Abstract
The heat shock response is an ancient and highly conserved process that is essential for surviving environmental stresses, including extremes of temperature. Fever is a more recently evolved response, during which organisms temporarily subject themselves to thermal stress in the face of infections. We review studies showing that fever is beneficial in the infected host. We show that core temperatures achieved during fever can activate the heat shock response and discuss some of the biochemical consequences of such an effect. We present data suggesting 4 possible mechanisms by which fever might confer protection: (1) directly killing or inhibiting growth of pathogens; (2) inducing cytoprotective heat shock proteins (Hsps) in host cells; (3) inducing expression of pathogen Hsps, an activator of host defenses; and (4) modifying and orchestrating host defenses. Two of these mechanisms directly involve the heat shock response. We describe how heat shock factor-1, the predominant heat-induced transcriptional enhancer not only activates transcription of Hsps but also regulates expression of pivotal cytokines and early response genes. The relationship between fever and the heat shock response is an illuminating example of how a more recently evolved response might exploit preexisting biochemical pathways for a new function.
INTRODUCTION
The heat shock response is an ancient and highly conserved biological process that is essential for surviving environmental stresses, including extremes of temperature, toxic chemicals, and high levels of radiation, conditions that cause denaturation of essential cellular proteins. During heat shock, the transcriptional and translational machinery of the cell is reprogrammed to express preferentially a set of stress-inducible heat shock proteins (Hsp). The Hsps interact with denatured proteins, either preserving them until the stress has ended or targeting the denatured protein for degradation and removal from the cell. While the heat shock response is a fail-safe mechanism for coping with unavoidable environmental stresses, fever is a complex physiologic response to infection or injury, during which organisms temporarily subject themselves to thermal stress. This paper will discuss whether fever is beneficial, if so, whether its benefit is achieved in part by inducing a heat shock response, and identify the physiological and biochemical consequences of increasing core temperature in the face of infection.
HEAT SHOCK RESPONSE AND FEVER, AN EVOLUTIONARY PERSPECTIVE
Genes encoding Hsps, the principal mediators of the heat shock response, are highly conserved and found in every species studied (Feder and Hofmann 1999). The presence of homologous Hsp genes in archebacteria, eubacteria, and eukaryotes suggests that they first arose at least 2.5 billion years ago (Feder and Hofmann 1999). The Hsp genes have persisted during evolution despite the appearance of alternative protective mechanisms against stress, including the ability of higher animals to withdraw from stressful environments. The persistence of Hsps argues for their fundamental importance in all organisms. A review of the molecular mechanisms through which Hsps exert protection is beyond the scope of this paper, but Hsp biochemistry has been comprehensively reviewed (Craig et al 1994; Morimoto et al 1997).
In the setting of a conserved heat shock response, fever arose as an additional response to infection in higher animals. Classically, fever is defined as an elevation in core temperature brought about by alterations in firing rate of thermoregulatory neurons by endogenous pyrogenic mediators. Such a change in neural output regulates the integrated behavioral, physiological, and biochemical processes that determine the balance between heat generation and elimination. This response is generally considered to be limited to endothermic animals. However, if one broadens the definition of fever to an increase in core temperature stimulated by infection or injury and achieved solely by seeking external sources of heat, the prevalence of fever expands to include many ectothermic vertebrates, arthropods, and annelids (Klatersky 1971). The ability of the same antipyretic drugs that are effective in mammals to block the heat-seeking behavior in infected fish (Reynolds 1977) and reptiles (Bernheim and Kluger 1976) suggests that the mechanisms of fever in ectothermic and endothermic animals might be related. Starks (Starks et al 2000) recently reported that honeybees increase hive-wide temperature following infection of the hive with the heat-sensitive pathogenic fungus Ascosphaera apis. This temperature increase, achieved through a communal increase in wing muscle activity by the adult bees, kills the fungal pathogen and preserves viability of the bee larvae. In this case, the febrile response has been adapted to a hive animal in which survival of the species depends on survival of the hive rather than viability of individuals. For the remainder of this paper, we will use the broader definition of fever.
The prevalence of fever in modern day members of the 2 major animal divisions, Deuterostomia (vertebrates) and Protostomia (arthropods and annelids), suggests that it must have first appeared approximately 600 millions years ago. The persistence of fever for over 600 million years despite its considerable metabolic cost offers persuasive evidence that it is protective in the infected host.
EXPERIMENTAL EVIDENCE THAT FEVER IS PROTECTIVE IN THE INFECTED HOST
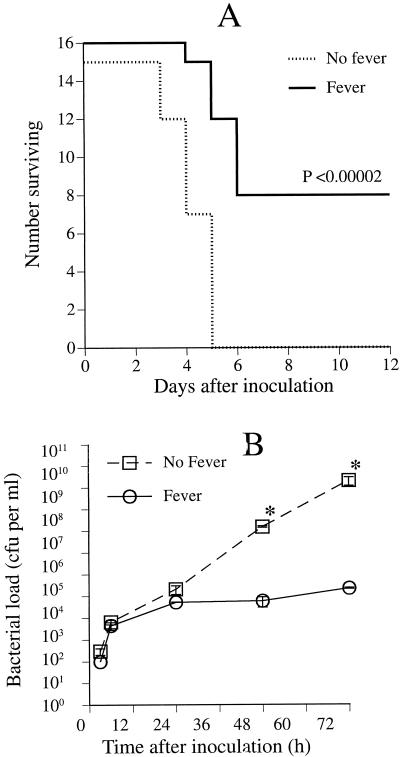
The assumption that fever is protective during infections is well supported by studies in diverse animal species. In 2 studies of ectothermic vertebrates, increases in core temperature from 38 to 40°C in the lizard Diposaurus dorsalis (Kluger et al 1975) and from 28 to 32.7°C in goldfish (Covert and Reynolds 1977) improves survival from 25% to 67% and from 64% to 100%, respectively, during infection with the same gram-negative pathogen, Aeromonas hydrophila. Similar results were found in infected sockeye salmon, rainbow trout, crickets, and grasshoppers (reviewed) (Kluger 1991). In a lethal Herpes simplex-infected mouse model, housing infected mice at 38°C for 6 days increased both their core temperature (by approximately 2°C) and their survival rate (from 0% to 85%) compared with infected mice housed at 23 to 26°C (Armstrong 1942). Schmidt et al confirmed these results in a similar model of Herpes simplex-infected mice (Schmidt and Rasmussen 1960). Bell and Moore (1974) reported a survival benefit of warming mice infected with rabies virus. In our own study of mice with experimental Klebsiella pneumoniae peritonitis, the survival rate improved from 0% to 50%, and the intraperitoneal bacterial load decreased 100 000-fold when core temperature (measured with a colonic thermistor probe) was raised from basal levels (36.5–37.5°C) to 39–39.5°C by housing mice at 35.5°C rather than 24°C (Fig 1) (Jiang et al 2000).
Fig 1.
