Abstract
Embryonic epiboly has become an important developmental model for studying the mechanisms underlying collective movements of epithelial cells. In the last couple of decades, most studies of epiboly have utilized Xenopus or zebrafish as genetically tractable model organisms, while the avian epiboly model has received virtually no attention. Here, we re-visit epiboly in quail embryos and characterize several molecular markers of epithelial-to-mesenchymal transition (EMT) in the inner zone of the extraembryonic Area Opaca and at the blastoderm edge. Our results show that the intermediate filament vimentin, a widely-used marker for the mesenchymal phenotype, is strongly expressed in the edge cells compared to the cells in the inner zone. Laminin, an extracellular matrix protein that is a major structural and adhesive component of the epiblast basement membrane and the inner zone of the Area Opaca, is notably absent from the blastoderm edge. While these expression profiles are consistent with a mesenchymal phenotype, several other epithelial markers, including cytokeratin, β-catenin, and E-cadherin, are present in the blastoderm edge cells. Moreover, the results of a BrDU proliferation assay strongly suggest that expansion of the edge cell population is primarily due to recruitment of cells from the inner zone, as opposed to proliferation. Taken together, our data show that the edge cells of the avian blastoderm have characteristics of both epithelial and mesenchymal cells, and that the avian epiboly model, which has been dormant for so many years, may yet again prove to be helpful as a unique developmental model for studying partial EMT in the context of collective epithelial cell migration.
Keywords: epiboly, epithelial migration, EMT, extracellular matrix, intermediate filaments, adhesion, motility
INTRODUCTION
Throughout the second half of the twentieth century and into the new millennium, studies of cell migration have primarily focused on uncovering the mechanisms involved in the motility of individual cells. In the past decade or so, investigators have increasingly turned their attention to the phenomenon of multi-cellular or collective cell migration, since cells move together as cohesive sheets or in continuous streams during many developmental and morphogenetic processes, including gastrulation, neural crest migration, and migration of heart primordia, in addition to some important pathophysiological situations, notably wound healing and cancer (Jacinto et al., 2001; Davidson et al., 2002; Keller et al., 2003; Christiansen and Rajasekaran, 2006; Zamir et al., 2008). Consequently, newer models, such as the posterior lateral line of the zebrafish and dorsal closure in Drosophila have emerged to address the unique mechanisms that drive and regulate collective cell migration (Kiehart et al., 2000; Jacinto et al., 2002; Ghysen and Dambly-Chaudiere, 2004).
The most widely studied collective cell behavior is the migration of an epithelium, and perhaps the best-known experimental model for epithelial migration is the classical “scratch wound” assay. The biophysical mechanisms involved in epithelial migration are complex (Rorth, 2009), and only recently, using techniques first developed for studies of individual cells, have some direct measurements of the forces contributed by individual cells within and at the “free edge” of a migrating epithelial monolayer been made in vitro (du Roure et al., 2005; Farooqui and Fenteany, 2005). In theory, there are two basic modes of epithelial migration or expansion. On one end of the spectrum, all the cells in the collective migrate cohesively and uniformly, with each cell contributing similar amounts of substrate traction force (du Roure et al., 2005). Alternatively, the cells constituting the free edge could generate the bulk of the traction force necessary for expansion, and, thus, “tow” the cells within the interior of the epithelium passively along for the ride (Omelchenko et al., 2003; Poujade et al., 2007). In the latter case, one can imagine that the interior epithelial cells rest on a basement membrane and are convected by the edge cells, which adhere to and crawl (autonomously) on an extracellular substrate. Clearly, it is important to distinguish between these two general cases, so that any particular model system can be characterized and studied appropriately, and the mechanisms underlying motility better understood.
Furthermore, it has been noted that the phenotype of individual cells within an epithelial monolayer in vitro varies widely and is often likely to be found in between the dogmatic notions of epithelial and mesenchymal (Revenu and Gilmour, 2009). Whereas cells in the interior of an epithelial monolayer display prototypical tight junctions, and express the classical epithelial markers cytokeratin and E-cadherin, the cells at the free edge may take on a more “mesenchymal-like” phenotype, exhibiting down-regulation of E-cadherin and β-catenin, as well as a flattened morphology and dynamic protrusions of lamellipodia and filopodia. For example, Chaffer et al. (2006) reported that different members of the TSU-Pr1 bladder carcinoma cell line can express both epithelial (E-cadherin and β-catenin) and mesenchymal markers (vimentin and matrix metalloproteases). Klymkowsky and Savagner (2009) refer to this dual nature as the “metastable phenotype,” which has also been called “partial EMT” (Leroy and Mostov, 2007; Arnoux et al., 2008; Revenu and Gilmour, 2009).
In this study, we re-visit the long dormant avian epiboly model and and show that it is a potentially useful system for studying mechanisms of epithelial migration and “partial EMT” (pEMT) in a natural developmental process. During avian epiboly, a thin ring of cells at the edge of the blastoderm migrates radially outward across the ovo-deposited (acellular) vitelline membrane, spreading the extraembryonic epithelium known as Area Opaca (AO), until it engulfs the entire yolk surface within a few days after laying. Owing to the large size of the egg yolk relative to, for example, Xenopus or zebrafish, avian epiboly requires expansion of the AO several hundred-fold over its initial area (New, 1959; Bellairs et al., 1967; Downie, 1976). Previous studies of avian epiboly several decades ago noted a spread morphology and loss of epithelial polarity of the blastoderm edge cells (Downie and Pegrum, 1971; Chernoff, 1989), prompting us to now investigate the extent to which these cells exhibit a “mesenchymal-like” or pEMT phenotype, using several EMT-informative markers. Thus, as a first step in this direction, we performed whole-mount immunofluorescence and high-resolution confocal microscopy to examine the spatial distribution and expression of vimentin, cytokeratin, β-catenin, E-cadherin, and laminin (major component of basal lamina) throughout the AO of early gastrula-stage (1-day-old) quail embryos, in which the extraembryonic mesodermal vasculature has not yet formed.
