eur Heart J. 2000;21:880. doi: 10.1053/euhj.1999.1880. [DOI] [PubMed] [Google Scholar]
- 30.Li ZJ. Wu JC. Sheikh AY. Kraft D. Cao F. Xie XY. Patel M. Cambhir SS. Robbins RC. Cooke JP. Wu JC. Differentiation, survival, and function of embryonic stem cell-derived endothelial cells for ischemic heart disease. Circulation. 2007;116:46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng L. Xiao Q. Margariti A. Zhang Z. Zampetaki A. Patel S. Capogrossi MC. Hu Y. Xu Q. HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells. J Cell Biol. 2006;174:1059–1069. doi: 10.1083/jcb.200605113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson L. Jones DR. Scotting P. Sottile V. Adult mesenchymal stem cells: differentiation potential and therapeutic applications. J Postgrad Med. 2007;53:121–127. doi: 10.4103/0022-3859.32215. [DOI] [PubMed] [Google Scholar]
- 33.Kondo K. Shintani S. Shibata R. Murakami H. Murakami R. Imaizumi M. Kitagawa Y. Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61–66. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 34.Lin H. Shabbir A. Molnar M. Yang J. Marion S. Canty JM., Jr. Lee T. Adenoviral expression of vascular endothelial growth factor splice variants differentially regulates bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2008;214:434–441. doi: 10.1002/jcp.21414. [DOI] [PubMed] [Google Scholar]
- 35.Xu J. Liu X. Jiang Y. Chu L. Hao H. Liu Z. Verfaillie C. Zweier J. Gupta K. Liu Z. MAPK/ERK signaling mediates VEGF-induced bone marrow stem cell differentiation into endothelial cell. J Cell Mol Med. 2008;12:2395–2406. doi: 10.1111/j.1582-4934.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Coppi P. Bartsch G., Jr. Siddiqui MM. Xu T. Santos CC. Perin L. Mostoslavsky G. Serre AC. Snyder EY. Yoo JJ. Furth ME. Soker S. Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 37.Zhang P. Baxter J. Vinod K. Tulenko TN. Dimuzio P. Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli. Stem Cells Dev. 2009;18:1299–1308. doi: 10.1089/scd.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang BH. Zheng JZ. Aoki M. Vogt P. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. PNAS. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang J. Wang J. Kong X. Yang J. Guo L. Zheng F. Zhang L. Huang Y. Wan Y. Vascular endothelial growth factor promotes cardiac stem cell migration via the PI3K/Akt pathway. Exp Cell Res. 2009;315:3521–3531. doi: 10.1016/j.yexcr.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 40.Cao Y. Sun Z. Liao L. Meng Y. Han Q. Zhao R. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 41.Wang H. Riha GM. Yan S. Li M. Chai H. Yang H. Yao Q. Chen C. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25:1817–1823. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto K. Takahashi T. Asahara T. Ohura N. Sokabe T. Kamiya A. Ando J. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol. 2003;95:2081–2088. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 43.Wu CC. Chao YC. Chen CN. Chien S. Chen YC. Chien CC. Chiu JJ. Yen BL. Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J Biomech. 2008;41:813–821. doi: 10.1016/j.jbiomech.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez P. Bourget C. Bareille R. Daculsi R. Bordenave L. Gen response in endothelial cells cultured on engineered surfaces is regulated by shear stress. Tissue Eng. 2007;13:1607–1614. doi: 10.1089/ten.2006.0399. [DOI] [PubMed] [Google Scholar]
- 45.Yang Z. Wang JM. Wang LC. Chen L. Tu C. Luo CF. Tang AL. Wang SM. Tao J. In vitro shear stress modulates antithrombogenic potentials of human endothelial progenitor cells. J Thromb Thrombolysis. 2007;23:121–127. doi: 10.1007/s11239-006-9045-0. [DOI] [PubMed] [Google Scholar]
- 46.Hashi CK. Zhu Y. Yang GY. Young WL. Hsiao BS. Wang K. Chu B. Li S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. PNAS. 2007;104:11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Planat-Bernard V. Silvestre J-S. Cousin B. Andre M. Nibbelink M. Tamarat R. Clergue M. Manneville C. Saillan-Barreau C. Duriez M. Tedgui A. Levy B. Penicaud L. Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 48.Miranville A. Heeschen C. Sengenes C. Curat CA. Busse R. Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto T. Kano K. Kondo D. Fukuda N. Iribe Y. Tanaka N. Matsubara Y. Sakuma T. Satomi A. Otaki M. Ryu J. Mugishima H. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210–222. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- 50.Madonna R. Geng YJ. De Caterina R. Adipose tissue-derived stem cells: characterization and potential for cardiovascular repair. Arterioscler Thromb Vasc Biol. 2009;29:1723–1729. doi: 10.1161/ATVBAHA.109.187179. [DOI] [PubMed] [Google Scholar]
- 51.Urbich C. Walter DH. Zeiher AM. Dimmeler S. Laminar shear stress upregulates integrin expression role in endothelial cell adhesion and apoptosis. Circ Res. 2000;87:683–689. doi: 10.1161/01.res.87.8.683. [DOI] [PubMed] [Google Scholar]
- 52.Chappell DC. Varner SE. Nerem RM. Medford RM. Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998;82:532–539. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- 53.Urbich C. Dernbach E. Reissner A. Vasa M. Zeiher AM. Dimmeler S. Shear stress–induced endothelial cell migration involves integrin signaling via the fibronectin receptor subunits α5 and β1. Arterioscler Thromb Vasc Biol. 2002;22:69–75. doi: 10.1161/hq0102.101518. [DOI] [PubMed] [Google Scholar]