Influence of core temperature on experimental K pneumoniae peritonitis. (A) Survival after inoculation with K pneumoniae. Mice were inoculated intraperitoneally with 100 CFU Caroli strain of K pneumoniae, then placed in 23°C (no fever) or 35.5°C (fever) ambient temperatures and survival was followed over 12 days. Core temperatures were maintained at 36.5–37°C and 39–39.5°C in the 2 groups. (B) Influence of core temperature on bacterial clearance after inoculation with K pneumoniae. Mice were inoculated intraperitoneally with 100 CFU K pneumoniae strain 5055, then placed in 23° (no fever) or 35.5°C (fever) ambient temperatures. Six mice in each group were sacrificed at the indicated times and the bacterial CFUs in peritoneal lavage fluid were determined by plating on MacConkey agar. Mean ± SE; n = 6. *P < 0.05 compared with the controls at 23°C ambient temperature
In several other animal models, administration of antipyretic agents blocked fever and decreased survival (Bernheim and Kluger 1976; Esposito 1984; Kurosawa et al 1987; Van Miert et al 1978; Vaughn et al 1980) during bacterial infections. In A hydrophila-infected lizards, treatment with sodium salicylate blocked fever in 7 of 12 animals, all of which died, while all febrile animals survived (Bernheim and Kluger 1976). In experimental murine Streptococcus pneumoniae pneumonia, treatment with aspirin impairs bacterial clearance and reduces the LD50 from 6.3 × 106 colony-forming units (CFU) to 3.3 × 105 CFU (Esposito 1984). While these studies demonstrate that an elevation in core temperature improves survival in several experimental models of infection, the mechanisms through which this occurs are incompletely understood. Four possible mechanisms are discussed below.
DIRECT EFFECTS ON PATHOGEN VIABILITY
Exposure to febrile temperatures might be directly cytotoxic or cytostatic for invading microbial pathogens, thereby accelerating pathogen clearance and shortening disease duration. This is the case for some human pathogens, including Cryptococcus hominis (Kuhn 1949), Spneumoniae (Rich and McKee 1936), Neisseria gonorrhoeae (Carpenter et al 1933), and Mycobacterium leprae (Rodbard et al 1980). However, the growth rate of most pathogenic bacteria, including Staphylococcus aureus, Escherichia coli (Mackowiak 1991), K pneumoniae (Jiang et al 2000), Pasteurella multocida (Kluger and Vaughn 1978), and A hydrophila (Covert and Reynolds 1977) varies little with temperature changes within the normal febrile range.
INDUCTION OF HEAT SHOCK IN HOST CELLS
The second way in which fever might confer protection during infection is by inducing Hsp expression in host cells, thereby increasing their resistance to the chemical stresses generated in the infected microenvironment (Perdrizet 1995). Cytoprotection conferred by exposure to supraphysiological temperatures (42–45°C) is mediated in part by 4 families of Hsps. These proteins, induced by thermal and biochemical stresses, preserve essential cellular components. The reader is referred to 2 recent reviews for a description of the biological activities of these proteins (Craig et al 1994; Moseley 1998). In addition to Hsps, heat shock also activates at least 2 other cytoprotective genes, Cu/Zn superoxide dismutase (SOD) (Yoo et al 1999) and hemoxygenase-1 (Ewing and Maines 1991). Many studies have clearly demonstrated associations between the magnitude of Hsp expression following heat shock (41–42°C) and resistance to a subsequent injury, including 2 models of sepsis, the lipopolysaccharide (LPS)-challenged mouse (Hotchkiss et al 1993), and the rat cecal ligation and puncture model (Villar et al 1994). Directly increasing intracellular concentrations of Hsp using genetic techniques have consistently conferred protection against cellular injury. For example, overexpressing Hsp70 protected rat coronary endothelial cells from hypoxia/reoxygenation injury (Suzuki et al 1998) and the H9C2 rat cardiomyocyte cell line from oxidative injury (Chong et al 1998). Transgenic mice that overexpress Hsp70 suffer smaller cerebral infarcts following experimental cerebral ischemia (Rajdev et al 2000). While these data clearly show that Hsps are cytoprotective in the face of injuries, including those encountered during infections, the relevance of these effects to fever depends on whether Hsp generation is activated by temperatures within the febrile range.
Most studies show that induction of heat shock in mammals requires >4°C increase in temperature above basal levels (reviewed) (Feder and Hofmann 1999; Ray 1999). However, smaller increases in temperature can activate the stress response in some in vivo models. For example, lysergic acid diethylamide-induced hyperthermia activated the heat-inducible transcription factor, heat shock factor, HSF-1, in brain and kidney while increasing core temperature by only 2.5°C (Brown and Rush 1996); however, Hsp expression was not measured in this study. Work in our own laboratory using an externally warmed mouse model showed that maintaining a 3°C increase in core temperature for 3 hours stimulated the appearance of Hsp70 protein in kidney and liver (Jiang et al 1999a). Su (Su et al 1999) showed that exposing isolated rat myoblast cultures to hyperthermia in vitro (39°C for 24–48 h) also confers protection against subsequent oxidative injury, but induces expression of the constitutive Hsp, HSC73, rather than the inducible Hsps. The lower thermal threshold for heat shock in some in vivo models may reflect the capacity of some inflammatory mediators, including interferon (IFN)α/β (Morange et al 1986) and arachidonic acid (Jurivich et al 1994), to reduce the threshold for inducing the stress response. This interaction between inflammatory mediators and body temperature in activating the stress response might allow animals to activate selectively the response during fever rather than during episodes of hyperthermia caused by extreme exertion or exposure to elevated environmental temperatures.
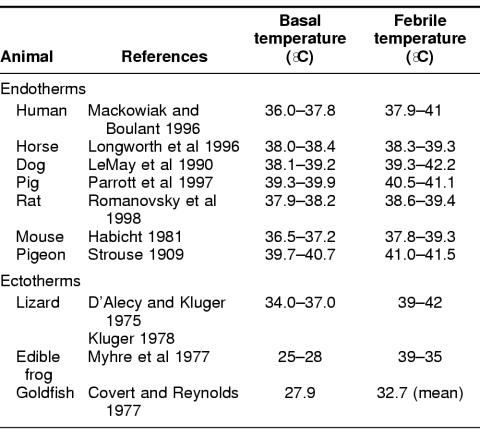
The core temperature increase generated during febrile responses to infection is remarkably consistent in higher animals. Despite having distinct basal temperatures, most mammals and birds, as well as many ectothermic vertebrates generate similar 1.5 to 5°C elevations in core temperature during infections (Table 1), a range that overlaps with the threshold for the heat shock response. These data suggest that fever is capable of activating at least some components of the heat shock response.
Table 1.