Our results show, to our knowledge, for the first time, robust co-expression of the mesenchymal marker vimentin and the epithelial markers β-catenin, E-cadherin, and cytokeratin at the free edge of an intact migrating epithelium in an embryonic model system. The results of a BrDU incorporation assay strongly suggest that the cells at the edge of the avian blastoderm are not proliferative, and moreover, that expansion of the edge cell population is due exclusively to recruitment of cells from the adjacent inner epithelial zone of the AO. These data appear to resolve a decades-old question of how the edge cell population is maintained during avian epiboly (Downie, 1976). We discuss the implications of these novel results in the context of evolutionary divergence of epiboly across different species, and, more generally, cellular mechanisms underlying epithelial migration and the pEMT phenotype.
RESULTS
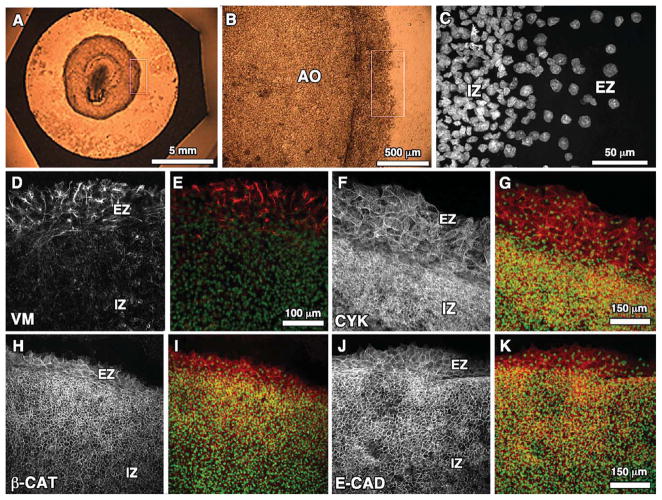
The primary anatomical area of interest in this study was the outer most region of the Area Opaca, which includes the blastoderm edge cells (Fig. 1A–C). The edge cell zone is easily distinguished from the cells in the interior by its much lower cell density (Fig. 1C), and, thus, all immunofluorescence images are accompanied by nuclear labeling to establish the edge zone region as a reference for each marker. It is important to note that the size or width of the edge cell region varies from a few nuclei (Fig. 1I) to over a dozen between different embryos (Fig. 1G). It has been suggested in previous studies that the edge zone size may be developmentally regulated (Bellairs et al., 1967; Downie, 1976), but we observed a significant amount of variation of edge zone size even for embryos of approximately similar stages and even for different areas of the same embryo (Fig. 2). In other words, the edge zone width is not strictly uniform around the entire embryo. Along with nuclear (Sytox) labeling, representative wide-field immunofluorescence images for each of the cellular markers (excluding laminin) that were studied are shown in Figure 1.
Fig. 1.
A–C: Progressive zoom-ins of the blastoderm edge zone of the quail embryo indicated by the white box in A. C: Sytox-labeled nuclei for region outlined in B. D, F, H, J: Representative wide-field (20×) confocal immunoflourescent images for vimentin (VM), cytokeratin (CYK), β-catenin (β-cat), and E-cadherin (E-cad), respectively, at the edge zone (EZ) and innner zone (IZ) of Stage-4 quail blastoderm. E, G, I, K: Each marker is shown merged with Sytox nuclear label.
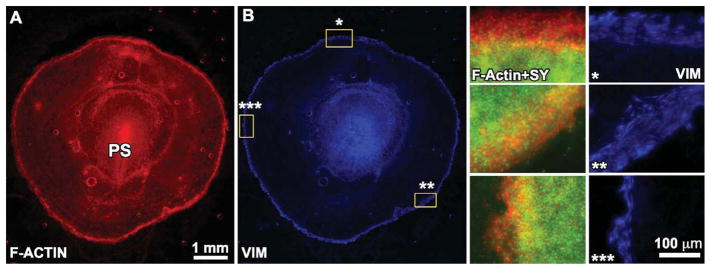
Fig. 2.
A,B: A wide-field (2.5×) montage of F-actin (rhodamine-phalloidin) and vimentin immunofluorescence for a stage-4 quail embryo. The starred regions in B at different regions around the embryo perimeter are shown at higher resolution on the right side. Note the variability in the width of the edge zone in these different regions, which is made clear by the edge-restricted vimentin expression. PS, primitive streak.
Intermediate Filaments (Vimentin and Cytokeratin)
Intermediate filaments (IF) link cells through “junctional complexes” known as desmosomes (Green and Gaudry, 2000), and are thought to play an important role in coordinating mechanical forces and maintenance of cellular integrity in embryonic development, growth, and maturation of specific tissues (Chen et al., 2004; Parry et al., 2007). In this study, we examined the expression pattern of vimentin (VM) and cytokeratin (CYK), which are the two major mammalian cytoplasmic IFs generally associated with, respectively, the mesenchymal and epithelial phenotypes. We performed initial tests on chick embryo fibroblasts using two different monoclonal antibodies, AMF-17b (Isaacs et al., 1989) and H5 (Herman et al., 1993), raised against chick VM. H5 was found to be significantly more intense by indirect immunofluorescence (data not shown), and, thus, was chosen for performing the whole-mount embryo studies described here. For CYK labeling, we used a monoclonal antibody, 1h5 (Klymkowsky et al., 1992), raised against Xenopus Type II basic CYK and tested positively against chicken (according to DSHB datasheet).
