Abstract
We have previously reported that low concentrations of interferon (IFN)-activated monocytes exert near-eradicative cytocidal activity against low concentrations of several human tumor cells in vitro. In the present study, we examined 7 human tumor cell lines and 3 diploid lines in the presence or absence of 10 ng/mL IFNα2a and monocytes. The results confirmed strong cytocidal activity against 4 of 7 tumor lines but none against 3 diploid lines. To model larger in vivo tumors, we increased the target cell concentration and determined the concentration of IFNα2a and monocytes, required for cell death. We found that increasing the tumor cell concentration from 10- to 100-fold (105 cells/well) required an increase in the concentration of IFNs by over 100-fold and monocytes by 10-fold. High concentrations of monocytes could sometimes kill tumor or diploid cells in the absence of IFN. We may conclude that killing of high concentrations of tumor or diploid cells required high concentrations of monocytes that could sometimes kill in the absence of IFN. Thus, high concentrations of tumor cells required high concentrations of IFN and monocytes to cause near eradication of tumor cells. These findings may have clinical implications.
Introduction
Interferons (IFNs) have been shown to exhibit antiviral, antiproliferative, and immunomodulatory effects (Isaacs 1963; Gresser 1982; Bekisz and others 2004). Activated monocytes incompletely kill tumor cells both in vitro and in vivo (Chen and Koren 1985; Martinet and others 1992; Galligioni and others 1993; Khammari and others 2007). We have previously reported that low concentrations (105 per microtiter well) of IFN-activated monocytes exert near-eradicative cytocidal activity against low concentrations of a number of human tumor cells in vitro (103–4 per well) (Baron and others 2007). Diploid cells are resistant in these conditions and may lack monocyte-signaling molecules (Utsugi and others 1991; Galligioni and others 1993; Shi and others 2005; Baron and others 2007). To model the high tumor cell concentrations found in vivo, we increased the target cell concentration 100-fold (105 cells/well) and determined the concentration of monocytes, with and without IFNs, required for target cell eradication.
Materials and Methods
Tumor cells were cultured in RPMI 1640 containing 10% fetal bovine serum, 1% l-glutamine, and 50 units/mL of penicillin and streptomycin. Diploid cells were cultured in Eagle's minimum essential media (EMEM) containing 10% fetal bovine serum, 1% l-glutamine, and 50 units/mL of penicillin and streptomycin. Elutriated monocytes were obtained from healthy adult donors by the National Institutes of Health Blood Bank using the Gambro Elutra method (Chen and others 2008). The preparation contained over 90% monocytes, <10% neutrophils, and <5% lymphocytes. As reported earlier, the level of contaminating lymphocytes and neutrophils in the monocyte preparation do not contribute to the antitumor activity observed (Schaider and others 2003; Otten and others 2005; Baron and others 2007). IFNα2a was obtained from Hoffmann LaRoche (Nutley, NJ). IFNγ was obtained from Intermune Pharmaceutical (Brisbane, CA). A549 lung, human osteosarcoma, LOX melanoma, SNB19 brain tumor, and OVCAR3 ovarian tumor lines were provided by the Developmental Therapeutics Program NCI/NIH and are listed among the 60 human cancer cell lines (NCI 60) maintained by NCI-Frederick. IgrOV1 ovarian tumor line was provided by Dr. Raj Puri of the Center for Biologics Evaluation and Research at the FDA. Diploid lines MRC5, WI38, and FS-4 were purchased from ATCC (Manassas, VA).
We treated low (103–4 per well) in 0.1 mL and high (105) concentrations of tumor and diploid cells, with low (105 per well) or high (106) concentrations of monocytes with and without IFN, and then quantified cell death after 3–5 days of incubation by measuring target cell confluence and dye uptake. Specifically, the cells were plated in 96-well plates at varying concentrations and were incubated at 37°C for 4 h to attain adherence. The cells were then treated with varying concentrations of monocytes and IFN. After 3–5 days, the plates were decanted, washed, and stained with crystal violet (2.3% in ethanol) to quantify cell death by dye uptake, colony counts, and/or microscopic percent confluence. Fold inhibition was calculated by comparison with medium control cultures (Baron and others 2007). As we reported previously, in a comparison of colony counts, visual confluence, and crystal violet or [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT) vital dye uptake (Baron and others 2007), the live target cells both adhere to the plastic and are stained, whereas monocytes and all dead target cells are washed away during the staining procedure. In the present study, to measure crystal violet dye uptake, 0.1 mL ethanol was added to each well to dissolve the crystal violet. Dye absorbance was measured at 570 nm using a VICTOR3 plate reader (Perkin Elmer, Waltham, MA).
