Summation
The MUC1 and MUC4 membrane mucins are each composed of a large alpha (α) and a small beta (β) subunit. The α subunits are fully exposed at the cell surface and contain variable numbers of repeated amino acid sequences that are heavily glycosylated. In contrast, the β subunits are much smaller and are anchored within the cell membrane, with their amino-terminal portions exposed at the cell surface and their carboxy-terminal tails facing the cytosol. Studies over the last several years are challenging the long-held belief that α subunits play the predominant role in cancer by conferring cellular properties that allow tumor cells to evade immune recognition and destruction. Indeed, the β subunits of MUC1 and MUC4 have emerged as oncogenes, as they engage signaling pathways responsible for tumor initiation and progression. Thus, a switch in the emphasis from the large α to the small β subunits offers attractive possibilities for successful clinical application. Such a focus shift is further supported by the absence of allelic polymorphism and variable glycosylation in the β subunit as well as by the presence of the β subunit in most MUC1 and MUC4 isoforms expressed by tumors. MUC1α, also known as CA15.3, is a Food and Drug Administration-approved serum biomarker for breast cancer, but its use is no longer recommended by the American Society of Clinical Oncology. However, comparison of β subunit expression in normal and malignant breast tissues may offer a novel approach to the exploitation of membrane mucins as biomarkers, as MUC1β-induced gene signatures with prognostic and predictive values in breast cancer have been reported. Preclinical studies with peptides that interfere with MUC1β oncogenic functions also look promising.
Key words: β subunit, biomarker, breast cancer, MUC1, MUC4, oncogene
Introduction
Mucins were originally defined as large O-glycoproteins with peptide cores rich in serine and threonine residues and carbohydrate contents of >50% by weight, present in mucus secreted by glandular epithelia. Given this structure, their function was assumed to be limited to the lubrication and protection of epithelial surfaces. However, research over the past several decades has allowed to substantially refine the understanding of mucins and the roles they play in epithelial biology and neoplasia. In the 1970–1980s, the development of monoclonal antibody (MAb) technology led to the discovery of mucins as tumor-associated antigens (TAAs), prompting intense interest in their exploitation as markers and therapeutic targets. The emergence of molecular methods in the 1980s allowed the cloning, conceptual translation, and expression of the first and most well-studied mucin, MUC1.1 Further molecular characterization of mucin genes revealed that these glycoproteins actually fall into two classes, secreted and membrane-bound mucins. According to the Human Genome Nomenclature Committee, the mucin gene family currently contains 18 members (MUC1 to MUC21 with MUC9–11 missing), of which 11 are membrane bound. All mucins contain a variable number of repeated peptides rich in serine and threonine residues that are modified by O-linked glycans. These variable numbers of tandem repeats (VNTRs) are the hallmark of mucins. Although all mucins are encoded by single genes, the membrane-bound mucins function at the cell surface as heterodimers composed of a large VNTR-containing extracellular subunit and a smaller subunit that contains an extracellular segment, a single transmembrane domain, and a carboxy-terminal cytosolic tail. In normal cells, mucin expression is restricted to the apical/luminal side of cells. In contrast, loss of apical restriction and elevated protein levels are common features of mucins expressed by carcinomas, which represent the vast majority of cancers in humans. This update will focus on the roles of two membrane-bound mucins—MUC1 and MUC4—in breast cancer and will emphasize the potential of their small carboxy terminal or β subunits as potential biomarkers.
Structure and Characteristics of MUC1 and MUC4
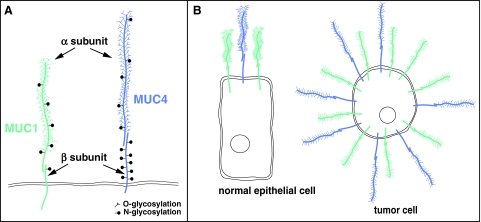
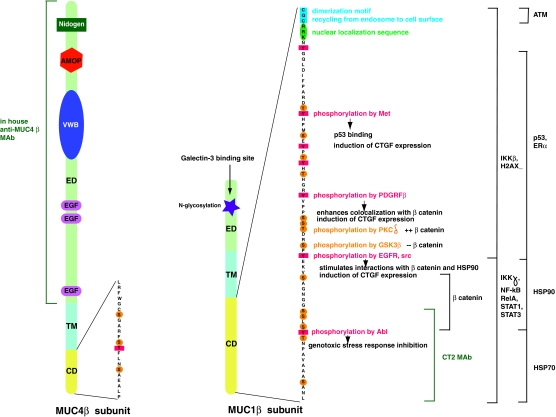
The human MUC1 gene contains eight exons and occupies about 4.4 kbp at locus 1q21. The number of VNTR elements is allele dependent, and in the case of MUC1, it varies between 20 and 125. The full-length MUC1 mRNA is not represented among the seven MUC1 mRNA variants annotated in RefSeq. The human MUC4 gene with 25 exons spans ∼70 kbp at locus 3q29. In this case, the full-length MUC4 mRNA is one of the three MUC4 mRNA variants annotated in RefSeq. Variability in numbers of the MUC1 and MUC4 transcripts results from alternative splicing taking place primarily in their amino-terminal or α subunit regions; the carboxy-terminal regions that include the β subunits remain unperturbed. To simplify the denomination of the MUC1 and MUC4 subunits, here the biochemical convention will be used and mucin subunits will be referred to as α and β. The overall structure of MUC1 and MUC4 proteins at the epithelial cell surface is illustrated in Fig. 1A. As reflected in the sizes of their respective genes, MUC4 is a much larger molecule than MUC1. While the α subunit of both mucins protrudes at the cell surface with a very high negative net charge as a result of the O-glycosylation and sulfation, that of MUC4 is more elongated because of a greater VNTR content. MUC4β is also longer than MUC1β, mainly because of its larger extracellular segment. In contrast, MUC1β has a larger cytoplasmic tail that is rich in serine, threonine, and tyrosine residues (Fig. 2). See Table 1 for additional comparisons between overall characteristics of MUC1 and MUC4.
FIG. 1.
MUC1 and MUC4 transmembrane mucins. (A) Overall structure of the MUC1 and MUC4 proteins at the epithelial cell surface. (B) Expression of MUC1 and MUC4 on normal epithelial cells versus tumor cells.
FIG. 2.
MUC1 and MUC4 β subunits. Structural domains, extracellular domain (ED), transmembrane domain (TM), and cytoplasmic domain (CD) of the MUC1 and MUC4 β subunits are illustrated, and the respective CD amino acid sequences are shown. MUC4β has a large ED with functional subdomains including a von Willebrand factor D (vWD) domain and two epidermal growth factor (EGF)-like domains and is specifically recognized by an in-house monoclonal antibody (MAb). MUC4β CD contains four potential phosphorylation sites. The small ED of MUC1β contains a galectin-3 binding site. MUC1β CD contains numerous phosphorylation sites, some of which, indicated, are crucial to its functions. MUC1β CD subregions involved in binding with various indicated effectors and the commercially available anti-MUC1α CT2 MAb are delimited. Dimerization and nuclear localization motifs required for MUC1β nuclear and mitochondrial import are highlighted.