Basal and febrile temperature ranges in modern animals
INDUCTION OF HSP GENERATION IN THE PATHOGENS
Another way in which fever might protect the infected host is by stimulating the pathogens to generate Hsps, many of which are potent activators of host innate immune defenses. For example, Legionella pneumophilia Hsp60, E coli GroEL, Mycobacterium tuberculosis Hsp70, M leprae HSP65, and Mycobacterium bovis Hsp65 each induced mouse peritoneal macrophages to express interleukin (IL)-1α, IL-1β, IL-6, tumor necrosis factor (TNF)α, and granulocyte–macrophage colony-stimulating factor mRNA (Retzlaff et al 1994). Recently, Kol et al (2000) reported that chlamydial Hsp65 and human Hsp60 apparently activate mononuclear phagocytes through the same pattern recognition receptor, CD14, that mediates macrophage activation by bacterial endotoxin. However, because CD14 is a phosphatidylinositol-anchored surface protein, Hsp must be either cell free or expressed on the bacterial surface to be accessible to this macrophage protein. This has been shown to occur during in vitro culture of 2 bacterial pathogens, Helicobacter pylori (Phadnis et al 1996) and Haemophylus ducreyi (Frisk et al 1998). Asea (Asea et al 2000) reported that CD14 is also essential for macrophage activation by human Hsp70. Hsp65 from Mbovis also directly activates human endothelial cells to increase adhesiveness for monocytes and granulocytes (Verdegaal et al 1996), an early step in the recruitment of these inflammatory cells to sites of infection. By increasing synthesis and release of Hsps from bacterial pathogens, fever might provide an early signal for activation of innate defenses. Furthermore, by stimulating Hsp generation in pathogens, fever might help target-activated host defenses to sites of infection and thus limit a potentially counterproductive systemic inflammatory response.
THE IMMUNOREGULATORY EFFECTS OF FEVER
A fourth way in which fever might improve survival during infections is by helping to orchestrate and optimize the host immune response through mechanisms that may utilize components of the heat shock response. Exposure to elevated temperatures has been reported to exert both stimulatory and inhibitory effects on components of the immune response.
Human polymorphonuclear cell (PMN) motility (Bryant et al 1971; Nahas et al 1971) and phagocytosis (Ellingson and Clark 1942; van Oss et al 1980) are potentiated at temperatures within the human febrile range, but PMN chemotaxis is not enhanced, and bactericidal capacity is only weakly and inconsistently augmented by exposure to these temperatures (Roberts and Steigbigel 1977; Sebag et al 1977). At temperatures above the human febrile range (>41°C) bacterial phagocytosis and killing by PMNs are reduced (Leijh et al 1979; Peterson et al 1976, 1977; Sebag et al 1977; van Oss et al 1980). Thus, enhancement of human PMN functions in vitro and optimal survival in clinical infections occur at similar temperatures (Hodgin and Sanford 1965; Bryant et al 1971; Mackowiak et al 1980).
In murine macrophages, several functions that are required for microbicidal activity are enhanced at febrile range temperatures, including expression of Fc receptors, phagocytosis, pinocytosis, and reduction of nitroblue tetrazolium (Yoshioka et al 1990; Bruggen et al 1991), a measure of oxygen radical generation and killing of intracellular bacteria (Berman and Neva 1981). However, like PMNs, murine and human macrophages have markedly reduced function at temperatures >41°C (Leijh et al 1981; Yoshioka et al 1990; Bruggen et al 1991).
Exposing human lymphocytes to febrile-range temperatures (38–41°C) in vitro enhances their L-selectin-mediated binding to lymphatic endothelium (Wang et al 1998), an important early step in lymphocyte recruitment. Several groups have shown that exposing human T lymphocytes to febrile temperatures also enhances their proliferative response to nonspecific mitogens (Ashman and Nahmias 1977; Roberts and Steigbigel 1977; Manzella and Roberts 1979; Narvanen et al 1986), and allogeneic lymphocytes (Smith et al 1978), IL-1, and IL-2 (Duff and Durum 1982; Lederman et al 1987). However, like PMNs and macrophages, human T lymphocytes exhibit a reduced proliferative response when exposed to temperature ≥41°C (Roberts et al 1985; Lederman et al 1987). In mice, T helper cell potentiation of the B cell antibody response (Jampel et al 1983a, 1983b; Saririan and Nickerson 1982) and generation of cytotoxic T lymphocytes against allogeneic cells (Smith et al 1978) and virus-infected cells (Mullbacher 1984; Owen et al 1988) are also enhanced by exposure to febrile temperatures. Taken together, the studies of neutrophils, macrophages, and lymphocytes in mice and humans demonstrate a similar pattern of temperature-responsiveness in which many of the functions that are essential for microbicidal activities are enhanced at febrile temperature (38–41°C) but are attenuated at temperatures >41°C.
Antimicrobial defenses are orchestrated, at least in part, by a structurally and functionally diverse group of proteins called cytokines. Such cytokines have complex biological activities, sometimes overlapping and sometimes antagonistic, that influence immune cell functions. Some cytokines, notably IL-1, TNFα, and the IFNs, are required for optimal host defense (Cross et al 1989, 1995; Marino et al 1997), and yet, when dysregulated, appear to participate in the pathogenesis of sepsis (Ward and Lentsch 1999). Thus, the net effect of these cytokines on survival during sepsis is determined by the magnitude, timing, and pattern of their collective expression.
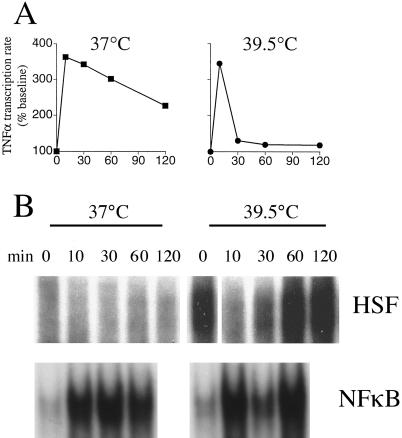
A growing body of literature has shown that expression of these cytokines is modified by exposure to elevated temperatures, but these effects are complex and are influenced by the magnitude and timing of the temperature change and the cellular source of the cytokines. For example, exposing murine peritoneal macrophages to 42–43°C for 1 hour beginning immediately after LPS stimulation caused profound inhibition of TNFα secretion, while a similar exposure to 40.5°C had no effect on TNFα secretion (Tomasovic et al 1989). In contrast, if the 1-hour warming to 42°C was delayed until 2 to 4 hours after LPS stimulation, TNFα secretion was increased 3- to 6-fold. If the 1-hour exposure to 42°C was followed by a 4-hour recovery at 37°C before adding LPS, the inhibition of TNFα seceretion was reversed, but the same 37°C recovery failed to restore TNFα expression after exposure to 43°C. In the U-373 astrocytoma cell line, continuous exposure to 40°C coincident with LPS stimulation reduced the accumulation of TNFα and IL-1β mRNA and attenuated cytokine secretion (Velasco et al 1991). In human monocyte-derived macrophages (Ensor et al 1994), the Raw 264.7 murine macrophage cell line (Ensor et al 1995; Jiang et al 2000; Singh et al 2000), murine Kupffer cells, liver slices, and peritoneal macrophages (Jiang et al 1999b), continuous exposure to 39.5–40°C beginning 30 minutes prior to stimulation with LPS reduced TNFα secretion. We recently reported that the temperature-dependent inhibition of TNFα expression in Raw 264.7 cells and murine peritoneal macrophages was caused by an early and abrupt termination of TNFα transcriptional activation in the warmer (39.5°C) cells (Fig 2A) (Singh et al 2000).