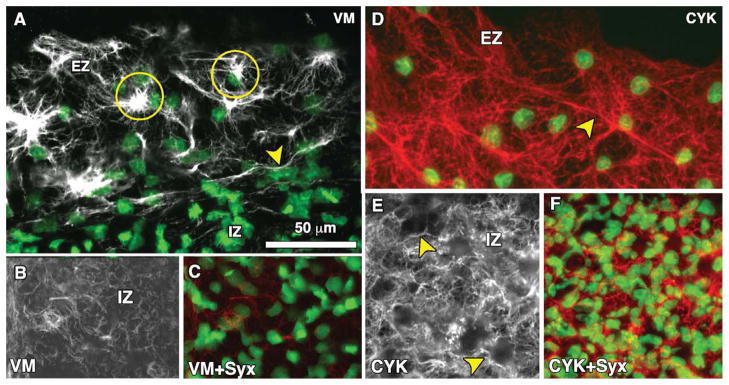
To our initial surprise, VM was found to be strongly expressed at the blastoderm edge (Figs. 1D, 2B), and much more weakly, yet still positive, throughout the interior region of the AO epiblast (Figs. 1D, 2B). The intensity of VM at the edge appears similar to that of the mesoderm in the region of the primitive streak (Fig. 2B). In general, an abrupt transition was observed between the inner epithelial and edge zones. The edge cells display a dense interconnected filamentous network and bright clusters or foci, which often appear to be “cupped” around one side of the nuclei (Fig 3A). We also observed individual or bundled VM filaments in the edge cells that extend several microns, sometimes tens of microns, which is significantly longer than the reported persistence length of ~1 μm (Mucke et al., 2004; Schopferer et al., 2009), suggesting that VM filaments in the edge cells bear significant mechanical loading (tension). In contrast to the edge zone, VM expression within the interior of the AO consisted mostly of tortuous “squiggles” or “whirls” (Fig. 3B). Moreover, we did not observe dense VM clusters adjacent to the nuclei in the inner zone. See Supplemental Movies S1 and S2, which are available online, for high-resolution confocal z-stacks of vimentin immunofluorescence.
Fig. 3.
Representative high-magnification (60×) confocal images for vimentin (A–C) and cytokeratin (D–F) immunofluorescence at the edge zone (EZ) and inner zone (IZ) of Stage-4 quail blastoderm. A: Vimentin expression (grayscale) is shown merged with Sytox nuclear labeling (green) at the edge of the blastoderm. The arrowhead shows a thick vimentin filament running circumferentially (parallel to the direction of the edge) that appears to delineate the transition between the EZ and IZ. Also shown are dense vimentin clusters or foci, which typically appear to be closely associated with edge cell nuclei (circles). B,C: Vimentin expression is shown in the IZ alone (B) and merged with Sytox nuclear labeling (C). In the IZ region, vimentin appears primarily as “squiggles” (circle). Also note the absence of the dense vimentin foci found in the EZ. See Supplemental movies S1 and S2 for high-resolution confocal z-stacks of vimentin immunofluorescence at the EZ and IZ. D: Cytokeratin at the EZ (red) is shown merged with Sytox nuclear labeling (green). The arrowhead indicates a prominent cytokeratin fibril. Note the highly interconnected “web-like” network visible throughout the EZ. E,F: Cytokeratin expression in the IZ. Arrowheads denote appearance of fibrils. Scale bar in A is the same for all panels.
The cytokeratins have been suggested to serve a function in maintenance of epithelial integrity (Magin et al., 2007). We observed strikingly different CYK expression patterns compared to VM. Importantly, in contrast to the obvious greater expression of VM in edge cells, we did not observe significant differences in CYK levels between the edge zone and interior cells. In the edge cells, CYK exhibits a “web-like” filamentous network with no apparent perinuclear localization (Figs. 1F, 3D). Within the inner region of the AO, CYK filaments appeared to form dense networks (Fig. 3E, F). A similar observation has been made for CYK networks in vitro, which generally display a pan-cytoplasmic distribution extending from the nucleus to the cell periphery, making contact at sites of cell-cell adhesion (DePianto and Coulombe, 2004).
Adherens Junction Proteins (E-Cadherin and β-Catenin)
Adherens junctions (AJ) link cells together through transmembrane adhesive proteins called cadherins, the most common of which in epithelial cells is E-cadherin. E-cadherin is coupled to the actin cytoskeleton through β-catenin, which binds to its cytoplasmic tail (Jou et al., 1995; Huber et al., 2001). The loss of E-cadherin expression at adherens junctions and translocation of β-catenin to the nucleus (where it acts as a transcription factor) are the hallmarks of the EMT (Cano et al., 2000; Hay, 2005; Lee et al., 2006; Baum et al., 2008). As expected from previous studies in chick embryos (Roeser et al., 1999), both β-catenin and E-cadherin expression are restricted to well-defined cell borders within the epiblast cells of the inner region of the AO (Fig. 1H,J). At the blastoderm edge, E-cadherin and β-catenin are also clearly localized to cell-cell junctions, but appear to be absent from the “free” or “leading” edge where a cell has no adjacent neighboring cell (Fig. 4A,B). We did not observe nuclear β-catenin localization; however, there appears to be some cytoplasmic or plasma membrane distribution for both β-catenin and E-cadherin not associated with cell junctions (Fig.4A,B, asterisks). Therefore, we cannot rule out at the present time nuclear localization of β-catenin. Supplemental movies S3–4 show 3-D projections for β-catenin and E-cadherin at the edge.
Fig. 4.