Table 1.
Comparison Between MUC1 and MUC4 Attributes
| MUC1 | MUC4 | |
|---|---|---|
| Transmembrane mucin | Yes | Yes |
| Number of VNTR | 20–125 | 146–396 |
| VNTR motif | GSTAPPAHGVTSAPDTRPAP | TSSASTGHATPLPVTD |
| Oncogenic subunit | Yes | Yes |
| MAbs against α subunit | Multiple | None |
| MAbs against β subunit | 2 | Few, but most lack specificity |
| IHC with MAbs against α subunit | +++++ | − |
| IHC with MAbs against β subunit | + | +++ |
| Expression pattern on normal epithelial cells | Apical | Apical |
| Expression pattern on malignant cells | Apical, entire membrane, cytoplasmic | Apical, entire membrane, cytoplasmic |
| Shedding | Yes | Yes |
| Signal-transducing β subunit | Yes | Yes |
| Length of β subunit extracellular domain | 58 aa | 725 aa |
| Length of cytoplasmic domain | 72 aa | 22 aa |
| β Subunit-like splice variants Y and Z | Yes | Yes |
VNTR, variable numbers of tandem repeat; MAbs, monoclonal antibodies; IHC, immunohistochemistry; aa, amino acid.
Alterations of MUC1 and MUC4 in Tumor Cells
MUC1 and MUC4, with heavily O-glycosylated α subunits, are present at the apical face of normal, polarized epithelial cells (Fig. 1B). MUC1 expression in hematopoietic and stem/progenitor cells has been also reported. In contrast, MUC1 and MUC4 in malignant cells display aberrant expression, glycosylation, and cell surface distribution (Fig. 1B).
Overexpression
MUC1 is overexpressed in the majority of carcinomas as well as in hematologic malignancies. Current knowledge suggests that overexpression of MUC1 is achieved at the genetic and transcriptional levels of gene expression control.2 Amplification of the MUC1 gene locus (1q21) has been observed in breast cancer cells. MUC1, through associations between MUC1β and other transcription factors such as signal transducer and activator of transcription 1 or 3 (STAT1 or 3), upregulates its own promoter activity. MUC4 overexpression has been also reported in a variety of carcinomas, including tumors of the lung, breast, colon, and ovary; however, the mechanisms responsible for its overexpression remain to be delineated.
MUC1 splice variants preferentially expressed by tumors have been also described.3 MUC1/Y and MUC1/Z (also called MUC1/X) arise through splicing events that remove the MUC1 VNTR region; MUC1/Z is 54 bp longer, but otherwise identical to MUC1/Y. The MUC1/Y and MUC1/Z transcripts encode proteins that are identical in the β subunit to full-length MUC1 and contain some additional non-VNTR sequences that are shared with MUC1α. The retention of the amino-terminal signal peptide in these transcripts ensures that MUC1/Y and MUC1/Z are synthesized at the endoplasmic reticulum and likely transported to the cell surface. MUC4 is similarly differentially spliced in tumors, and like MUC1, two of the major splice variants (MUC4/X and MUC4/Y) lack the VNTR region.4
Altered glycosylation
In general, mucins carry a large percentage of their bulk as O-linked glycans and far fewer N-linked glycans. As serine and threonine residues are the targets of O-linked glycosylation, each VNTR motif of MUC1 and MUC4 can in principle carry up to five and six glycans, respectively (Table 1). Studies of MUC1 O-glycosylation in the mammary gland show that normal cells synthesize MUC1 with more elongated and highly branched glycans, whereas tumor cells produce MUC1 with fewer glycans, and these tend to be shorter and less branched and often differ in carbohydrate composition; Tn, sialyl Tn, and the oncofetal Thomson-Friedenreich antigens are frequently present within tumor-associated MUC1.5
Mislocalization
In tumor cells that have lost their polarity, MUC1 and MUC4 proteins are no longer restricted to the apical domain and are spread over the entire cell surface (Fig. 1B). The loss of polarity in malignant cells enables interactions between membrane-bound mucins and other plasma membrane proteins, such as the human epidermal receptor (HER) family of receptor tyrosine kinases (RTKs) that normally reside within the basolateral domain of polarized cells. Interactions between mucins and RTKs lead to activation of signaling pathways resulting in cell proliferation and survival.6 Such interactions are transient in normal epithelial cells during the reversible loss of polarity induced by stress, whereas they are constitutive in malignant cells because of a permanent loss of polarity and are frequently enhanced through overexpression of the interaction partners.
Functions of the α Subunits
In general, the α subunits of MUC1 and MUC4 carry out the canonical mucin functions of epithelial surface lubrication and protection. The extensive O-glycosylation of the α subunit, coupled with the propensity of these subunits to oligomerize, results in the formation of gels at the apical cell surface that are selectively permeable to ions, molecules, particles, and other cells.
