Abstract
Background
We evaluated scar lesions following initial and repeat catheter ablation of atrial fibrillation (AF) and correlated these regions to low-voltage tissue on repeat electroanatomical (EA) mapping. We also identified gaps in lesion sets that could be targeted and closed during repeat procedures.
Methods and Results
One hundred forty-four patients underwent AF ablation and received a delayed-enhancement MRI (DE-MRI) at three months post-ablation. The number of pulmonary veins (PV) with circumferential lesions were assessed and correlated with procedural outcome. Eighteen patients with AF recurrence underwent repeat ablation. MRI scar regions were compared to electroanatomical (EA) maps during the repeat procedure. Regions of incomplete scar around the PVs were then identified and targeted during repeat ablation in order to ensure complete circumferential lesions. Following the initial procedure, complete circumferential scarring of all 4 PVA was achieved in only 7% of patients, with the majority of patients (69%) having <2 completely scarred PVA. After the first procedure, the number of PVs with complete circumferential scarring and total LA wall scar burden was associated with better clinical outcome. Patients with successful AF termination had higher average total LA wall scar of 16.4% ± 9.8 (p = 0.004) and percent PVA scar of 66.2 ± 25.4 (p = 0.01) compared to patients with AF recurrence who had an average total LA wall scar 11.3% ± 8.1 and PVA percent scar 50.0 ± 24.7. In patients who underwent repeat ablation, the PV antra scar percentage was 56.1% ± 21.4 after the first procedure compared to 77.2% ± 19.5 after the second procedure. The average total LA scar after the first ablation was 11.0% ± 4.1, while the average total LA scar after second ablation was 21.2% ± 7.4. All patients had increased number of completely scarred pulmonary vein antra after the second procedure. MRI scar after the first procedure and low voltage regions on EA obtained during repeat ablation demonstrated a positive quantitative correlation of R2 = 0.57.
Conclusions
Complete circumferential PV lesions is difficult to achieve but is associated with better clinical outcome. DE-MRI can accurately define scar lesions following AF ablation and can be used to target breaks in lesion sets during repeat ablation.
Keywords: Atrial Fibrillation, Catheter Ablation, MRI, Pulmonary Vein Antrum Isolation, Left Atrial Scar Lesions
Background
Pulmonary vein antrum (PVA) isolation is a treatment option for atrial fibrillation (AF) patients, which uses high-frequency energy to induce myocardial necrosis at anatomical landmarks within the left atrium (LA).1,2 This strategy aims at creating contiguous and confluent ablation lesions around the PVA in order to electrically isolate the LA from arrhythmogenic foci located in the pulmonary veins (PV).3 Procedure failure is often attributed to resumption of conduction between the PV’s and LA and thought to be due to incomplete ablation lesion sets.4
Delayed-enhancement MRI (DE-MRI) is a noninvasive imaging modality used to visualize radiofrequency-induced scar in the left atrial (LA) wall following AF ablation.5–8 Regions of nonviable or scarred myocardium have increased image intensity in DE-MRI scans due to the slow washout kinetics of the gadolinium-based contrast agents in injured tissue.9=11 Three-dimensional models of the LA can be generated to display the anatomical location and size of ablation-induced scar lesions following catheter-based procedures.5 However, there are no reports verifying the accuracy of DE-MRI in depicting the location and extent of ablation lesions. Although there is a prior pilot study demonstrating a correlation between the number of PVs with circumferential lesions and outcome this was obtained in a limited patient series and has not been studied thoroughly.12 Moreover, no studies exist using DE-MRI to identify breaks in ablation lesion sets that can be targeted and closed during repeat procedure, as well as examining outcome data following repeat procedures.
The aim of this study was the following: (1) correlate anatomical scar data including total LA scar percentage, total PVA scar percentage and number of complete scarred PVA following initial and repeat AF ablation procedures; (2) correlate DE-MRI scar lesions at following AF ablation with regions of low-voltage tissue obtained using electroanatomical (EA) mapping during repeat procedure; (2) examine the ability to identify regions of interrupted ablation-lesion sets that could be targeted and closed during repeat ablation.
Methods
Study Population
Between October 2006 and April 2008, 235 patients presented to the University of Utah for catheter ablation of AF. Of theses patients 144 received an interpretable DE-MRI scan three months following ablation. Twenty-four patients with AF recurrence elected to undergo repeat ablation procedures. Eighteen of these patients met the following criteria: (1) quality DE-MRI 3 months following the initial procedure (2) quality DE-MRI 3 months following the repeat procedure and (3) six month follow-up after the second ablation procedure. Six patients were excluded from our study for the following reasons: 3 were ineligible for MRI and received CT scans; 2 patients had poor quality DE-MRI scan after the first procedure; 1 patient had a poor quality DE-MRI scan after the second procedure. Of the 18 patients, 13 had quality EA maps (defined as >100 points). Table 1 lists the clinical characteristics of the 144 patients included.
Table 1.
