Abstract
Heat shock proteins like gp96 (grp94) are able to induce specific cytotoxic T-cell (CTL) responses against cells from which they originate and are currently studied in clinical trials for use in immunotherapy of tumors. We have recently demonstrated that gp96 binds to at least one yet unidentified receptor restricted to antigen-presenting cells (APCs) like dendritic cells (DCs) but not to T cells. Moreover we have shown, that for CTL activation by gp96-chaperoned peptides receptor-mediated uptake of gp96 by APCs is required. Lately, we have discovered a second function of gp96 when interacting with professional APCs. Gp96 is able to mediate maturation of DCs as determined by up-regulation of MHC class II, CD86 and CD83 molecules, secretion of pro-inflammatory cytokines IL-12 and TNF-α and enhanced T-cell simulatory capacity. Furthermore, the gp96 receptor(s) are down-regulated on mature DCs, suggesting that the gp96 receptor(s) behave similar to other endocytic receptors like CD36, mannose receptor etc. Our findings now provide additional evidence for the remarkable immunogenicity of gp96: first, the existence of specific gp96 receptors on APCs and second, the capacity to activate dendritic cells which is strictly required to enable these highly sophisticated APCs to prime CTL responses.
INTRODUCTION
To activate naive T cells, the adaptive immune system has evolved a very specialized and powerful tool, the antigen-presenting cell (APC). Not only do APCs present peptide ligands bound to their cell surface major histocompatibility complex (MHC) molecules, they are also equipped with a large set of costimulatory molecules such as CD80, CD86, and CD40. The recognition of both MHC-peptide complexes and costimulators is required for the activation of naive T cells. The ability of the APC to carefully regulate the cell surface expression levels of costimulatory and MHC molecules puts these sophisticated cells into the position of most important regulators of the immune system's T-cell limb.
ANTIGEN PROCESSING: THE DICHOTOMY OF MAJOR HISTOCOMPATIBILITY COMPLEX CLASS I AND II PATHWAYS
Pivotal to antigen presentation is the display of peptides in the context of major histocompatibility complex (MHC) class I and II molecules, which allows specific recognition by the T-cell receptor (TCR). The antigen-processing apparatus of the antigen-presenting cell (APC) can be grouped into 2 distinct pathways involving the degradation of protein antigens into short peptide fragments finally embedded in the polymorphic binding domains of the MHC molecules. For MHC class I molecules, cytosolic antigen is degraded by the machinery of the 20S and 26S proteasomes. Both multiprotein complexes generate sets of peptides (Emmerich et al 2000), which in many cases already have the right C-terminus to bind to their corresponding MHC class I molecule (Stoltze et al 2000). These peptides are then transported to the endoplasmic reticulum (ER) via the ER membrane-resident transporter associated with antigen presentation (TAP) 1 and 2 molecules. The lumen of the ER provides a specialized environment that allows these peptides to be finally, presumably N-terminally, processed and associated to MHC class I molecules. MHC class I–peptide complexes are then shuttled toward the cell surface via the secretory pathway for recognition by CD8+ T cells (for review, see Rock and Goldberg 1999). On the other hand, MHC class II molecules associate with longer peptides generated by exopeptidases in the acidic milieu of endosomal compartments. The resulting MHC class II–peptide complexes are exported to the cell surface, where they can be recognized by TCRs of CD4+ T cells (for review, see Watts 1997). In most cases, peptides associated to MHC class I are derived from intracellular ligands of MHC class II from extracellular proteins.
HEAT SHOCK PROTEINS AS INTRACELLULAR AND EXTRACELLULAR PEPTIDE CARRIERS
Molecular chaperones such as heat shock proteins (Hsps) are peptide-binding molecules that facilitate folding and refolding of proteins by preventing their aggregation and stabilizing high-energy folding intermediates. Recently, it has been shown by several groups that chaperones and cochaperones can be associated with pathways of antigen processing at various points (Lammert et al 1997; Spee and Neefjes 1997; Panjwani et al 1999; Luders et al 2000). It seems to be an attractive idea that Hsps are involved in the shuttling of peptides between the different antigen-processing compartments inside the cell (Srivastava et al 1994). Moreover, once the Hsps are released from cells by necrosis or possibly even secretion, they can function as peptide carriers in an even wider sense—from cell to cell. This extraordinary function of Hsps was discovered by purification of Hsp-peptide complexes and their use in eliciting CD8+ cytotoxic T-cell responses. In pioneering experiments, Srivastava and coworkers demonstrated that the Hsps gp96 (Grp94), Hsc70 (the constitutive form of the Hsp70 protein), and Hsp90 are able to confer immunity against autologous tumor preparations from which these Hsps had been isolated before (reviewed by Srivastava et al 1998; Schild et al 1999).
