Abstract
Human adipose-derived stromal cells (hASCs) have the proven capacity to ossify skeletal defects. The mechanisms whereby hASCs stimulate bone repair are not fully understood. In this study, we examined the potential for hASCs to stimulate autogenous repair of a mouse calvarial defect. Immunofluoresence, osteogenic stains, and surface electron microscopy were used to demonstrate osteogenic differentiation of hASCs. hASCs were engrafted into 4 mm calvarial defects in athymic mice using an osteoconductive scaffold. Analysis included microcomputed tomography, histology, in situ hybridization, and quantitative real-time–polymerase chain reaction. Next, the in vitro interaction between hASCs and mouse calvarial osteoblasts (mOBs) was assessed by the conditioned medium and coculture assays. The medium was supplemented with Hedgehog signaling modifiers, including recombinant N-terminal Sonic hedgehog, smoothened agonist, and cyclopamine. Finally, cyclopamine was delivered in vivo to hASC-engrafted defects. Significant calvarial healing was observed among hASC-engrafted defects compared with control groups (no treatment or scaffold alone) (*P < 0.05). hASCs showed evidence of stimulation of host mouse osteogenesis, including (1) increased expression of bone markers at the defect edge by in situ hybridization, and (2) increased host osteogenic gene expression by species-specific quantitative real-time polymerase chain reaction. Using the conditioned medium or coculture assays, hASCs stimulated mOB osteogenic differentiation, accompanied by Hedgehog signaling activation. N-terminal Sonic hedgehog or smoothened agonist replicated, while cyclopamine reversed, the pro-osteogenic effect of the conditioned medium on mOBs. Finally, cyclopamine injection arrested bone formation in vivo. hASCs heal critical-sized mouse calvarial defects, this is, at least in part, via stimulation of autogenous healing of the host defect. Our studies suggest that hASC-derived Hedgehog signaling may play a paracrine role in skeletal repair.
Introduction
As both the U.S. and global populations continue to age, so does the associated incidence of diseases and deficits afflicting the musculoskeletal system. This stems from postsurgical, post-traumatic, and degenerative etiologies. In 2007, it was estimated that diseases of the musculoskeletal system cost over 26 billion dollars with an average annual growth of 8.5% [1]. In the face of such staggering numbers, surgeons struggle with current techniques for bone repair, as none harnesses the ability of living tissue to dynamically remodel in response to environmental cues [2]. Ultimately, we hope that reconstructive strategies can shift from one of simple tissue repair to one of tissue regeneration [3].
Recent clinical studies have focused on the use of human adipose-derived stromal cells (hASCs) to replace bone loss [4]. In case reports, defects of the cranium [5], maxilla [6], and mandible [7] have been either healed or enabled to heal faster with the use of hASCs [8]. Such reconstructions eliminate the need for alloplastic materials, and thus reduce the risk of infection, breakdown, or rejection. In our laboratory, we have observed that ASCs, whether derived from mouse or human origin, contribute to osseous healing of mouse cranial defects [9,10]. For example, a critical-sized nude mouse calvarial defect shows no healing without ASC engraftment even up to 16 weeks postinjury [9,10]. Upon hASC engraftment, however, significant bony healing is observed in as little as 4 weeks postinjury [10].
Despite accumulating translational research, there is a paucity of data defining the mechanisms through which hASCs influence an osseous defect. It is unclear if hASCs directly form bone to heal a skeletal defect or, in polar opposition, if engrafted hASCs function as efficient factories to produce potent pro-osteogenic cytokines. In the case of our calvarial defect model, careful examination of calvarial defects engrafted with ASCs yields some profitable insights into the potential derivation of healing. For example, bone is often observed to mineralize from the edges of a cranial defect inward, which suggests that the host calvarium may contribute to the bony regenerate. Alternatively, small, isolated islands of bone are often observed early postoperatively, which suggest either contribution from engrafted ASCs or alternatively from host dura mater (a cell type of significant osteogenic potential) [11–13]. Finally, uniform and thorough healing of an hASC-engrafted defect suggests primary osseous healing derived from the engrafted donor cells themselves. It is likely that donor ASCs and host calvarial osteoblasts contribute to bony healing. In the current study, we postulated that the cell–cell interaction between engrafted ASCs and host calvarial osteoblasts is important for the healing of calvarial defects.
To examine this potential interaction, we set out to perform a series of in vivo and in vitro experiments. First, the edge of the hASC-engrafted defect site was examined as the probable site of hASC–mouse osteoblast (mOB) interaction by routine histology, in situ hybridization, and species-specific quantitative real-time polymerase chain reaction (qRT-PCR). Next, to further define this paracrine interaction, the conditioned medium (CM) and coculture experiments were performed with mOBs and hASCs. Moreover, hASCs and mOBs stimulate osteogenesis of the opposing cell type either through the use of CM or the coculture technique. This was accompanied and modulated by bone morphogenetic protein (BMP) and Hedgehog signaling. In addition, we observed engrafted hASCs to stimulate healing within calvarial defects. Finally, application of the hedgehog antagonist cyclopamine blocked this pro-osteogenic interaction, both in vitro and in vivo.
One signaling pathway we found to be significant in the interaction between osteoblasts and hASCs was Hedgehog signaling. Hedgehog proteins are secreted intercellular signaling molecules that exist in 3 mammalian types: Sonic, Indian, and Desert [14]. Hedgehog signaling is initiated by interaction with the receptor patched 1 (Ptc1), a transmembrane protein on the surface of hedgehog-responsive cells. Upon hedgehog stimulation of Ptc1, Smoothened (Smo; the transducer of a hedgehog signal) is released from inhibition by Ptch1 and activates members of the Glioma-associated oncogene (Gli) family of transcription factors: Gli1, Gli2, and Gli3. Gli1 and Gli2 are in general considered transcriptional activators, whereas Gli3 is a transcriptional repressor. Hedgehog signaling is important for skeletal patterning, and for the induction and maintenance of cellular differentiation [15]. Exogenous supplementation of Hedgehog protein has been shown to induce osteogenic differentiation in various bone forming cells in vitro [16–20]. Further, mouse strains with aberrant Hedgehog signaling demonstrate a severe skeletal phenotype [21,22]. Given the prominent role of Hedgehog signaling in skeletogenesis and bone biology, we hypothesized that Hedgehog signaling plays a role in the regeneration of bone in a critical-sized calvarial defect seeded with hASCs.
Materials and Methods
Chemicals, supplies, and animals
Dulbecco's modified Eagle's medium, minimum essential medium (α-MEM), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from Gibco Life Technologies. N-terminal Sonic hedgehog (Shh-N) was purchased from R&D Systems. Cell culture wares were purchased from Corning, Inc. Unless otherwise specified, all other chemicals were purchased from Sigma-Aldrich. CD-1 wild-type mice, CD-1 nude mice (Crl:CD-1 Foxn1nu), and CD-1 mice expressing Luciferase transgene under a GAPDH promoter were obtained from Charles Rivers.
Cell harvest
hASCs were harvested from human lipoaspirate derived from 8 women between the ages of 36 and 55, with an average age of 42.0 and average body mass index of 26.0 (Supplementary Table S1, available online at www.liebertonline.com/scd). Lipoaspirate was digested with a type II collagenase solution at 37°C. Cells were pelleted via centrifugation and filtered at 100 μm pore size, and primary cultures established at 37°C, 5% CO2 in Dulbecco's modified Eagle's medium and 10% FBS. Mouse ASCs (mASCs) were harvested via a similar fashion from CD-1 mice (3 weeks old) expressing Luciferase transgene [23]. Mouse parietal bone nonsuture associated calvarial-derived osteoblasts were obtained from p30 CD-1 wild-type mice as previously described [24]. Briefly, cranial sutures, dura mater, and periosteum were meticulously dissected off, specimens were minced, and digested in a collagenase A/dispase II solution (Roche Diagnostics Corporation). The fourth through sixth digestions were collected and primary cultures established in α-MEM, 10% FBS. For all assays, first passage cells only were used.
In vitro differentiation and assessments
For osteogenic differentiation, mOBs and hASCs were seeded either in coculture experiments or in CM experiments. For coculture assays, mOBs were seeded on 12-well transwell inserts (0.22 μm pore size) at a density of 35,000 cells per insert. hASCs were seeded underneath in 12-well plates at a density of 50,000 cells per well. Upon attachment, transwell inserts were combined and the medium was replenished with the osteogenic differentiation medium (ODM) [25]. All assays were performed in triplicate wells.
CM was obtained as follows. Briefly, 5 × 106 mOBs or hASCs were plated in 100 mm plates. After attachment, cells were washed with phosphate-buffered saline (PBS), and the serum-free medium was added and collected after 24 h. The medium was concentrated 10 times using Centricon filters (Centricon-3, 3000 NMWL; Millipore Corporation), and volumes were then normalized by cell number so that an equal volume of CM was produced by either hASCs or mOBs.