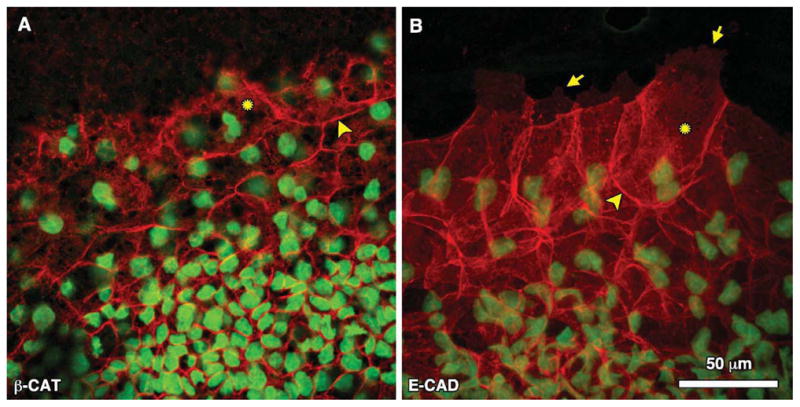
Representative high-magnification (60×) confocal images of (A) β-catenin and (B) E-cadherin immunofluorescence at the blastoderm edge. Arrowheads point to localization at cell-cell junctions. Arrows indicate the extent of edge cell lamellipodia where there is notable absence of adherens junctions. Asterisks denote regions of positive immunofluorescence that may represent either cytoplasmic or membrane localization. See Supplemental movies S3 and S4 for 3-D confocal projections.
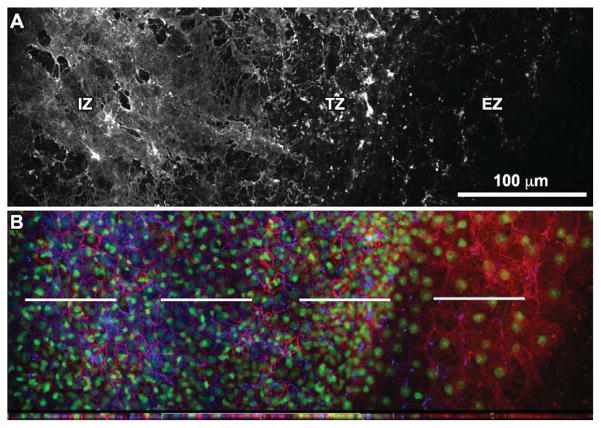
Extracellular Matrix (Laminin)
In the AO, a nascent basal lamina lies subjacent to the extraembryonic epiblast. Here, we performed immunofluorescent localization of the extracellular matrix protein laminin (LM), a major component of the basal lamina that is critical for gastrulation (Smyth et al., 1998; Miner et al., 2004; Miner and Yurchenco, 2004). We observed three morphologically distinct regions of LM immunofluorescence in the AO. In the “edge zone” (EZ), LM expression is punctate and sparse (Fig. 5A). In the “transition zone” (TZ) just behind the edge, long tightly packed individual laminin fibrils originating from the basal lamina are apparent (Fig. 5A). Additionally, the cell density is higher in the TZ than the EZ. Further towards the “interior zone” (IZ) of the AO, the LM expression appears to be more continuous (Fig. 5A). See Supplemental Movies S5–7 for high-resolution confocal stacks of combined LM and β-catenin expression in the three different zones.
Fig. 5.
Laminin expression at the edge of the quail blastoderm. A: High-magnification (60×) confocal panoramic representation of laminin immunofluorescence reveals an edge zone (EZ), transition zone (TZ), and inner zone (IZ) of progressively increasing expression. The image is composed of four overlapping high-resolution 60× images to create a montage effect. B: For the same region, β-catenin (red) and nuclear (Sytox Green) staining is shown together with laminin (blue). Below the merged image is a cross-section taken at the level of the dashed white lines. Confocal z-stacks for each of the three regions are included in Supplemental Movies S5–S7.
Cell Proliferation Assay
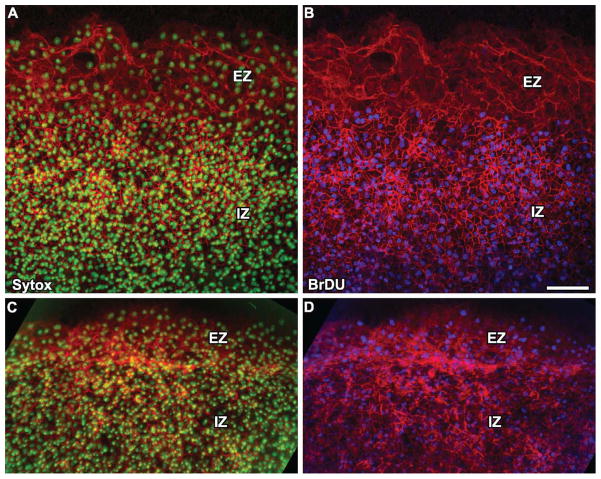
A previous study by Downie (1976) had noted that while there were mitotic figures in the inner zone of the AO, there were none observed at the edge of the blastoderm, which suggested that edge cells are non-proliferative. To confirm these earlier findings, we used BrDU incorporation to label cell nuclei undergoing DNA synthesis, which has since proven to be a more robust proliferation assay than simply counting mitotic figures. We performed two types of experiments: (1) BrDU pulse followed by immediate fixation (pulse-fix); (2) BrDU pulse followed by additional incubation. In the pulse-fix experiment, embryos were incubated with BrDU for 25 min followed by a washout at room temperature and subsequent fixation and embryo processing for anti-BrDU immunofluorescence. The results of the pulse-fix experiment show that while there are numerous BrDU-positive nuclei in the AO inner zone, there appear to be virtually no positively labeled at the edge of the blastoderm (Fig. 6B). If cells are non-proliferative at the edge, how does the edge cell population expand as the size of the embryo increases during epiboly? We hypothesize that cells are recruited to the edge by translocation from the inner zone of the AO. To test this hypothesis, we performed a pulse-follow experiment. As before, embryos were first incubated for 25 min with BrDU in the media followed by washing out. Next, embryos were incubated overnight for an additional 20–24 hr to allow the embryo to undergo expansion. After incubation, embryos were fixed and processed for immunoflourescence. In contrast to the pulse-fix, we saw numerous anti-BrDU-positive nuclei in these embryos at the blastoderm edge (Fig. 6D). These results provide strong, if indirect, evidence that cells translocate from the inner zone to the edge as the embryo expands.
Fig. 6.
