Abstract
Background
Systemic ethanol administration increases neuroactive steroid levels that increase ethanol sensitivity. Acetaldehyde is a biologically active compound that may contribute to behavioral and rewarding effects of ethanol. We investigated the role of acetaldehyde in ethanol–induced elevations of 3α-hydroxy-5α-pregnan-20-one (3α,5α-THP) levels in cerebral cortex.
Methods
Male Sprague-Dawley rats were administered ethanol and plasma acetaldehyde concentrations were measured by gas chromatography to determine relevant concentrations. Rats were then administered acetaldehyde directly, acetaldehyde plus cyanamide to block its degradation, or ethanol in the presence of inhibitors of ethanol metabolism, to determine effects on 3α,5α-THP levels in cerebral cortex.
Results
Ethanol administration (2 g/kg) to rats results in a peak acetaldehyde concentration of 6-7 μM at 10 minutes that remains stable for the duration of the time points tested. Direct administration of acetaldehyde eliciting this plasma concentration does not increase cerebral cortical 3α,5α-THP levels and inhibition of ethanol-metabolizing enzymes to modify acetaldehyde formation does not alter ethanol–induced 3α,5α-THP levels. However, higher doses of acetaldehyde (75 and 100 mg/kg), in the presence of cyanamide to prevent its metabolism, are capable of increasing cortical 3α,5α-THP levels.
Conclusions
Physiological concentrations of acetaldehyde are not responsible for ethanol-induced increases in 3α,5α-THP, but a synergistic role for acetaldehyde with ethanol may contribute to increases in 3α,5α-THP levels and ethanol sensitivity.
Keywords: Acetaldehyde, Neuroactive steroids, Ethanol, GABA-A receptors
INTRODUCTION
Neuroactive steroids produce their effects on membrane receptors that regulate central nervous system activity rather than nuclear receptors that regulate gene expression. The 3α,5α-reduced pregnan steroids, including 3α-hydroxy-5α-pregnan-20-one (3α,5α-THP) and 3α,21-dihydroxy-5α-pregnan-20-one (3α,5α-THDOC), are endogenous modulators of GABA-A receptors and produce rapid changes in central nervous system activity (for review Belelli and Lambert, 2005). The GABA-A receptor system is the primary inhibitory receptor system in the brain and is responsible for many behavioral effects of ethanol. Systemic ethanol administration increases plasma and brain levels of neuroactive steroids (Morrow et al., 1999; VanDoren et al., 2000) that can act with nanomolar potency on GABA-A receptors (Morrow et al., 1987; Puia et al., 1990).
Neuroactive steroids can be synthesized de novo in the brain or produced peripherally in the adrenals and gonads. While ethanol-induced increases in neuroactive steroids can originate from both adrenal glands and brain, the adrenal glands are a major source of neuroactive steroids and their precursors. Indeed, adrenalectomy reduces neuroactive steroid levels in plasma and brain and prevents ethanol-induced elevations. However, administration of 5α-dihydroprogesterone, the immediate precursor of the potent GABAergic neurosteroid 3α,5α-THP, to adrenalectomized animals restores ethanol-induced elevations of cortical 3α,5α-THP (Khisti et al., 2003) suggesting an important role for both adrenal and brain steroidogenesis. In addition, studies have demonstrated brain steroidogenesis in adrenalectomized animals given time for recovery (Follesa et al., 2006) as well as in cell culture (Hu et al., 1987). Indeed, all of the steroidogenic biosynthetic enzymes are present in brain and have been shown to colocalize in specific cell types (King et al., 2002).