Cells were maintained for 7 days in ODM. To assess early osteogenesis, alkaline phosphatase (ALP) staining was performed at 3 days [26]. To assess bone nodule formation, alizarin red (AR) staining was performed at 7 days [26]. Specific gene expression was assayed by qRT-PCR (Supplementary Table S2, available online at www.liebertonline.com/scd). For select experiments, Shh-N, smoothened agonist (SAG), or cyclopamine were added to ODM. Concentrations used were based on data obtained from mASCs [27]. Vehicle control for Shh-N was 0.01% bovine serum albumin; for cyclopamine, 0.1% dimethyl sulfoxide.
Adipogenic differentiation was performed utilizing standard adipogenic differentiation components [28]. Oil red O staining was performed after 7 days [23].
Preparation of scaffolds
Apatite-coated poly(lactic-co-glycolic acid) (PLGA) scaffolds were fabricated from PLGA 85/15 by solvent casting and a particulate leaching process. Briefly, PLGA/chloroform solutions were mixed with 200–300-m-diameter sucrose to obtain 92% porosity (volume fraction), and compressed into thin sheets in a Teflon mold. After freeze-drying overnight, scaffolds were immersed in ddH2O to dissolve the sucrose, and gently removed from the Teflon plate for disinfection and drying.
For apatite coating, simulated body fluid (SBF) solution was prepared by sequentially dissolving CaCl2, MgCl2·6H2O, NaHCO3, and K2HPO4·3H2O in ddH2O. Solution pH was lowered to 6 by adding 1 M hydrochloric acid to increase the solubility. Na2SO4, KCl, and NaCl were added and the final pH was adjusted to 6.5 (SBF 1). Mg2+ and HCO3−-free SBF (SBF 2) was prepared by adding CaCl2 and K2HPO4 · 3H2O in ddH2O and pH was lowered to 6. KCl and NaCl were added and the final pH was adjusted to 6.8. All solutions were sterile filtered through a 0.22 μm polyethersulfone (PES) membrane (Nalgene). The obtained PLGA scaffolds were incubated in SBF 1 for 12 h and changed to Mg2+ and HCO3−-free SBF 2 for another 12 h at 37°C under gentle stirring. Coated scaffolds were washed with ddH2O to remove excess ions and lyophilized before further studies.
Creation of calvarial defects
Nonhealing, critical-sized (4 mm) calvarial defects were created in the right parietal bone of adult (60 days old) male CD-1 nude mice. After anesthesia, the surgical site was cleaned, an incision was made just off the sagittal midline, and pericranium was removed from the right parietal bone. A unilateral 4-mm full-thickness defect was created using diamond-coated trephine bits in the nonsuture associated right parietal bone, leaving dura mater undisturbed.
In preparation for cell engraftment, scaffolds were seeded with hASCs or mASCs 24 h before implantation. Cells (150,000) were placed on scaffolds in 125 μL of medium in 96-well culture plates and incubated for 24 h. Before implantation, scaffolds were copiously rinsed with PBS. Animals were divided equally into 4 treatment groups: (1) empty defects in which a 4 mm defect was created but left empty (n = 5), (2) scaffold only, in which a PLGA scaffold without cells was placed in the defect site (n = 5), (3) hASCs on a scaffold, in which hASCs were impregnated in a scaffold and this was then placed in the defect site (n = 5), and (4) luciferase mASCs on a scaffold (n = 5). Finally, the skin was sutured and animal monitored per established postoperative protocols.
In additional experiments, cyclopamine was delivered postoperatively. Briefly, on postoperative days 1–3 animals were sedated and a sterile saline suspension of cyclopamine was injected subcutaneously directly overlying the site of hASC engraftment (50 μL of a 20 μM solution). Animals were injected with saline only as a control.
In vivo imaging
Micro-computed tomography (CT) was performed on live animals in a serial manner postoperatively (through 8 weeks healing), using a high-resolution MicroCAT II™ (ImTek, Inc.) small animal imaging system, with the following settings: X-ray voltage of 80 kVp, anode current of 500 μA, and an exposure time of 500 ms for each of the 360 rotational steps. The 2D projection images were used to reconstruct tomograms with a Feldkamp algorithm, using a commercial software package (Cobra EXXIM; EXXIM Computing Corporation), resulting into a resolution of 80 μm. Three-dimensional reconstructions were generated by MicroView software (GE Healthcare). Fraction bony healing was quantified using Adobe Photoshop, comparing scans to those on the same animal taken immediately postoperatively.
For in vivo imaging system (IVIS), mice were anesthetized and luciferin (150 mg/kg in 200 μL) injected into the peritoneal cavity. After 10 min, animals were then placed in the IVIS 200B™ imaging system and imaged for 3 min. Animals were then allowed to recover.
Histologic analyses
From 1 to 8 weeks postoperatively, animals were sacrificed for histology. Calvaria were harvested, formalin fixed, decalcified in 19% EDTA, paraffin imbedded, and sectioned at 8 μm thickness. Aniline blue staining was performed on every 10th section throughout the sample. Histomorphometry of each aniline blue section was performed to obtain an average pixel number of new bone per histological field [29]. Next, select slides were stained with pentachrome, in which bone appears bright yellow. ALP and saffranin O staining was performed on select slides [23,29]. ALP expression was quantified on select slides utilizing the magic wand tool in Adobe Photoshop (n = 5 slides per group). In situ hybridization was performed on select slides for mouse Runt-related transcription factor-2 (Runx2), osteocalcin (Ocn), and Ptc1 [29]. Nonspecific binding was minimized by high stringency hybridization conditions, for all assays sense probes were used side-by-side with minimal background.
Surface electron microscopy
To examine cellular morphology, surface electron microscopy (SEM) was performed on hASCs after 3 days in culture (either in the standard growth medium, adipogenic medium, or osteogenic medium). Cells were fixed on glass cover-slips (12 mm circular, 15,000 cells) with 4% paraformaldehyde, 2% glutaraldehyde in 0.1 M sodium cacodylate buffer. As well, cell attachment on PLGA scaffolds was confirmed by SEM observation. Next, samples were rinsed in the same buffer and postfixed for 1 h with 1% aqueous OsO4. After dehydration in an ascending ethanol series, samples were critical point dried with liquid CO2 in a Tousimis Autosamdri-814 apparatus (Tousimis), mounted on adhesive carbon films on 15 mm aluminum stubs (Ted Pella), and sputter-coated with 100A of Au/Pd using a Denton Desk 11 Sputter Coater. Observation was performed with a Hitachi S-3400N variable pressure SEM operated at 15 kV, working distance 7–8 mm, and secondary electron detection under high-vacuum conditions (<1 Pa).
Immunofluorescent staining
Cells were fixed with 2% formaldehyde in PBS for 2 min, permeabilized with 0.5% Triton X-100 in PBS for 10 min, and blocked with 5% bovine serum albumin in PBS for 1 h. Cells were then stained with appropriate primary antibodies and AlexaFluor-conjugated secondary antibodies (Invitrogen). The primary antibodies for OCN (Santa Cruz Biotechnology), RUNX-2 (Santa Cruz Biotechnology), and BMP-2 (Santa Cruz Biotechnology) were used in the staining.
Polymerase chain reaction
Total RNA was isolated from cells and tissue as previously described [25,29]. For cells, after 2 washes in cold, sterile PBS, cells were lysed with a cell scraper, followed by isolation with the RNeasy Mini Kit (Qiagen Sciences). For tissue, RNA was isolated from formalin-fixed, paraffin-embedded slides using the RecoverAll RNA kit (Ambion; Cat No. AM1975). The defect site of 6 slides was meticulously dissected (∼24 sections), which was used as tissue starting material. Reverse transcription was performed with 1 μg RNA using TaqMan Reverse Transcription Reagents (Applied Biosystems). qRT-PCR was carried out using the Applied Biosystems Prism 7900HT Sequence Detection System and Sybr Green PCR Master Mix (Applied Biosystems). Specific primers for the genes examined were based on their PrimerBank sequence and are listed in Supplementary Table S2. Genes assays included standard osteogenic markers, such as ALP and RUNX2, and extracellular matrix components, such as OCN, osteopontin (OPN), and type I collagen (COL1A1). Genes involved in transduction of Hedgehog signaling were also assayed, including the transmembrane receptor for Hedgehog signaling PTC1, and the effectors of Hedgehog signaling, Glioma-associated oncogene transcription factors, GLI1, GLI2, and GLI3. Transcription of PTC1 is positively regulated by Hedgehog signaling, and can be considered a surrogate marker of Hedgehog activation. GLI1 and GLI2 are in general considered transcriptional activators, whereas GLI3 is a transcriptional repressor. Finally, individual BMP family members were evaluated. The most commonly studied, pro-osteogenic BMP ligands were assayed in a candidate fashion, BMP2, BMP4, and BMP7. The levels of gene expression were determined by normalizing to values of mouse or human Gapdh and performed in triplicate. The PCR product was run out on a 2% agarose gel to verify appropriate product size.