Results
Killing of high concentrations of OVCAR3 tumor cells by monocytes requires higher concentrations of IFNs
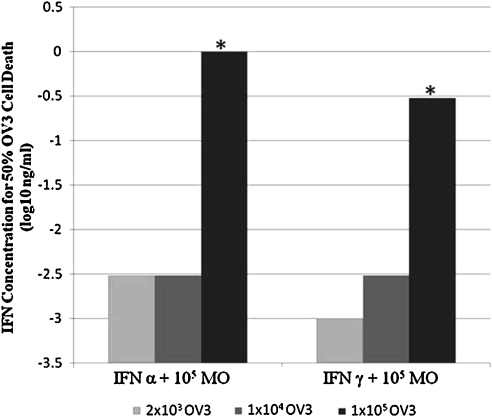
Generally, an increased concentration of tumor cells is reported to require stronger antitumor treatments to achieve tumoricidal effects (Khammari and others 2007). To determine whether increased concentrations of OVCAR3 cells would similarly require higher concentrations of IFNs to activate the monocytes' cytocidal activity, we treated ∼10-fold incremental concentrations of OVCAR3 cells with 10-fold increments of IFNα2a or IFNγ in the presence of 105 monocytes/well. After incubation for 4 days, the unattached monocytes were washed away and the adherent tumor cells were stained with crystal violet to determine the 50% cell death endpoint. The findings were confirmed by microscopic determination of confluency and, in some experiments, by MTT assay. IFN-α2a and γ-activation of monocytes consistently achieved at least 10-fold higher tumor killing than IFN-α2a or -γ alone (data not shown). Further, monocytes alone exhibited only partial (<45%) cell killing for 2 × 103 OV3/0.1 mL in microtiter wells and had no visible effect on 1 × 104 or 1 × 105 OVCAR3 cells/well (data not shown). As shown in Fig. 1, increasing the OVCAR3 tumor cell concentration from 103–4 to 105 cells/well strongly increased the concentration of IFNs needed to achieve 50% cell death. The IFN alone control and the monocyte alone control showed no cell inhibition. This finding indicates that to effectively kill higher concentrations of tumor cells, substantially higher concentrations of IFN and/or monocytes are required.
FIG. 1.
Killing of high concentrations of OVCAR3 tumor cells by monocytes requires higher concentrations of IFNs. The interferon alone and the monocyte alone controls showed no cell inhibition. *P < 0.01 by Student's t-test comparing 1 × 105 OVCAR-3 concentrations with 2 × 103 and 1 × 104. IFN, interferon.
Expanded studies of in vitro modeling of low concentrations of target cells (103–4 per well) and low concentrations of monocytes (105 per well)
We confirmed and expanded the previous study by using low concentrations of monocytes and target cells (Baron and others 2007) to include a total of 7 human tumor cell lines and 3 diploid lines in the presence or absence of 10 ng/mL IFNα2a and the stated number of monocytes. In these experiments, the 10 ng/mL IFNα2a alone demonstrated ∼50% killing for the OVCAR3 ovarian tumor cell line and no killing for the other tumor and diploid lines (data not shown). Monocytes alone resulted in 45%–75% killing of 3 of 7 tumor lines, but none of the 3 diploid lines were killed (Table 1). Also, as shown in Table 1, addition of IFNα2a to the monocytes greatly increased the killing (P < 0.01) of the 3 sensitive lines and one insensitive line to near-eradicative levels but did not kill the diploid lines. IFN alone showed no killing. Thus, these expanded studies of killing involving low concentrations of monocytes, IFNα2a, and target cells confirmed and extended earlier studies reporting near eradication of several tumor lines, but not diploid lines.
Table 1.