Fig 2.
Effect of incubation temperature on TNFα transcription and HSF-1 and NFκB activation. (A) TNFα transcription in Raw 264.7 cells. Cells were preincubated for 30 minutes and then stimulated with 100 ng/ml LPS at the indicated temperature and the TNFα and glyceraldehyde phosphate dehydrogenase (GAPDH) transcription rates were measured by nuclear run-on assay. Background signal (insertless plasmid) was subtracted, and the TNFα/GAPDH ratio was calculated for each time point and normalized to pre-LPS levels at each temperature. Mean ± SE; *P < 0.05 compared with 37°C. (B) An electrophoretic mobility shift assay (EMSA) analysis of HSF and NFκB. EMSA was performed using an HRE sequence probe from the human Hsp70 promoter (5′-GATCTCGGCTGGAATATTCCCGACCTGGCAGCCGA-3′) (upper) or an NFκB response element probe from the murine TNFα promoter (5′-ACAGGGGGCTTTCCCTCCT-3′). Raw 264.7 nuclear extracts were obtained after incubation with 100 ng/ml LPS at the indicated temperature and for the indicated time. For the 39.5°C extracts, cells were preincubated at 39.5°C for 30 minutes prior to adding LPS
The direct effects of elevated temperature on IFN generation are variable and appear to depend on the type of IFN studied, the magnitude of the temperature increase, and the stimulus used to induce IFN production. Incubating human peripheral blood mononuclear cells (PBMC) at 39°C for 24 hours reduces IFNγ in LPS-stimulated cells (Kappel et al 1991), but warming the cells to 40.7°C for 24 hours failed to inhibit IFNγ secretion (Roberts 1986). Exposing human PBMC to 42.7°C for 24 hours reduces generation of IFNα in mitogen-stimulated, but not in influenza virus-infected cells (Roberts 1986). By contrast, increasing in vivo core temperature of humans and monkeys to febrile levels by external warming prior to collecting PBMC increases their capacity for generating IFNγ after stimulation with phytohemagglutinin in vitro (Downing et al 1987, 1988).
A CENTRAL ROLE FOR HSF-1 IN MEDIATING THE EFFECTS OF FEVER
Hsp genes are regulated by a family of transcription factors called HSFs. A family of 4 HSFs has been described in vertebrates, of which HSF-1 is the predominant stress-activated member (Tanabe et al 1997). The biology of HSF-1 relevant to its function as a transcriptional activator has been recently reviewed (Morimoto 1998). HSF-1 is a complex molecule comprising several domains, including an amino-terminal transcription activation domain, a carboxy-terminal DNA binding domain, and a trimerization domain mediating its homotypic polymerization. Under basal conditions, HSF-1 exists as a monomer bound to Hsp90 in a multichaperone complex (Zou et al 1998) and is incapable of stable DNA binding. Following stress, HSF-1 trimerizes, translocates to the nucleus, and is phosphorylated. Once activated, HSF-1 attains the capacity to bind to its cognate heat shock response element (HRE) binding site comprised of inverted dyad repeats of the pentanucleotide nGAAn (Perisic et al 1989) and to activate transcription (Sarge et al 1993). Phosphorylation of HSF-1 can either activate or inactivate its DNA binding and transactivating activities depending on which serine and threonine residues are phosphorylated. While the protein kinases mediating stress-induced HSF-1 activation have not been clearly identified, several members of the mitogen-activated protein (MAP) kinase family have been shown to inactivate HSF-1, including extracellular signal-regulated kinase (ERK)-1 (Mivechi and Giaccia 1995; Knauf et al 1996). HSF-1 can also be inactivated by binding to Hsp90 and p23 (Zou et al 1998), Hsp70 (Kim et al 1995), and HSFBP (Satyal et al 1998).
Recent studies of HSF-1 indicate that besides activating transcription of Hsp genes, it also inhibits transcription of certain cytokine and early response genes, including IL-1β (Cahill et al 1996), c-fos (Chen et al 1997), and TNFα (Singh et al 2000). In the case of IL-1β, the access of the C/EBP-β/NF-IL-6 transcriptional enhancer to its cognate binding site in the IL-1β promoter is blocked by HSF-1 binding to a contiguous HRE in the heat-shocked THP-1 human promonocyte cell line (Cahill et al 1996). In the case of c-fos, the ability of HSF-1 mutants that are deficient in DNA binding and transactivation activities to inhibit its transcription and the absence of an HRE in the c-fos promoter indicates that HSF-1 represses c-fos and IL-1β through different molecular mechanisms (Chen et al 1997). In studying how febrile temperatures inhibited TNFα expression in the murine Raw 264.7 macrophage cell line, we found (1) that HSF-1 is partially activated at febrile temperatures (39–39.5°C) to a DNA binding form that lacks transactivating activity, (2) that this heat-activated form of HSF-1 can bind to the TNFα promoter and/or 5′ untranslated region sequence (−85 to +138 bp of the murine TNFα gene), and (3) that overexpression of HSF-1 represses TNFα transcription (Singh et al 2000). Although this region of the TNFα gene does not contain HREs, it does contain multiple HRE half-sites (nGAAn) in critical locations, including sites (1) adjacent to an essential Sp1 binding site, (2) at the transcription start site, and (3) a 5 half-site array 35–75 nucleotides downstream of the transcription start site. These observations suggest that HSF-1 activated during febrile states might repress TNFα transcription by blocking the binding of Sp1, by interfering with assembly of the general transcription complex, and by preventing transcript elongation. This proposition was further supported by Xiao (Xiao et al 1999), who showed that mice deficient in HSF-1 have exaggerated TNFα production and increased mortality following endotoxin challenge.
In Raw 264.7 macrophages, LPS treatment in the presence of activated HSF-1 (achieved by preincubating the cells at 39.5°C for 30 min) stimulates TNFα transcriptional activation of comparable magnitude but of much shorter duration than in 37°C Raw 264.7 cell cultures (Fig 2A) (Singh et al 2000). This raises the question of how transcriptional activation could occur at all in the presence of this putative repressor. A parallel analysis of HSF-1 by electrophoretic mobility shift assay shows that activated HSF-1 is lost within 10 minutes of adding LPS (Fig 2B). This loss is coincident with activation of the transcriptional enhancer NFκB and with transcriptional activation of TNFα. Activated HSF-1 subsequently reappeared coincident with a rapid inactivation of TNFα transcription and a reduction in levels of NFκB. The mechanisms through which LPS causes the loss of activated HSF-1 are unkown, but immunoblot analysis suggests that it is caused by inactivation of HSF-1 rather than reduction in the level of HSF-1 protein (Singh et al 2000). LPS is a potent inducer of MAP kinases, including ERK-1, which in turn can inactivate HSF-1 (Mivechi and Giaccia 1995), suggesting at least one way in which LPS may transiently derepress TNFα transcription in the presence of HSF-1.