Statistical analysis
Means and standard deviations were calculated from numerical data, as presented in the text, figures, and figure legends. In figures, bar graphs represent means, whereas error bars represent one standard deviation. Statistical analysis was performed using an appropriate analysis of variance when >2 groups were compared, followed by a Student's t-test to directly compare 2 groups. The exact statistical analysis for each dataset is described in the figure legends. Inequality of standard deviations was excluded by employing the Levene's test. *P ≤ 0.05 was considered to be significant.
Results
Characterization of hASCs
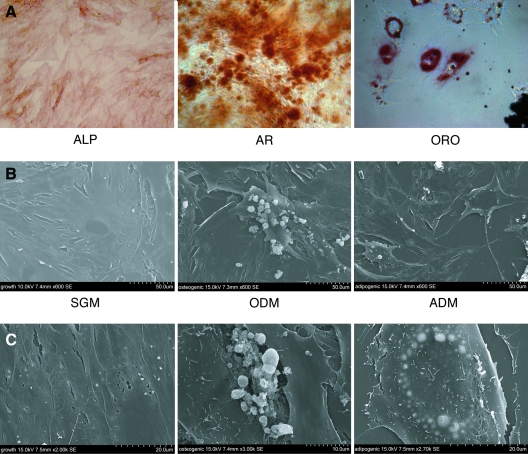
First, we verified that hASCs retained the ability to differentiate toward both osteoblast and adipocyte lineages (Fig. 1A). ALP staining and AR staining at 3 and 7 days, respectively, in ODM was performed, showing robust osteogenesis in the absence of exogenous growth factors (left and middle, Fig. 1A). Contrarily, after 7 days in the adipogenic differentiation medium (ADM), significant intracellular lipid accumulation was observed by Oil red O staining (right, Fig. 1A). Next, cell surface morphology was examined by SEM (Fig. 1B). After 3 days in the standard growth medium, hASCs had a flattened, broad cell surface with some stellate cell surface projections (Fig. 1B, left 600×, left 2,000×). After 3 days in ODM, cell edges appeared more roughened and scalloped, whereas bone nodules appeared in clusters both on top and between hASCs (Fig. 1B, middle 600×, middle below 3,000×). After 3 days in ADM, hASCs exhibited a more star-shaped appearance and the majority of cells showed accumulating intracellular lipid (appearing light) in a circumferential pattern about the nucleus (Fig. 1C, right 600×, right 2,700×). Thus, hASCs when isolated and cultured from lipoaspirate retain the ability to differentiate toward both osteo- and adipocytic phenotypes.
FIG. 1.
Characterization of human adipose-derived stromal cells (hASCs). (A) Differentiation of hASCs toward osteoblast and adipocyte cell fates. (A, left) Alkaline phosphatase (ALP) staining after 3 days differentiation, demonstrating early osteoblast differentiation, 10×. (A, middle) Alizarin red (AR) staining after 7 days differentiation, indicating terminal osteoblast differentiation, 10×. (A, right) Oil red O (ORO) staining after 7 days adipogenic differentiation, showing intracellular lipid accumulation, 40×. (B, C) Surface electron microscopy of passage 1 hASCs after 3 days in the standard growth medium (SGM), osteogenic, or adipogenic differentiation medium (ODM or ADM). In SGM (left), hASCs have a smooth cell surface, while the appearance of bone nodules (center) or intracellular lipids (right) were appreciable with ODM and ADM, respectively, 600×–3,000× magnification. Color images available online at www.liebertonline.com/scd.
Lastly, we demonstrate an increase in osteogenic gene expression during osteogenic differentiation by immunofluorescence of hASCs in culture (Supplementary Fig. S1, available online at www.liebertonline.com/scd). At day 0, there are low levels of RUNX-2, a gene of early osteogenic differentiation, and OCN and BMP-2, genes of late osteogenic differentiation (Supplementary Fig. S1A, B left column, available online at www.liebertonline.com/scd). At day 3, as expected, we see an increase of RUNX-2 expression, which is localized in the nucleus (Supplementary Fig. S1A middle column). At day 7, there was an increase in expression of OCN in the cytoplasm and continued expression of RUNX-2 (Supplementary Fig. S1A right column) with both negative controls demonstrating an absence of stain (Supplementary Fig. S1A left). We also demonstrate an increase in BMP-2 expression by immunofluoresence during osteogenic differentiation (Supplementary Fig. S1B).
ASCs engraftment in a calvarial defect model
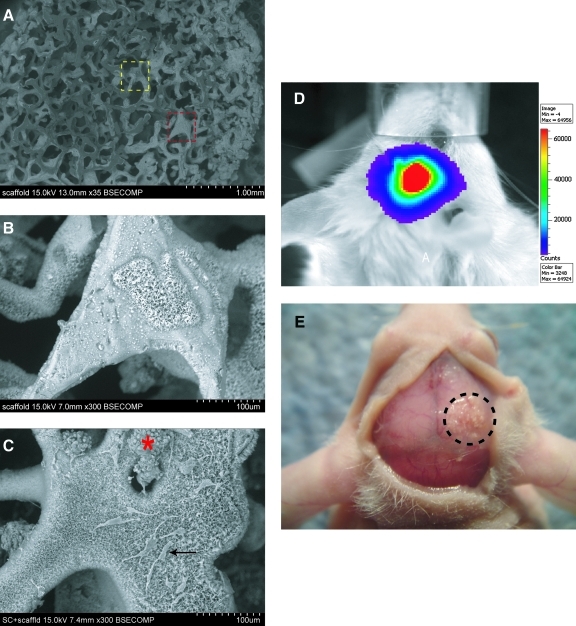
A critical-sized (4 mm) calvarial defect model was employed as previously described [9]. First, hASCs attached to an osteoconductive scaffold [hydroxyapatite (HA)-PLGA] was verified by direct observation (Fig. 2A–C). As depicted in Fig. 2A, a porous discoid scaffold was created to fit in the defect site (35×). At high magnification, the coral-like appearance of the HA coating was apparent (Fig. 2A yellow square and Fig. 2B, 300×). ASCs showed preferential attachment to the HA-coated surfaces, either spreading in single cell distributions (black arrow) or combining in large clusters (red asterisk) (Fig. 2A red square and Fig. 2C, 300×). Next, transplanted ASC survival was verified within a calvarial defect site. For this purpose, mASCs were harvested from Firefly Luc+ transgenic mice, such that Luc was constitutively expressed by live mASCs. Luciferase detection was performed using IVIS (Fig. 2D). At 2 weeks postoperatively, a strong, localized signal was observed within the defect site (Fig. 2D). Moroever, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining on histological sections at this time point showed very sparse positive staining within the defect site (data not shown). Finally, gross analysis at 2 and 8 weeks revealed that scaffolds remained in place within the right parietal bone defect (encircled with dashes, Fig. 2E).
FIG. 2.
ASCs are engrafted and survive in a mouse cranial defect. (A) Appearance of hydroxyapatite-coated PLGA scaffold by surface electron microscopy, 35×. Higher magnification images of the areas within the dotted yellow and red lines can be seen in (B) and (C), respectively. (B) Scaffold appearance at 300×, hydroxyapatite appears coral-like with fine pore size. (C) hASCs attached on scaffold, 300×. Cells either spread out individually (black arrow) or remain in cell clusters (red asterisk). (D) Image of luciferase activity of a mouse 2 weeks postoperatively, after engraftment with Luc+ mouse ASCs. Scale on the right indicates intensity of signal, with red being the strongest signal. (E) Gross photographic image of nude CD-1 mouse 8 weeks after engraftment of hASCs. Encircled area shows the previous 4 mm parietal defect that was treated with a HA coated PLGA scaffold seeded with hASCs. Color images available online at www.liebertonline.com/scd.