Demographic Data
| Total | Patients without Recurrence (102) | Patients with Recurrence (42) | P-Value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 102 | 64 | 38 | 0.212 |
| Female | 42 | 30 | 12 | |
|
| ||||
| Age (years) | 63 ± 13 | 63 ± 14 | 64 ± 9 | 0.598 |
|
| ||||
| AF Type | ||||
| Paroxysmal | 57 | 40 | 17 | 0.516 |
| Persistent | 87 | 62 | 25 | |
| Average Follow up (days) | 330 ± 160 | 340 ± 164 | 330 ± 180 | 0.709 |
|
| ||||
| RF Burn Time (seconds) | 2800 ± 890 | 2700 ± 950 | 2800 ± 600 | 0.658 |
|
| ||||
| Myocardial Infarction | 5 | 3 | 2 | 0.456 |
| Coronary Artery Disease | 20 | 14 | 6 | 0.559 |
| Valve Surgery | 3 | 3 | 0 | 0.352 |
| Smoker | 26 | 16 | 10 | 0.180 |
| Hypertension | 73 | 51 | 22 | 0.470 |
| Diabetes | 15 | 11 | 4 | 0.543 |
| Congestive Heart Failure | 10 | 7 | 3 | 0.601 |
Pulmonary Vein Antrum Isolation, Posterior Wall and Septal Debulking
The methods for PVAI with posterior wall and septal debulking under intracardiac echocardiogram (ICE) guidance have been described previously.5 The technique is briefly summarized below. After venous access was obtained, a 14-pole coronary sinus catheter was placed into the coronary sinus via the right internal jugular access (TZ Medical Inc., Portland, OR, or Bard EP, Lowell, MA) for use as a mapping reference. A phased-array ultrasound catheter was positioned in the mid-right atrium (Siemens AG Inc., Malvern, PA, USA) and used to guide a double transseptal puncture, through which was placed a 10-pole circular mapping catheter (Biosense Webster Inc) and a 3.5-mm Thermocool irrigated tip ablation catheter (Biosense Webster Inc). Using fluoroscopy and electroanatomic mapping (CARTOMERGE, Biosense-Webster, Inc.) for catheter navigation, intracardiac potentials in the PV antra, the posterior wall and septum were mapped during sinus rhythm or AF and were targeted for ablation. Circular mapping-guided RF delivery was performed, using circular mapping electrogram artifacts to confirm ablation catheter tip location relative to the substrate of interest. Lesions were delivered using 50 W with a 50°C temperature limit, for a duration of 10 seconds (maximum 15 seconds), with the endpoint being abolition of local electrograms. When all targets had been ablated, the entire region was re-surveyed for any return of electrical activity and any such regions were retreated. In addition, entry block in all four pulmonary veins was confirmed with the circular mapping catheter after debulking was accomplished. At the end of all re-do procedures 15 mcg/min of Isopretrenol in addition to burst atrial pacing at 200 ms was performed to rule out initiation of AF or atrial flutter.
Post-Ablation Imaging
At three months following both the initial and repeat ablation procedure, a DE-MRI scan was performed to assess LA scar using methods previously described.5 Three months post-procedure was chosen as the time point for lesion assessment as it has previously been shown to indicate chronic scar formation.6 Briefly, patients underwent a delayed enhancement MRI sequence on a 1.5 Tesla Avanto clinical scanner (Siemens Medical Solutions, Erlangen, Germany) using a TIM phased-array receiver coil. High resolution DE-MRI was acquired approximately 15 minutes after contrast agent injection (0.1 mmol/kg, Multihance (Bracco Diagnostic Inc., Princeton, NJ)) using a 3D inversion recovery, respiration navigated, ECG-gated, gradient echo pulse sequence. Typical acquisition parameters were: free-breathing using navigator gating, a transverse imaging volume with voxel size = 1.25 × 1.25 × 2.5 mm (reconstructed to 0.625 × 0.625 × 1.25 mm), TR/TE = 5.5/2.3 ms, inversion time (TI) = 270–310 ms, GRAPPA with R=2 and 50 reference lines. ECG gating was used to acquire a small subset of phase encoding views during the diastolic phase of the LA cardiac cycle. The time interval between the R- peak of the ECG and the start of data acquisition was defined using the cine images of the LA. To preserve magnetization preparation in whole image volume, navigator was acquired immediately after data acquisition block. Fat saturation was used to suppress fat signal. The TE of the scan (2.3 ms) was chosen such that fat and water are out of phase and the signal intensity of partial volume fat-tissue voxels was reduced allowing improved delineation of the LA wall boundary. The TI value for the DE-MRI scan was identified using a scout scan and was chosen to minimize intensity of normal myocardium. Typical scan time for the DE-MRI study was 5–10 minutes depending on subject respiration and heart rate. If the first acquisition of 3D DE-MRI did not have an optimal TI or had sub-optimal image quality, the scan was repeated.
DE-MRI Image Processing
Following acquisition of the delayed enhancement scan, images were processed into three-dimensional models by manually segmenting the LA in the maximum intensity projection and volume rendering the image. A smooth table opacity and application of a color-look-up-table (CLUT) was then applied to better visualize scar regions. The LA model was then used for analysis of scar patterns around each PV antrum and to identify regions of interrupted lesions. Each patient had images generated of the right and left pulmonary veins with contoured outlines of the right superior PV, right inferior PV, left superior PV, left inferior PV. The number of pulmonary veins that exhibited complete and contiguous scarring as agreed upon by two independent and blinded reviewers was then recorded. The extent of PVA scar percentage was obtained by dividing each PVA into eight equal segments and then estimating the extent containing scar lesions within each segment. Quantification of scar in the LA wall was then performed by tracing the epicardial and endocardial borders using custom image display and then analyzed by software written in Matlab (The Mathworks Inc. Natick, MA). Normal and injured tissue were defined based on a bimodal distribution of pixel intensities within the LA wall. The first mode of lower pixel intensities was chosen as normal tissue. Injured tissue was defined at 3 standard deviations above the normal tissue mean pixel intensity. Regions defined as lesion were visualized independently to ensure appropriateness of lesion detection. Three-months following the second ablation procedure, DE-MRI was again performed and the 3D models of the LA were generated to analyze PVA scarring from second procedure similar to the methods described above.
Correlation between DE-MRI and Electroanatomical Mapping
A quantitative and qualitative analysis was performed to correlate low voltage regions on EA maps and enhancement on DE-MRI using methods previously described.13 Thirteen patients with high quality CartoXP maps (defined as greater than 100 voltage points evenly spread throughout the atrium) were selected. The LA on the EA map and 3D DE-MRI was subdivided into 18 specific regions; 9 on the posterior wall and 9 on the anterior and septal wall. Four blinded reviewers (two experts in cardiac MRI and two experts in AF ablation) scored the MRI models and EA maps on a 0 to 3 scale. For MRI models, 0 was no enhancement, 1 was mild, 2 was moderate, and 3 was extensive enhancement. For the EA maps, 0 was considered healthy tissue (voltage > 1 mV, purple on EA maps), 1 was mildly decreased tissue voltage (voltage between > 0.1 mV and < 0.5 mV), 2 was moderate decreased tissue voltage (presence of low voltage tissue [voltage > 0.1 mV and < 0.5 mV] as well as fibrotic scar [voltage < 0.1 mV]), and 3 was extensively decreased tissue voltage (voltage < 0.1 mV, red on EA maps). The overall score was an average sum of all nine regions for both the posterior wall and the septum.