MUC1α is a tumor-associated antigen (TAA)
MUC1 has multiple names, including epithelial membrane antigen, polymorphic epithelial mucin, polymorphic urinary mucin, DF3 antigen, CD227, and episialin. This multiplicity of names reflects the multitude of MAbs that have been independently raised against the MUC1α subunit, using tumor cells or their membranes, human milk fat globule (HMFG) proteins, peptides, or oligosaccharides as immunogens. The investigation of the reactivity and specificity of 56 such MAbs led to the following conclusions: 34 MAbs are directed toward epitopes located within the VNTR motif; 16 MAbs showed evidence for involvement of carbohydrate moieties within their epitopes; no obvious relationship was found between the type of immunogen and the specificity of each MAb; and the hydrophilic sequence PDTRPAP was always present either in part or full in epitopes within the MUC1 protein core.7 The presence of the PDTRPAP sequence in peptide epitopes is consistent with the identification by nuclear magnetic resonance of PDTR, which forms a knob-like structure, as the immunodominant peptide in each VNTR.8
The characterization of these MAbs provided two key lines of evidence that established MUC1 as a TAA. First, immunohistochemical studies demonstrated that tumor cells express higher amounts of MUC1 than normal cells. Second, the ability of SM3, an MAb raised against the MUC1 protein core stripped of its glycans, to discriminate between MUC1 on normal and tumor cells prompted the notion that tumor-associated MUC1 is aberrantly glycosylated.5 The heavy glycosylation of MUC1 in normal tissue prevents SM3 binding to its PDTRP epitope; the more efficient binding of this antibody to tumor cells strongly suggested that new epitopes are exposed on tumor-associated MUC1 as a consequence of suppressed glycosylation. As MUC1 is both overexpressed and alternately post-translationally modified by tumor cells, it satisfies two criteria for qualification as a TAA.9
Antiadhesive properties of MUC1α
The adhesive and antiadhesive properties of MUC1α are believed to promote cell migration, a requirement for metastasis. On one hand, the structure of MUC1α—large, cell surface protruding, rigid, and highly negatively charged—confers antiadhesive properties to MUC1-overexpressing cells that interfere with cell–cell interactions.10 On the other hand, tumor-associated MUC1α gains adhesive properties upon binding to adhesion molecules. The binding of MUC1α to adhesion molecules expressed on endothelial cells, such as selectin-like molecules and intracellular adhesion molecule 1, has long been known to contribute to the hematogenous metastatic spread of MUC1-overexpressing cells,3 and galectin-3 might be a major player in this process. By binding to Thomson-Friedenreich antigens present on tumor-associated MUC1α, galectin-3, a lectin found upregulated in many cancers, induces a repolarization of MUC1 at the cell surface, resulting in the exposure of heterotypic cell–cell adhesion molecules such a CD44 and E-selectin, which are otherwise concealed by the elongated structure of MUC1. Exposure of these cell adhesion molecules enables interaction with endothelial cells, eventually contributing to metastasis. By the same token, in the bloodstream of patients, exposure of homotypic adhesion molecules such as E-cadherin favors tumor cell aggregation and prevents cell death, again promoting metastasis.11 Modulation of the immune system and immune response are additional recognized functions of MUC1α. MUC1α is a self-antigen that triggers both humoral and cellular responses in cancer patients.9 Mechanisms by which MUC1α interferes with the cellular immune response include inhibition, through its antiadhesive properties, of attachment of cytotoxic T-lymphocytes (CTLs) to target tumor cells.3 The MUC1α-induced humoral response is not cancer specific, as anti-MUC1α antibodies have been also described in healthy women.12
Antiadhesive properties of MUC4α
A major function of the MUC4α subunit in tumor cells is in the prevention or disruption of cell–cell and cell–substratum interactions. Inducible expression of rat Muc4 (formerly called sialomucin complex) caused cells to detach from the culture dish and from each other.13 This process was strictly dependent on the extent of Muc4 O-glycosylation; expression of an Muc4 form harboring eight VNTR elements very efficiently disrupted cellular adhesion, whereas forms expressing five, three, or one repeat were progressively diminished in their antiadhesive capacity. Obviously, the antiadhesive activity of MUC4 must be kept in check in normal epithelial cells, probably by limiting MUC4 protein levels and restricting its localization to the apical surface. However, it is likely that tumor cells exploit the antiadhesive properties of MUC4 to facilitate their release from the primary tumor mass and their hematogenous dissemination during metastasis.
Functions of the β Subunits
The α subunits of MUC1 and MUC4 are responsible for their functions as mucins, and the β subunits appear to contribute to cellular growth signaling. The biological relevance of cellular signaling by membrane mucins remains a subject of some conjecture; it is not immediately obvious why a protein, whose primary function is in the physical protection of the exposed surface of terminally differentiated epithelial cells, should additionally influence cellular growth. One popular model is that, as the primary sensors of assaults to the mucus layer and thus the apical surface, membrane mucins are uniquely positioned to initiate healing responses in the form of cellular survival, proliferation, and motility.14 Exploitation of membrane mucin signaling function by premalignant or tumor cells, via overexpression or mislocalization, could facilitate transitions to progressively more malignant states of disease.
MUC1β is an oncogene
MUC1 emerged as an oncogene nearly three decades after its initial molecular characterization. Findings that overexpression of full-length MUC1 promotes transformation in vitro (3Y1 fibroblasts) and in vivo (mice) were quickly followed by the demonstration that overexpression of the MUC1 cytoplasmic domain (MUC1-CD) alone is sufficient to induce anchorage-independent growth and tumorigenicity in vitro.2 The MUC1 β subunit carries the 72-amino-acid (aa)-long MUC1-CD, which is highly conserved (>95%) across mammalian species. Saying that the cytoplasmic domain of MUC1 is a busy very place15 sums up well the still growing number of mechanisms by which MUC1β promotes tumor progression and metastasis. Biochemical studies in cell lines and genetic studies in transgenic mice have implicated MUC1-CD in a variety of oncogenic pathways.
MUC1β signaling at the cell membrane is initiated upon phosphorylation. MUC1-CD contains seven tyrosine residues and is a substrate for RTKs, including all four members of the HER family, the fibroblast growth factor receptor 3, the platelet-derived growth factor β, and MET.2 N-glycosylation of the single asparagine residue present in the extracellular region of MUC1β provides a binding site for galectin-3. It is believed that galectin-3 serves as a bridge to associate MUC1β with the epidermal growth factor receptor (EGFR) and possibly other RTKs, and that N-glycosylated MUC1β increases levels of galectin-3 mRNA by suppressing the expression of miR-322.2 Activated EGFR phosphorylates MUC1-CD on Tyr 60 (Fig. 2) and thereby enables binding of the adapter protein Grb2 and the ras guanine nucleotide exchange protein SOS, which results in the activation of the Ras and mitogen-activated protein kinases. Intracellular proto-oncogenic kinases such as PKCδ, src, and glycogen synthase kinase 3β (GSK3β), through phosphorylation of serine and threonine residues (Fig. 2), regulate the interactions between MUC1-CD and other intracellular binding partners. Through its interaction with EGFR, MUC1β potentiates receptor signaling by increasing recycling rather than degradation after endocytosis; delivery of internalized receptor factors to the cell surface results in re-exposure to growth factor and thus enhanced signaling.16 In pancreatic cells, galectin-3 appears to regulate endocytosis of both, MUC1 and EGFR.17
MUC1β is detected in the cytoplasm and nucleus of cancer cells overexpressing MUC1. However, whether the entire MUC1β subunit or only MUC1-CD is released from the plasma membrane or, alternatively, whether cytoplasmic and nuclear MUC1β are even inserted into membranes during synthesis are questions that remain to be resolved. Nevertheless, the trafficking of MUC1-CD in cells exposed to stress and/or overexpressing MUC1 is well established in vitro. Dimerization of MUC1-CD, via the CQC motif immediately adjacent to the transmembrane domain (Fig. 2), is required for import of MUC1-CD into mitochondria and the nucleus. This same motif regulates the recycling of MUC1 from endosomes to the plasma membrane, suggesting that oligomerization may play an important role in this process as well. Association with importin β targets MUC1-CD to the nucleus, where it acts as a powerful transcriptional regulator.18 In association with β catenin, MUC1-CD coactivates the transcription of Wnt target genes, including cyclin D1 and connective tissue growth factor (CTGF).19 By inducing the expression of CTGF, a potent mediator of extracellular matrix remodeling and angiogenesis, MUC1-CD facilitates the creation of a reactive tumor microenvironment; MUC1-CD-dependent induction of CTGF requires tyrosine phosphorylation of MUC1-CD (Fig. 2).18 MUC1β directly binds to p53 and regulates p53 transcription in response to genotoxic stress.2 In breast cancer cells, MUC1β associates with estrogen receptor α (ERα) to enhance the expression of estrogen-responsive genes. In association with STAT1 or STAT3, MUC1β upregulates the expression of prosurvival genes that include MUC1 itself.20 MUC1β promotes the nuclear localization of EGFR, and interactions between MUC1β and EGFR in the nucleus contribute to accumulation of chromatin-bound EGFR and higher expression of cyclin D1.21
The cell survival advantage conferred by overexpression of MUC1β is further enhanced by the ability of MUC1β to block cell death mediated by both intrinsic and extrinsic apoptotic pathways. Binding of MUC1β dimers to heat shock proteins 70 or 90 is required for MUC1β import into the outer membrane of mitochondria, where MUC1β abolishes apoptotic responses to genotoxic, oxidative, hypoxic and metabolic stresses by blocking the loss of the mitochondrial transmembrane potential.2 MUC1β prevents radiation-induced cell death by associating with ataxia-telangiectasia-mutated protein and its substrate H2AX to promote DNA double-strand repair.22 Direct transcriptional repression of the p53 tumor suppressor gene by p53-bound MUC1β, MUC1β/STAT1/3-induced expression of apoptosis inhibitors, as well as suppression of the proapoptotic function of the Abl tyrosine kinase through MUC1β/Abl interactions in the cytoplasm further inhibit the intrinsic apoptotic pathway. By associating with caspase 8, MUC1β prevents death-receptor-induced cell death. Activation of the nuclear factor κB pathway, via MUC1β binding to I Kappa Kinase β and γ, and RelA (p65) in the cytoplasm, reinforces the inhibition of the extrinsic apoptotic pathway.