In Vitro Modeling of Low Concentrations of Target Cells (103–4 per Well) and Low Concentrations of Monocytes (105 per Well) on Cell Viability
| |
Monocytes alone treatment |
Monocytes + IFNα2a treatment |
||
|---|---|---|---|---|
| Target cells | % Killing | Fold killing | % Killing | Fold killing |
| OVCAR3 ovarian | 75a | 4a | 99a | 100a |
| Osteosarcoma | 50 | 2 | 98a | 50a |
| A549 lung | 45 | 2 | 95a | 20a |
| LOX melanoma | 0 | 0 | 99a | 100a |
| IgrOV1 ovarian | 0 | 0 | 0 | 0 |
| Huh7 liver | 0 | 0 | 0 | 0 |
| SNB19 | 0 | 0 | 0 | 0 |
| WI38 diploid | 0 | 0 | 0 | 0 |
| MRC5 diploid | 0 | 0 | 0 | 0 |
| FS-4 diploid | 0 | 0 | 0 | 0 |
P < 0.01 by Student's t-test comparing the variously treated cell lines with their appropriate interferon and monocyte control cultures.
IFN, interferon.
Effect of high concentrations of target cells (105 per well) and high concentrations of monocytes (106 per well) on cell killing
High concentrations of monocytes (106 per well) without IFN achieved near eradication of all tumor lines tested and overcame resistance to killing by the IgrOV1 ovarian and the SNB19 glioblastoma cell lines and by 1 of 2 diploid lines (Table 2). The addition of IFNα2a could not enhance the already complete killing by monocytes alone but did enhance killing of 1 diploid cell line. Viabilities of both tumor cells and monocytes were confirmed by colony count and MTT colorimetric cell viability assay, respectively (data not shown).
Table 2.
In Vitro Modeling of High Concentrations of Target Cells (105 per Well) and High Concentrations of Monocytes (106 per Well) on Cell Viability
| |
Monocytes alone treatment |
Monocytes + IFNα2a treatment |
||
|---|---|---|---|---|
| Target cells | % Killing | Fold killing | % Killing | Fold killing |
| OVCAR3 ovarian | 99a | 100a | 99a | 100a |
| A549 lung | 98a | 50a | 98a | 50a |
| IgrOV1 ovarian | 98a | 50a | 98a | 50a |
| SNB19 glioblastoma | 95a | 20a | 95a | 20a |
| WI38 diploid | 98a | 50a | 98a | 50a |
| MRC5 diploid | 0 | 0 | 98a | 50a |
P < 0.01 by Student's t-test comparing the variously treated cell lines with their appropriate control cultures.
Discussion
We reported that low concentrations of IFN-activated monocytes (105 cells per microtiter well) exerted strong cytocidal activity against low concentrations (103–4 tumor cells per microtiter well) of some human tumor cells in vitro. To model larger in vivo tumors, we increased the target cell concentration 100-fold (105 tumor cells) and determined the concentration of monocytes, with and without IFNα2a required for cell death. We found that increasing the tumor cell concentration from 10- to 100-fold increased the required cytocidal concentration of IFNs by over 100-fold and the required concentration of monocytes by 10-fold. Unexpectedly, killing of tumor cells at higher concentrations of monocytes did not require activation by IFN. Also unexpectedly, at the 10-fold higher concentration of monocytes, the 2 diploid cell lines studied were sensitive to the cytocidal activity of monocytes, but activation by IFN was needed to kill 1 of the 2 diploid lines. These complex results at the high concentrations of target cells and monocytes suggest that many unidentified variables involving monocytes, IFNs, and tumor cells have complex interactions that need to be identified. Despite these unidentified variables, some biological conclusions can be made, such as, (a) for the first time, activated monocytes achieved near eradication of high concentrations of tumor cells, (b) activation by IFNs strongly increased monocyte cytocidal activity under many conditions, and (c) cell death of 2 diploid lines studied occurred only at the highest concentration of monocytes.
Possible mechanisms of cytocidal activity by monocytes
A number of mechanisms by which monocytes kill tumor cells have been identified. These include cytokines such as tumor necrosis factor, proteases, oxidative compounds such as nitric oxide and peroxides, TRAIL, and thymidines (Drysdale and others 1988; Pak and Fidler 1991; Vanderheyde and others 2004; Tsuno and others 2009). However, the pathways of killing of tumor cells by IFN-activated monocytes have not been determined (Kopper and Lapis 1985). We plan such studies in the future.