Post-Ablation Management
Following the procedure, all patients were placed on a telemetry unit for 24-hour observation. Patients were discharged with patient-triggered and auto-detected event monitoring for 8 weeks. Patients continued anti-coagulation therapy with warfarin (international normalized ratio of 2.0–3.0) for a minimum of three months. Patients were assessed for AF recurrence at three months, six months, one year and then every year after the procedure with eight-day Holter monitoring and ECG while at clinic. Patients in this study required a minimum of six months follow up after their second procedure. Procedural success was defined according to recent Heart Rhythm Society Guidelines as freedom from AF, atrial tachycardia, and atrial flutter while off of anti-arrhythmic medications two months following ablation (i.e., two month blanking period).14
Statistical Analysis
Normal continuous variables are presented as mean ± standard deviation. Differences were considered statistically significant with a two-sided p < 0.05. Statistical Analysis was performed using the SPSS 17.0 Statistical Package (SPSS Inc.; Chicago, IL), and Microsoft Excel 2007 (Microsoft corporation; Redmond, WA). A quantitative analysis of the relationship between DE-MRI and EA maps was performed using linear regression. Kaplan Meier analysis for AF recurrence was performed on PVA scar percentage post-ablation and (4) number of pulmonary veins with complete antral scarring post-ablation.
Results
Patient Population
One hundred forty-four patients underwent PVAI with posterior wall and septum debulking for the treatment of AF. Of these patients, forty-two (28.2%) experienced recurrence of AF after their ablation procedure. Average time to AF recurrence was 5.11± 2.84 months. The average follow-up in this study was 10.23 ± 5.14 months (range = 6 to 20 months). There was no statistical difference between the two groups with regards to gender, age, AF type, radiofrequency burn time, as well as other baseline patient characteristics (Table 1).
Eighteen patients underwent a repeat ablation procedure. There were 14 males and 4 females, with an average age of 66.0 years ± 8.7 among males and 70.0 years ± 2.5 among females (Table 2). Three patients had paroxysmal and fifteen patients had persistent atrial fibrillation. Average ejection fraction was 51.1 % ± 7.8. Of the 18 patients, five (27.8%) had recurrence of atrial fibrillation following the second procedure. Although all patients have varying degrees of pre-ablation enhancement, no patients had significant enhancement to register with the scar quantification code used to detect post-ablation scarring.
Table 2.
Repeat Ablation Patients
| Patient | Age (yrs) | Sex | AF Type | Procedure 1 | Procedure 2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scar % | # PV Isolated | PV Scar % | Scar % | # PVs Isolated | PV Scar % | AF Recurrence | ||||
| 1 | 50 | M | Persistent | 11.5 | 0 | 44 | 34.8 | 2 | 72 | Yes |
| 2 | 71 | F | Paroxysmal | 11.1 | 1 | 40 | 17.9 | 1 | 64 | No |
| 3 | 73 | F | Persistent | 9.8 | 1 | 40 | 11.1 | 1 | 45 | Yes |
| 4 | 68 | F | Persistent | 3.5 | 0 | 32 | 13.4 | 3 | 86 | No |
| 5 | 73 | M | Persistent | 18.3 | 3 | 92 | 14.9 | 4 | 100 | No |
| 6 | 54 | M | Persistent | 7.9 | 1 | 50 | 18.1 | 2 | 64 | Yes |
| 7 | 59 | M | Persistent | 16.3 | 2 | 90 | 22.4 | 4 | 100 | No |
| 8 | 69 | M | Persistent | 4.3 | 0 | 20 | 24.3 | 1 | 44 | No |
| 9 | 64 | M | Paroxysmal | 9.9 | 1 | 65 | 18.3 | 2 | 77 | No |
| 10 | 67 | M | Persistent | 7.8 | 1 | 44 | 17.5 | 4 | 100 | No |
| 11 | 71 | M | Persistent | 5.3 | 1 | 66 | 19.4 | 3 | 86 | No |
| 12 | 63 | M | Persistent | 14 | 1 | 62 | 18.9 | 3 | 80 | Yes |
| 13 | 64 | M | Persistent | 11.8 | 2 | 93 | 28.1 | 4 | 100 | No |
| 14 | 78 | M | Persistent | 8.9 | 1 | 56 | 31.6 | 4 | 100 | No |
| 15 | 68 | F | Persistent | 10.1 | 0 | 34 | 11.7 | 1 | 56 | No |
| 16 | 59 | M | Persistent | 11.5 | 1 | 69 | 22.4 | 2 | 73 | Yes |
| 17 | 74 | M | Paroxysmal | 15.9 | 1 | 43 | 20.7 | 2 | 57 | No |
| 18 | 79 | M | Persistent | 17.9 | 1 | 70 | 36.7 | 2 | 75 | No |
Ablation-Scarring after the First Procedure and Outcome
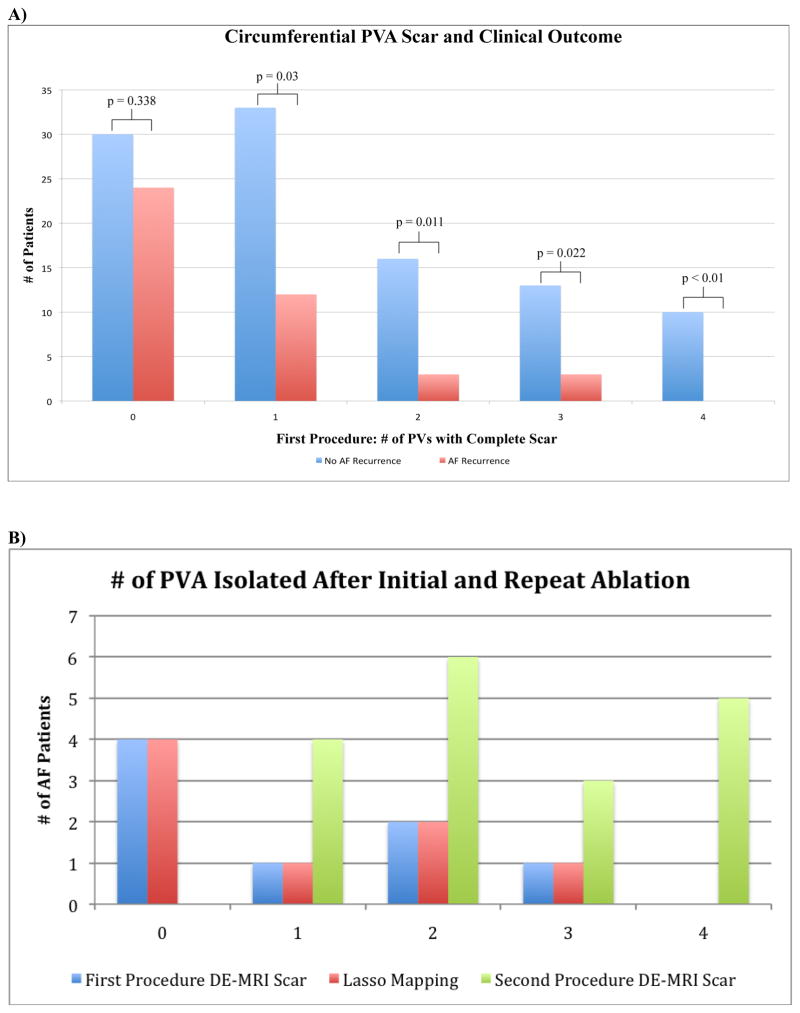
Following the initial procedure, patients with 4 completely scarred PVA (ten patients) had 100% procedure success, whereas patients with 3 PVA (16 patients, 76.9% successful outcome), 2 PVA (19 patients, 84.2% procedure success), 1 PVA (45 patients, 73.3% procedure success, and zero PVA (54 patients, 55.5% procedure success) all had lower success rates. Figure 1a depicts this relationship.