Effects hASCs and osteoblasts in vivo healing of a calvarial defect
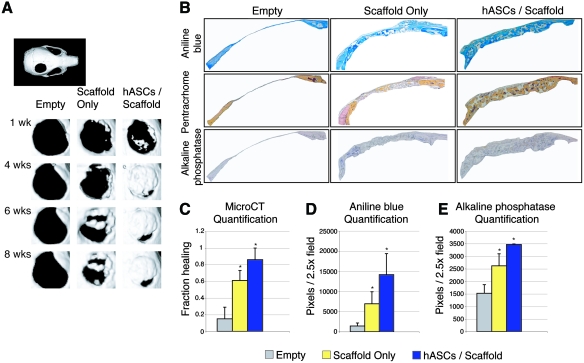
Having demonstrated successful engraftment, we confirmed next that hASCs were capable of healing a critical sized calvarial defect (Fig. 3), as previously observed [10]. In as little as 1 week, hASCs showed patchy bone formation (top row, Fig. 3A). By 4 weeks, the majority of the hASC-engrafted defects showed robust healing (second row, Fig. 3A). These findings were comparable to defects left empty or scaffold-only engrafted defects, which showed significantly less healing. Results were quantified and presented as average fraction healing of the original defect size (Fig. 3C). After 8 weeks serial CT scans, animals were sacrificed and histologic analysis was performed. Aniline blue staining and quantification, in which osteoid appears dark blue, recapitulated findings by micro-CT: hASC-engrafted scaffolds demonstrated significant bone formation within the defect site compared with other groups (Fig. 3B, top row; Fig. 3D). Adjacent slides were stained with both pentachrome and ALP (Fig. 3B middle and bottom row; Fig. 3E), which appear yellow and purple, respectively. Significant bone formation was again observed throughout hASC-treated defects, which was observed to completely bridge the defect site. These data suggest that hASC-engrafted HA-PLGA scaffolds can be successfully utilized to ossify a nonhealing calvarial defect, yet the mechanism of hASC-mediated repair remained obfuscated.
FIG. 3.
hASCs heal mouse critical-sized defects. (A) Micro-computed tomography (CT) of defect sites postoperatively. Defects were either left empty or treated with scaffold only or hASCs with scaffold (n = 5 per group). Micro-CT images were obtained at stratified time points up to 8 weeks postoperatively. In upper left, a whole skull is provided for orientation. (B) After 8 weeks, mice were sacrificed for histological analysis. From left to right, defects shown include those left empty, treated with scaffold only, or treated with scaffold and hASCs. From top to bottom, staining includes aniline blue, pentachrome, and ALP, with bone appearing blue, yellow, and purple, respectively. (C) At 8 weeks, healing was quantified by micro-CT and Adobe Photoshop, expressed as average fraction osseous healing of the original defect size. (D) At 8 weeks, bone formation was quantified by Adobe Photoshop measurement of average aniline blue positive bone per 2.5 × field, n = 50 slides per group, 5 animals per group. (E) At 8 weeks, bone activity was quantified by Adobe Photoshop measurement of average ALP-positive bone per 2.5 × field, n = 5 slides per group, 5 animals per group. A one-factor analysis of variance (ANOVA) was utilized, followed by a post hoc Student's t-test to assess significance (*P < 0.05). Color images available online at www.liebertonline.com/scd.
hASCs upregulate osteogenic gene expression at the osteoblast/hASC junction
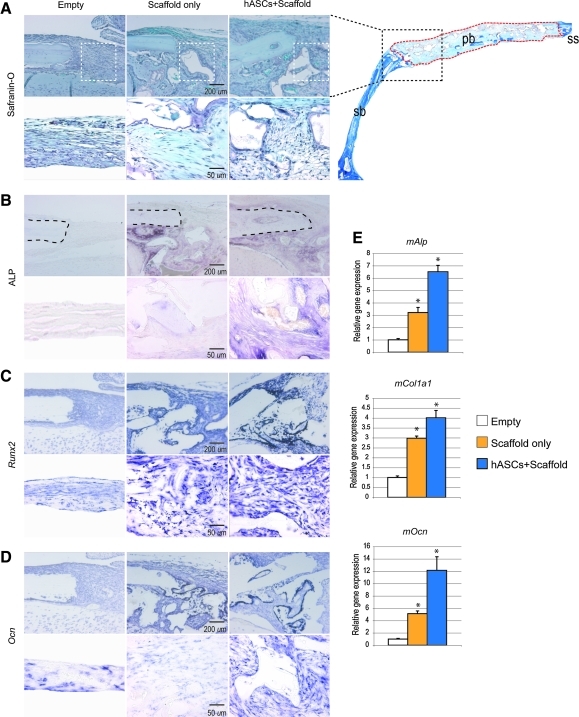
We next sought to define the interaction between engrafted hASCs and the host defect site and defect-derived osteoblasts. This was performed by in situ hybridization and qRT-PCR analysis specific for mouse genes. Histology was obtained after 2 weeks postoperatively, the time we identified as representing the most active healing period (data not shown). For orientation, a low magnification aniline blue stain of the entire defect is shown (Fig. 4A, right). Safranin-O staining was performed in which the bone appears light blue (top row, Fig. 4A). At this early time point, very little expression of ALP activity was noted among empty defects (left column, Fig. 4B; dashed lines show defect margin). Both defects treated with scaffold only (middle column) and hASC-engrafted scaffolds (right column) showed upregulation of ALP activity at the defect edge (second row, Fig. 4B). Next, in situ for mouse Runx2 and Ocn was performed. Again both scaffold alone and hASC-engrafted scaffolds were observed to increase expression of these osteogenic genes in the mouse. Importantly, the most robust staining was consistently observed among hASC-treated defects (right column, Fig. 4C, D). Sense probes detected minimal background staining in all cases (data not shown). Next we sought to quantify these differences that we observed histologically. RNA was harvested from the defects and pooled for analysis at 2 weeks postoperatively. Consistent with our in situ analysis, osteogenic genes specific for mouse showed significant upregulation among hASC-treated defects (Fig. 4E; *P < 0.05). This included both early and late markers of osteogenesis, including mAlp, mCol1a1, and mOcn. Collectively, these data suggested that hASC engraftment stimulates autogenous mOB activity within a cranial defect site. We next turned to in vitro cell culture to more thoroughly define this relationship.
FIG. 4.
hASCs stimulate mouse osteoblast (mOB) activity in a calvarial defect. (A) Histological analysis of the defect edge of defects left empty, treated with scaffold only, or treated with scaffold/hASCs at 2 weeks postoperatively. In the upper right corner, a low magnification image (2×) of the defect site in the right parietal bone (pb) and surrounding calvaria is shown for orientation purposes. The black dotted line depicts the edge of the defect site in the top panel of (A–D). The red dotted line outlines the defect site. Higher magnification images of the defect site, area outlined in white dotted lines in top panel of (A), can be seen in the bottom panel of (A–D). (A) Safranin-O staining, in which osteoid appears light blue. An absence of cartilage was noted in all sections, which would appear pink. (B) ALP staining, nonspecific for mouse versus human activity. (C) Runt-related transcription factor-2 (Runx2) expression by in situ hybridization, specific for mouse. (D) Osteocalcin (Ocn) expression by in situ hybridization, specific for mouse. (E) Quantitative real-time polymerase chain reaction analysis of RNA derived from cranial defects at 2 weeks postoperatively, specific for mouse genes. A significant induction of mAlp, mCol1a1, and mOcn was observed with hASC engraftment. n = 5 animals per group, *P < 0.05. A one-factor ANOVA was utilized, followed by a post hoc Student's t-test to assess significance. Sagittal suture (ss) and squamosal bone (sb). Color images available online at www.liebertonline.com/scd.
hASCs induce osteogenic differentiation of mOBs in vitro
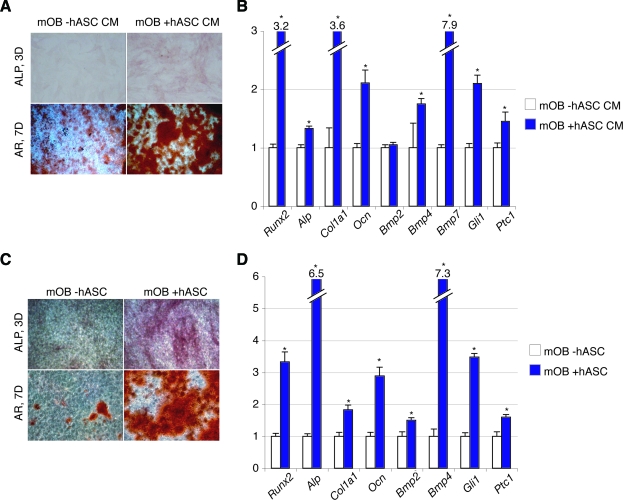
Given our in vivo findings, we next sought to identify the molecular underpinnings of this cell–cell interaction. First, we hypothesized that hASCs could stimulate mOB osteogenic differentiation by paracrine means. We tested this both with the use of CM (Fig. 5, top) and coculture assays (Fig. 5, bottom). CM obtained from hASCs increased mOB bone nodule formation after 7 days, as assessed by AR staining (Fig. 5A). Likewise, osteogenic markers such as mRunx2, mAlp, mCol1a1, and mOcn were increased with the addition of hASCs CM to ODM (Fig. 5B). A similar phenomenon was observed with coculture of mOBs with hASCs (Fig. 5, bottom). ALP staining at 3 days differentiation showed a marked increase in the intensity of staining upon hASC coculture with mOBs (Fig. 5C, top). Similarly, bone nodule formation showed increased intensity with coculture (Fig. 5C, bottom). As with CM, results were validated by the examination of mouse osteogenic gene expression by qRT-PCR, showing an increase in all markers upon coculture with hASCs (mRunx2, mAlp, mCol1a1, and mOcn, *P < 0.05; Fig. 5D).