Based on these data, we propose the hypothesis that once the febrile state is established the presence of activated HSF-1 in macrophages represses TNFα transcription. The interaction between LPS-induced signaling pathways and HSF-1 changes the kinetics of subsequent TNFα expression to one characterized by shorter bursts rather than sustained TNFα synthesis. Such a change in cytokine expression pattern might retain the immunostimulatory activity of TNFα while reducing the risk of host tissue injury that can be caused by sustained exposure to high levels of TNFα (Tracey et al 1986).
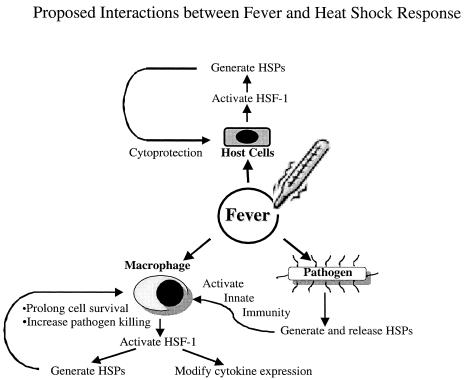
In summary, we have reviewed studies showing that fever is beneficial in the infected host. We showed that fever can activate the heat shock response and discussed some of the biochemical consequences of such an effect. We presented data suggesting 4 possible mechanisms through which fever might confer protection. Two of these mechanisms directly involve the heat shock response in host cells or by the pathogen, and a third mechanism, the regulation of immune defenses, that might share some components of the heat shock response (Fig 3). We described how HSF-1, the predominant heat-induced transcriptional enhancer not only activates transcription of HSPs but also regulates expression of pivotal cytokines and early response genes. The relationship between fever and the heat shock response is an intriguing example of how a more recently evolved biological response might exploit preexisting biochemical pathways for a new purpose.
Fig 3.
Proposed interactions between fever and heat shock response through which protection is conferred to the infected host
Acknowledgments
We thank Drs Rose Viscardi, Phil Mackowiak, and Sheldon E. Greisman for their intellectual input and for reviewing this manuscript. This work was supported by PHS grant RO1 AI42117 (J.D.H.) and a VA Merit Review grant (J.D.H.).
REFERENCES
- Armstrong C. Some recent research in the field of neurotropic viruses with especial reference to lymphocytic choriomeningitis and herpes simplex. Milit Surg. 1942;91:129–145. [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nature Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Ashman RB, Nahmias AJ. Enhancement of human lymphocyte responses to phytomitogens in vitro by incubation at elevated temperatures. Clin Exp Immunol. 1977;29:464–467. [PMC free article] [PubMed] [Google Scholar]
- Bell JF, Moore GF. Effects of high ambient temperature on various stages of rabies virus infection in mice. Infect Immun. 1974;10:510–515. doi: 10.1128/iai.10.3.510-515.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JD, Neva FA. Effect of temperature on multiplication of Leishmania amastigotes within human monocyte-derived macrophages in vitro. Am J Trop Med Hyg. 1981;30:318–321. doi: 10.4269/ajtmh.1981.30.318. [DOI] [PubMed] [Google Scholar]
- Bernheim HA, Kluger MJ. Fever: effect of drug-induced antipyresis on survival. Science. 1976;193:237–239. doi: 10.1126/science.935867. [DOI] [PubMed] [Google Scholar]
- Brown IR, Rush SJ. In vivo activation of neural heat shock transcription factor HSF1 by a physiologically relevant increase in body temperature. J Neurosci Res. 1996;44:52–57. doi: 10.1002/(SICI)1097-4547(19960401)44:1<52::AID-JNR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Bruggen IV, Robertson TA, Papadimitriou JM. The effect of mild hyperthemia on the morphology and function of murine resident peritoneal macrophages. Exp Mol Pathol. 1991;55:119–134. doi: 10.1016/0014-4800(91)90047-2. [DOI] [PubMed] [Google Scholar]
- Bryant RE, Hood AF, Hood CE, Koenig MG. Factors affecting mortality of gram-negative rod bacteremia. Arch Intern Med. 1971;127:120–128. [PubMed] [Google Scholar]
- Cahill CM, Warerman WR, Xie Y, Auron PE, Calderwood SK. Transcriptional repression of the prointerleukin 1β gene by heat shock factor 1. J Biol Chem. 1996;271:24874–24879. [PubMed] [Google Scholar]
- Carpenter CM, Boak RA, Mucci LA, Warren SL. Clinical and experimental studies of the physiological effects of fever temperatures. The thermal death time of Neisseria gonorrheae in vitro with special reference to fever temperatures. J Lab Clin Med. 1933;18:981–990. [Google Scholar]
- Chen C, Xie Y, Stevenson MA, Auron PE, Calderwood SK. Heat shock factor 1 represses ras-induced transcriptional activation of the c-fos gene. J Biol Chem. 1997;272:26803–26806. doi: 10.1074/jbc.272.43.26803. [DOI] [PubMed] [Google Scholar]
- Chong KY, Lai CC, Lille S, Chang C, Su CY. Stable overexpression of the constitutive form of heat shock protein 70 confers oxidative protection. J Mol Cell Cardiol. 1998;30:599–608. doi: 10.1006/jmcc.1997.0623. [DOI] [PubMed] [Google Scholar]
- Covert JB, Reynolds WW. Survival value of fever in fish. Nature. 1977;267:43–45. doi: 10.1038/267043a0. [DOI] [PubMed] [Google Scholar]
- Craig EA, Weissman JS, Horwich AL. Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell. 1994;78:365–372. doi: 10.1016/0092-8674(94)90416-2. [DOI] [PubMed] [Google Scholar]
- Cross A, Asher L, Seguin M, Yuan L, Kelly N, Hammack C, Sadoff J. The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense mechanism in mice against extraintestinally invasive Escherichia coli. J Clin Invest. 1995;96:676–686. doi: 10.1172/JCI118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AS, Sadoff JC, Kelly N, Bernton E, Gemski P. Pretreatment with recombinant murine tumor necrosis factor a/cachectin and murine interleukin 1a protects mice from lethal bacterial infection. J Exp Med. 1989;169:2021–2027. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alecy LG, Kluger MJ. Avian febrile response. J Physiol (Lond) 1975;253:223–232. doi: 10.1113/jphysiol.1975.sp011188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing JF, Martinez-Valdez H, Elizondo RS, Walker EB, Taylor MW. Hyperthermia in humans enhances interferon-γ synthesis and alters the peripheral lympocyte population. J Interferon Res. 1988;8:143–150. doi: 10.1089/jir.1988.8.143. [DOI] [PubMed] [Google Scholar]
- Downing JF, Taylor MW, Wei KM, Elizondo RS. In vivo hyperthermia enhances plasma antiviral activity and stimulates peripheral lymphocytes for increased synthesis of interferon-γ. J Interferon Res. 1987;7:185–193. doi: 10.1089/jir.1987.7.185. [DOI] [PubMed] [Google Scholar]