CROSS-PRESENTATION: BREAKING THE CLASSIC MHC CLASS I/II DICHOTOMY
It was established that Hsp-mediated immunity requires the presence of APCs (Udono et al 1994; Suto and Srivastava 1995) in line with the idea that Hsp-peptide complexes are taken up by APCs, which in a subsequent step elicit a cytotoxic T-lymphocyte (CTL) response. Hence, peptides that were associated to Hsps are transferred to MHC class I molecules for recognition by CD8+ T cells. Specific activation of CTLs is independent from the MHC haplotype of the cells from which the Hsp-peptide complexes originate (Arnold et al 1995). This phenomenon of cross-priming was originally discovered by Bevan (1976). Today, the term cross-presentation is used as a more general description for the re-presentation of exogenously derived, cell-associated antigens (Carbone et al 1998).
The phenomenon of cross-presentation requires the existence of processing pathways distinct from the classic dichotomy in antigen processing from endogenous and exogenous origin. Several pathways for presentation of exogenous antigen on MHC class I molecules have been described (reviewed by Rock 1996; Jondal et al 1996), which can be put into 2 fundamentally different processing groups: one involving transport of antigen from endosomes into the cytosol and then further processing similar to the classic MHC class I processing pathway in a proteasome- and TAP-dependent fashion. In the other pathway, recycled MHC class I molecules are directed to endosomes similar to processing of MHC class II molecules.
THE DENDRITIC CELL: WELL EQUIPPED FOR CROSS-PRIMING
The nature of the APC possessing cross-priming abilities is still not completely unveiled. However, accumulating evidence suggests that bone marrow–derived dendritic cells (DCs) are the only cells that are capable of efficiently stimulating resting, naive T cells and inducing CTL responses in vivo (reviewed by Banchereau and Steinman 1998; Banchereau et al 2000). Immature DCs usually reside in nonlymphoid tissue such as skin, where they are specialized in endocytosis and antigen processing. To use these antigens for T-cell stimulation, the DC must undergo a differentiation process called maturation or activation. Several stimuli, such as pathogens, microbial products such as lipopolysaccharide (LPS), and tissue damage, induce their initial maturation and migration to the T-cell areas of the secondary lymphoid organs (Cyster 1999). Mature DCs lose their capability to efficiently capture and process antigen while becoming highly specialized in antigen presentation. MHC class II molecules, now loaded with antigen from the endocytosed material, are forced to move to the cell surface for presentation to CD4+ T-helper cells (Cella et al 1997; Pierre et al 1997). Besides MHC molecules, costimulatory molecules such as CD40, CD80, and CD86 are up-regulated (Caux et al 1994; Inaba et al 1994), which deliver a second signal to CD4+ T-helper cells recognizing antigen on MHC class II molecules. In turn, the T cell can further activate the DC via CD40 ligand (CD40L)–CD40 interactions (Ridge et al 1998; Schoenberger et al 1998). Such a fully activated DC is able to secrete cytokines like interleukin (IL) 12 to instruct T-helper cells to differentiate along the Th1 or Th2 pathway (Macatonia et al 1995; Cella et al 1996; Koch et al 1996). More importantly, activated DCs are capable of cross-priming naive CD8+ T cells to effector CTLs (Heath and Carbone 1999).