Figure 1.
A) Correlation of Circumferential PVA Scar from First Ablation Procedure and Recurrence. B) Patients with PVA Isolated Following Initial and Repeat Ablation Procedures.
Patients with successful AF termination had higher average total LA wall scar of 16.4% ± 9.8 (p = 0.004) and percent PVA scar of 66.2 ± 25.4 (p = 0.01) compared to patients with AF recurrence who had an average total LA wall scar 11.3% ± 8.1 and PVA percent scar 50.0 ± 24.7.
Frequency of Complete Circumferential PVA Lesions
Only 10/144 (6.9%) patients had circumferential scarring of all four PVA, 16/144 (11.1%) had 3 completely scarred PVA, 19/144 (13.2) had 2 completely scarred PVA, 45/144 (31.3%) had 1 completely scarred PVA and 54/144 (37.5%) had no completely scarred PVA (Table 3). Figure 1b shows the frequency of PVA scarring. The LIPV was most frequently circumferentially scarred PV occurring in 71/144 (49.3%) patients. The LSPV had complete scarring in 43/144 (29.9%) patients. The RIPV had complete scar in 32/144 (22.2%) patients. The RSPV was the most difficulty PV to achieve circumferential scarring occurring in only 17/144 (11.8%) patients.
Table 3.
Pulmonary Vein Antrum Scar Analysis
| Total | No AF Recurrence (102) | AF Recurrence (42) | P Value | ||
|---|---|---|---|---|---|
| Pulmonary Veins Isolated | 0 | 54 | 30 | 24 | 0.012 |
| 1 | 45 | 33 | 12 | ||
| 2 | 19 | 16 | 3 | ||
| 3 | 16 | 13 | 3 | ||
| 4 | 10 | 10 | 0 | ||
|
| |||||
| LIPV Isolated | 71 | 55 | 16 | 0.049 | |
| LSPV Isolated | 43 | 39 | 4 | 0.001 | |
| RIPV Isolated | 32 | 25 | 7 | 0.211 | |
| RSPV Isolated | 17 | 15 | 2 | 0.079 | |
|
| |||||
| LIPV Scar | 73.6% ± 30.0 | 77.9% ± 27.6 | 63.2% ± 33.2 | 0.007 | |
| LSPV Scar | 56.4% ± 33.7 | 63.4% ± 32.9 | 39.4% ± 29.4 | 0.001 | |
| RIPV Scar | 52.6% ± 30.6 | 55.8% ± 30.8 | 45.0% ± 29.0 | 0.054 | |
| RSPV Scar | 44.5% ± 30.0 | 47.6% ± 30.7 | 36.9% ± 27.2 | 0.056 | |
Ablation Lesions Prior to and Following Repeat Procedure
Eighteen patients underwent repeat ablation. Following the initial ablation, complete circumferential scarring was demonstrated around no PVA in four patients (22.2%), one PVA in eleven patients (61.1%), around 2 PVA in two patients (11.1%), and around three PVA in one patient (5.5%). No patients had complete scarring of all four PVA after the initial procedure. Following the repeat procedure, all patients had increased number of completely scarred PVA with five patients (27.8%) experiencing complete circumferential scarring of all four PVA and three patients (16.7%) with complete circumferential scarring of three PVA. Five patients (33.3%) had complete circumferential scarring in two PVA and four patients (22.2%) had complete scarring of one PVA. The numbers of veins scarred after the first and second procedures are demonstrated in Figure 1b.
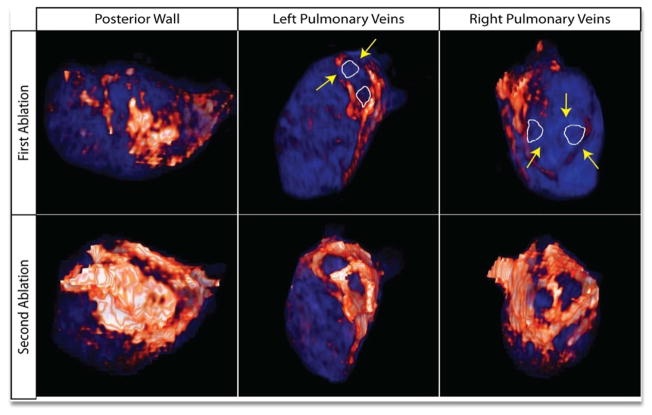
Figure 2 depicts a patient with incomplete isolation of all four PVA (top row) after his first procedure. Prior to the repeat ablation the regions of interrupted ablation lesions were targeted (yellow arrows) and given to the electrophysiologists prior to the procedure. Electrical recovery was present in all four PVA. Following the procedure, DE-MRI was obtained (bottom row) which demonstrated complete circumferential lesions around all four PVA.
Figure 2.