FIG. 5.
hASCs stimulate mOB differentiation in vitro. (A, B) mOBs were placed in osteogenic differentiation with or without supplementation of the hASC-conditioned medium (hASC CM). (A) ALP and AR staining of mOBs with or without hASC CM at 3 and 7 days differentiation, respectively. (B) Gene expression profile of mOBs after 3 days differentiation with or without hASC CM. Markers examined include osteoblast-specific genes, bone morphogenetic protein (BMP), and Hedgehog genes. (C, D) mOBs were place in osteogenic differentiation alone or in coculture of hASCs. (C) ALP and AR staining at 3 and 7 days, respectively, either alone or in coculture with hASCs. (D) Gene expression profile of mOBs after 3 days differentiation alone or in coculture with hASCs. n = 3 wells for all assays, *P < 0.05. A two-tailed Student's t-test was performed to assess significance. Color images available online at www.liebertonline.com/scd.
Signaling pathways of importance in osseous differentiation were next assessed on the transcriptional level among mOBs, either with stimulation of CM or with coculture (right, Fig. 5B, D). BMPs, first described for their ability to induce ectopic bone formation, have been demonstrated to be of significant importance in the osseous differentiation of ASCs. We assayed individual BMPs, including mBmp2, mBmp4, and mBmp7. The majority of BMP transcripts were observed to significantly increase upon either addition of hASC CM to ODM, or alternatively via the coculture of mOBs with hASCs. Hedgehog signaling has been shown to be important in the osteogenic differentiation of both unipotent and multipotent cells, including ASCs [27]. We therefore next analyzed the expression profile of 2 reporters of Hedgehog signaling activity, mGli1 and mPtc1. Again, either hASC CM or hASC coculture led to an increase in these markers of Hedgehog pathway activity. Thus, in aggregate, by both the condition medium and coculture techniques, hASCs induced the in vitro osteogenic differentiation of mOBs. This was accompanied by a global increase in BMP and Hedgehog signaling pathways.
mOBs induce osteogenic differentiation of hASCs in vitro due to Sonic signaling
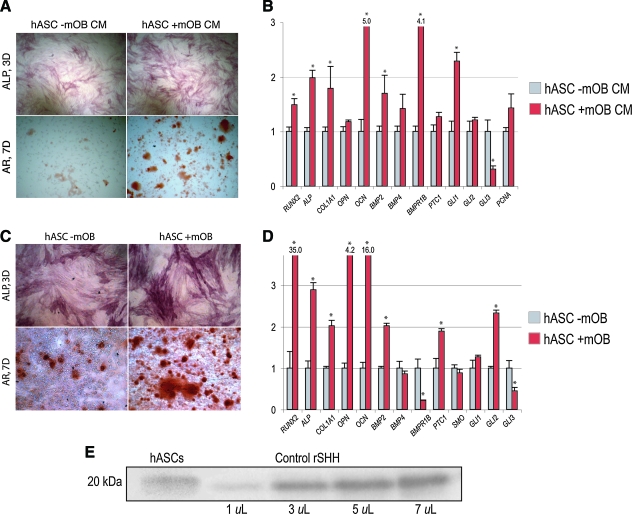
Having observed the paracrine effects of hASCs on mOBs, the converse relationship was assayed. Utilizing the same techniques of CM and coculture, the effects of mOBs on hASC osteogenic differentiation were assessed (Fig. 6). AR staining at 7 days differentiation showed that mOB CM had a significant pro-osteogenic effect on hASCs (Fig. 6A). Results were verified by qRT-PCR, showing overall an increase in human osteogenic gene markers upon addition of mOB CM to ODM (hRUNX2, hALP, hCOL1A1, hOPN, and hOCN, *P < 0.05) (Fig. 6B). Likewise, coculture of hASCs with mOBs yielded similar results (Fig. 6, bottom), including an upregulation of ALP and AR staining as well as many human genes of osteogenic differentiation (Fig. 6C, D).
FIG. 6.
mOBs stimulate hASC differentiation in vitro. (A, B) hASCs were placed in ODM with or without supplementation of the mOB-conditioned medium (mOB CM). (A) ALP and AR staining of hASCs with or without mOB CM at 3 and 7 days differentiation, respectively. (B) Gene expression profile of hASCs after 3 days differentiation with or without mOB CM. Markers examined include osteoblast-specific genes, BMP, and Hedgehog genes. (C, D) hASCs were placed in osteogenic differentiation alone or in coculture of mOBs. (C) ALP and AR staining at 3 and 7 days, respectively, either alone or in coculture with mOBs. (D) Gene expression profile of hASCs after 3 days differentiation alone or in coculture with mOBs. n = 3 for all assays, *P < 0.05. A two-tailed Student's t-test was performed to assess significance. (E) Western immunoblot analysis of hASCs for Sonic hedgehog (SHH) demonstrating expression of the 20 kDa protein (left). This was run concurrently with different concentrations of recombinant human SHH as a positive control (right). Color images available online at www.liebertonline.com/scd.
Individual components of BMP and Hedgehog signaling pathways among hASCs were again assessed by qRT-PCR in both CM and coculture environments (hBMP2, hBMP4, hBMPR1B, hGLI1, hGLI2, hGLI3, and hPTC1). BMP2 transcript abundance increased with either CM or coculture mOB stimulation (*P < 0.05). Transcriptional activators of Hedgehog signaling were increased in expression, including GLI1 and GLI2, whereas the negative regulator GLI3 was significantly decreased in expression upon either mOB CM or coculture exposure (*P < 0.05). Thus, in aggregate, a reciprocal relationship was observed between hASCs and OBs in culture: both cell types were observed to enhance the osseous differentiation of the other cell type. This was in both cases accompanied by increased BMP and Hedgehog signaling.
Lastly, we set to demonstrate that untreated hASCs express SHH protein by immunoblotting and thus looked at total lysate from hASCs. Since we do not pretreat the hASCs with the osteogenic medium, we analyzed untreated hASCs. Indeed, we note that untreated hASCs do express SHH protein (Fig. 6E).
hASC-mediated mOB osteogenesis can be modulated by Hedgehog signaling
Our in vivo and in vitro experiments suggested that the hASC–mOB interaction resulted in a profitable enhancement of osteogenic differentiation, which ultimately resulted in a well-healed osseous defect. In both instances of CM and coculture enhancement of mOB osteogenesis, an increase in Hedgehog signaling among mOBs was an associated finding. We next set out to examine if enhanced Hedgehog signaling in fact underlie rather than simply accompany the pro-osteogenic effect of this paracrine relationship.
Next, we verified as others have reported [17,18] that Hedgehog signaling increases markers of differentation in calvarial-derived osteoblasts (Supplementary Fig. S2, available online at www.liebertonline.com/scd). Either SAG or recombinant Shh-N were added ODM. Concentrations of Shh-N (250–500 ng/mL) were utilized based on previous observation in mASCs; a dose-response curve was performed for SAG [27]. Briefly, either the addition of SAG and Shh-N to the osteogenic medium significantly increased all markers of differentiation examined. This included ALP activity (Supplementary Fig. S2A, B, available online at www.liebertonline.com/scd), osteogenic gene expression (Runx2 and Alp) and bone nodule formation (Supplementary Fig. S2D, available online at www.liebertonline.com/scd).
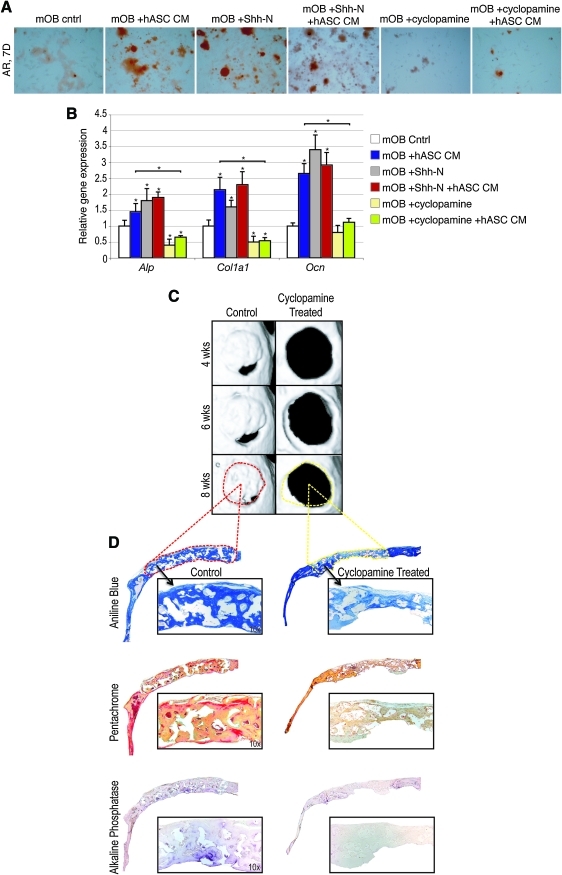
We then postulated that the osteogenic effect of hASC CM on mOB would be replicated by the addition of Shh-N and conversely antagonized by the addition of cyclopamine. Concentrations were based on previous observation in mASCs [27]. AR staining was performed at 7 days differentiation (Fig. 7A). Concordantly, RNA was analyzed under identical conditions after 3 days differentiation (Fig. 7B). Our results demonstrated that indeed Shh-N enhanced the osteogenic differentiation of mOBs to a similar degree as hASC CM (eg, compare blue and gray bars, Fig. 7B). Conversely, cyclopamine inhibited osteogenesis in mOBs and fully reversed the pro-osteogenic effect of hASC CM addition to ODM (yellow and green bars, Fig. 7B). Thus, in summary, the pro-osteogenic effect of SHH on osteoblasts and hASC CM on mOBs was (1) accompanied by an increase in Hedgehog signaling, (2) could be replicated by the addition of recombinant Shh protein to the medium, and (3) could be fully antagonized by the Hedgehog antagonist cyclopamine.