The unique position of DCs among APCs has been confirmed by various in vitro antigen re-presentation systems. Bhardwaj and colleagues were able to show that apoptotic cells can be endocytosed by macrophages and DCs via CD36, but only DCs, which coexpress the αvβ5 integrins, can stimulate T cells with antigen derived from the apoptotic vesicles (Albert et al 1998a, 1998b). Amigorena and coworkers have demonstrated that uptake of ovalbumin (OVA)–immune complexes by Fcγ receptors on the surface of APCs only leads to re-presentation of an OVA epitope on MHC class I molecules in DCs but not in macrophages or B cells (Amigorena and Bonnerot 1999; Rodriguez et al 1999). Soluble antigen could also be cross-presented but had to be present at significantly higher concentrations in the surrounding medium. Interestingly, the latter group discovered a transport route of the antigen from endosomes to the cytosol specific for DCs. It seems possible that DCs possess either special endosomal proteins that facilitate this antigen transfer or a subset of specialized translocator endosomes. In both cases—apoptotic bodies and immune complexes—DCs use receptors to internalize antigen for cross-presentation. The advantage of receptor-mediated endocytosis does not only lie in a much more efficient and specific uptake in comparison to unspecific means such as macropinocytosis. Receptors might also shuttle the antigen into the right pathway for antigen presentation.
Because immunization and cross-priming in mice with gp96-peptide complexes work very effectively even in the nanomolar range, Srivastava and coworkers speculated a few years ago that gp96 and other Hsps might be taken up by receptors found on the surface of professional APCs (Srivastava et al 1994). The existence of such Hsp-specific receptors would also account for the cross-priming abilities of Hsps such as gp96. For instance, the ER chaperone protein disulfide isomerase (PDI) displays much better peptide-binding capacities than gp96 as demonstrated by TAP translocation and cross-linking experiments (Lammert et al 1997) but is not able to induce specific CTL responses (unpublished observation), possibly because APCs do not possess receptors for PDI.
EVIDENCE FOR EXISTENCE OF ONE OR MORE gp96 RECEPTORS ON APCs
First indications for specific interactions of Hsps with APCs were available from electron microscope studies (Arnold-Schild et al 1999). Gold-labeled gp96 and Hsc70 (the constitutive form of Hsp70) were found to bind to macrophage and monocytic cell lines. Both Hsps were specifically localized in clathrin-coated pits on the cell surface, indicating receptor-mediated binding. This binding was not observed on fibroblasts. Using a large excess of unlabeled gp96 or Hsc70, binding of gold-labeled Hsps to clathrin-coated plasma membrane regions could be blocked. Later, Nicchitta and coworkers were also able to demonstrate binding of fluorescein isothiocyanate (FITC)–labeled gp96 molecules to peritoneal macrophages but not to COS or CHO cells (Wassenberg et al 1999).
There are several criteria for specific receptor-mediated binding of molecules to cells. The binding must be saturable, since the number of receptors per cell is finite. Furthermore, the binding of the labeled molecule should be blocked by the same unlabeled molecule, and this competition should be titratable, showing a function corresponding to saturation. Blocking of binding of the labeled molecules should already work at low concentrations of unlabeled competitor. Theoretically, if the labeled molecule is added to the cells in saturating concentration, a 1:1 ratio of labeled-unlabeled protein should reduce the binding of the labeled protein by 50%. In many cases, such optimal blocking is rarely seen because of additional unspecific binding of the labeled molecule. The fraction of unspecific binding is largely dependent on the binding buffer and the quality of the protein and its labeling.
In our case, FITC-labeled gp96 molecules (kindly provided by Immatics Biotechnologies) were bound to bone marrow–derived mouse DCs. Saturation of binding was reached at 30 to 50 μg/mL of gp96. At the latter concentration, 45% inhibition of binding was reached with a 1:1 ratio of labeled vs unlabeled gp96 molecules. Binding of gp96-FITC was observed for mouse splenic and human blood monocytes and macrophages; B cells showed less but still significant binding. In no case was binding to T cells and natural killer cells observed; hence the expression of gp96 receptor(s) is restricted to APCs (Singh-Jasuja et al 2000b).