Some possible therapeutic approaches using activated monocytes
Our findings show the unanticipated eradicative antitumor potency of homologous human monocytes, compared with the previously reported 30%–80% tumor cell inhibition (Manna and Mohanakumar 2002; Vanderheyde and others 2004). An initial consideration is the safety of large numbers of autologous monocytes needed for antitumor effects. The safety of human monocytes from blood banks has been reported in several phase 1 human trials (Faradji and others 1991a, 1991b; Hossne and others 2009). In addition to the safety of monocytes, FDA-approved IFNs are available for clinical studies (Bekisz and others 2010). Thus, studies of clinical antitumor efficacy of IFN-activated monocytes seem feasible and relevant.
As the contact of activated monocytes with tumor cells is important, an initial clinical study of IFN-activated monocytes might be done in patients with tumors localized to the skin and accessible by injection. Subsequent clinical trials of tumors localized to the brain might be considered.
Additional future studies might compare activation by IFNs with other strong activators such as cytosine-phosphate-guanine (CpG) and lipopolysaccharide (LPS) (Manna and Mohanakumar 2002; Sugiyama and others 2005) for antitumor potency. Other studies might evaluate the addition of monocyte-recruiting cytokines such as interleukin-8, interleukin-18, and monocyte chemotactic protein-1 (MCP-1) (Papadopoulou and others 2008; Ruth and others 2010) to IFN-activated monocytes to determine whether recruiting activated monocytes to tumor sites in vivo can provide protection. Importantly, the recruiting cytokines can often also function as activators of monocytes in addition to recruiting (Drysdale and others 1988). Another important study might be to determine whether circulating monocytes, injected intravenously, can migrate to metastatic sites and provide protection. Many of these questions can be addressed in mouse models of human tumors (Kioi and others 2006). Preclinical studies in this mouse model have been started.
Can monocytes provide natural surveillance against tumors?
An important question is whether the strong cytocidal activity of IFN-activated monocytes reflects the existence of a natural surveillance mechanism against some tumors. Unfortunately, this possibility is not supported by our findings and the literature. Against the possibility that monocytes within tumors are activated, there is the finding that most tumors do not produce activators of monocytes (Wojtowicz-Praga 1997; Mocellin and others 2001). Actually, many tumors produce inhibitors of monocytes (Mocellin and others 2001; Dirkx and others 2006). In addition, the concentrations of monocytes and macrophages that naturally infiltrate tumors are generally too low to cause eradication (An and others 1987; Yamashiro and others 1994; Ohta and others 2003; Baron and others 2007). Further, the concentration of tumor-infiltrating macrophages is often correlated not with antitumor protection but rather with angiogenesis and poor prognoses (Dirkx and others 2006; Shieh and others 2009). Thus, available evidence favors the interpretation that monocytes and macrophages do not function as natural surveillance mechanisms against tumors. However, our studies indicate that normal, circulating, nonactivated, autologous monocytes are available for therapeutic activation in clinical trials.
Conclusions
This study is the first report of the conditions for near eradication of a number of human tumors in the presence of human monocytes and IFN. In vivo studies in murine models of human tumors have been planned.