FIG. 7.
Hedgehog signaling modulates the pro-osteogenic effect of hASC–mOB paracrine relationship. (A) mOBs were seeded in differentiation assays with N-terminal Sonic hedgehog (Shh-N) (250 ng/mL) or cyclopamine (20 mM) added to ODM, either alone or in combination with hASC CM. (A) AR staining at 7 days differentiation of mOBs under various conditions. (B) Osteogenic gene expression as assayed by quantitative real-time polymerase chain reaction at 3 days differentiation under various conditions. (C, D) Effects of cyclopamine administration to hASC-engrafted defects. A 50 μL suspension of cyclopamine (20 mM) was delivered by subcutaneous injection directly overlying the defect site for the first 3 postoperative days. (C) Micro-CT images were obtained at stratified time points up to 8 weeks postoperatively. Dotted red line represents area of histology for control below. Yellow dotted line depicts histology of cyclopamine-treated defect below. (D) Staining for aniline blue (above), pentachrome (middle), and ALP (below) was performed after 8 weeks healing. n = 3 for all assays, *P < 0.05. A 2-factor ANOVA was utilized (with or without hASC CM, with or without additional factors), followed by a post hoc Student's t-test to assess significance. Color images available online at www.liebertonline.com/scd.
These results strongly suggested that the in vitro pro-osteogenic effect of hASCs unto mOBs as illustrated by CM assays was at least in part produced by and dependent on paracrine Hedgehog signaling. We next set out to test our hypothesis in vivo. To assess Shh expression pattern of mouse in vivo, we first looked at Ptc1 as a reporter for Hedgehog signaling in defect sites either left empty or treated with scaffold only or scaffold with hASCs at 1 week postoperatively (Supplementary Fig. S2F, available online at www.liebertonline.com/scd). Results showed a small and limited increase in mouse Ptc1 expression at the defect site among defects left empty (left, Supplementary Fig. S2F, available online at www.liebertonline.com/scd). The distribution of mouse Ptc1 staining was expanded at the defect site edge among hASC-engrafted defects (right, Supplementary Fig. S2F).
Next, we sought to regulate the Shh pathway in vivo by adding the hedgehog antagonist cyclopamine. After hASC calvarial engraftment, hedgehog signaling was blocked by the addition of cyclopamine via subcutaneous injection (days 1–3 postoperatively) (Fig. 7C). This short application time of the alkaloid had significant long-term effects on defect ossification: at 8 weeks, while control injected mice showed significant bony healing, those injected with cyclopamine did not. Representative slides are shown, stained with aniline blue, pentachrome, and ALP (Fig. 7D). Thus, the addition of cyclopamine interfered with the pro-osteogenic effect of hASCs, whether in vitro or in vivo.
Discussion
Multiple hurdles exist between current use of hASCs in the laboratory setting and their future translation to bedside application. For example, standard components of the cell growth medium such as FBS, for example, have been studied and observed to potentially harbor oncogenic properties [30,31], as have other cytokines commonly studied in ASC biology [32–34]. Consequently, the duration and magnitude of exposure to such agents must be tightly controlled. Next, the procedure of hASC engraftment must be optimized for each tissue engineering purpose. For example, hASC basic biology differs significantly based on gender, patient age, and site of anatomic derivation [28,35,36]. In the future, a surgeon must take these differences into account when planning autologous reconstruction of skeletal or soft tissue defects. Finally, the defect site of engraftment must be considered as well. We have observed in the present study what would likely intuite: namely, that hASCs when engrafted into a defect site will interact with their surroundings. This may be beneficial in the case of an acute cranial defect, such that the hASC–mOB interaction results in a profitable increase in osteogenic differentiation among both cell types.
In our study, we identified in a candidate fashion 2 pathways, which may play a role in the pro-osteogenic paracrine relationship between hASCs and mOBs: BMP and Hedgehog signaling. BMP signaling, first recognized to stimulate ectopic bone formation, has since been shown to be important in osteogenic differentiation of ASCs [37–41]. In fact, BMP signaling via the receptor Bmpr1b has been shown to be necessary for mASC osteogenic differentiation, as siRNA-mediated knockdown inhibits osteogenic differentiation [39]. As well, the BMP antagonist Noggin functions to inhibit the osteogenic differentiation of mASCs [42,43]. In our laboratory, exogenous addition of either rBMP-2 or rBMP-4 significantly enhances the osteogenic differentiation of hASCs [44]. In the present study, we observed increased BMP expression to accompany either CM or coculture stimulation of mOBs and may underlie this pro-osteogenic relationship in combination with other paracrine factors.
Next, our data suggest strongly that Hedgehog signaling plays a role in the mOB–hASC interaction observed. The effect of Shh-N on osteogenic differentiation has been well described in vitro, in cell lines (including C3H10T1/2 and KS483 cells) [16,19], primary calvarial osteoblasts [19], and bone marrow mesenchymal cells [40]. Recently, we observed this to be the case in mASCs as well, in which Hedgehog signaling was observed to regulate the balance of osteo- and adipogenic differentiation of mASCs, as observed in other cell types and model systems [19,27,45]. In the present study we confirmed that human-like mASCs produce Shh. We then observed that manipulation of Hedgehog signaling, via either addition of exogenous Shh-N or the antagonist cyclopamine, significantly altered the effect of hASC CM. Moreover, the in vivo addition of the Smoothened antagonist cyclopamine impaired hASC-mediated defect healing (Fig. 7D), in a manner similar to other published reports [46,47]. Collectively, these data suggested that the pro-osteogenic effect of the paracrine hASC–mOB relationship relied at least in part on intact Hedgehog signaling. Of course, BMP and Hedgehog pathways do not operate independently, and many coordinate relationships have been observed to exist both within and outside the field of bone biology [18,48–52].
Our study focused specifically on the manner in which hASCs may stimulate endogenous osteoblast differentiation of the host mouse calvarium. We have previously found that hASCs remain at detectable levels in a defect for at least 2 weeks during the acute bone formation stage but then decrease significantly to the point that they are difficult to detect by 8 weeks (data not shown). No doubt there are other intriguing paracrine interactions that may be explored. First, the underlying dura mater is of course a source of osteoprogenitor cells and may contribute to osseous regeneration either with or without hASC engraftment [53–56]. In our calvarial defect model, we pay close attention to leaving the dura mater intact, both to ensure mouse survival and to optimize bony regeneration. It may be that dura mater–hASC interaction may yield an equally potent pro-osteogenic admixture. Second and importantly, the mOB–hASC in vivo interaction is also within an acute wound environment, as hASCs are engrafted immediately after surgical defect creation. Thus, other cell types are attracted to the acute wound environment, including polymorphonuclear cells and macrophages among others. Moreover, pro-osteogenic cytokines are known to be upregulated in an acute skeletal injury, including but not limited to Interleukins and BMPs. In this light, the degree to which hASCs stimulate autogenous repair may be predicated on the presence of an acute skeletal wound.
Several limitations do exist toward the broader generalization of these results to the application for regenerative medicine purposes. First, it does appear that experimental variability exists by patient in terms of their osteogenic differentiation. In our hands, in vivo hASC-mediated calvarial repair does indeed vary between hASC source. Differences in age of the patient, gender, anatomic site of ASC derivation, passage number, and freeze–thaw conditions have all been shown to create disparity in the osteogenic in vitro differentiation of ASCs [35,57–60]. We have attempted to counteract this apparent heterogeneity by selecting similar patients for hASC derivation. For our study, we isolated hASCs from 8 female patients. All patients were under 50 years of age and all hASCs were derived from the flank and thigh regions, and all were first passage on usage. In doing so, it is possible that our results cannot be yet extrapolated to all hASC sources with disparate age, gender, and general health. Nevertheless, our present study sheds light on potential mechanisms of hASC-mediated bony repair.