- Duff GW, Durum SK. Fever and immunoregulation: hyperthermia, interleukins 1 and 2, and T-cell proliferation. Yale J Biol Med. 1982;55:437–442. [PMC free article] [PubMed] [Google Scholar]
- Ellingson HV, Clark PF. The influence of artificial fever on mechanisms of resistance. J Immunol. 1942;43:65–83. [Google Scholar]
- Ensor JE, Crawford EK, Hasday JD. Warming macrophages to febrile range destabilizes tumor necrosis factor-α mRNA without inducing heat shock. Am J Physiol. 1995;269:C1140–C1146. doi: 10.1152/ajpcell.1995.269.5.C1140. [DOI] [PubMed] [Google Scholar]
- Ensor JE, Wiener SM, McCrea KA, Viscardi RM, Crawford EK, Hasday JD. Differential effects of hyperthermia on macrophage interleukin-6 and tumor necrosis factor-α expression. Am J Physiol Cell Physiol. 1994;266:C967–C974. doi: 10.1152/ajpcell.1994.266.4.C967. [DOI] [PubMed] [Google Scholar]
- Esposito AL. Aspirin impairs antibacterial mechanisms in experimental pneumococcal pneumonia. Am Rev Respir Dis. 1984;130:857–862. doi: 10.1164/arrd.1984.130.5.857. [DOI] [PubMed] [Google Scholar]
- Ewing JF, Maines MD. Rapid induction of heme oxygenase 1 mRNA and protein by hyperthermia in rat brain: heme oxygenase 2 is not a heat shock protein. Proc Natl Acad Sci USA. 1991;88:5364–5368. doi: 10.1073/pnas.88.12.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Frisk A, Ison CA, Lagergard T. GroEL heat shock protein of Haemophilus ducreyi: association with cell surface and capacity to bind to eukaryotic cells. Infect Immun. 1998;66:1252–1257. doi: 10.1128/iai.66.3.1252-1257.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habicht GS. Body temperature in normal and endotoxin-treated mice of different ages. Mech Aging Dev. 1981;16:97–104. doi: 10.1016/0047-6374(81)90037-3. [DOI] [PubMed] [Google Scholar]
- Hodgin UG, Sanford JP. Gram-negative rod bacteremia. An analysis of 100 patients. Am J Med. 1965;39:952–960. doi: 10.1016/0002-9343(65)90118-x. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R, Nunnally I, Lindquist S, Taulien J, Perdrizet G, Karl I. Hyperthermia protects mice against the lethal effects of endotoxin. Am J Physiol. 1993;265:R1447–R1457. doi: 10.1152/ajpregu.1993.265.6.R1447. [DOI] [PubMed] [Google Scholar]
- Jampel HD, Duff GW, Gershon RK, Atkins E, Durum SK. Fever and immunoregulation. J Exp Med. 1983a;157:1229–1238. doi: 10.1084/jem.157.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jampel HD, Duff GW, Gershon RK, Atkins E, Durum SK. Fever and immunoregulation. III. Hyperthermia augments the primary in vitro humoral immune response. J Exp Med. 1983b;157:1229–1238. doi: 10.1084/jem.157.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Cross AS, Singh IS, Chem TT, Viscardi RM, Hasday JD. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun. 2000;68:1265–1270. doi: 10.1128/iai.68.3.1265-1270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, DeTolla L, Kalvakolanu I, Fitzgerald B, Hasday JD. Fever upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am J Physiol. 1999a;276:R1653–R1660. doi: 10.1152/ajpregu.1999.276.6.R1653. [DOI] [PubMed] [Google Scholar]
- Jiang Q, DeTolla L, Kalvakolanu I, et al. Febrile range temperature modifies early systemic TNFα expression in mice challenged with bacterial endotoxin. Infect Immun. 1999b;67:1539–1546. doi: 10.1128/iai.67.4.1539-1546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurivich DA, Sistonen L, Sarge KD, Morimoto RI. Arachidonate is a potent modulator of human heat shock gene transcription. Proc Natl Acad Sci USA. 1994;91:2280–2284. doi: 10.1073/pnas.91.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappel M, Diamant M, Hansen MB, Klokker M, Pedersen BK. Effects of in vitro hyperthermia on the proliferative response of blood mononuclear cell subsets, and detection of interleukins 1 and 6, tumour necrosis factor-alpha and interferon-gamma. Immunology. 1991;73:304–308. [PMC free article] [PubMed] [Google Scholar]
- Kim D, Ouyang H, Li GC. Heat shock protein hsp70 accelerates the recovery of heat-shocked mammalian cells through its modulation of heat shock transcription factor HSF1. Proc Natl Acad Sci USA. 1995;92:2126–2130. doi: 10.1073/pnas.92.6.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatersky J. Etude experimentale et clinique des effets favorables et defavorables de la fievre et de l'administration de corticoides au cours d'infectons bacteriennes. Acta Clin Belg. 1971;26(0) [PubMed] [Google Scholar]
- Kluger MJ, Ringler DH, Anver MR. Fever and Survival. Science. 1975;188:166–168. [PubMed] [Google Scholar]
- Kluger MJ. The evolution and adaptive value of fever. Am Sci. 1978;66:38–43. [PubMed] [Google Scholar]
- Kluger MJ 1991 The adaptive value of fever. In: Fever: Basic Mechanisms and Management, ed Mackowiak PA. Raven Press, New York, 105–124. [Google Scholar]
- Kluger MJ, Vaughn LK. Fever and survival in rabbits infected with Pasteurella multocida. J Physiol. 1978;282:243–251. doi: 10.1113/jphysiol.1978.sp012460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf U, Newton EM, Kyriakis J, Kingston RE. Repression of human heat shock factor 1 activity at control temperature by phosphorylation. Genes & Dev. 1996;10:2782–2793. doi: 10.1101/gad.10.21.2782. [DOI] [PubMed] [Google Scholar]
- Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- Kuhn LR. Effect of elevated body temperature on cryptococcus in mice. Proc Soc Exp Biol Med. 1949;71:341–343. doi: 10.3181/00379727-71-17185. [DOI] [PubMed] [Google Scholar]
- Kurosawa S, Kobune F, Okuyama K. Effects of antipyretics in rinderpest virus infection in rabbits. J Infect Dis. 1987;155:991–997. doi: 10.1093/infdis/155.5.991. [DOI] [PubMed] [Google Scholar]
- Lederman HM, Brill CR, Murphy PA. Interleukin-1 driven secretion of interleukin-2 is highly temperature-dependent. J Immunol. 1987;138:3808–3811. [PubMed] [Google Scholar]
- Leijh PCJ, van den Barselaar MT, van Furth R. Kinetics of phagocytosis and intracellular killing of Staphylococcus aureus and Escherichia coli by human monocytes. Scand J Immunol. 1981;13:159–174. doi: 10.1111/j.1365-3083.1981.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Leijh PCJ, van den Barselaar M, van Zwet TL, Dubbeldeman-Rempt I, van Furth R. Kinetics of phagocytosis of Staphylococcus aureus and Escherichia coli by human granulocytes. Immunology. 1979;37:453–465. [PMC free article] [PubMed] [Google Scholar]