Finally, MUC1β contributes to cell migration by interacting with β catenin in the cytoplasm of cells. MUC1β competes with E-cadherin for the binding of β catenin. MUC1-CD phosphorylation by PKCδ, src, and EGFR enhances MUC1β/β catenin interactions, whereas glycogen synthase kinase 3β-mediated MUC1-CD phosphorylation inhibits interactions (Fig. 2). E-cadherin is a major component of adherens junctions that contributes to epithelial cell polarity. E-cadherin/β catenin complexes direct the formation of an actin filament network through the epithelial cell layer. The MUC1β-mediated decrease of E-cadherin/β catenin complexes results in an antiadhesive effect that disrupts the epithelial sheet and promotes cell migration.
Cellular signaling by MUC4β
Like MUC1, the β subunit of MUC4 appears to be involved in oncogenic signaling. However, in contrast to MUC1, its cytoplasmic tail has been thus far not demonstrated to play a prominent role, and signaling instead appears to be mediated by the extracellular region of the MUC4β subunit. The MUC4 extracellular region contains three epidermal growth factor (EGF)-like domains, one of which exhibits very high homology to EGF and other members of the family of growth factors that activate members of the HER family of RTKs. It has been demonstrated that MUC4 and HER2 associate when the two proteins are coexpressed in the same cells, and the EGF-like domain most homologous to the active growth factors is necessary for this interaction.23 Expression of MUC4 in some cell lines elevates HER2 tyrosine phosphorylation, leading to the speculation that MUC4 is an activating ligand for HER2. These observations are particularly interesting, because conventional wisdom suggests that, despite its robust tyrosine kinase activity, HER2 does not have a cognate ligand and signals exclusively by heterodimerizing with other members of the HER family.
It should be noted that there are some observations that challenge the interpretation that MUC4 is an activating ligand for HER2. First, elevation of HER2 tyrosine phosphorylation upon MUC4 expression does not occur in all cells, suggesting that other components may be necessary for HER2 stimulation by the mucin. Also, soluble forms of MUC4 containing the required EGF-like domain do not appear to bind to or activate HER2, pointing to a strictly cell autonomous mechanism of action. In this regard, it is interesting that one of the major outcomes of MUC4 interaction with HER2 appears to be the heightened response of the HER2/HER3 receptor heterodimer to the HER3-binding growth factor neuregulin-1.23,24 Coupled with observations that MUC4 relocalizes receptors from intracellular pools to the cell surface,24 a simple model consistent with all observations is that MUC4-mediated HER receptor relocalization delivers receptors to cellular sites where they are accessible for autocrine growth factor stimulation.
MUC4-potentiated HER2/HER3 signaling through canonical growth factor signaling pathways such as PI3K-Akt and ras-Erk accounts for some cellular responses to MUC4 stimulation, including augmented proliferation and survival. However, it has been reported that MUC4 elevates the survival of cell lines that lack either HER receptor expression or activation upon MUC4 expression.25 These observations point to the existence of at least one other pathway through which MUC4 influences cellular growth control. Importantly, deletion of the MUC4 intracellular domain does not affect its ability to promote cellular survival,25 suggesting that MUC4 interaction with a cell surface or extracellular protein is essential for this mechanism of signaling. The identification of this protein and the elucidation of the associated survival mechanism is a key question for the future.
Expression of MUC1 and MUC4 in Breast Cancer
The suspected heterogeneity of breast tumors has been definitively confirmed by global gene expression analyses. At least five breast tumor subtypes can be distinguished: ER+ and luminal A or B, ER− and normal-like or HER2+, or basal-like. Most importantly, these breast cancer subtypes are associated with different clinical outcomes.26 Indeed, the ability to predict risk of tumor metastasis and disease recurrence is critical in the treatment of breast cancer patients. Currently, lymph node status remains the most reliable low-cost prognostic factor available,27 but disease will recur in about 20%–30% of lymph node negative patients. Availability of additional and reliable biomarkers is expected to enable further stratification of the disease and improve risk assessment.
MUC1 expression in breast tumors, in most instances, refers to expression of MUC1α. Reasons for this are threefold: first, many antiMUC1α MAbs are available; second, until roughly 2003, MUC1α was believed to be essential in MUC1's role in cancer; and third, in the run-up to the approval of circulating MUC1α as a prognostic and predictive marker for breast cancer (see below), MUC1 expression studies were driven by the biomarker potential of MUC1α. Findings from early MUC1α expression studies conducted by immunohistochemistry in fewer than 80 breast carcinomas uncovered several trends regarding expression patterns, tumor characteristics, and patient prognosis. First, the presence of MUC1α in the majority of tumor cells is associated with better tumor differentiation and improved prognosis.28 Second, some studies found a direct correlation between MUC1α staining and ER status and an inverse correlation between MUC1 staining and tumor grade.28 Third, MUC1α expression patterns in tumor cells include apical, entire membrane and cytoplasmic expression, and localization in the cytoplasm and/or the entire membrane is associated with worse prognosis.28 More recently, these findings were confirmed by MUC1α expression studies conducted on larger numbers of tumor samples present on tissue microarrays. Examination of 1447 invasive breast carcinomas with long-term patient follow-up information led to the conclusion that negative expression of MUC1α, observed in about 9% of tumors, was significantly associated with regional recurrence and distant metastasis and with borderline significance in the presence of lymph node metastasis.29 In tumors with high MUC1α expression, MUC1α was associated with ER+ status. Expression of MUC1α alone was not predictive of patient outcome, but there was an association between its cellular localization and overall survival; MUC1α cytoplasmic and/or membranous expression was associated with poorer survival compared with apical and combined apical and cytoplasmic localization. A relation between MUC1α expression patterns and clinical outcome was further confirmed by analysis of 243 primary invasive carcinomas.30 MUC1α expression was found in 93.2% of cases. Negative MUC1 expression, observed in 6.8% of tumors, was determined as an independent risk factor for poor recurrence-free survival and overall survival and was associated with negative ER, PR, and cyclin D1.