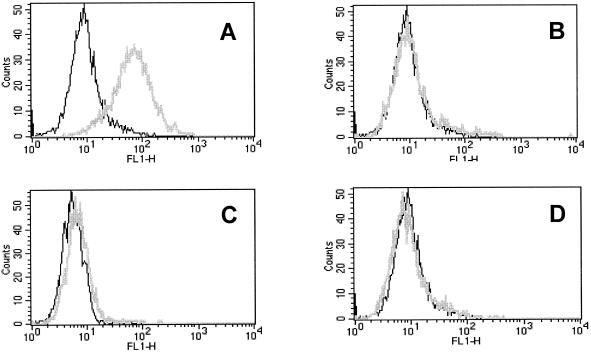
Furthermore, we were interested to know whether or not PDI, which has very strong peptide-binding abilities, would also bind to cells. Figure 1 shows binding of gp96-FITC compared with PDI-FITC (PDI kindly provided by C. Nicchitta, Duke University, NC) to D2SC/1 cells, a splenic DC precursor cell line. In no case was binding of PDI-FITC observed, indicating that a cell surface receptor does not exist for PDI. This might explain why PDI molecules cannot be used for immunization in mice (Lammert et al, unpublished observation; Nair et al 1999).
Fig 1.
The Hsp gp96 but not PDI binds to APC lines. Binding of 30 μg/mL of FITC-labeled gp96 (gray line) compared with control protein OVA-FITC (black line) on the DC precursor cell line D2SC/1 at 4°C (A) and competition of this binding with an excess of 100-fold unlabeled gp96 (B). gp96-FITC does not bind to the T-cell line RMA (C). Up to 100 μg/mL of PDI-FITC do not bind either to D2SC/1 (D) or RMA (not shown)
ENDOCYTOSED gp96 COLOCALIZES WITH RECYCLED MHC CLASS I MOLECULES IN DCs
Confocal microscopy allows a high-resolution visualization of the binding of gp96-FITC to cells. Gp96-FITC molecules bound to bone marrow–derived mouse DCs with a characteristic patched staining on the cell surface. We could demonstrate that receptor-bound gp96—when chased at 37°C—is endocytosed into compartments containing MHC class I and II molecules (Singh-Jasuja et al 2000b). Figure 2 shows binding on and endocytosis into D2SC/1 cells. After 15 minutes of endocytosis, gp96 is found in early endosomes—visualized by colocalization with endocytosed transferrin—together with MHC class I molecules. However, no colocalization between gp96 and MHC class I molecules is observed on the surface. This suggests that peptide transfer from gp96 to MHC class I does not happen on the cell surface but requires endocytosis. Interestingly, it has been reported recently that internalized cell surface MHC class I molecules, like class II molecules, are able to bind their antigen in endosomal compartments, suggesting that these vesicles are indeed putative MHC class I and class II loading compartments for exogenous antigen.
Fig 2.
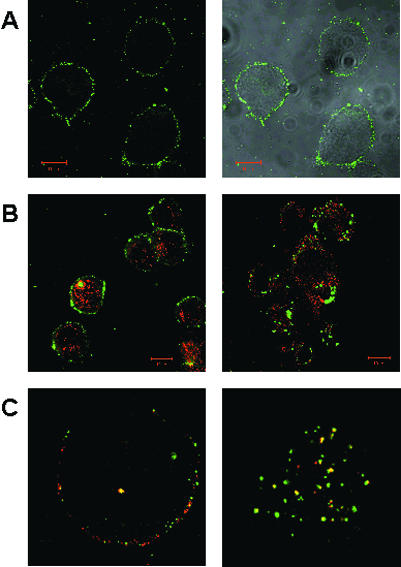
Confocal micrographs of D2SC/1 cells. (A) Binding of gp96-FITC to the surface of D2SC/1 cells at 4°C shown as single-channel picture (left panel) and overlap with transmission picture (right panel). (B) Costaining with lysosomes (left panel) and no colocalization with endocytosed gp96-FITC after 15 minutes of treatment at 37°C. gp96-FITC do not colocalize with MHC class I molecules on the surface of D2SC/1 cells (left panel of C) but do after 15 minutes of endocytosis (right panel) in early endosomes identified with transferrin (not shown). gp96-FITC is displayed in green, lysosomes (stained by α-Lamp1) and MHC class I molecules (stained by α-H2-Kd) in red, and colocalization in yellow
Recently, Germain and coworkers were able to demonstrate that Hsp70 is taken up by peritoneal macrophages in a receptor-dependent manner (Castellino et al 2000). Subsequently, antigen associated to Hsp70 is introduced into the MHC class I processing pathway via a endosomal or cytosolic route, depending on the sequence context of the antigenic peptide bound to the Hsp. If the C-terminal anchor residue fits already for association to MHC class I molecules, an endosomal route can be taken. In the other case, the antigen has to be released into the cytosol and requires processing by the proteasome and translocation by TAP. Whether or not gp96 as well might transport its cargo to MHC molecules via distinct processing pathways, depending on further processing requirement of the associated peptides, is currently under investigation.