In conclusion, hASCs heal critical-sized calvarial defects. This is, at least in part, via stimulation of autogenous healing of the host defect. In vitro assays suggest that hASC-derived Hedgehog signaling may coordinately stimulate bone formation.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health, National Institute of Dental and Craniofacial Research Grant 1 R21 DE019274-01, 1 RC2 DE020771-01, the Oak Foundation and Hagey Laboratory for Pediatric Regenerative Medicine and the National Endowment for Plastic Surgery to M.T.L. B.L was supported by the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 1F32AR057302-02.
Author Disclosure Statement
The authors have no financial interest in any of the products, devices, procedures, or anything else connected with the article. There was no internal or external funding received to complete this study. University of Stanford IRB approval was obtained before commencement of the study (IRB No. 2188, 9999).
References
- 1.HCUP. Agency for Healthcare Research and Quality; 2007. Healthcare cost and utilization project. [PubMed] [Google Scholar]
- 2.Fong KD. Nacamuli RP. Song HM. Warren SM. Lorenz HP. Longaker MT. New strategies for craniofacial repair and replacement: a brief review. J Craniofac Surg. 2003;14:333–339. doi: 10.1097/00001665-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Nacamuli RP. Longaker MT. Bone induction in craniofacial defects. Orthod Craniofac Res. 2005;8:259–266. doi: 10.1111/j.1601-6343.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- 4.Halvorsen YC. Wilkison WO. Gimble JM. Adipose-derived stromal cells—their utility and potential in bone formation. Int J Obes Relat Metab Disord. 2000;24(Suppl 4):S41–S44. doi: 10.1038/sj.ijo.0801503. [DOI] [PubMed] [Google Scholar]
- 5.Lendeckel S. Jodicke A. Christophis P. Heidinger K. Wolff J. Fraser JK. Hedrick MH. Berthold L. Howaldt HP. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Mesimaki K. Lindroos B. Tornwall J. Mauno J. Lindqvist C. Kontio R. Miettinen S. Suuronen R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Kulakov AA. Goldshtein DV. Grigoryan AS. Rzhaninova AA. Alekseeva IS. Arutyunyan IV. Volkov AV. Clinical study of the efficiency of combined cell transplant on the basis of multipotent mesenchymal stromal adipose tissue cells in patients with pronounced deficit of the maxillary and mandibulary bone tissue. Bull Exp Biol Med. 2008;146:522–525. doi: 10.1007/s10517-009-0322-8. [DOI] [PubMed] [Google Scholar]
- 8.Mao JJ. Giannobile WV. Helms JA. Hollister SJ. Krebsbach PH. Longaker MT. Shi S. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta DM. Kwan MD. Slater BJ. Wan DC. Longaker MT. Applications of an athymic nude mouse model of nonhealing critical-sized calvarial defects. J Craniofac Surg. 2008;19:192–197. doi: 10.1097/scs.0b013e31815c93b7. [DOI] [PubMed] [Google Scholar]
- 10.Levi B. James AW. Nelson ER. Vistnes D. Wu B. Lee M. Gupta A. Longaker M. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS ONE. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley JP. Levine JP. McCarthy JG. Longaker MT. Studies in cranial suture biology: regional dura mater determines in vitro cranial suture fusion. Plast Reconstr Surg. 1997;100:1091–1099. doi: 10.1097/00006534-199710000-00001. discussion; 1100–1102. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald JA. Mehrara BJ. Spector JA. Chin GS. Steinbrech DS. Saadeh PB. Luchs JS. Paccione MF. Gittes GK. Longaker MT. Biomolecular mechanisms of calvarial bone induction: immature versus mature dura mater. Plast Reconstr Surg. 2000;105:1382–1392. doi: 10.1097/00006534-200004040-00018. [DOI] [PubMed] [Google Scholar]
- 13.Levine JP. Bradley JP. Roth DA. McCarthy JG. Longaker MT. Studies in cranial suture biology: regional dura mater determines overlying suture biology. Plast Reconstr Surg. 1998;101:1441–1447. doi: 10.1097/00006534-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Ma G. Xiao Y. He L. Recent progress in the study of Hedgehog signaling. J Genet Genomics. 2008;35:129–137. doi: 10.1016/S1673-8527(08)60019-3. [DOI] [PubMed] [Google Scholar]
- 15.Ehlen HW. Buelens LA. Vortkamp A. Hedgehog signaling in skeletal development. Birth Defects Res C Embryo Today. 2006;78:267–279. doi: 10.1002/bdrc.20076. [DOI] [PubMed] [Google Scholar]
- 16.van der Horst G. Farih-Sips H. Lowik CW. Karperien M. Hedgehog stimulates only osteoblastic differentiation of undifferentiated KS483 cells. Bone. 2003;33:899–910. doi: 10.1016/j.bone.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Jemtland R. Divieti P. Lee K. Segre GV. Hedgehog promotes primary osteoblast differentiation and increases PTHrP mRNA expression and iPTHrP secretion. Bone. 2003;32:611–620. doi: 10.1016/s8756-3282(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 18.Yuasa T. Kataoka H. Kinto N. Iwamoto M. Enomoto-Iwamoto M. Iemura S. Ueno N. Shibata Y. Kurosawa H. Yamaguchi A. Sonic hedgehog is involved in osteoblast differentiation by cooperating with BMP-2. J Cell Physiol. 2002;193:225–232. doi: 10.1002/jcp.10166. [DOI] [PubMed] [Google Scholar]
- 19.Spinella-Jaegle S. Rawadi G. Kawai S. Gallea S. Faucheu C. Mollat P. Courtois B. Bergaud B. Ramez V. Blanchet AM. Adelmant G. Baron R. Roman-Roman S. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci. 2001;114:2085–2094. doi: 10.1242/jcs.114.11.2085. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T. Aikawa T. Iwamoto-Enomoto M. Iwamoto M. Higuchi Y. Pacifici M. Kinto N. Yamaguchi A. Noji S. Kurisu K. Matsuya T. Induction of osteogenic differentiation by hedgehog proteins. Biochem Biophys Res Commun. 1997;237:465–469. doi: 10.1006/bbrc.1997.7156. [DOI] [PubMed] [Google Scholar]
- 21.Koyama E. Ochiai T. Rountree RB. Kingsley DM. Enomoto-Iwamoto M. Iwamoto M. Pacifici M. Synovial joint formation during mouse limb skeletogenesis: roles of Indian hedgehog signaling. Ann N Y Acad Sci. 2007;1116:100–112. doi: 10.1196/annals.1402.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vortkamp A. Lee K. Lanske B. Segre GV. Kronenberg HM. Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y. Hammerick KE. James AW. Carre AL. Leucht P. Giaccia AJ. Longaker MT. Inhibition of histone deacetylase activity in reduced oxygen environment enhances the osteogenesis of mouse adipose-derived stromal cells. Tissue Eng Part A. 2009;15:3697–3707. doi: 10.1089/ten.tea.2009.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y. Malladi P. Zhou D. Longaker MT. Molecular and cellular characterization of mouse calvarial osteoblasts derived from neural crest and paraxial mesoderm. Plast Reconstr Surg. 2007;120:1783–1795. doi: 10.1097/01.prs.0000279491.48283.51. [DOI] [PubMed] [Google Scholar]
- 25.James AW. Xu Y. Wang R. Longaker MT. Proliferation, osteogenic differentiation, and fgf-2 modulation of posterofrontal/sagittal suture-derived mesenchymal cells in vitro. Plast Reconstr Surg. 2008;122:53–63. doi: 10.1097/PRS.0b013e31817747b5. [DOI] [PubMed] [Google Scholar]
- 26.James AW. Levi B. Commons GW. Glotzbach J. Longaker M. Paracrine interaction between adipose-derived stromal cells and cranial suture-derived mesenchymal cells. Plast Reconstr Surg. 2009;3:806–822. doi: 10.1097/PRS.0b013e3181e5f81a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James AW. Leucht P. Levi B. Carre AL. Xu Y. Helms JA. Longaker MT. Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng Part A. 16:2605–2616. doi: 10.1089/ten.tea.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levi B. James AW. Glotzbach J. Wan D. Commons GW. Longaker MT. Depot specific variation in the osteogenic and adipogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg. 2010;3:822–835. doi: 10.1097/PRS.0b013e3181e5f892. [DOI] [PubMed] [Google Scholar]