- LeMay DR, LeMay LG, Kluger MJ, D'Alecy LG. Plasma profiles of IL-6 and TNF with fever-inducing doses of lipopolysaccharide in dogs. Am J Physiol. 1990;259:R126–R132. doi: 10.1152/ajpregu.1990.259.1.R126. [DOI] [PubMed] [Google Scholar]
- Longworth KE, Smith BL, Staub NC, Steffey EP, Serikov VB. Use of detergent to prevent initial responses to endotoxin in horses. Am J Vet Res. 1996;57:1063–1066. [PubMed] [Google Scholar]
- Mackowiak PA 1991 Direct effects of physiologic variations in temperature on pathogenic microorganisms. In: Fever: Basic Mechanisms and Management, ed Mackowiak P. Raven Press, New York, 167–182. [Google Scholar]
- Mackowiak PA, Boulant JA. Fever's glass ceiling. Clin Infect Dis. 1996;22:525–536. doi: 10.1093/clinids/22.3.525. [DOI] [PubMed] [Google Scholar]
- Mackowiak PA, Browne RH, Southern PM Jr, Smith JW. Polymicrobial sepsis: an analysis of 184 cases using log linear models. Am J Med Sci. 1980;280:73–80. doi: 10.1097/00000441-198009000-00002. [DOI] [PubMed] [Google Scholar]
- Manzella JP, Roberts NJ Jr. Human macrophage and lymphocyte responses to mitogen stimulation after exposure to influenza virus, ascorbic acid, and hyperthermia. J Immunol. 1979;123:1940–1944. [PubMed] [Google Scholar]
- Marino MW, Dunn A, Grail D, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mivechi NF, Giaccia AJ. Mitogen-activated protein kinase acts as a negative regulator of the heat shock response in NIH3T3 cells. Cancer Res. 1995;55:5512–5519. [PubMed] [Google Scholar]
- Morange M, Dubois MF, Bensaude O, Lebon P. Interferon pretreatment lowers the threshold for maximal heat-shock response in mouse cells. J Cell Physiol. 1986;127:417–422. doi: 10.1002/jcp.1041270310. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes & Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29. [PubMed] [Google Scholar]
- Moseley PL. Heat shock proteins and the inflammatory response. Ann NY Acad Sci. 1998;856:206–213. doi: 10.1111/j.1749-6632.1998.tb08327.x. [DOI] [PubMed] [Google Scholar]
- Mullbacher A. Hyperthermia and the generation and activity of murine influenza-immune cytotoxic T cells in vitro. J Virol. 1984;52:928–931. doi: 10.1128/jvi.52.3.928-931.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre K, Cabanac M, Myhre G. Fever and behavioural temperature regulation in the frog Rana esculenta. Acta Physiol Scand. 1977;101:219–229. doi: 10.1111/j.1748-1716.1977.tb06001.x. [DOI] [PubMed] [Google Scholar]
- Nahas GG, Tannieres ML, Lennon JF. Direct measurement of leukocyte motility: effect of pH and temperature. Proc Soc Exp Biol Med. 1971;138:350–352. doi: 10.3181/00379727-138-35894. [DOI] [PubMed] [Google Scholar]
- Narvanen O, Jokinen I, Poikonen K, Rasanen L, Arvilommi H. Effect of elevated temperature on human immunoglobulin synthesis, lymphokine production and lymphocyte proliferation in vitro. Acta Pathol Microbiol Immunol Scand [C] 1986;94:239–244. doi: 10.1111/j.1699-0463.1986.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Owen JA, Dudzik KI, Klein L, Dorer DR. The kinetics of generation of influenza-specific cytotoxic T-lympocyte precursor cells. Cell Immunol. 1988;111:247–252. doi: 10.1016/0008-8749(88)90067-6. [DOI] [PubMed] [Google Scholar]
- Parrott RF, Vellucci SV, Goode JA, Lloyd DM, Forsling ML. Interrelated adrenocortical and neurohypophysial responses associated with fever in endotoxin-treated pigs. Am J Physiol. 1997;273:R1046–R1052. doi: 10.1152/ajpregu.1997.273.3.R1046. [DOI] [PubMed] [Google Scholar]
- Perdrizet GA. Heat shock and tissue protection. New Horiz. 1995;3:312–320. [PubMed] [Google Scholar]
- Perisic O, Xiao H, Lis JT. Stable binding of drospophila heat shock factor to head-to-head and tail-tail repeats of a conserved 5 bp recognition unit. Cell. 1989;59:797–806. doi: 10.1016/0092-8674(89)90603-x. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Verhoef J, Quie PG. Influence of temperature on opsonization and phagocytosis of staphylococci. Infect Immun. 1977;15:175–179. doi: 10.1128/iai.15.1.175-179.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson PK, Verhoef J, Sabath LD, Quie PG. Extracellular and bacterial factors influencing staphylococcal phagocytosis and killing by human polymorphonuclear leukocytes. Infect Immun. 1976;14:496–501. doi: 10.1128/iai.14.2.496-501.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadnis SH, Parlow MH, Levy M, Ilver D, Caulkins CM, Connors JB, Dunn BE. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun. 1996;64:905–912. doi: 10.1128/iai.64.3.905-912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–791. [PubMed] [Google Scholar]
- Ray PK. Stress genes and species survival. Mol Cell Biochem. 1999;196:117–123. [PubMed] [Google Scholar]
- Retzlaff C, Yamamoto Y, Hoffman PS, Friedman H, Klein TW. Bacterial heat shock proteins directly induce cytokine mRNA and interleukin-1 secretion in macrophage cultures. Infect Immun. 1994;62:5689–5693. doi: 10.1128/iai.62.12.5689-5693.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds WW. Fever and antipyresis in the bluegill sunfish, Lepomis macrochirus. Comp Biochem Physiol C. 1977;57:165–167. doi: 10.1016/0306-4492(77)90065-x. [DOI] [PubMed] [Google Scholar]
- Rich AR, McKee CM. The mechanism of a hitherto unexplained form of native immunity to the type III pneumococcus. Bull Johns Hopkins Hosp. 1936;59:171–207. [Google Scholar]
- Roberts NJ, Steigbigel RT. Hyperthermia and human leukocyte functions: effects on response of lympocytes to mitogen and antigen and bactericidal capacity of monocytes and neutrophils. Infect Immun. 1977;18:673–679. doi: 10.1128/iai.18.3.673-679.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts NJ Jr. Differential effects of hyperthermia on human leukocyte production of interferon-α and interferon-γ. Proc Soc Exp Biol Med. 1986;183:42–47. doi: 10.3181/00379727-183-42383. [DOI] [PubMed] [Google Scholar]
- Roberts NJ Jr, Lu ST, Michaelson SM. Hyperthermia and human leukocyte functions: DNA, RNA, and total protein synthesis after exposure to <41°C or >42.5°C hyperthermia. Cancer Res. 1985;45:3076–3082. [PubMed] [Google Scholar]