Ethanol increases rat plasma and brain concentrations of the neuroactive steroids 3α,5α-THP and 3α,5α-THDOC, which are potent GABAergic modulators that can elicit many of the same effects as ethanol (Barbaccia et al., 1999; Morrow et al., 1999). The maximal effect of ethanol on neuroactive steroid levels is observed at 2.5 g/kg ethanol. Ethanol-induced increases in GABAergic neuroactive steroids are requisite for anxiolytic (Hirani et al., 2005) and anticonvulsant (VanDoren et al., 2000) effects of ethanol and contribute to sedative-hypnotic actions (Khisti et al., 2003) and spatial learning impairment (Matthews et al., 2002). In addition, electrophysiological effects of ethanol in medial septal and hippocampal neurons are dependent upon ethanol-induced increases in the GABAergic neuroactive steroids (Tokunaga et al., 2003; VanDoren et al., 2000). These steroids substitute for ethanol in discrimination studies in rodents and monkeys (Grant et al., 1996; Hodge et al., 2001; Shannon et al., 2005) and exogenous administration can alter ethanol drinking patterns (Ford et al., 2007; Janak et al., 1998; Morrow et al., 2001). All these studies have suggested that neuroactive steroids mediate several behavioral effects of ethanol and contribute to ethanol sensitivity.
The primary metabolite of ethanol, acetaldehyde, can also produce behavioral effects that are similar to ethanol (for review Quertemont et al., 2005). For example, systemic acetaldehyde administration causes a depression in locomotor activity (Myers et al., 1987), impairment of spatial memory (Abe et al., 1999; Quertemont et al., 2004), ethanol-like discrimination (Redila et al., 2002) and hypnotic effects (Quertemont et al., 2004). These observations raise the possibility that acetaldehyde may contribute to GABAergic effects of ethanol mediated by neuroactive steroids. While many behavioral effects have been studied, few studies have examined acetaldehyde’s anxiolytic or anticonvulsant properties that are dependent upon elevations of neurosteroids. Moreover, many of the experiments involving acetaldehyde have relied upon direct administration of acetaldehyde into the brain or systemic administration of high concentrations unlikely to be found physiologically. Thus, the present study was designed to determine if physiologically relevant concentrations of acetaldehyde contribute to the ethanol-induced increases in neuroactive steroid levels.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats weighing between 250-350 g were used for all experiments (Harlan, Indianapolis, IN). The animals were group housed (3 per cage) and maintained in standard light and dark (lights on, 7:00 A.M. to 7:00 P.M.) conditions with food and water available ad libitum. Rats were acclimated to the handling procedure for one week before the test day. All experiments were conducted in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of North Carolina Chapel Hill.
Drug administration
For steroid measurements, animals were sacrificed by decapitation 45 minutes after i.p. administration of saline, ethanol (2 g/kg, 20% v/v), or acetaldehyde (20, 50, 75, 100 mg/kg). 4-methylpyrazole (200 mg/kg i.p.) was administered 60 minutes prior to ethanol to inhibit alcohol dehydrogenase. Animals receiving catalase inhibitor, similar to prior studies (Manrique et al., 2005), were administered sodium azide (10 mg/kg i.p.) 30 minutes prior to an acute ethanol injection (2 g/kg, 20% v/v). To inhibit aldehyde dehydrogenase, animals were administered cyanamide (50 mg/kg i.p.) 60 minutes prior to an acute ethanol injection (2 g/kg, 20% v/v) (Jamal et al., 2005). Saline pretreated animals were used for controls in both experiments. All experiments utilized a minimum of six animals per group.
Plasma ethanol and acetaldehyde measurements by gas chromatography
A 6 μl aliquot of plasma was analyzed for ethanol levels using a SRI 8610c gas chromatograph (Torrance, CA). Acetaldehyde levels were determined using 100 μl of plasma and in both instances similar volumes were used to establish a standard curve. The ethanol standard curve ranged from 0 to 400 mg/dl and the acetaldehyde standard curve ranged from 0 to 250 μM. Samples were distributed to tubes containing 375 μl of water and 0.5 g NaCl. Samples were heated in a water bath at 60 °C for 10 minutes and a 1.5 ml sample of headspace gas was removed and injected directly into the GC. Samples were run at 140 °C through a Hayesep D column and detected with a flame ionization detector. Hydrogen gas, carrier gas, and internal air generator flow rates were 13.3, 25, and 250 ml/min respectively. Areas under the curve were analyzed with SRI PeakSimple software and converted to mg/dl for ethanol and μM for acetaldehyde based on the standard curves.