Only one study conducted analysis of MUC1 expression using MAbs directed against both MUC1 α and β subunits.31 Of the 96 invasive carcinomas examined in this study, 93% reacted with the anti-MUC1β MAb CT2 (Fig. 2), and 73.5% with the anti-MUC1α MAb C595. All previously reported MUC1α expression patterns were found with CT2. Thus, in comparison to detection of MUC1α, detection of MUC1β results in nearly 20% more tumors being positive for MUC1 expression. This difference in detection may be attributed to different affinities of the MAbs for their respective epitope. Alternatively, expression of MUC1β protein isoforms, such as MUC1/Y and MUC1/Z proteins, lacking the VNTR region recognized by the anti-MUC1α MAbs, is only detected by the anti-MUC1β MAb. The authors of the study concluded that analysis of MUC1 expression with an MAb directed against MUC1β constitutes a better indicator for MUC1 expression, as epitope glycosylation is not an issue with intracellular MUC1β.31
The strength of the anti-MUC1α MAbs as diagnostic agents is related to the presence of multiple epitopes in the MUC1 VNTR region, offering the possibility of very high sensitivity in detecting the MUC1 protein in patient tumor samples. However, because the number of tandem repeats can markedly vary among individuals, and because glycosylation is known to interfere with antigen recognition and can also markedly vary from one patient's tumor to the next, interpretation of expression results across a range of patient samples using these antibodies can be challenging.
MUC4β expression has been reported in primary tumors from 95% of breast cancer patients29; however, this study employed an antibody preparation that has been observed to cross react with other cellular proteins, to such an extent that MUC4β is only a minor component of the total proteins recognized by this antibody. Using an in-house preparation of the MAb 1G8 on breast tissue microarrays encompassing patient-matched normal breast tissue, primary tissue, and lymph node metastases, it was observed that MUC4β expression levels are reduced during the transition from normal to primary tumor in almost 60% of patients.32 These observations are consistent with the notion that MUC4 is a marker of terminal differentiation, and its expression is suppressed as tumor cells dedifferentiate. Further, it was observed that MUC4 protein levels are markedly elevated in lymph node metastases relative to primary tumor, pointing to the possibility that MUC4 plays an active role in the primary tumor-to-metastasis transition. In this regard, it might be predicted that the population of patients whose primary tumors overexpress MUC4β relative to normal (roughly 10%–15% of patients) may suffer a particularly poor prognosis.
Clinical Applications of MUC1 and MUC4
Translation of knowledge acquired at the bench on the roles of MUC1 and MUC4 in cancer into clinical applications is the driving force behind much of the research focused on these mucins. Biomarker and therapeutic applications are naturally more advanced for MUC1, as its association with cancer precedes that of MUC4.
MUC1 and MUC4 as cancer biomarkers
Biomarkers are needed to assist with disease prognosis (prognostic markers) as well as evaluate treatment efficacy (predictive markers). Circulating MUC1, best known as cancer antigen 15.3 (CA15.3), is an established but no longer recommended prognostic and predictive marker in breast cancer. The discovery of MUC1 overexpression on breast cancer cells prompted the detection of the MUC1 TAA in the serum of cancer patients. Circulating MUC1, also called shed MUC1, corresponds to the MUC1α subunit that is released from the cell membrane through proteolytic action of tumor necrosis factor α converting enzyme (TACE or ADAM 17) and metalloproteinase MT1.15 Two antibody-based assays, CA15.3 and CA27.29, were approved by the US Food and Drug Administration for the detection of circulating MUC1α in the serum of breast cancer patients. In the CA15.3 test, MUC1α is detected in a radioactive, colorimetric, or fluorescent immunoassay with two anti-MUC1 MAbs, 115 D8 and DF3. The CA27.29 test is an automated competitive chemiluminescent immunoassay, based on the use of the anti-MUC1α B27.29 MAb and purified B27.29 antigen.33 However, the value of MUC1α as a serum marker in breast cancer patients is controversial, because it has become clear that levels of circulating MUC1 are variable in women with or without cancer. Citing a lack of conclusive data, the American Society of Clinical Oncology no longer recommends the use of CA15.3 and CA27.29 tests in screening, diagnostic, and staging tests or to detect disease recurrence after primary breast cancer therapy. The American Society of Clinical Oncology only recommends the use of these tests in conjunction with diagnostic imaging, history, and physical examination for monitoring patients with metastatic breast cancer during active therapy.34 The failure of the CA15.3 and CA27.29 tests to accurately predict cancer and response to therapy might be attributed to the poor ability of the MAbs used in these tests to discriminate between heavily glycosylated MUC1α shed from normal cells and hypoglycosylated MUC1α shed from malignant cells.
Targeting of MUC1α has also been used for the radioimmunolocalization of metastatic breast cancer. In a phase I trial, metastatic lesions in breast cancer patients were successfully imaged by using the BrE3-111Indium immunoconjugate35; BrE3 is another anti-MUC1α MAb raised against HMFG. In addition to antibodies and their fragments, aptamers that target the VNTR region of MUC1α have been described as MUC1-targeting agents.
The large α subunit of transmembrane mucins, long believed to be essential for function in cancer, has overshadowed the evaluation of their small β subunit as biomarkers. The authors of the current article propose that assessing the expression of the MUC1β and MUC4β subunits in breast cancer will prove a more valuable endeavor. Indeed, compared with MUC1α, immunodetection of the β subunit will provide more accuracy, because it will include most MUC1 protein isoforms and is not influenced by interindividual sequence polymorphism, multiple epitopes, or variable glycosylation. Antibodies currently available against MUC1β are fewer in comparison to those against MUC1α. DMC209, an MAb recognizing the amino terminus of the MUC1β extracellular domain,36 as well as polyclonal antibodies against the amino- and carboxy-terminal regions of MUC1β have been described, but CT2, an MAb recognizing the C terminus of MUC1β, is the only antibody available commercially (Fig. 2 and Table 1).
The β subunits of MUC1 and MUC4 are likely to provide both prognostic and predictive marker information in breast cancer. Expression patterns of the β subunits in cells from biopsies and/or their detection on circulating tumor cells could be used as prognostic tools. The potential of the β subunit as a predictive marker is inferred from observations that cancer cells overexpressing either MUC1β or MUC4β become resistant to cell death induced by chemotherapeutic agents (see above functions of the β subunit), as well as to herceptin therapy mediated via mechanisms including steric hindrance.37–39 Recently, MUC1-induced tumorigenesis signatures were shown to predict outcome in breast and lung cancer patients and response to breast cancer treatment.2
MUC1 and MUC4 as therapeutic targets
Although the MUC1 TAA has been the focus of passive and active immunotherapeutic studies for over 20 years, neither approach has yet been met with overwhelming success in clinical trials.