Acknowledgment
This work was supported by the Intramural Research Program of the NIAID, NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- An T. Sood U. Pietruk T. Cummings G. Hashimoto K. Crissman JD. In situ quantitation of inflammatory mononuclear cells in ductal infiltrating breast carcinoma. Relation to prognostic parameters. Am J Pathol. 1987;128:52–60. [PMC free article] [PubMed] [Google Scholar]
- Baron S. Hernandez J. Bekisz J. Poast J. Goldman N. Clouse K. Fields K. Bacot S. Wang J. Zoon K. Clinical model: interferons activate human monocytes to an eradicative tumor cell level in vitro. J Interferon Cytokine Res. 2007;27:157–163. doi: 10.1089/jir.2006.0083. [DOI] [PubMed] [Google Scholar]
- Bekisz J. Baron S. Balinsky C. Morrow A. Zoon KC. Antiproliferative properties of type I and type II interferon. Pharmaceuticals (Basel) 2010;3:994–1015. doi: 10.3390/ph3040994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekisz J. Schmeisser H. Hernandez J. Goldman ND. Zoon KC. Human interferons alpha, beta and omega. Growth Factors. 2004;22:243–251. doi: 10.1080/08977190400000833. [DOI] [PubMed] [Google Scholar]
- Chen AR. Koren HS. Impaired oxidative burst does not affect human monocyte tumoricidal activity. J Immunol. 1985;134:1909–1913. [PubMed] [Google Scholar]
- Chen Y. Hoecker P. Zeng J. Dettke M. Combination of Cobe AutoPBSC and Gambro Elutra as a platform for monocyte enrichment in dendritic cell (DC) therapy: clinical study. J Clin Apher. 2008;23:157–162. doi: 10.1002/jca.20173. [DOI] [PubMed] [Google Scholar]
- Dirkx AE. Oude Egbrink MG. Wagstaff J. Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- Drysdale BE. Agarwal S. Shin HS. Macrophage-mediated tumoricidal activity: mechanisms of activation and cytotoxicity. Prog Allergy. 1988;40:111–161. [PubMed] [Google Scholar]
- Faradji A. Bohbot A. Frost H. Schmitt-Goguel M. Siffert JC. Dufour P. Eber M. Lallot C. Wiesel ML. Bergerat JP. Phase I study of liposomal MTP-PE-activated autologous monocytes administered intraperitoneally to patients with peritoneal carcinomatosis. J Clin Oncol. 1991a;9:1251–1260. doi: 10.1200/JCO.1991.9.7.1251. others. [DOI] [PubMed] [Google Scholar]
- Faradji A. Bohbot A. Schmitt-Goguel M. Dumont S. Eischen A. Wiesel ML. Stierle A. Follea G. Eber M. Bergerat JP. Apheresis-elutriation program for adoptive immunotherapy with autologous activated monocytes in cancer patients. Int J Artif Organs. 1991b;14:304–312. others. [PubMed] [Google Scholar]
- Galligioni E. Quaia M. Spada A. Favaro D. Santarosa M. Talamini R. Monfardini S. Activation of cytolytic activity in peripheral blood monocytes of renal cancer patients against non-cultured autologous tumor cells. Int J Cancer. 1993;55:380–385. doi: 10.1002/ijc.2910550307. [DOI] [PubMed] [Google Scholar]
- Gresser I. How does interferon inhibit tumour growth? Philos Trans R Soc Lond B Biol Sci. 1982;299:69–76. doi: 10.1098/rstb.1982.0107. [DOI] [PubMed] [Google Scholar]
- Hossne NA., Jr. Invitti AL. Buffolo E. Azevedo S. Rodrigues de Oliveira JS. Stolf NG. Cruz LE. Sanberg PR. Refractory angina cell therapy (ReACT) involving autologous bone marrow cells in patients without left ventricular dysfunction: a possible role for monocytes. Cell Transplant. 2009;18:1299–1310. doi: 10.3727/096368909X484671. [DOI] [PubMed] [Google Scholar]
- Isaacs A. Foreign nucleic acids. Sci Am. 1963;209:46–50. doi: 10.1038/scientificamerican1063-46. [DOI] [PubMed] [Google Scholar]
- Khammari A. Nguyen JM. Pandolfino MC. Quereux G. Brocard A. Bercegeay S. Cassidanius A. Lemarre P. Volteau C. Labarriere N. Long-term follow-up of patients treated by adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2007;56:1853–1860. doi: 10.1007/s00262-007-0340-1. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioi M. Kawakami M. Shimamura T. Husain SR. Puri RK. Interleukin-13 receptor alpha2 chain: a potential biomarker and molecular target for ovarian cancer therapy. Cancer. 2006;107:1407–1418. doi: 10.1002/cncr.22134. [DOI] [PubMed] [Google Scholar]
- Kopper L. Lapis K. What's new in macrophage-tumor cell interaction? Pathol Res Pract. 1985;179:652–655. doi: 10.1016/S0344-0338(85)80212-0. [DOI] [PubMed] [Google Scholar]