CROSS-PRESENTATION OF gp96-ASSOCIATED ANTIGEN ON MHC CLASS I MOLECULES REQUIRES RECEPTOR-MEDIATED ENDOCYTOSIS
To investigate whether unspecific uptake or receptor-mediated endocytosis of gp96 molecules leads to presentation of the associated peptides on MHC class I molecules, we purified gp96 from RMA-S SigE1b cells, a cell line expressing the adenovirus-5 E1b epitope (Toes et al 1995) exclusively in the ER. Such in vivo–generated gp96-E1B complexes were able to elicit a CTL response in mice immunized with these complexes, demonstrating the association of E1B to gp96 molecules. Incubation of the mouse DC line D1 or bone marrow–derived DCs (unpublished data) together with gp96-E1b resulted in activation of E1B-specific CTL clones as measured by intracellular interferon-γ staining of CTLs. Gp96 associated with no or irrelevant peptides or a CTL clone recognizing the different E1A epitope was used as the control and showed no CTL activation in any case. Furthermore, the addition of excess irrelevant gp96 to gp96-E1B complexes was able to completely block activation (Singh-Jasuja et al 2000b). These results demonstrate that the gp96 receptor(s) on APCs are strictly required for cross-presentation of gp96-associated peptides on MHC class I molecules. Unspecific endocytosis, which cannot be blocked by low amounts of competitor gp96 molecules, is not able to do so. Similar results were also obtained by Germain and colleagues (Castellino et al 2000). Antigenic precursor peptides associated to Hsp70 in vitro were taken up by macrophages in a saturable fashion. They were re-presented on MHC class I molecules assayed by IL-2 secretion of CTLs specific for the epitope sequence embedded in the precursor peptide. As shown for gp96, these experiments, too, demonstrated blocking of cross-presentation by unloaded Hsp70 molecules as competitors.
gp96 IS ABLE TO INDUCE MATURATION OF DCs
It has been shown recently that other Hsps, such as Hsp60 (Chen et al 1999; Kol et al 2000) and Hsp70 (Asea et al 2000; Moroi et al 2000), can induce the activation of monocytes via interaction with CD14. Thus, Hsps may serve not only as a vehicle for antigenic peptides but also as a danger signal to the innate and specific immune system as suggested for other endogenous activators of DCs (Matzinger 1998). Activated, mature DCs display their full costimulatory potential and are able to communicate very effectively with naive T cells. Only activated DCs have the ability to prime CTL responses. Thus, we investigated whether gp96 was able to deliver a maturation signal to DCs.
DCs were derived in vitro from mouse bone marrow stem cells or CD14+ monocytes from human blood by incubation with granulocyte-macrophage colony-stimulating factor or granulocyte-macrophage colony-stimulating factor and IL-4, respectively, according to existing protocols (Inaba et al 1992; Bender et al 1996).
After 12 hours, up to 10 ng/mL of IL-12 and tumor necrosis factor α (TNF-α) had accumulated in the medium supernatant of the DCs activated with gp96 or LPS. Controls, including boiled gp96, did not exceed 0.1 ng/mL of IL-12 and 1 ng/mL of TNF-α, whereas the activity of LPS could not be diminished by boiling. Thus, we were able to demonstrate that DCs treated with gp96 were activated as shown by secretion of proinflammatory cytokines. This effect was not due to contaminations of the protein preparations by endotoxins such as LPS. Furthermore, 48 hours after initial treatment of immature DCs, we observed up-regulation of costimulatory molecules such as CD86 (B7.2), MHC class II molecules, and, in the case of human DCs, CD83.