3D MRI model of the LA following failed PVAI (1) and repeat successful PVAI (2). After the initial failed ablation, all four PV’s showed incomplete PVA scar as evident by lack of continuous scar (orange/white) around each pulmonary vein ostia (white outline). Gap lesions of healthy myocardium (blue) were identified and targeted (yellow arrows) prior to repeat ablation. Following the successful repeat procedure, all four PVA had continuous scar lesions.
In the study, all patients had an increase in total LA scar percentage following the second procedure. The average circumferential PV antral scar after first ablation was 56.1% ± 21.4, while the average PV antral scar after the second ablation was 77.2% ± 19.5. The average total LA scar after the first ablation was 11.0% ± 4.1, while the average total LA scar after second ablation was 21.2% ± 7.4. The increase in posterior wall scarring of three patients is shown in Figure 3. Thirteen of the eighteen patients (72.2%) had successful suppression of AF following the second ablation procedure. All five patients with AF recurrence after the second procedure demonstrated at least one incomplete circumferential PVA lesion. Figure 4 demonstrates two patient examples of patients with incomplete ablation lesion sets who suffered AF recurrence following the second ablation procedure.
Figure 3. Increased Posterior Wall Scar Formation.
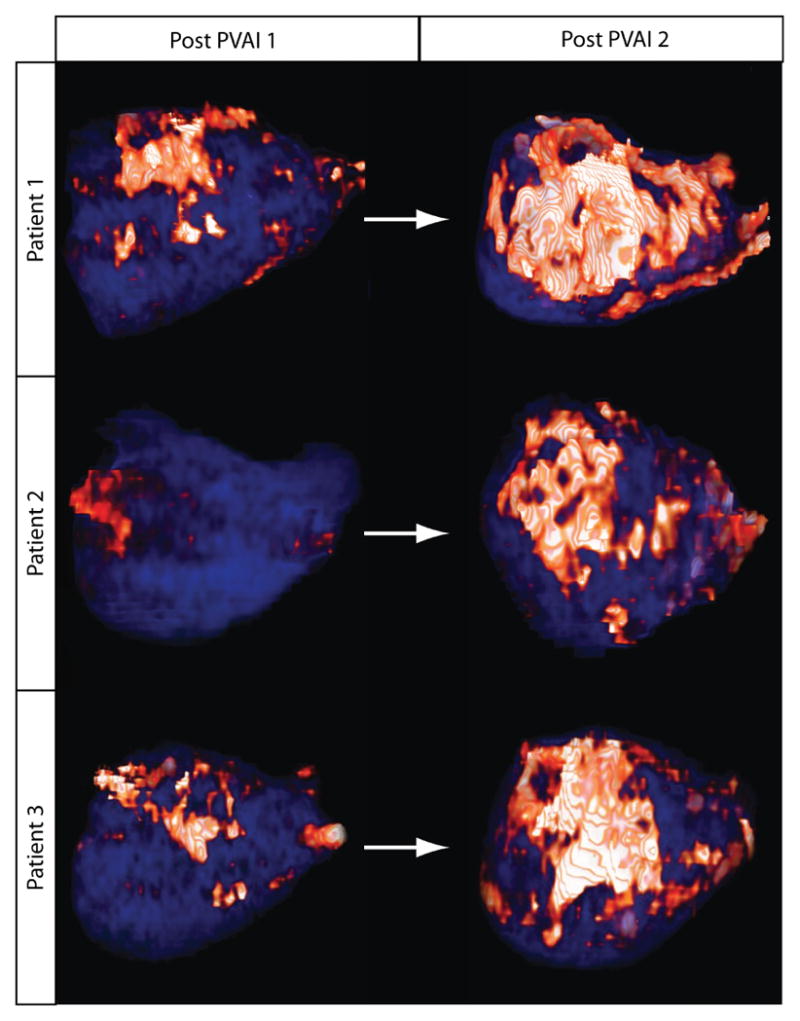
The first column (left) represents posterior wall scar (orange/white) following the first procedure in three different patients. The second column (right) represents the scar formation following the second ablation procedure. The repeat procedure induced significantly more posterior wall scarring during the second debulking procedure. This was associated with increased total LA wall scar in each patient.
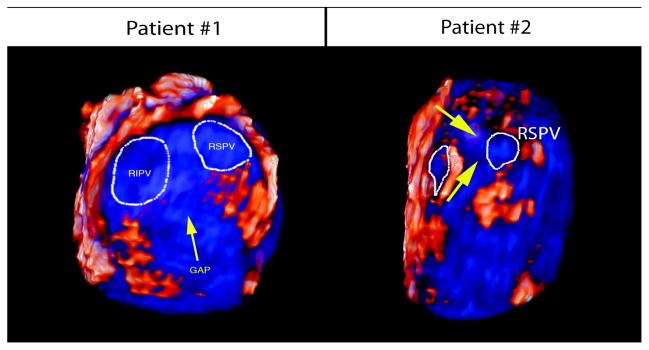
Figure 4. Identification of Gap Lesions following Procedure Failure.
In our patient series, five patients had recurrence of AF following the second procedure. All patients had significant gap lesions on their follow up MRI. Below is an example of two patients who experienced significant gap lesions (blue tissue, marked by yellow arrow) on their right pulmonary veins.