MUC1-based active immunotherapy is supported by the observations that the MUC1 TAA is capable of inducing anti-MUC1 CTLs and antibodies in cancer patients. Active immunotherapy of tumors aims at triggering innate and adaptive immune responses via vaccination against a particular TAA. Mice are the primary preclinical model for immunotherapy studies, and for MUC1 such studies have benefited from the development of a transgenic mouse expressing human MUC1. Nevertheless, extrapolations of immune responses between mice and humans remain challenging, as demonstrated by the many MUC1 vaccines deemed successful in mice that did not progress further than phase I in the clinic.40 Initial MUC1 vaccines concentrated on the use of unglycosylated MUC1 tandem repeat peptides since the MUC1 TAA is hypoglycosylated in comparison to the heavily glycosylated MUC1 present on normal cells. While a majority of unglycosylated peptide formulations failed at efficiently inducing humoral and cellular immune responses in humans, Stimuvax was an exception. The Stimuvax vaccine consists of a 25 aa long MUC1 tandem repeat peptide in a liposome formulation with a Toll-like receptor 4 agonist. It is currently under evaluation in phase III trials in patients with non–small cell lung cancer, as well as in hormone receptor-positive, locally advanced, recurrent or metastatic breast cancer patients (www.oncothyreon.com/clinical/stimuvax.html). More recent studies have shown that glycosylated MUC1 tandem repeat peptides are more efficient at inducing humoral and cellular responses. However, in humans not all glycopeptides generate antibodies that consistently react with breast cancers or breast cancer cell lines.9 Induction of humoral and cellular immune responses effective against tumors when using MUC1-Tn glycopeptides in mice was attributed to the fact that such peptides are not recognized as self and are preferentially cross presented on dendritic cells.41 Another glycopeptide, a 100 aa long MUC1 peptide carrying oxidized mannan showed promise in humans up to a phase III study. In this case, the presence of mannan contributes to antigen uptake via the mannose receptor present on dendritic cells and macrophages. Dendritic cells are the most potent antigen-presenting cells. MUC1 immunotherapy with dendritic cells, loaded ex vivo with MUC1 peptides or fused to tumor cells, has exhibited some success in humans.9 TG4010, a vaccine made of a replication-deficient vaccinia virus expressing MUC1 and interleukin 2, successfully induced antitumor immune responses in breast cancer and non–small cell lung cancer patients.9
An effective antitumor vaccine must evoke cellular immunity for the generation of CTLs. For the binding of CTLs to tumor cells to occur, CTL receptors have to recognize the exact same antigenic determinants on tumor cells against which CTLs have been primed. The challenge for any vaccine is to provide exogenous antigens from which antigenic determinants present on tumor cells will be generated through proteolytic processing for exposure on antigen-presenting cells. Thus, designing an effective vaccine against the MUC1 TAA is extremely challenging because of its variable glycosylation, and VNTR polymorphisms, including amino acid substitutions. Further, although little is known about the MUC1 isoforms lacking the VNTR region, an anti-MUC1 TAA vaccine would be ineffective against them.
Passive immunotherapy of tumors relies on MAbs (IgG) for the killing of tumor cells. Passive immunotherapy against the MUC1 TAA seemed obvious at first, given the availability of many mouse anti-MUC1α MAbs that can be humanized to reduce their immunogenicity in humans. Despite some preclinical success, efficacy of this approach in the clinic remains elusive. The major obstacle to MUC1 passive immunotherapy appears to be the target itself: internalization of antibody-bound MUC1 has been described for several antibodies. Effective passive immunotherapy involves recruitment of immune effector cells via the Fc portion of the cell surface-bound antibody to develop antibody-dependent cellular cytotoxicity and possibly complement dependent cytotoxicity. The lack of Fc function for an antibody can be overcome by conjugation with a toxic payload such a radiation, toxin, or drug. The HMFG MAb, even when humanized and conjugated to 90Y, a radionuclide emitting high-energy β particles and causing cell death in ways similar to high doses of X rays, performed poorly in a phase III trial in ovarian cancer patients. Reasons for this are mostly related to the MUC1 TAA.9 Internalization is a requirement for toxin or drug immunoconjugates, but for radioimmunoconjugates internalization limits radiation exposure of nontargeted neighboring cells, known as the bystander effect and believed to greatly contribute to killing of tumor cells. In addition, circulating MUC1α or CA15.3 may significantly decrease the concentration of MAb reaching the tumor, further affecting tumor penetration that is already hindered by the size of the antibody. Better tumor penetration can be achieved by using antibody fragments that retain the binding specificity of IgGs; bispecific antibodies, with one arm binding the MUC1 TAA and the other to an epitope present on T-cell receptors to trigger cellular immunity, have been tested in mice. Finally, the fact that the MUC1 TAA is variable in structure, because of glycosylation and interindividual polymorphism of the VNTR region, is also an obstacle for MUC1 passive immunotherapy. The use of antibody fragments and aptamers as MUC1α-targeting agents for targeted drug delivery to MUC1-expressing cells has been also explored in preclinical studies.42
Emergence over the past 10 years of the β subunit of transmembrane mucins as the oncogenic factor has prompted a focus shift from the α to the β subunit as potential therapeutic targets. Further, lower genetic and structural polymorphisms of β subunits in comparison to α subunits make them more attractive targets.
MUC1β and MUC4β transduce signals that promote cell proliferation and survival. In addition, MUC1β acts as a powerful transcriptional regulator capable of reorchestrating gene expression profiles in tumors and their microenvironment. Inhibition of these β subunit functions is now possible, because at least some of the cellular mechanisms involved have been unraveled.
At present, therapeutic targeting of MUC1β has focused on the evaluation of small molecules capable of penetrating into cells; two classes of small molecules have shown success in preclinical studies: peptides and small molecule inhibitors. PMIP, a peptide mimicking the interaction domain of MUC1 for both EGFR and β catenin, significantly affected breast cancer progression by abolishing direct interactions between MUC1/EGFR and MUC1/β catenin. In mice, after intraperitoneal injection, PIMP inhibited primary tumor growth, tumor spread, and recurrence of tumors in a xenograft breast cancer model and inhibited tumor growth in a transgenic model of human breast cancer.43 Peptides GO-201 and GO-202, which block the oligomerization of MUC1β and thereby prevent its nuclear and mitochondrial import, have been shown to induce the growth arrest and death of human breast cancer cells. Oral administration of the GO-201 peptide to mice bearing human breast tumor xenografts resulted in loss of tumorigenicity and prolonged tumor regression as a consequence of extensive necrosis.44 The absence of toxicity associated with the use of PMIP or GO-201 peptides in mice is encouraging for their evaluation in humans, where their potential immunogenicity is a concern. In contrast, small molecule inhibitors are not immunogenic. Apigenin was isolated by screening small molecule libraries for compounds that block the dimerization of the MUC1 cytoplasmic domain.45 In breast cancer cells overexpressing MUC1, apigenin promotes similar phenotypes to those observed with the GO-201 and GO-202 peptides. Interestingly, this small molecule inhibitor is an orally bioavailable flavone in animals and humans and has been widely studied for its anti-inflammatory properties as well as a cancer chemopreventive agent.45
Determining whether the antibodies that have been described to target the MUC1β extracellular domain36,46 can block MUC1β signaling and/or interfere with its possible release from the cell membrane could provide mechanistic information and possibly an additional therapeutic option. MicroRNAs (miRNAs) are small noncoding RNA molecules involved in post-transcriptional suppression of gene expression. Three miRNAs are currently known to target MUC1: miR-145, miR-1226, and miR-125b. miR-125 inhibits cell invasion and metastasis of MUC1-overexpressing breast cancer cells,47 and miR-1226 and miR-125b act as tumor suppressors in breast cancer cells at least in part by suppressing the translation of the MUC1 oncoprotein.48,49 The therapeutic potential of these miRNAs, which by binding to the 3′ untranslated region of MUC1 mRNAs suppress the translation of all MUC1 variants, remains to be assessed.