To test whether these mature DCs were also able to activate T cells better than immature DCs, we incubated mouse and human DCs treated with gp96 for 48 hours (as well as all controls mentioned above) with mouse or human T cells of different haplotype or donor, respectively, to observe the activation of allogeneic T cells. Proliferation of T cells was assayed after 5 days by incorporation of 3H thymidine. Indeed, proliferation of T cells incubated with gp96- or LPS-activated DCs was 2 to 3 times higher than of T cells incubated with immature DCs (Singh-Jasuja et al 2000a). At the same time proliferation of activated DCs was substantially lower compared with nonactivated DCs, confirming that activated DCs grow slower than immature DCs.
ACTIVATED DCs DOWN-REGULATE THEIR gp96 RECEPTOR(S)
As shown previously, human and mouse DCs are able to bind gp96 in a specific, receptor-mediated manner. Furthermore, receptor-mediated endocytosis of gp96 is required for activation of CTLs specific for gp96-associated antigen (Singh-Jasuja et al 2000b). We investigated whether the gp96 receptor(s), like other endocytic receptors, are down-regulated on mature DCs, which are specialized rather in presentation than uptake and processing of antigen (Albert et al 1998a). We found that activated mouse and human DCs that express high levels of CD86 and, in the case of human DCs, express CD83 no longer bound gp96. Hence, the gp96 receptor behaves similar to other endocytic receptors, such as the scavenger receptor CD36, integrins αvβ3 and αvβ5, and Fc receptors (Singh-Jasuja et al 2000a).
Hsps AS IDEAL CROSS-PRIMING VEHICLES
These latest results provide some important information on the nature of gp96-mediated and inflammatory immunity in a more general way. Indeed, gp96 and other Hsps could provide one missing link to the question what triggers inflammation induced by necrosis. Necrotic cells that result from tissue damage or viral infection release their contents, including gp96, into the extracellular fluid. Surrounding immature DCs could take up gp96 and other Hsps in a receptor-mediated fashion for presentation of gp96-bound antigen on MHC class I molecules. At the same time, gp96 would deliver a maturation signal, enabling the DCs to up-regulate their costimulatory molecules, such as CD86. Presentation of antigen in the context of MHC class I molecules and costimulators together would lead to priming of CD8+ T cells to cytotoxic effector cells and the induction of a proinflammatory cytokine response. In this model, recognition of antigen on MHC class II molecules by CD4+ T cells is not required, since the activation signal is already delivered by the antigen carrier gp96 itself. The question regarding the requirement of CD4+ T-cell help in Hsp immunization is still not clear; it has been shown that depletion of CD4+ T cells in the effector but not in the priming phase abrogates gp96-mediated immunity (Udono et al 1994).
Until now, debates about the nature of the cross-priming antigen carrier have included apoptotic vesicles (Albert et al 1998b), Hsps, immune complexes (Amigorena and Bonnerot 1998; Regnault et al 1999), and exosomes (Zitvogel et al 1998). Apoptotic cells, which occur rather frequently not only in embryonal development, were originally distinguished from necrotic cells on the basis of morphological differences and the ability of necrotic but not apoptotic cells to induce a proinflammatory cytokine response. Although necrosis is associated with release of proinflammatory cytokines such as TNF-α and IL-12 by APCs, apoptotic cells induce anti-inflammatory cytokines such as transforming growth factor β and IL-10 (Fadok et al 1998). Apoptotic bodies can be engulfed by DCs; the antigens they contain can be effectively processed and cross-presented on MHC class I molecules to activate T cells. Uptake of apoptotic cells, however, does not result in activation of DCs, whereas necrotic cells do so (Sauter et al 2000). Hence, apoptotic cells, although shown to be effective antigen carriers for cross-presentation, do not trigger costimulator expression and would be, on their own, ineffective for cross-priming. On the contrary, presentation of antigen to T cells lacking costimulators inactivates T cells by inducing peripheral tolerance (Steinman et al 2000).