Radioimmunoassay (RIA) of neuroactive steroid 3α,5α-THP
RIAs were conducted as previously described (Janis et al., 1998). Briefly, brain samples were weighed and suspended in 2.5 ml of 0.3N NaOH, homogenized with a sonic dismembrator, and extracted three times with 3 ml aliquots of 10% ethyl acetate in heptane (vol/vol). Extraction recovery was monitored by the addition of 2000 cpm of [3H] 3α,5α-THP. The brain extracts were purified using solid phase silica columns (Burdick and Jackson, Muskegon, MI) and subsequently dried. Samples were reconstituted and assayed in duplicate by the addition of [3H] 3α,5α-THP and anti-3α,5α-THP antibody. Total binding was determined in the absence of unlabeled 3α,5α-THP and nonspecific binding was determined in the absence of antibody. The antibody binding reaction was allowed to equilibrate for 2 hours and cold dextran coated charcoal was used to separate bound from unbound steroid. Bound radioactivity was determined by liquid scintillation spectroscopy. Steroid levels in the samples were extrapolated from a concurrently run standard curve and corrected for their respective extraction efficiencies.
Data analysis
Results are expressed as mean ± S.E.M. Steroid levels are expressed as ng/g for brain tissue. Plasma ethanol levels are expressed as mg/dl and plasma acetaldehyde concentrations are expressed in μM units. Significance was determined by one way ANOVA followed by post hoc Newman Keuls test or the Student’s t test as appropriate. Analyses were performed using the software GraphPad Prism version 4.
RESULTS
To determine the role of acetaldehyde in ethanol-induced increases in cerebral cortical 3α,5α-THP levels, we first sought to ascertain the concentrations of acetaldehyde in plasma following ethanol administration to rats. Rats were administered ethanol (2 g/kg) since this dose produces near maximal effects on GABAergic neuroactive steroids and produces prominent behavioral effects of ethanol. Plasma ethanol and acetaldehyde levels were measured at various time points following ethanol administration. Plasma ethanol levels peaked around 228 mg/dl (~50 mM) as quickly as 10 minutes post ethanol administration and steadily declined over time (Fig. 1A). Plasma acetaldehyde levels remained relatively constant at approximately 6-7 μM across the same time frame, while ethanol was continuously metabolized (Fig. 1B). Furthermore, plasma and cerebral cortical 3α,5α-THP levels were elevated at 45 minutes, corresponding to peak elevations in ethanol-induced acetaldehyde levels (Fig. 1C).
Fig. 1. Time-course of plasma ethanol and acetaldehyde concentrations following ethanol administration.
Animals were administered a 2 g/kg dose of ethanol and blood was collected at varying time points. (A) Plasma ethanol and (B) acetaldehyde levels were measured via gas chromatography. Ethanol and acetaldehyde are rapidly increased and then acetaldehyde levels stabilize as ethanol is metabolized. (C) 3α,5α-THP levels were increased in both the plasma and the cerebral cortex 45 minutes after ethanol administration. Plasma 3α,5α-THP levels are measured as ng/ml and brain as ng/g. *p<0.01 compared to controls, n=4-8 in duplicate.