Correlation of DE-MRI Scar with Electroanatomical Mapping and Recovery of Conduction
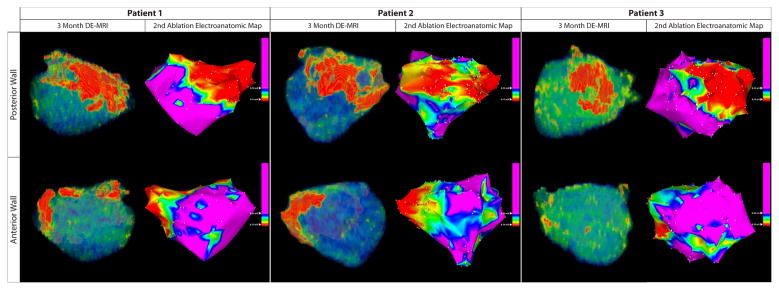
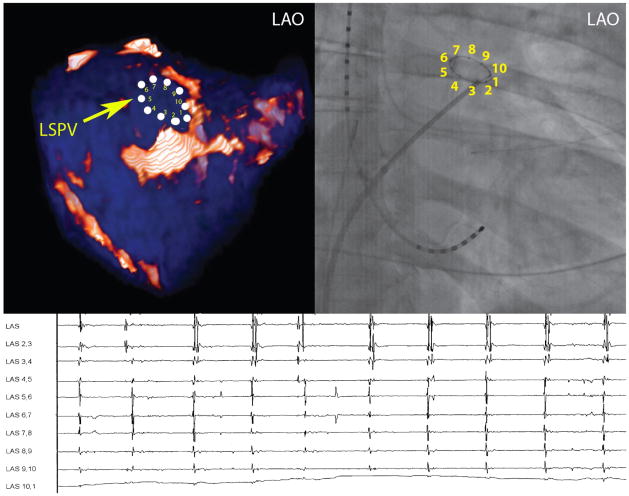
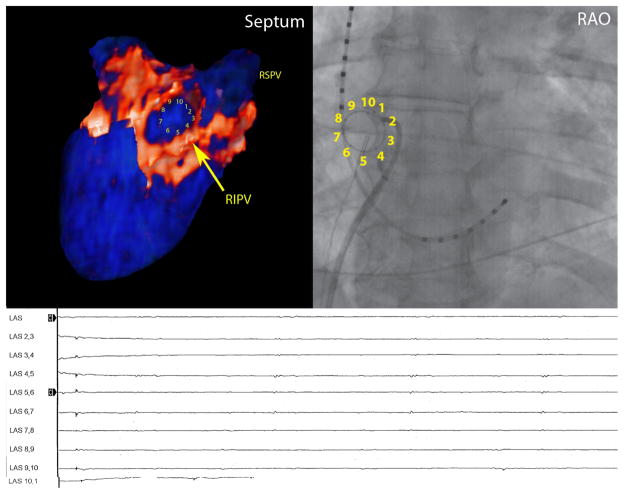
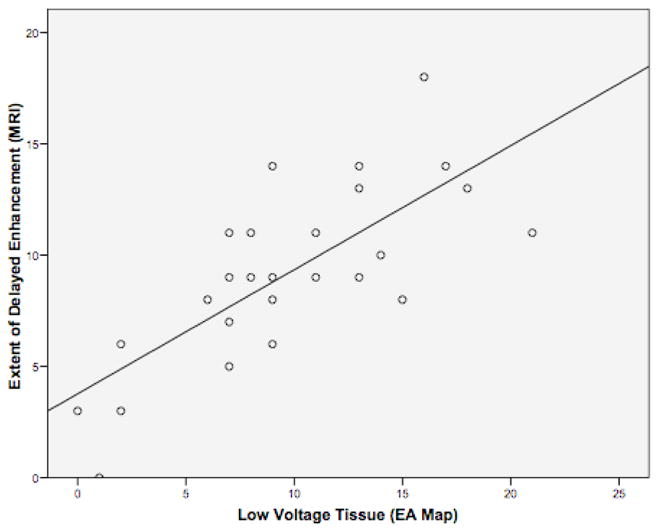
A qualitative correlation between regions of enhancement on DE-MRI and low voltage regions on EA maps was seen in all patients. Figure 5 demonstrates three patients. Patients one and three had DE-MRI scar (red) located along the posterior wall and right PVA, which correlated with the distribution of low voltage tissue (red) on EA maps. Patient 2 had minimal scar following the first procedure and had normal voltage tissue on the EA map. Quantitative analysis of this relationship demonstrated a positive correlation of R2 = 0.57 (Figure 6).
Figure 5.
Correlation between DE-MRI following a failed ablation procedure with the electroanatomical map (EAM) obtained during the repeat procedure for three patients. The images on the left demonstrate PA (top) and AP (bottom) views of the DE-MRI color model scar patterns. The images on the right are the EAM obtained during repeat procedure. There is a strong a correlation in the size and distribution of MRI scar (red tissue) with low voltage regions (<0.1mV; red) of the EAM.
Figure 6. Quantitative Correlation between DE-MRI Scar and Low-Voltage Tissue on Electroanatomical Mapping.
Recovery of Electrical Potential and Gap Lesions
All patients with incomplete ablation sets marked by identifiable gap lesions had recovery of electrical activity on repeat electrophysiological (EP) study. Figure 7 shows recovery of conduction into the left superior PV as demonstrated by pulmonary vein potentials within the PV, which was abolished during the initial procedure. This recovery occurs in a vein with only minimal scarring and incomplete circumferential lesions. In contrast, Figure 8 represents a patient with complete circumferential lesion (orange/white) of the right inferior PV with no evidence of electrical activity during the repeat EP study. No patients in the series had complete circumferential scar around the PVA and electrical recovery.
Figure 7. Recovery of Electrical Potentials that Correlate with Incomplete Scar Lesions.
In this patient example, the upper left picture demonstrates incomplete PVA scarring of the left superior pulmonary vein. This vein demonstrated recovery of previously isolated electrical potentials that correlated with the lack of complete anatomical scarring.
Figure 8. Complete Anatomical and Electrical Isolation.
Patients who demonstrated complete anatomical scar on DE-MRI had no recovery of electrical potentials on repeat exam. In this example, the upper left picture demonstrates a lesion with complete anatomical scarring of the right superior pulmonary vein. This vein demonstrated no recovery of electrical potentials on repeat electrophysiology study.
Discussion
This report describes the analysis of LA scar lesions following initial and repeat AF ablation. Our study indicates the number of circumferentially scarred PVA is associated with better clinical outcome and confirm earlier studies that total LA scar burden is associated with AF termination. However, although associated with excellent procedure outcomes, only a small minority of patients (6.9%) had complete and contiguous scarring of all four PVA. We found that DE-MRI can accurately depict the location and extent of scar lesions based on the correlation of these lesions with low voltage LA regions on EA maps. We also demonstrate that DE-MRI can be used to identify the location of breaks in ablation lesions, which correlates with recovery of electrical conduction that could be responsible for AF recurrence post-ablation. Completing circumferential PV antra scarring and re-debulking the PW during repeat procedure appears to improve procedural success. This novel MRI based method could be used as a measure to guide a repeat AF ablation procedure.