From the current viewpoint, cross-priming mediated by Hsp-antigen complexes seems to be more attractive than by apoptotic bodies. It appears that Hsps and apoptotic cells, rather than being competitors in cross-priming, fulfill not only different but also opposing tasks. Interestingly, in this model, both processes—cross-priming by gp96 and cross-tolerance by apoptotic cells—require receptors for their initiation. Although the gp96 receptor is essential for cross-presentation of associated antigen (Singh-Jasuja et al 2000b), Fadok and colleagues have recently identified the phosphatidylserine receptor on bone marrow–derived macrophages to mediate the uptake of apoptotic cells (Fadok et al 2000). A few years ago, the same group was already able to show that late-stage apoptotic cells expose the lipid phosphatidylserine, which is normally localized to the inner leaflet of the plasma membrane, on the outer membrane leaflet (Fadok et al 1992).
The Hsp gp96 has been described years ago by Srivastava and colleagues as an effective antigen carrier that has some potential in immunotherapy of tumors. Today, we are able to understand the nature of the immune response elicited by gp96 much better, because a number of attributes meanwhile have been associated with this multifunctional molecule. First, the existence of one or more gp96 receptors enables the very efficient uptake of gp96 from the extracellular fluid. Second, the ability of gp96 to induce up-regulation of costimulatory activity and down-regulation of endocytic activity (such as the gp96 receptor) in DCs makes these cells ideally suited to prime T-cell responses. Third, the ability of gp96 to elicit the release of proinflammatory cytokines, hereby providing an effective danger signal also to the innate immune system, further supports the efficient induction of immune responses. Fourth, recent observations (Hilf et al, unpublished data) point into the direction that gp96 can bind specifically to platelets, and might play an additional role in wound healing.
Acknowledgments
We would like to thank Niels Emmerich for reading the manuscript and Pieter Spee for help with confocal microscopy. This work was supported by grants from the Deutsche Forschungsgemeinschaft Leibnizprogram to H.G.R. (Ra369/4-1), Sonderforschungsbereich (510, C1 to H.S.), and the European Union (Biomed 95-1627). The research of R.E.M. Toes has been made possible by a fellowship of the Royal Netherlands Academy of Arts and Sciences.
REFERENCES
- Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998a;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998b;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Amigorena S, Bonnerot C. Role of B-cell and Fc receptors in the selection of T-cell epitopes. Curr Opin Immunol. 1998;10:88–92. doi: 10.1016/s0952-7915(98)80037-x. [DOI] [PubMed] [Google Scholar]
- Amigorena S, Bonnerot C. Fc receptor signaling and trafficking: a connection for antigen processing. Immunol Rev. 1999;172:279–284. doi: 10.1111/j.1600-065x.1999.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med. 1995;182:885–889. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H, Schild H. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760. [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- Bevan MJ. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J Immunol. 1976;117:2233–2238. [PubMed] [Google Scholar]
- Carbone FR, Kurts C, Bennett SR, Miller JF, Heath WR. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol Today. 1998;19:368–373. doi: 10.1016/s0167-5699(98)01301-2. [DOI] [PubMed] [Google Scholar]
- Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, Germain RN. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191:1957–1964. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C, Vanbervliet B, Massacrier C, Azuma M, Okumura K, Lanier LL, Banchereau J. B70/B7-2 is identical to CD86 and is the major functional ligand for CD28 expressed on human dendritic cells. J Exp Med. 1994;180:1841–1847. doi: 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–3219. [PubMed] [Google Scholar]
- Cyster JG. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J Exp Med. 1999;189:447–450. doi: 10.1084/jem.189.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerich NP, Nussbaum AK, Stevanovic S, Priemer M, Toes RE, Rammensee HG, Schild H. The human 26 S and 20 S proteasomes generate overlapping but different sets of peptide fragments from a model protein substrate. J Biol Chem. 2000;275:21140–21148. doi: 10.1074/jbc.M000740200. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Cytotoxic T lymphocyte activation by cross-priming. Curr Opin Immunol. 1999;11:314–318. doi: 10.1016/s0952-7915(99)80050-8. [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Witmer-Pack M, Inaba M, et al. The tissue distribution of the B7–2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M, Schirmbeck R, Reimann J. MHC class I-restricted CTL responses to exogenous antigens. Immunity. 1996;5:295–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]