A, B: Pulse-fix BrDU proliferation assay shows that the edge zone (EZ) of the blastoderm is virtually non-proliferative in comparison to the inner zone (IZ). The counter-stain is β-catenin (red). C, D: Pulse-incubate experiment shows that BrDU-positive nuclei are present in the EZ after 24 hr of additional incubation time following an initial BrDU pulse, which suggests that cells translocate from the IZ to the EZ during epiboly. Scale bar = 100 μm.
DISCUSSION
The major result of this study is the surprising and novel finding that the intermediate filament vimentin, a classical marker of the mesenchymal phenotype, is robustly expressed in the motile edge cells of the avian blastoderm during epiboly (the rapid expansion of the extraembryonic epithelium). Previous vimentin studies in chick did not report blastoderm edge expression (Erickson et al., 1987; Page, 1989). We believe the vimentin expression pattern may be unique to the avian embryo, since a similar expression pattern was not reported for Xenopus or zebrafish (Cerda et al., 1998) epiboly in previous immunofluorescence studies. Our results also show that E-cadherin, β-catenin, and cytokeratin are co-expressed along with vimentin in edge cells; however, the epiblast basal lamina, as shown by laminin immunofluorescence, appears sparse and punctate at the blastoderm edge. Taken together, these data suggest that the edge cells of the avian extraembryonic AO employ vimentin in a cellular or functional manner. We speculate that loss of basal lamina contact causes up-regulation of vimentin expression or polymerization specifically at the blastoderm edge.
Cytokeratin is generally thought of as the main intermediate filament type expressed in epithelial cells. However, vimentin is often up-regulated in epithelial cells in vitro (Klymkowsky, 1982), and has been shown to be up-regulated in several wound healing models and in certain epithelial tissues during development, such as the neural tube (Erickson et al., 1987; Viebahn et al., 1988). It should be noted that in the neural tube, vimentin expression is found without co-expression of cytokeratin (Viebahn et al., 1988), and that N-cadherin is the predominant cadherin isoform. Indeed, in most embyronic tissues the expression patterns of vimentin and cytokeratin are mutually exclusive, with a few notable exceptions, including the lateral plate mesoderm (Erickson et al., 1987). It has been suggested that models of epiboly are relevant to studies of wound healing, since the process involves spreading or migration of an epithelium (New, 1959; Chernoff, 1989; Weliky and Oster, 1990). Using MCF10A cells in a human mammary epithelium wound healing model, Gilles et al. (1999) showed that vimentin is up-regulated in cells located proximal to or at the free edge, which were involved in active migration towards the center of a lesion. Using the same cell line, Polette et al. (2007) showed that in addition to vimentin up-regulation, β-catenin and the tight junction protein ZO-1 display cytosplasmic and/or nuclear localization at the leading edge, indicative of the metastable EMT phenotype. We did not observe nuclear localization of β-catenin, nor significant down-regulation of E-cadherin at the blastoderm edge, which raises the question whether vimentin expression is being regulated independently. It makes sense that edge cells would maintain strong cell-cell contacts to promote cohesive and uniform expansion of the blastoderm. Developing new techniques for genetic manipulation (perhaps, via electroporation) and isolation of edge cells will likely be required to address this issue further.
According to Downie (1976), the blastoderm radius doubles approximately every 24 hr over the first few days of chick development, eventually engulfing the entire yolk surface. This expansion represents a several-hundred fold increase in surface area. Clearly, as the perimeter of the blastoderm increases, there must be a concomitant increase in cell number at the migrating edge. The two most probable mechanisms for the addition of new edge cells during avian epiboly are: (1) proliferation of edge cells; and (2) “recruitment” of cells from the inner zone to the edge (Downie, 1976). To address this question, Downie (1976) blocked cells in metaphase using colcemid treatement, and reported that there were no mitotic figures in the edge zone over a period of 6 hr, strongly suggesting that proliferation is not the primary mechanism for cellular expansion of the edge zone. Our new studies using BrDU incorporation confirm that edge cells rarely, if ever, enter the DNA synthesis phase of the cell cycle, and, thus, strongly suggest that proliferation is not the primary mechanism for expansion of the edge cell population. Instead, it seems more likely to us that edge cells are recruited from the inner zone, which is highly proliferative. The results of a pulse-follow experiment show anti-BrDU-positive nuclei in the edge after embryos were incubated overnight following initial BrDU treatment, thus supporting the recruitment hypothesis. Cells undergoing translocation from the inner zone to the edge would then exhibit several behaviors that are typical of the EMT process, including: (1) Increase in cellular motility; (2) Loss of apico-basal polarity; (3) Increase in vimentin expression; and (4) Degradation of basement membrane and concomitant decrease in laminin expression. We hypothesize that the changes in cell phenotype exhibited during an edge cell recruitment process represent a particular subclass of pEMT that is critical for maintaining epiboly in the avian embryo. Further studies will be necessary to understand the cell-cycle control mechanisms occurring in the edge zone, and to identify the molecular mechanisms that underly the recruitment process. With recent progress in generating transgenic quail lines, it may be possible to directly observe the motility of fluorescently-labeled cells at the blastoderm edge in the near future (Sato et al., 2010).