- 29.James AW. Theologis AA. Brugmann SA. Xu Y. Carre AL. Leucht P. Hamilton K. Korach KS. Longaker MT. Estrogen/estrogen receptor alpha signaling in mouse posterofrontal cranial suture fusion. PLoS One. 2009;4:e7120. doi: 10.1371/journal.pone.0007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emerman JT. Fiedler EE. Tolcher AW. Rebbeck PM. Effects of defined medium, fetal bovine serum, and human serum on growth and chemosensitivities of human breast cancer cells in primary culture: inference for in vitro assays. In Vitro Cell Dev Biol. 1987;23:134–140. doi: 10.1007/BF02623594. [DOI] [PubMed] [Google Scholar]
- 31.Dahl JA. Duggal S. Coulston N. Millar D. Melki J. Shahdadfar A. Brinchmann JE. Collas P. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int J Dev Biol. 2008;52:1033–1042. doi: 10.1387/ijdb.082663jd. [DOI] [PubMed] [Google Scholar]
- 32.Korc M. Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 2009;9:639–651. doi: 10.2174/156800909789057006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gotoh N. Control of stemness by fibroblast growth factor signaling in stem cells and cancer stem cells. Curr Stem Cell Res Ther. 2009;4:9–15. doi: 10.2174/157488809787169048. [DOI] [PubMed] [Google Scholar]
- 34.Li JL. Harris AL. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Front Biosci. 2009;14:3094–3110. doi: 10.2741/3438. [DOI] [PubMed] [Google Scholar]
- 35.Aksu AE. Rubin JP. Dudas JR. Marra KG. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg. 2008;60:306–322. doi: 10.1097/SAP.0b013e3180621ff0. [DOI] [PubMed] [Google Scholar]
- 36.Samulin J. Lien S. Grindflek E. Berget I. Ruyter B. Sundvold H. Depot specific differences during adipogenesis of porcine stromal-vascular cells. Cell Biol Int. 2008;32:525–531. doi: 10.1016/j.cellbi.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Skillington J. Choy L. Derynck R. Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J Cell Biol. 2002;159:135–146. doi: 10.1083/jcb.200204060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dragoo JL. Lieberman JR. Lee RS. Deugarte DA. Lee Y. Zuk PA. Hedrick MH. Benhaim P. Tissue-engineered bone from BMP-2-transduced stem cells derived from human fat. Plast Reconstr Surg. 2005;115:1665–1673. doi: 10.1097/01.prs.0000161459.90856.ab. [DOI] [PubMed] [Google Scholar]
- 39.Wan DC. Shi YY. Nacamuli RP. Quarto N. Lyons KM. Longaker MT. Osteogenic differentiation of mouse adipose-derived adult stromal cells requires retinoic acid and bone morphogenetic protein receptor type IB signaling. Proc Natl Acad Sci U S A. 2006;103:12335–12340. doi: 10.1073/pnas.0604849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehlhorn AT. Niemeyer P. Kaschte K. Muller L. Finkenzeller G. Hartl D. Sudkamp NP. Schmal H. Differential effects of BMP-2 and TGF-beta1 on chondrogenic differentiation of adipose derived stem cells. Cell Prolif. 2007;40:809–823. doi: 10.1111/j.1365-2184.2007.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knippenberg M. Helder MN. Zandieh Doulabi B. Wuisman PI. Klein-Nulend J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem Biophys Res Commun. 2006;342:902–908. doi: 10.1016/j.bbrc.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 42.Wan DC. Pomerantz JH. Brunet LJ. Kim JB. Chou YF. Wu BM. Harland R. Blau HM. Longaker MT. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem. 2007;282:26450–26459. doi: 10.1074/jbc.M703282200. [DOI] [PubMed] [Google Scholar]
- 43.Cho HH. Park HT. Kim YJ. Bae YC. Suh KT. Jung JS. Induction of osteogenic differentiation of human mesenchymal stem cells by histone deacetylase inhibitors. J Cell Biochem. 2005;96:533–542. doi: 10.1002/jcb.20544. [DOI] [PubMed] [Google Scholar]
- 44.Panetta NJ. Gupta DM. Lee JK. Wan DC. Commons GW. Longaker MT. Human adipose-derived stromal cells respond to and elaborate bone morphogenetic protein-2 during in vitro osteogenic differentiation. Plast Reconstr Surg. 2010;125:483–493. doi: 10.1097/PRS.0b013e3181c82d75. [DOI] [PubMed] [Google Scholar]
- 45.Edwards PC. Ruggiero S. Fantasia J. Burakoff R. Moorji SM. Paric E. Razzano P. Grande DA. Mason JM. Sonic hedgehog gene-enhanced tissue engineering for bone regeneration. Gene Ther. 2005;12:75–86. doi: 10.1038/sj.gt.3302386. [DOI] [PubMed] [Google Scholar]
- 46.Zunich SM. Douglas T. Valdovinos M. Chang T. Bushman W. Walterhouse D. Iannaccone P. Lamm ML. Paracrine sonic hedgehog signalling by prostate cancer cells induces osteoblast differentiation. Mol Cancer. 2009;8:12. doi: 10.1186/1476-4598-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwamoto M. Enomoto-Iwamoto M. Kurisu K. Actions of hedgehog proteins on skeletal cells. Crit Rev Oral Biol Med. 1999;10:477–486. doi: 10.1177/10454411990100040401. [DOI] [PubMed] [Google Scholar]
- 48.Kim HJ. Rice DP. Kettunen PJ. Thesleff I. FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development. 1998;125:1241–1251. doi: 10.1242/dev.125.7.1241. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka Y. Okada Y. Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- 50.Gimeno L. Martinez S. Expression of chick Fgf19 and mouse Fgf15 orthologs is regulated in the developing brain by Fgf8 and Shh. Dev Dyn. 2007;236:2285–2297. doi: 10.1002/dvdy.21237. [DOI] [PubMed] [Google Scholar]
- 51.Kawai S. Sugiura T. Characterization of human bone morphogenetic protein (BMP)-4 and -7 gene promoters: activation of BMP promoters by Gli, a sonic hedgehog mediator. Bone. 2001;29:54–61. doi: 10.1016/s8756-3282(01)00470-7. [DOI] [PubMed] [Google Scholar]
- 52.Jacob S. Wu C. Freeman TA. Koyama E. Kirschner RE. Expression of Indian Hedgehog, BMP-4 and Noggin in craniosynostosis induced by fetal constraint. Ann Plast Surg. 2007;58:215–221. doi: 10.1097/01.sap.0000232833.41739.a5. [DOI] [PubMed] [Google Scholar]
- 53.Spector JA. Greenwald JA. Warren SM. Bouletreau PJ. Crisera FE. Mehrara BJ. Longaker MT. Co-culture of osteoblasts with immature dural cells causes an increased rate and degree of osteoblast differentiation. Plast Reconstr Surg. 2002;109:631–642. doi: 10.1097/00006534-200202000-00033. discussion 643–644. [DOI] [PubMed] [Google Scholar]
- 54.Spector JA. Greenwald JA. Warren SM. Bouletreau PJ. Detch RC. Fagenholz PJ. Crisera FE. Longaker MT. Dura mater biology: autocrine and paracrine effects of fibroblast growth factor 2. Plast Reconstr Surg. 2002;109:645–654. doi: 10.1097/00006534-200202000-00035. [DOI] [PubMed] [Google Scholar]
- 55.Warren SM. Greenwald JA. Nacamuli RP. Fong KD. Song HJ. Fang TD. Mathy JA. Longaker MT. Regional dura mater differentially regulates osteoblast gene expression. J Craniofac Surg. 2003;14:363–370. doi: 10.1097/00001665-200305000-00015. [DOI] [PubMed] [Google Scholar]
- 56.Roth DA. Bradley JP. Levine JP. McMullen HF. McCarthy JG. Longaker MT. Studies in cranial suture biology: part II. Role of the dura in cranial suture fusion. Plast Reconstr Surg. 1996;97:693–699. doi: 10.1097/00006534-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 57.van Beek EA. Bakker AH. Kruyt PM. Hofker MH. Saris WH. Keijer J. Intra- and interindividual variation in gene expression in human adipose tissue. Pflugers Arch. 2007;453:851–861. doi: 10.1007/s00424-006-0164-4. [DOI] [PubMed] [Google Scholar]
- 58.Schipper BM. Marra KG. Zhang W. Donnenberg AD. Rubin JP. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60:538–544. doi: 10.1097/SAP.0b013e3181723bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valeri CR. Pivacek LE. Effects of the temperature, the duration of frozen storage, and the freezing container on in vitro measurements in human peripheral blood mononuclear cells. Transfusion. 1996;36:303–308. doi: 10.1046/j.1537-2995.1996.36496226141.x. [DOI] [PubMed] [Google Scholar]
- 60.Thirumala S. Gimble JM. Devireddy RV. Cryopreservation of stromal vascular fraction of adipose tissue in a serum-free freezing medium. J Tissue Eng Regen Med. 2009;4:224–232. doi: 10.1002/term.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.