Complete Circumferential Anatomical Isolation
PVAI is a technically challenging procedure requiring confluent ablation lesions within the complex and heterogeneous LA-PV anatomy.15,16 The use of circumferential mapping and anatomical guidance with electroanatomical maps can assist in creating contiguous scar lesions around the PVA. However, these modalities have questionable accuracy17 and creating continuous ablation scar around each antrum remains a difficult task to achieve.18 Our study was consistent with prior reports as only 6.9% of patients had complete circumferential scarring of all four PVA. This indicates that obtaining complete circumferential isolation is a difficult procedure endpoint to achieve and might be occurring less frequently than previously suspected, even if initial pulmonary vein isolation was achieved. Our data does support the notion that the greater the number of veins with complete anatomical scarring increases the likelihood of successful procedure outcome. However, many patients remained in normal sinus rhythm even though they had incomplete PVA scarring, suggesting other factors influencing AF recurrence post-ablation.
Left Atrial Substrate Modification
There is substantial evidence that the mechanisms of AF are multifactorial.19 Triggers outside of the PVs, including the LA posterior wall appear to contribute to both AF initiation and maintenance.20–24 Modified ablation techniques that have been adapted to address the role of the posterior wall in the chronic fibrillatory process.25 Substrate modification relies on decreasing the amount of viable LA tissue capable of harboring AF by debulking significant portions of the LA posterior wall.26 Pappone et al have speculated that the extent of left atrial ablation (>30%) is an important prognostic indicator for procedure success than PV isolation.2 This finding was supported in a recent analysis of scar patterns performed by our group in which LA scar burden was a significant predictor of AF termination.5 This study confirmed these earlier findings in a larger patient population. Likewise, in this study, each patient experienced an increase in total LA wall scar burden, primarily located within the left atrial posterior wall (Figure 6) following the second procedure.
Accuracy of Delayed Enhancement MRI
Delayed enhancement MRI was recently introduced as a non-invasive technique to visualize the effects of the ablation procedure.5–8 Our study is the first study to examine whether or not the hyper-enhanced tissue identified by DE-MRI correlates with low voltage detected using invasive EA mapping. In our study, all patients demonstrated similar distribution in the extent and location of low voltage tissue on EA maps and scar tissue on DE-MRI. Although some patients’ examples demonstrated scar tissue on EA maps that did not correlate with MRI findings, this was often in regions not targeted during the initial ablation procedure. Many physiological processes other than ablation-induced scar could account for the low voltage LA regions, such as pre-procedural fibrosis from LA structural remodeling.13
Identification of Breaks in Ablation Lesion Sets
Numerous prior reports have detailed the relationship between resumption of PV-LA conduction and AF recurrence following PVAI.4,27 These authors speculate interrupted ablation lesions play a contributing factor to procedural failure. Our study demonstrated that all patients who experienced AF recurrence following the initial and repeat procedures demonstrated significant gaps between lesions, which correlated with recovery of local EGMs or PV electrical conduction. The ability of DE-MRI to non-invasively and accurately evaluate the integrity of scar lesions could provide a valuable feedback tool to assess whether successful lesion placement was achieved. This imaging modality could potentially aid electrophysiologists in identifying regions of healthy myocardium following failed ablation procedures that can be targeted during repeat ablation in order to close all lesion sets. As demonstrated in this manuscript, the ability to locate regions of incomplete or recovered scarring prior to the repeat procedure did help focusing our ablation lesions around the PVA and the left atrial posterior wall and septum, hence increasing the amount of scarring in those areas as demonstrated in Figure 8.
Study Limitations
The limited number of patients who underwent repeat ablation in this series is a limitation of our paper. Due to the similar procedural technique used for all patients, we were unable to distinguish which scar patterns (substrate modification versus anatomical isolation) is most responsible for procedure success. Additional studies that examine this topic would be beneficial in understanding the most appropriate and efficacious ablation strategy. Likewise, additional studies that used a control group that did not use DE-MRI assessment of scar for repeat ablation would be of benefit in comparing clinical outcome. It is also acknowledged that asymptomatic AF is a difficult to detect and that may influence the reported ablation success rates. Studies examining segmental analysis of early electrical activation and propagation with MRI scar in antral regions are also needed for further validation of the correlation between electrical activity and ablation scar detected by MRI. Likewise, additional studies comparing voltage areas and MRI scar specifically within in the antrum regions are needed for a better comparison of the utility of MRI in guiding ablation procedures.
Conclusion
We report using DE-MRI to correlate LA scar patterns after initial and repeat AF ablation. We found that the number of circumferential PVA lesions and total LA scar was important in procedure success. Moreover, we found that obtaining complete circumferential PVA lesions around the PV is a difficult endpoint to achieve long term. However, we found that DE-MRI can display gaps or recovered sites within ablation lesions that can be targeted during repeat procedures. We postulate this imaging modality can help assist in achieving complete and contiguous PVA scarring following repeat ablation procedures.
Acknowledgments
Funding sources: Drs. Kholmovski and Marrouche are partially funded by Surgivision Inc.