Chernoff (1989) previously reported that laminin is absent from the ventral and basal surfaces of the edge region, with both becoming progressively more complete closer to the embryo interior (e.g., Area Pellucida); however, the whole-mount immunofluorescent images provided in that study were not convincing evidence to our eyes, and did not show the laminin expression in relation to the cell nuclei, as we have provided here. Our new immunofluorescence data clearly show the absence of laminin at the edge zone. If epithelial cells in the interior region of the AO epiblast are indeed recruited to the edge zone, as our data suggest, then it seems logical that laminin degradation plays a role in this process, as would occur in a classical EMT. During wound healing, cytokines secreted at the free edge of the epithelium promote the production of matrix metalloproteases (MMPs), which subsequently act to degrade the basemement membrane and increase motility of the cells at the leading edge (Madlener et al., 1996; Rechardt et al., 2000; Salmela et al., 2004). A similar process may occur at the edge of the blastoderm during epiboly. Alternatively, given the rapid expansion of the blastoderm, and the previously reported presence of tension during avian epiboly (Downie, 1976), it is possible that some of the observed basement membrane degradation is due to mechanical force generated by the edge zone itself. As the edge cells migrate radially outward, they exert tensile (pulling) forces on the basement membrane, causing it to physically detach or become disrupted in the interior zone, which may then initiate the pEMT process and recruitment of edge cells. In effect, the edge cells would be like the proverbial magician pulling out the table cloth from underneath the dinnerware. Our observation that vimentin filaments in the edge cells appear to be longer than the persistence length of vimentin polymers found in vitro (Mucke et al., 2004), suggests that vimentin filaments are, indeed, under considerable tension, and is consistent with the idea that the edge cells exert large towing forces on the blastoderm. Future studies characterizing vimentin expression and the physical state of the basement membrane in the other major model organisms, namely Xenopus and zebra-fish, are needed to conclusively show that expression of the markers examined here is unique, and should yield novel insights into possible shared and divergent biophysical mechanisms required for driving epiboly across different species.
EXPERIMENTAL PROCEDURES
Quail Embryo Culture
Japanese quail (coturnix coturnix) eggs were purchased from the Ozark Egg Company (Stover, MO) and incubated for 2.5 hr in a humidified chamber at 37°C. The embryos were cultured using a modified New culture technique (Chapman et al., 2001) with 9/16″ filter paper rings on semi-solid agar/albumen media, and incubated overnight for a total incubation time of approximately 24 hr until embryos reached Hamburger-Hamil-ton (HH) stage 4–5 (Hamburger and Hamilton, 1951).
Whole Mount Immunofluorescence
Embryos were fixed in 3% paraformaldehyde (PFA) for 30–60 min at 4°C, and permeablized for 45 min using 0.5 or 1% Triton X-100 also at 4°C. Ice cold methanol (100%) was added for 30 min. A series of ethanol solutions of 90, 70, 50, 30, and 15% were applied for 15 min each to rehydrate specimens. We would like to note that in routine whole mount immunofluorescence studies in chick embryos, either detergent or methanol is used alone for the permeabilization step. In our hands, we found that using these solutions in combination resulted in embryos that were much cleaner and more free of lipid yolk droplets, resulting in better imaging, especially at the thin blastoderm edge. Furthermore, using methanol as the primary permeabilization step often resulted in loss of preservation of the blastoderm edge, which was obviously critical for this study. Blocking buffer (3% BSA in PBS) was applied from 8 hr to overnight, followed by application of primary antibodies overnight, a second round of blocking for at least 4 hr, and finally secondary antibodies were applied overnight in blocking buffer. Subsequently, to label nuclei, embryos were incubated in a solution containing Sytox Green (500 nM in PBS) for 15 min. All incubation steps were carried out on a gentle rocker, which greatly improved immunofluorescence by minimizing yolk particles. It should be noted that previous studies have suggested that cytokeratin is particularly susceptible to loss of antigenicity upon PFA fixation (Klymkowsky et al., 1987); however, we found that by using relatively brief PFA application (30 min) at 4°C, immunofluorescence labeling was preserved. For actin filament labeling, rhodamine-phalloidin (Cytoskeleton Inc., Denver, CO) was diluted 1:1,000 from 14 μM stock and applied for 4 hr.
To mount embyros for confocal microscopy, excess PBS and yolk was absorbed gently using a Kimwipe and the embryos were placed ventral side down on a glass slide. The paper ring surrounding the embryo was carefully cut away, leaving the blastoderm attached to the vitelline membrane. This step has to be done very carefully to avoid excessive wrinkling of the vitelline membrane and folding of the blastoderm edge onto the AO. Any remaining fluid was again absorbed, and approximately 20 μL of Prolong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA) was gently applied to the embryo, followed by an 18-mm cover slip. After remaining covered at room temperature overnight, clear nail polish was placed around the edges of the cover slip. This procedure ensured very flat specimens, without having to use xylenes or clearing agents.
Vimentin (H5), laminin (3H11), fibronectin (B3/D6), cytokeratin (1h5), and BrDU monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank (The University of Iowa, Iowa City, IA); polyclonal anti-β-catenin (ab6302) was obtained from Abcam (Cambridge, MA); monoclonal anti-E-cadherin (36/E) was obtained from BD Transduction Labs (Franklin Lakes, NJ); Sytox Green dead cell stain was obtained from Invitrogen (Carlsbad, CA). Secondary antibodies were purchased from Jackson ImmunoResearch Labs (West Grove, PA) and ThermoFisher Scientific (Waltham, MA). All antibodies were diluted to a concentration of 1 μg/mL.
Microscopy and Image Processing
A Nikon C1 confocal microscope system was used for all immunofluorescence imaging, except for the wide-field images shown in Figure 2, which were captured with a Leica DMI6000 inverted epifluorescent microscope. Nikon 20×Plan Apo (dry) or Nikon 60×Plan Apo (oil) were used exclusively on the confocal setup.
BrDU Proliferation and Recruitment Assays
BrDU (Sigma Aldrich, St. Louis, MO) was diluted to 100 μM in agar-albumen culture media, and embryos were incubated initially for a 25-min pulse at 37°C. After the initial pulse, the BrDU was then washed out by transferring embryos to untreated culture plates for an additional 4 hr at room temperature. In some experiments, embryos were incubated overnight for 20–24 hr to determine whether BrDU-positive nuclei translocated to the blastoderm edge. Embryos were then fixed in 3% PFA and processed for immunofluorescent BrDU labeling using the protocol described above. Before application of the primary antibody, specimens were incubated in DNase reaction buffer (Promega) at 25 U/mL for 1 hr at 37°C to expose the BrDU epitope.