- Koch F, Stanzl U, Jennewein P, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- Lammert E, Arnold D, Nijenhuis M, et al. The endoplasmic reticulum-resident stress protein gp96 binds peptides translocated by TAP. Eur J Immunol. 1997;27:923–927. doi: 10.1002/eji.1830270418. [DOI] [PubMed] [Google Scholar]
- Lammert E, Stevanovic S, Brunner J, Rammensee HG, Schild H. Protein disulfide isomerase is the dominant acceptor for peptides translocated into the endoplasmic reticulum. Eur J Immunol. 1997;27:1685–1690. doi: 10.1002/eji.1830270714. [DOI] [PubMed] [Google Scholar]
- Luders J, Demand J, Hohfeld J. The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J Biol Chem. 2000;275:4613–4617. doi: 10.1074/jbc.275.7.4613. [DOI] [PubMed] [Google Scholar]
- Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- Moroi Y, Mayhew M, Trcka J, et al. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc Natl Acad Sci USA. 2000;97:3485–3490. doi: 10.1073/pnas.070550797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Wearsch PA, Mitchell DA, Wassenberg JJ, Gilboa E, Nicchitta CV. Calreticulin displays in vivo peptide-binding activity and can elicit CTL responses against bound peptides. J Immunol. 1999;162:6426–6432. [PubMed] [Google Scholar]
- Panjwani N, Akbari O, Garcia S, Brazil M, Stockinger B. The HSC73 molecular chaperone: involvement in MHC class II antigen presentation. J Immunol. 1999;163:1936–1942. [PubMed] [Google Scholar]
- Pierre P, Turley SJ, Gatti E, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- Regnault A, Lankar D, Lacabanne V, et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T- helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Rock KL. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–137. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- Rock KL, Goldberg AL. Degradation of cell proteins and the generation of MHC class I- presented peptides. Annu Rev Immunol. 1999;17:739–779. doi: 10.1146/annurev.immunol.17.1.739. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–368. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild H, Arnold-Schild D, Lammert E, Rammensee HG. Stress proteins and immunity mediated by cytotoxic T lymphocytes. Curr Opin Immunol. 1999;11:109–113. doi: 10.1016/s0952-7915(99)80019-3. [DOI] [PubMed] [Google Scholar]
- Schoenberger SP, Toes RE, van d V, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes REM, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000a;30:2211–2215. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Singh-Jasuja H, Toes RE, Spee P, et al. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000b;191:1965–1974. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spee P, Neefjes J. TAP-translocated peptides specifically bind proteins in the endoplasmic reticulum, including gp96, protein disulfide isomerase and Calreticulin. Eur J Immunol. 1997;27:2441–2449. doi: 10.1002/eji.1830270944. [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Ménoret A, Basu S, Binder RJ, McQuade KL. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltze L, Nussbaum AK, Sijts A, Emmerich NP, Kloetzel PM, Schild H. The function of the proteasome system in MHC class I antigen processing. Immunol Today. 2000;21:317–319. doi: 10.1016/s0167-5699(00)01665-0. [DOI] [PubMed] [Google Scholar]
- Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein- chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- Toes RE, Offringa R, Blom RJ, Brandt RM, van der Eb AJ, Melief CJ, Kast WM. An adenovirus type 5 early region 1B-encoded CTL epitope-mediating tumor eradication by CTL clones is down-modulated by an activated ras oncogene. J Immunol. 1995;154:3396–3405. [PubMed] [Google Scholar]
- Udono H, Levey DL, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc Natl Acad Sci USA. 1994;91:3077–3081. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg JJ, Dezfulian C, Nicchitta CV. Receptor mediated and fluid phase pathways for internalization of the ER Hsp90 chaperone GRP94 in murine macrophages. J Cell Sci. 1999;112:2167–2175. doi: 10.1242/jcs.112.13.2167. [DOI] [PubMed] [Google Scholar]
- Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]