Abbreviations
- AF
Atrial fibrillation
- PVA
Pulmonary vein antrum
- DE-MRI
Delayed enhancement MRI
- PV
Pulmonary vein
- LA
Left atrium
- RIPV
Right inferior pulmonary vein
- RSPV
Right superior pulmonary vein
- LIPV
Left inferior pulmonary vein
- LSPV
Left superior pulmonary vein
- EA
Electroanatomical
Footnotes
Conflict of Interest Disclosures: none
References
- 1.Marrouche NF, Guenther J, Segerson NM, Daccarett M, Rittger H, Marschang H, Schibgilla V, Schmidt M, Ritscher G, Noelker G, Brachmann J. Randomized comparison between open irrigation technology and intracardiac-echo-guided energy delivery for pulmonary vein antrum isolation: procedural parameters, outcomes, and the effect on esophageal injury. J Cardiovasc Electrophysiol. 2007;18:583–588. doi: 10.1111/j.1540-8167.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 2.Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, Salvati A, Dicandia C, Calabro MP, Mazzone P, Ficarra E, Di Gioia C, Gulletta S, Nardi S, Santinelli V, Benussi S, Alfieri O. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001;104:2539–2544. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 3.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. New England Journal of Medicine. 1998;339:656–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 4.Verma A, Kilicaslan F, Pisano E, Marrouche NF, Fanelli R, Brachmann J, Geunther J, Potenza D, Martin DO, Cummings J, Burkhardt JD, Saliba W, Schweikert RA, Natale A. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005;112:627–635. doi: 10.1161/CIRCULATIONAHA.104.533190. [DOI] [PubMed] [Google Scholar]
- 5.McGann CJ, Kholmovski EG, Oakes RS, Blauer JJ, Daccarett M, Segerson N, Airey KJ, Akoum N, Fish E, Badger TJ, DiBella EV, Parker D, MacLeod RS, Marrouche NF. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52:1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 6.Badger TJ, Oakes RS, Daccarett M, Burgon NS, Akoum N, Fish EN, Blauer JJ, Rao SN, Adjei-Poku Y, Kholmovski EG, Vijayakumar S, DiBella EV, MacLeod RS, Marrouche NF. Temporal left atrial lesion formation after ablation of atrial fibrillation. Heart Rhythm. 2009;6:161–168. doi: 10.1016/j.hrthm.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 7.Reddy VY, Schmidt EJ, Holmvang G, Fung M. Arrhythmia recurrence after atrial fibrillation ablation: can magnetic resonance imaging identify gaps in atrial ablation lines? J Cardiovasc Electrophysiol. 2008;19:434–437. doi: 10.1111/j.1540-8167.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 8.Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, Josephson ME, Manning WJ. Detection of Pulmonary Vein and Left Atrial Scar after Catheter Ablation with Three-Dimensional Navigator-Gated Delayed Enancement MR Imaging: Initial Experience. Radiology. 2007;243:690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 9.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 10.De Cobelli F, Pieroni M, Esposito A, Chimenti C, Belloni E, Mellone R, Canu T, Perseghin G, Gaudio C, Maseri A, Frustaci A, Del Maschio A. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol. 2006;47:1649–1654. doi: 10.1016/j.jacc.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 11.Rochitte CE, Tassi EM, Shiozaki AA. The emerging role of MRI in the diagnosis and management of cardiomyopathies. Curr Cardiol Rep. 2006;8:44–52. doi: 10.1007/s11886-006-0010-5. [DOI] [PubMed] [Google Scholar]
- 12.Peters DC, Wylie JV, Hauser TH, Nezafat R, Han Y, Woo JJ, Taclas J, Kissinger KV, Goddu B, Josephson ME, Manning WJ. Recurrence of Atrial Fibrillation Correlates with Extent of Post-Procedural Late Gadolinium Enhancement. J Am Coll Cardiol Img. 2009;2:308–16. doi: 10.1016/j.jcmg.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oakes RS, Badger TJ, Kholmovski E, Segerson NM, DiBella EVR, Fish E, Blauer JJE, Akoum N, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of low voltage left atrial tissue using delayed enhancement MRI in patients with atrial fibrillation. Circulation. 2009;119:1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calkins H, Brugada J, Packer D, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Personnel, Policy, Procedures and Follow-UpA report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Scharf C, Sneider M, Case I, Chugh A, Lai SW, Pelosi F, Jr, Knight BP, Kazerooni E, Morady F, Oral H. Anatomy of the pulmonary veins in patients with atrial fibrillation and effects of segmental ostial ablation analyzed by computed tomography. J Cardiovasc Electrophysiol. 2003;14:150–155. doi: 10.1046/j.1540-8167.2003.02444.x. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Quintana D, Cabrera JA, Climent V, Farre J, Mendonca MC, Ho SY. Anatomic relations between the esophagus and left atrium and relevance for ablation of atrial fibrillation. Circulation. 2005;112:400–1405. doi: 10.1161/CIRCULATIONAHA.105.551291. [DOI] [PubMed] [Google Scholar]
- 17.Klemm HU, Steven D, Johnsen C, Ventura R, Rostock T, Lutomsky B, Risius T, Meinertz T, Willems S. Catheter motion during atrial ablation due to the beating heart and respiration: impact on accuracy and spatial referencing in three-dimensional mapping. Heart Rhythm. 2007;4:587–592. doi: 10.1016/j.hrthm.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Ernst S, Ouyang F, Lober F, Antz M, Kuck KH. Catheter-induced linear lesions in the left atrium in patients with atrial fibrillation: an electroanatomic study. J Am Coll Cardiol. 2003;42:1271–1282. doi: 10.1016/s0735-1097(03)00940-9. [DOI] [PubMed] [Google Scholar]
- 19.Allessie MA, Boyden PA, Camm AJ, Kleber AG, Lab MJ, Legato MJ, Rosen MR, Schwartz PJ, Spooner PM, Van Wagoner DR, Waldo AL. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103:769–777. doi: 10.1161/01.cir.103.5.769. [DOI] [PubMed] [Google Scholar]
- 20.Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach D, Pandit S, Vikstrom KL, Ploutz-Snyder R, Talkachou A, Atienza F, Guiraudon G, Jalife J, Berenfeld O. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. 2006;113:626–633. doi: 10.1161/CIRCULATIONAHA.105.575340. [DOI] [PubMed] [Google Scholar]
- 21.Todd DM, Skanes AC, Guiraudon G, Guiraudon C, Krahn AD, Yee R, Klein GJ. Role of the posterior left atrium and pulmonary veins in human lone atrial fibrillation: electrophysiological and pathological data from patients undergoing atrial fibrillation surgery. Circulation. 2003;108:3108–3114. doi: 10.1161/01.CIR.0000104567.72914.BF. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol. 1997;273:H805–816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- 23.Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13 (Suppl 1):37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 24.Katritsis D, Ioannidis JP, Anagnostopoulos CE, Sarris GE, Giazitzoglou E, Korovesis S, Camm AJ. Identification and catheter ablation of extracardiac and intracardiac components of ligament of Marshall tissue for treatment of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2001;12:750–758. doi: 10.1046/j.1540-8167.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 25.Sanders P, Hocini M, Jais P, Sacher F, Hsu LF, Takahashi Y, Rotter M, Rostock T, Nalliah CJ, Clementy J, Haissaguerre M. Complete isolation of the pulmonary veins and posterior left atrium in chronic atrial fibrillation. Long-term clinical outcome. Eur Heart J. 2007;28:1862–1871. doi: 10.1093/eurheartj/ehl548. [DOI] [PubMed] [Google Scholar]
- 26.Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F, Jr, Bates ER, Lehmann MH, Vicedomini G, Augello G, Agricola E, Sala S, Santinelli V, Morady F. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, Schaumann A, Chun J, Falk P, Hennig D, Liu X, Bansch D, Kuck KH. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005;111:127–135. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]