Abolishing the oncogenic functions of MUC1β by targeting intracellular interactions of its cytoplasmic domain has therapeutic potential in humans. However, acquired tumor resistance to targeted therapies is now a recurrent theme in the clinic. Therefore, designing clinical trials in patients with MUC1-overexpressing tumors to evaluate a cocktail of agents including molecules targeting MUC1β and MUC1α should be considered.
Conclusions
Like the forest hiding the tree, the large α subunits of the transmembrane mucins have overshadowed their small β subunit counterparts for a long time. However, the discovery of the oncogenic potential of the β subunit is shifting the focus from the large to the small, to the point that therapeutic targeting of β subunits is beginning to look like a more promising strategy than targeting α subunits. This β-versus-α, less-is-more concept may also hold true for biomarker potential; evaluation of β expression could provide much more robust information on the prognostic and predictive value of oncogenic transmembrane mucins.
Acknowledgments
H. Albrecht acknowledges Institutional Research Grant (IRG No. 95–125-07) funding. K. Carraway was supported by grants from the NIH (CA123541 and GM068994).
Disclosure Statement
No competing financial interests exist.
About the Authors

Huguette Albrecht is an assistant researcher in the Department of Public Health Sciences at the University of California Davis School of Medicine. She received her PhD in molecular and cellular biology from the Louis Pasteur University in Strasbourg, France. Her past work includes the development of recombinant antibody fragment-based radiopharmaceuticals for cancer therapy. Her current research focus and interests comprise studying the effects of low-dose radiation in humans and MUC1 oncogenesis, particularly as it applies to breast cancer.

Kermit Carraway, III, is a professor of biochemistry and molecular medicine at the University of California Davis School of Medicine. He received his PhD in biochemistry and cell biology from Cornell University. In addition to roles of mucins in signal transduction and cellular growth control, his research interests include the contribution of growth factor receptor signaling to cellular processes involved in tumor initiation and progression and roles for negative regulatory mechanisms and protein degradation pathways in controlling receptor function.
References
- 1.Gendler SJ. Lancaster CA. Taylor-Papadimitriou J, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286. [PubMed] [Google Scholar]
- 2.Kufe DW. Mucins in cancer: Function, prognosis and therapy. Nat Rev Cancer. 9:874. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia. 2001;6:339. doi: 10.1023/a:1011379725811. [DOI] [PubMed] [Google Scholar]
- 4.Moniaux N. Escande F. Batra SK. Porchet N. Laine A. Aubert JP. Alternative splicing generates a family of putative secreted and membrane-associated MUC4 mucins. Eur J Biochem. 2000;267:4536. doi: 10.1046/j.1432-1327.2000.01504.x. [DOI] [PubMed] [Google Scholar]
- 5.Burchell JM. Mungul A. Taylor-Papadimitriou J. O-linked glycosylation in the mammary gland: Changes that occur during malignancy. J Mammary Gland Biol Neoplasia. 2001;6:355. doi: 10.1023/a:1011331809881. [DOI] [PubMed] [Google Scholar]
- 6.Carraway KL., 3rd Funes M. Workman HC. Sweeney C. Contribution of membrane mucins to tumor progression through modulation of cellular growth signaling pathways. Curr Top Dev Biol. 2007;78:1. doi: 10.1016/S0070-2153(06)78001-2. [DOI] [PubMed] [Google Scholar]
- 7.Price MR. Rye PD. Petrakou E, et al. Summary report on the ISOBM TD-4 workshop: analysis of 56 monoclonal antibodies against the MUC1 mucin. Tumour Biol. 1998;19(suppl1):1. doi: 10.1159/000056500. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot JD. Tjandra N. Bu D. Ho C. Montelaro RC. Finn OJ. Biophysical characterization of one-, two-, and three-tandem repeats of human mucin (muc-1) protein core. Cancer Res. 1993;53:5386. [PubMed] [Google Scholar]
- 9.Beatson RE. Taylor-Papadimitriou J. Burchell JM. MUC1 immunotherapy. Immunotherapy. 2010;2:305. doi: 10.2217/imt.10.17. [DOI] [PubMed] [Google Scholar]
- 10.Taylor-Papadimitriou J. Burchell J. Miles DW. Dalziel M. MUC1 and cancer. Biochim Biophys Acta. 1999;1455:301. doi: 10.1016/s0925-4439(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Q. Barclay M. Hilkens J, et al. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol Cancer. 2010;9:154. doi: 10.1186/1476-4598-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croce MV. Isla-Larrain MT. Capafons A. Price MR. Segal-Eiras A. Humoral immune response induced by the protein core of MUC1 mucin in pregnant and healthy women. Breast Cancer Res Treat. 2001;69:1. doi: 10.1023/a:1012220902991. [DOI] [PubMed] [Google Scholar]
- 13.Komatsu M. Carraway CA. Fregien NL. Carraway KL. Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J Biol Chem. 1997;272:33245. doi: 10.1074/jbc.272.52.33245. [DOI] [PubMed] [Google Scholar]
- 14.Hollingsworth MA. Swanson BJ. Mucins in cancer: Protection and control of the cell surface. Nat Rev Cancer. 2004;4:45. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 15.Carson DD. The cytoplasmic tail of MUC1: a very busy place. Sci Signal. 2008;1:pe35. doi: 10.1126/scisignal.127pe35. [DOI] [PubMed] [Google Scholar]
- 16.Pochampalli MR. el Bejjani RM. Schroeder JA. MUC1 is a novel regulator of ErbB1 receptor trafficking. Oncogene. 2007;26:1693. doi: 10.1038/sj.onc.1209976. [DOI] [PubMed] [Google Scholar]
- 17.Merlin J. Stechly L. de Beauce S, et al. Galectin-3 regulates MUC1 and EGFR cellular distribution and EGFR downstream pathways in pancreatic cancer cells. Oncogene. 2011;30:2514. doi: 10.1038/onc.2010.631. [DOI] [PubMed] [Google Scholar]