Supplementary Material
Acknowledgments
Grant sponsor: Georgia Tech Research Corporation.
We thank the Georgia Tech Research Corporation for providing funding to E.A.Z. We also thank Ed Phelps and Nduke Enemchukwu in the Garcia Lab for providing training on the Nikon C1 system to M.A.F. Several monoclonal antibodies in this study were obtained from the Developmental Studies Hybridoma Bank (DSHB) developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242, including 1h5 (M. Klymkowsky), H5 (J.R. Sanes), and 3H11 (W. Halfter). The authors would like to acknowledge NIH grant R01GM065918 (A.J.G.) for funds that were used to acquire the confocal microscope used in this study.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Arnoux V, Nassour M, L’Helgoualc’h A, Hipskind RA, Savagner P. Erk5 controls Slug expression and keratinocyte activation during wound healing. Mol Biol Cell. 2008;19:4738–4749. doi: 10.1091/mbc.E07-10-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargagna-Mohan P, Hamza A, Kim YE, Khuan Abby Ho Y, Mor-Vaknin N, Wendschlag N, Liu J, Evans RM, Mar-kovitz DM, Zhan CG, Kim KB, Mohan R. The tumor inhibitor and anti-angiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem Biol. 2007;14:623–634. doi: 10.1016/j.chembiol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Bellairs R, Bromham DR, Wylie CC. The influence of the area opaca on the development of the young chick embryo. J Embryol Exp Morphol. 1967;17:195–212. [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Cerda J, Conrad M, Markl J, Brand M, Herrmann H. Zebrafish vimentin: molecular characterization, assembly properties and developmental expression. Eur J Cell Biol. 1998;77:175–187. doi: 10.1016/S0171-9335(98)80105-2. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dyn. 2001;220:284–289. doi: 10.1002/1097-0177(20010301)220:3<284::AID-DVDY1102>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Chen CS, Tan J, Tien J. Mechano-transduction at cell-matrix and cell-cell contacts. Annu Rev Biomed Eng. 2004;6:275–302. doi: 10.1146/annurev.bioeng.6.040803.140040. [DOI] [PubMed] [Google Scholar]
- Chernoff EA. Adhesion and fusion of the extraembryonic epiblast. Tissue Cell. 1989;21:735–746. doi: 10.1016/0040-8166(89)90082-7. [DOI] [PubMed] [Google Scholar]
- Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Hoffstrom BG, Keller R, DeSimone DW. Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin alpha(5)beta(1), fibronectin, and tissue geometry. Dev Biol. 2002;242:109–129. doi: 10.1006/dbio.2002.0537. [DOI] [PubMed] [Google Scholar]
- DePianto D, Coulombe PA. Intermediate filaments and tissue repair. Exp Cell Res. 2004;301:68–76. doi: 10.1016/j.yexcr.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Downie JR. The mechanism of chick blastoderm expansion. J Embryol Exp Morphol. 1976;35:559–575. [PubMed] [Google Scholar]
- Downie JR, Pegrum SM. Organization of the chick blastoderm edge. J Embryol Exp Morphol. 1971;26:623–635. [PubMed] [Google Scholar]
- du Roure O, Saez A, Buguin A, Austin RH, Chavrier P, Silberzan P, Ladoux B. Force mapping in epithelial cell migration. Proc Natl Acad Sci USA. 2005;102:2390–2395. doi: 10.1073/pnas.0408482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson CA, Tucker RP, Edwards BF. Changes in the distribution of intermediate-filament types in Japanese quail embryos during morphogenesis. Differentiation. 1987;34:88–97. doi: 10.1111/j.1432-0436.1987.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudiere C. Development of the zebrafish lateral line. Curr Opin Neurobiol. 2004;14:67–73. doi: 10.1016/j.conb.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Gilles C, Polette M, Zahm JM, Tournier JM, Volders L, Foidart JM, Birembaut P. Vimentin contributes to human mammary epithelial cell migration. J Cell Sci. 1999;112:4615–4625. doi: 10.1242/jcs.112.24.4615. [DOI] [PubMed] [Google Scholar]
- Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol. 2000;1:208–216. doi: 10.1038/35043032. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morphol. 1951:88. [PubMed] [Google Scholar]
- Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- Herman JP, Victor JC, Sanes JR. Developmentally regulated and spatially restricted antigens of radial glial cells. Dev Dyn. 1993;197:307–318. doi: 10.1002/aja.1001970408. [DOI] [PubMed] [Google Scholar]
- Huber AH, Stewart DB, Laurents DV, Nelson WJ, Weis WI. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J Biol Chem. 2001;276:12301–12309. doi: 10.1074/jbc.M010377200. [DOI] [PubMed] [Google Scholar]
- Isaacs WB, Cook RK, Van Atta JC, Red-mond CM, Fulton AB. Assembly of vimentin in cultured cells varies with cell type. J Biol Chem. 1989;264:17953–17960. [PubMed] [Google Scholar]
- Jacinto A, Martinez-Arias A, Martin P. Mechanisms of epithelial fusion and repair. Nat Cell Biol. 2001;3:E117–123. doi: 10.1038/35074643. [DOI] [PubMed] [Google Scholar]
- Jacinto A, Wood W, Woolner S, Hiley C, Turner L, Wilson C, Martinez-Arias A, Martin P. Dynamic analysis of actin cable function during Drosophila dorsal closure. Curr Biol. 2002;12:1245–1250. doi: 10.1016/s0960-9822(02)00955-7. [DOI] [PubMed] [Google Scholar]
- Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky MW. Vimentin and keratin intermediate filament systems in cultured PtK2 epithelial cells are interrelated. EMBO J. 1982;1:161–165. doi: 10.1002/j.1460-2075.1982.tb01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. Am J Pathol. 2009;174:1588–1593. doi: 10.2353/ajpath.2009.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky MW, Maynell LA, Polson AG. Polar asymmetry in the organization of the cortical cytokeratin system of Xenopus laevis oocytes and embryos. Development. 1987;100:543–557. doi: 10.1242/dev.100.3.543. [DOI] [PubMed] [Google Scholar]
- Klymkowsky MW, Shook DR, Maynell LA. Evidence that the deep keratin filament systems of the Xenopus embryo act to ensure normal gastrulation. Proc Natl Acad Sci USA. 1992;89:8736–8740. doi: 10.1073/pnas.89.18.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy P, Mostov KE. Slug is required for cell survival during partial epithelial-mesenchymal transition of HGF-induced tubulogenesis. Mol Biol Cell. 2007;18:1943–1952. doi: 10.1091/mbc.E06-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlener M, Mauch C, Conca W, Brauchle M, Parks WC, Werner S. Regulation of the expression of stromelysin-2 by growth factors in keratinocytes: implications for normal and impaired wound healing. Biochem J. 1996;320:659–664. doi: 10.1042/bj3200659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magin TM, Vijayaraj P, Leube RE. Structural and regulatory functions of keratins. Exp Cell Res. 2007;313:2021–2032. doi: 10.1016/j.yexcr.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- Mucke N, Wedig T, Burer A, Marekov LN, Steinert PM, Langowski J, Aebi U, Herrmann H. Molecular and biophysical characterization of assembly-starter units of human vimentin. J Mol Biol. 2004;340:97–114. doi: 10.1016/j.jmb.2004.04.039. [DOI] [PubMed] [Google Scholar]
- New DA. The adhesive properties and expansion of the chick blastoderm. J Embryol Exp Morphol. 1959;7:146–164. [PubMed] [Google Scholar]
- Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci USA. 2003;100:10788–10793. doi: 10.1073/pnas.1834401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. Changing patterns of cytokeratins and vimentin in the early chick embryo. Development. 1989;105:97–107. doi: 10.1242/dev.105.1.97. [DOI] [PubMed] [Google Scholar]
- Parry DA, Strelkov SV, Burkhard P, Aebi U, Herrmann H. Towards a molecular description of intermediate filament structure and assembly. Exp Cell Res. 2007;313:2204–2216. doi: 10.1016/j.yexcr.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Polette M, Mestdagt M, Bindels S, Nawrocki-Raby B, Hunziker W, Foidart JM, Birembaut P, Gilles C. Beta-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs. 2007;185:61–65. doi: 10.1159/000101304. [DOI] [PubMed] [Google Scholar]
- Poujade M, Grasland-Mongrain E, Hertzog A, Jouanneau J, Chavrier P, Ladoux B, Buguin A, Silberzan P. Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci USA. 2007;104:15988–15993. doi: 10.1073/pnas.0705062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechardt O, Elomaa O, Vaalamo M, Paak-konen K, Jahkola T, Hook-Nikanne J, Hembry RM, Hakkinen L, Kere J, Saarialho-Kere U. Stromelysin-2 is upregulated during normal wound repair and is induced by cytokines. J Invest Dermatol. 2000;115:778–787. doi: 10.1046/j.1523-1747.2000.00135.x. [DOI] [PubMed] [Google Scholar]
- Revenu C, Gilmour D. EMT 2.0: shaping epithelia through collective migration. Curr Opin Genet Dev. 2009;19:338–342. doi: 10.1016/j.gde.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Roeser T, Stein S, Kessel M. Nuclear beta-catenin and the development of bilateral symmetry in normal and LiCl-exposed chick embryos. Development. 1999;126:2955–2965. doi: 10.1242/dev.126.13.2955. [DOI] [PubMed] [Google Scholar]
- Rorth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–429. doi: 10.1146/annurev.cellbio.042308.113231. [DOI] [PubMed] [Google Scholar]
- Salmela MT, Pender SL, Karjalainen-Lindsberg ML, Puolakkainen P, Macdonald TT, Saarialho-Kere U. Collagenase-1 (MMP-1), matrilysin-1 (MMP-7), and stromelysin-2 (MMP-10) are expressed by migrating enterocytes during intestinal wound healing. Scand J Gastroenterol. 2004;39:1095–1104. doi: 10.1080/00365520410003470. [DOI] [PubMed] [Google Scholar]
- Sato Y, Poynter G, Huss D, Filla MB, Czirok A, Rongish BJ, Little CD, Fraser SE, Lansford R. Dynamic analysis of vascular morphogenesis using transgenic quail embryos. PLoS One. 2010;5:e12674. doi: 10.1371/journal.pone.0012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopferer M, Bar H, Hochstein B, Sharma S, Mucke N, Herrmann H, Wil-lenbacher N. Desmin and vimentin intermediate filament networks: their viscoelastic properties investigated by mechanical rheometry. J Mol Biol. 2009;388:133–143. doi: 10.1016/j.jmb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Meyer M, Frie C, Paulsson M, Edgar D. The targeted deletion of the LAMC1 gene. Ann N Y Acad Sci. 1998;857:283–286. doi: 10.1111/j.1749-6632.1998.tb10133.x. [DOI] [PubMed] [Google Scholar]
- Viebahn C, Lane EB, Ramaekers FC. Keratin and vimentin expression in early organogenesis of the rabbit embryo. Cell Tissue Res. 1988;253:553–562. doi: 10.1007/BF00219746. [DOI] [PubMed] [Google Scholar]
- Weliky M, Oster G. The mechanical basis of cell rearrangement. I. Epithelial morphogenesis during Fundulus epiboly. Development. 1990;109:373–386. doi: 10.1242/dev.109.2.373. [DOI] [PubMed] [Google Scholar]
- Zamir EA, Rongish BJ, Little CD. The ECM moves during primitive streak formation: computation of ECM versus cellular motion. PLoS Biol. 2008;6:e247. doi: 10.1371/journal.pbio.0060247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.