- Manna PP. Mohanakumar T. Human dendritic cell mediated cytotoxicity against breast carcinoma cells in vitro. J Leukoc Biol. 2002;72:312–320. [PubMed] [Google Scholar]
- Martinet N. Beck G. Bernard V. Plenat F. Vaillant P. Schooneman F. Vignaud JM. Martinet Y. Mechanism for the recruitment of macrophages to cancer site. In vivo concentration gradient of monocyte chemotactic activity. Cancer. 1992;70:854–860. doi: 10.1002/1097-0142(19920815)70:4<854::aid-cncr2820700422>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Mocellin S. Wang E. Marincola FM. Cytokines and immune response in the tumor microenvironment. J Immunother. 2001;24:392–407. [PubMed] [Google Scholar]
- Ohta M. Kitadai Y. Tanaka S. Yoshihara M. Yasui W. Mukaida N. Haruma K. Chayama K. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int J Oncol. 2003;22:773–778. [PubMed] [Google Scholar]
- Otten MA. Rudolph E. Dechant M. Tuk CW. Reijmers RM. Beelen RH. van de Winkel JG. van Egmond M. Immature neutrophils mediate tumor cell killing via IgA but not IgG Fc receptors. J Immunol. 2005;174:5472–5480. doi: 10.4049/jimmunol.174.9.5472. [DOI] [PubMed] [Google Scholar]
- Pak CC. Fidler IJ. Molecular mechanisms for activated macrophage recognition of tumor cells. Semin Cancer Biol. 1991;2:189–195. [PubMed] [Google Scholar]
- Papadopoulou C. Corrigall V. Taylor PR. Poston RN. The role of the chemokines MCP-1, GRO-alpha, IL-8 and their receptors in the adhesion of monocytic cells to human atherosclerotic plaques. Cytokine. 2008;43:181–186. doi: 10.1016/j.cyto.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth JH. Park CC. Amin MA. Lesch C. Marotte H. Shahrara S. Koch AE. Interleukin-18 as an in vivo mediator of monocyte recruitment in rodent models of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R118. doi: 10.1186/ar3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaider H. Oka M. Bogenrieder T. Nesbit M. Satyamoorthy K. Berking C. Matsushima K. Herlyn M. Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. Int J Cancer. 2003;103:335–343. doi: 10.1002/ijc.10775. [DOI] [PubMed] [Google Scholar]
- Shi J. Ikeda K. Fujii N. Kondo E. Shinagawa K. Ishimaru F. Kaneda K. Tanimoto M. Li X. Pu Q. Activated human umbilical cord blood dendritic cells kill tumor cells without damaging normal hematological progenitor cells. Cancer Sci. 2005;96:127–133. doi: 10.1111/j.1349-7006.2005.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh YS. Hung YJ. Hsieh CB. Chen JS. Chou KC. Liu SY. Tumor-associated macrophage correlated with angiogenesis and progression of mucoepidermoid carcinoma of salivary glands. Ann Surg Oncol. 2009;16:751–760. doi: 10.1245/s10434-008-0259-6. [DOI] [PubMed] [Google Scholar]
- Sugiyama T. Gursel M. Takeshita F. Coban C. Conover J. Kaisho T. Akira S. Klinman DM. Ishii KJ. CpG RNA: identification of novel single-stranded RNA that stimulates human CD14+CD11c+monocytes. J Immunol. 2005;174:2273–2279. doi: 10.4049/jimmunol.174.4.2273. [DOI] [PubMed] [Google Scholar]
- Tsuno T. Mejido J. Zhao T. Schmeisser H. Morrow A. Zoon KC. IRF9 is a key factor for eliciting the antiproliferative activity of IFN-alpha. J Immunother. 2009;32:803–816. doi: 10.1097/CJI.0b013e3181ad4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugi T. Schroit AJ. Connor J. Bucana CD. Fidler IJ. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991;51:3062–3066. [PubMed] [Google Scholar]
- Vanderheyde N. Vandenabeele P. Goldman M. Willems F. Distinct mechanisms are involved in tumoristatic and tumoricidal activities of monocyte-derived dendritic cells. Immunol Lett. 2004;91:99–101. doi: 10.1016/j.imlet.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Wojtowicz-Praga S. Reversal of tumor-induced immunosuppression: a new approach to cancer therapy. J Immunother. 1997;20:165–77. [PubMed] [Google Scholar]
- Yamashiro S. Takeya M. Nishi T. Kuratsu J. Yoshimura T. Ushio Y. Takahashi K. Tumor-derived monocyte chemoattractant protein-1 induces intratumoral infiltration of monocyte-derived macrophage subpopulation in transplanted rat tumors. Am J Pathol. 1994;145:856–867. [PMC free article] [PubMed] [Google Scholar]