- 18.Behrens ME. Grandgenett PM. Bailey JM, et al. The reactive tumor microenvironment: MUC1 signaling directly reprograms transcription of CTGF. Oncogene. 2010;29:5667. doi: 10.1038/onc.2010.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kufe D. Oncogenic function of the MUC1 receptor subunit in gene regulation. Oncogene. 2010;29:5663. doi: 10.1038/onc.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad R. Rajabi H. Kosugi M, et al. MUC1-C oncoprotein promotes STAT3 activation in an autoinductive regulatory loop. Sci Signal. 2011;4:ra9. doi: 10.1126/scisignal.2001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bitler BG. Goverdhan A. Schroeder JA. MUC1 regulates nuclear localization and function of the epidermal growth factor receptor. J Cell Sci. 2010;123(pt10):1716. doi: 10.1242/jcs.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L. Liao X. Beckett M, et al. MUC1-C Oncoprotein interacts directly with ATM and promotes the DNA damage response to ionizing radiation. Genes Cancer. 2010;1:239. doi: 10.1177/1947601910368059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carraway KL., 3rd Rossi EA. Komatsu M, et al. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem. 1999;274:5263. doi: 10.1074/jbc.274.9.5263. [DOI] [PubMed] [Google Scholar]
- 24.Funes M. Miller JK. Lai C. Carraway KL., 3rd Sweeney C. The mucin Muc4 potentiates neuregulin signaling by increasing the cell-surface populations of ErbB2 and ErbB3. J Biol Chem. 2006;281:19310. doi: 10.1074/jbc.M603225200. [DOI] [PubMed] [Google Scholar]
- 25.Workman HC. Sweeney C. Carraway KL., 3rd The membrane mucin Muc4 inhibits apoptosis induced by multiple insults via ErbB2-dependent and ErbB2-independent mechanisms. Cancer Res. 2009;69:2845. doi: 10.1158/0008-5472.CAN-08-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorlie T. Perou CM. Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzgibbons PL. Page DL. Weaver D, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 28.Rahn JJ. Dabbagh L. Pasdar M. Hugh JC. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer. 2001;91:1973. doi: 10.1002/1097-0142(20010601)91:11<1973::aid-cncr1222>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Rakha EA. Boyce RW. Abd El-Rehim D, et al. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005;18:1295. doi: 10.1038/modpathol.3800445. [DOI] [PubMed] [Google Scholar]
- 30.van der Vegt B. de Roos MA. Peterse JL, et al. The expression pattern of MUC1 (EMA) is related to tumour characteristics and clinical outcome of invasive ductal breast carcinoma. Histopathology. 2007;51:322. doi: 10.1111/j.1365-2559.2007.02757.x. [DOI] [PubMed] [Google Scholar]
- 31.Croce MV. Isla-Larrain MT. Rua CE. Rabassa ME. Gendler SJ. Segal-Eiras A. Patterns of MUC1 tissue expression defined by an anti-MUC1 cytoplasmic tail monoclonal antibody in breast cancer. J Histochem Cytochem. 2003;51:781. doi: 10.1177/002215540305100609. [DOI] [PubMed] [Google Scholar]
- 32.Workman HC. Miller JK. Ingalla EQ, et al. The membrane mucin MUC4 is elevated in breast tumor lymph node metastases relative to matched primary tumors and confers aggressive properties to breast cancer cells. Breast Cancer Res. 2009;11:R70. doi: 10.1186/bcr2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gion M. Mione R. Leon AE. Dittadi R. Comparison of the diagnostic accuracy of CA27.29 and CA15.3 in primary breast cancer. Clin Chem. 1999;45:630. [PubMed] [Google Scholar]
- 34.Harris L. Fritsche H. Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 35.Kramer EL. DeNardo SJ. Liebes L, et al. Radioimmunolocalization of metastatic breast carcinoma using indium-111-methyl benzyl DTPA BrE-3 monoclonal antibody: Phase I study. J Nucl Med. 1993;34:1067. [PubMed] [Google Scholar]
- 36.Rubinstein DB. Karmely M. Ziv R, et al. MUC1/X protein immunization enhances cDNA immunization in generating anti-MUC1 alpha/beta junction antibodies that target malignant cells. Cancer Res. 2006;66:11247. doi: 10.1158/0008-5472.CAN-06-1486. [DOI] [PubMed] [Google Scholar]
- 37.Nagy P. Friedlander E. Tanner M, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473. [PubMed] [Google Scholar]
- 38.Fessler SP. Wotkowicz MT. Mahanta SK. Bamdad C. MUC1* is a determinant of trastuzumab (herceptin) resistance in breast cancer cells. Breast Cancer Res Treat. 2009;118:113. doi: 10.1007/s10549-009-0412-3. [DOI] [PubMed] [Google Scholar]
- 39.Price-Schiavi SA. Jepson S. Li P, et al. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99:783. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 40.Tang CK. Apostolopoulos V. Strategies used for MUC1 immunotherapy: Preclinical studies. Expert Rev Vaccines. 2008;7:951. doi: 10.1586/14760584.7.7.951. [DOI] [PubMed] [Google Scholar]
- 41.Finn OO. Banchereau J. Cancer vaccines: The state of the art. Semin Immunol. 2010;22:103. [PubMed] [Google Scholar]
- 42.Orava EW. Cicmil N. Gariepy J. Delivering cargoes into cancer cells using DNA aptamers targeting internalized surface portals. Biochim Biophys Acta. 2010;1798:2190. doi: 10.1016/j.bbamem.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Bitler BG. Menzl I. Huerta CL, et al. Intracellular MUC1 peptides inhibit cancer progression. Clin Cancer Res. 2009;15:100. doi: 10.1158/1078-0432.CCR-08-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raina D. Ahmad R. Joshi MD, et al. Direct targeting of the mucin 1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69:5133. doi: 10.1158/0008-5472.CAN-09-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J. Rajabi H. Kufe DW. Muc1-C oncoprotein is a target for small molecule inhibitors. Mol Pharmacol. 2011;79:886. doi: 10.1124/mol.110.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahanta S. Fessler SP. Park J. Bamdad C. A minimal fragment of MUC1 mediates growth of cancer cells. PLoS One. 2008;3:e2054. doi: 10.1371/journal.pone.0002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sachdeva M. Mo YY. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010;70:378. doi: 10.1158/0008-5472.CAN-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin C. Rajabi H. Kufe D. miR-1226 targets expression of the mucin 1 oncoprotein and induces cell death. Int J Oncol. 2010;37:61. doi: 10.3892/ijo_00000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajabi H. Jin C. Ahmad R. McClary C. Joshi MD. Kufe D. Mucin 1 oncoprotein expression is suppressed by the miR-125b oncomir. Genes Cancer. 2010;1:62. doi: 10.1177/1947601909357933. [DOI] [PMC free article] [PubMed] [Google Scholar]