- Rodbard D, Wachslight-Rodbard H, Rodbard S. Temperature: a critical factor determining localization and natural history of infectious, metabolism, and immunological diseases. Perspect Biol Med. 1980;23:439–474. doi: 10.1353/pbm.1980.0062. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Simons CT, Kulchitsky VA. “Biphasic” fevers often consist of more than two phases. Am J Physiol. 1998;275:R323–R331. doi: 10.1152/ajpregu.1998.275.1.R323. [DOI] [PubMed] [Google Scholar]
- Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress [published errata appear in Mol Cell Biol 1993 May;13(5):3122–3 and 1993 Jun;13(6):3838–9] Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saririan K, Nickerson DA. Enhancement of murine in vitro antibody formation by hyperthermia. Cell Immunol. 1982;74:306–312. doi: 10.1016/0008-8749(82)90031-4. [DOI] [PubMed] [Google Scholar]
- Satyal SH, Chen D, Fox SG, Kramer JM, Morimoto RI. Negative regulation of the heat shock transcriptional response by HSBP1. Genes & Dev. 1998;12:1962–1974. doi: 10.1101/gad.12.13.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JR, Rasmussen AF Jr. The influence of environmental temperature on the course of experimental herpes simplex infection. J Infect Dis. 1960;107:356–360. doi: 10.1093/infdis/107.3.356. [DOI] [PubMed] [Google Scholar]
- Sebag J, Reed WP, Williams RC Jr. Effect of temperature on bacterial killing by serum and by polymorphonuclear leukocytes. Infect Immun. 1977;16:947–954. doi: 10.1128/iai.16.3.947-954.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IS, Calderwood S, Kalvakolanu I, Viscardi R, Hasday JD. Inhibition of tumor necrosis factor-α transcription in macrophages exposed to febrile range temperature: a possible role for heat shock factor-1. J Biol Chem. 2000;275:9841–9848. doi: 10.1074/jbc.275.13.9841. [DOI] [PubMed] [Google Scholar]
- Smith JB, Knowlton RP, Agarwal SS. Human lymphocyte responses are enhanced by culture at 40°C. J Immunol. 1978;121:691–694. [PubMed] [Google Scholar]
- Starks PT, Blackie CA, Seeley TD. Fever in honeybee colonies [in process citation] Naturwissenschaften. 2000;87:229–231. doi: 10.1007/s001140050709. [DOI] [PubMed] [Google Scholar]
- Strouse S. Experimental studies on pneumococcus infections. J Exp Med. 1909;11:743–761. doi: 10.1084/jem.11.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CY, Chong KY, Chen J, Ryter S, Khardori R, Lai CC. A physiologically relevant hyperthermia selectively activates constitutive hsp70 in H9c2 cardiac myoblasts and confers oxidative protection. J Mol Cell Cardiol. 1999;31:845–855. doi: 10.1006/jmcc.1998.0923. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sawa Y, Kaneda Y, Ichikawa H, Shirakura R, Matsuda H. Overexpressed heat shock protein 70 attenuates hypoxic injury in coronary endothelial cells. J Mol Cell Cardiol. 1998;30:1129–1136. doi: 10.1006/jmcc.1998.0678. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Nakai A, Kawaoe Y, Nagata K. Different thresholds in the responses of two heat shock transcription factors, HSF1 and HSF3. J Biol Chem. 1997;272:15389–15395. doi: 10.1074/jbc.272.24.15389. [DOI] [PubMed] [Google Scholar]
- Tomasovic SP, Barta M, Klostergaard J. Temporal dependence of hyperthermic augmentation of macrophage-TNF production and tumor cell-TNF sensitization. Int J Hyperthermia. 1989;5:625–639. doi: 10.3109/02656738909140486. [DOI] [PubMed] [Google Scholar]
- Tracey K, Beutler B, Lowry S, et al. Shock and tissue injury induced by recombinant human cachetin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Van Miert ASJPAM, Van Duin CT, Busser FJM. The effect of fluriprofen, a potent non-steroidal anti-inflammatory agent, upon Trypanosoma vivax infection in goats. J Vet Pharmacol Ther. 1978;1:69–76. [Google Scholar]
- van Oss CJ, Absolom DR, Moore LL, Park BH, Humbert JR. Effect of temperature on the chemotaxis, phagocytic engulfment, digestion and O2 consumption of human polymorphonuclear leukocytes. J Reticuloendothel Soc. 1980;27:561–565. [PubMed] [Google Scholar]
- Vaughn LK, Veale WL, Cooper KE. Antipyresis: its effect on mortality rate of bacterially infected rabbits. Brain Res Bull. 1980;5:69–73. doi: 10.1016/0361-9230(80)90285-3. [DOI] [PubMed] [Google Scholar]
- Velasco S, Tarlow M, Olsen K, Shay JW, McCracken GH, Nisen PD. Temperature-dependent modulation of lipopolysaccharide-induced interleukin-1β and tumor necrosis factor α expression in cultured human astroglial cells by dexamethasone and indomethacin. J Clin Invest. 1991;87:1674–1680. doi: 10.1172/JCI115184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdegaal ME, Zegveld ST, van Furth R. Heat shock protein 65 induces CD62e, CD106, and CD54 on cultured human endothelial cells and increases their adhesiveness for monocytes and granulocytes. J Immunol. 1996;157:369–376. [PubMed] [Google Scholar]
- Villar J, Ribeiro SP, Mullen JBM, Kuliszewski M, Post M, Slutsky AS. Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med. 1994;22:914–921. [PubMed] [Google Scholar]
- Wang WC, Goldman LM, Schleider DM, Appenheimer MM, Subjeck JR, Repasky EA, Evans SS. Fever-range hyperthermia enhances L-selectin-dependent adhesion of lymphocytes to vascular endothelium. J Immunol. 1998;160:961–969. [PubMed] [Google Scholar]
- Ward PA, Lentsch AB. The acute inflammatory response and its regulation. Arch Surg. 1999;134:666–669. doi: 10.1001/archsurg.134.6.666. [DOI] [PubMed] [Google Scholar]
- Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18:5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HY, Chang MS, Rho HM. The activation of the rat copper/zinc superoxide dismutase gene by hydrogen peroxide through the hydrogen peroxide-responsive element and by paraquat and heat shock through the same heat shock element. J Biol Chem. 1999;274:23887–23892. doi: 10.1074/jbc.274.34.23887. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Koga S, Maeta M, Shimizu N, Hamazoe R, Murakami A. The influence of hyperthermia in vitro on the functions of peritoneal macrophages in mice. Jpn J Surg. 1990;20:119–122. doi: 10.1007/BF02470725. [DOI] [PubMed] [Google Scholar]
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]