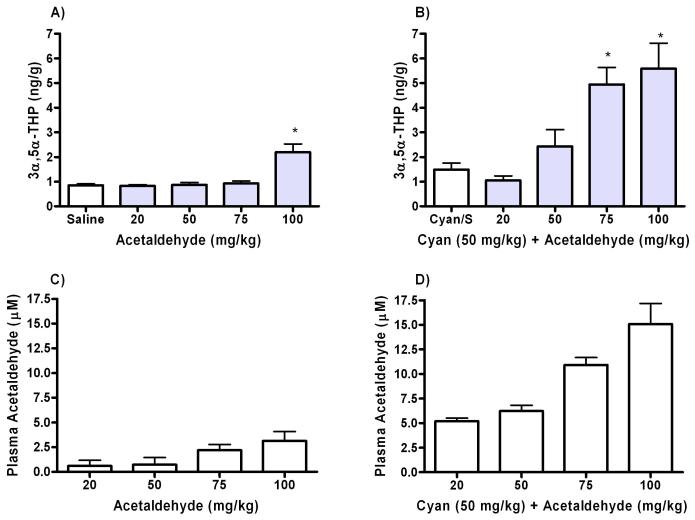
To determine if the concentration of acetaldehyde produced by ethanol (2 g/kg) is capable of producing an increase in 3α,5α-THP levels, we measured 3α,5α-THP in the cerebral cortices of rats administered various doses of acetaldehyde. Various doses of acetaldehyde were used because it was unknown what doses would produce relevant concentrations. Neuroactive steroid levels were measured at 45 minutes, as this represents the timeframe of the peak neurosteroid response to 2 g/kg ethanol. Figure 2 shows 3α,5α-THP and acetaldehyde levels 45 minutes post acetaldehyde administration from naïve animals (Fig. 2A,C) and animals pretreated with the aldehyde dehydrogenase (ALDH) inhibitor cyanamide (Fig. 2B,D). Cyanamide administration was necessary to delay acetaldehyde metabolism in order to achieve concentrations similar to those observed following ethanol administration. In this experiment, animals were pretreated with cyanamide 60 minutes prior to acetaldehyde administration and compared to control animals pretreated with the inhibitor prior to saline. Following cyanamide pretreatment, acetaldehyde administration increased 3α,5α-THP levels at both the 75 mg/kg and the 100 mg/kg doses of acetaldehyde. However, these treatments produced plasma acetaldehyde levels that were markedly higher than acetaldehyde concentrations found after ethanol administration alone (Fig. 2D). At 50mg/kg, acetaldehyde administration produced blood acetaldehyde concentrations similar to ethanol, but there was no effect on cerebral cortical 3α,5α-THP levels.
Fig. 2. Acetaldehyde administration to levels that mimic ethanol metabolism do not increase 3α,5α-THP levels in cortex.
(A) Acetaldehyde was administered in varying doses and brains were collected after 45 minutes to measure cortical levels of 3α,5α-THP. (B) The same concentrations of acetaldehyde were administered to rats pretreated with the ALDH inhibitor cyanamide (50 mg/kg) 60 minutes prior to acetaldehyde administration and cortical 3α,5α-THP was measured. Plasma acetaldehyde concentrations were measured in (C) animals receiving acetaldehyde alone and in (D) animals receiving the inhibitor prior to acetaldehyde administration. 3α,5α-THP levels were measured by RIA and acetaldehyde concentrations via gas chromatography. *p<0.001 compared to controls, n=6 for control and n=8 for acetaldehyde groups in duplicate.
While direct acetaldehyde administration is important to assess acetaldehyde’s effects, experiments examining its effects after ethanol administration are necessary to determine how acetaldehyde contributes to the ethanol response. Therefore, one strategy to study the role of acetaldehyde in ethanol-induced increases in neuroactive steroids is to alter its metabolism after ethanol administration. If acetaldehyde is involved, then systemic manipulation of the enzymes involved in its metabolism should alter affects on 3α,5α-THP levels following ethanol administration. Figure 3 shows cerebral cortical 3α,5α-THP levels and the corresponding plasma ethanol and acetaldehyde concentrations for animals receiving ethanol with and without prior inhibition of ALDH. 3α,5α-THP levels were increased following ethanol administration compared to their respective controls regardless of ALDH inhibition (Fig. 3A). Pretreatment with the ALDH inhibitor prior to ethanol administration did not alter 3α,5α-THP levels compared to ethanol alone. In addition, the cyanamide pretreatment did not significantly alter steroid levels compared to saline pretreated controls. Acetaldehyde levels were measured to confirm that the inhibitor was effective. Acetaldehyde concentrations were increased 386.8 ± 35.1% following ALDH inhibition compared to ethanol alone (Fig. 3C). Plasma ethanol levels were not significantly altered by ALDH inhibition (Fig. 3B).
Fig. 3. Effect of pretreatment with the aldehyde dehydrogenase inhibitor cyanamide on ethanol-induced 3α,5α-THP elevation.
Animals were administered cyanamide (50 mg/kg, i.p.) 60 minutes prior to an acute injection of ethanol or saline and steroids were measured 45 minutes following the injections. (A) 3α,5α-THP levels were increased in the rat cerebral cortex after acute ethanol (2 g/kg, i.p.) administration and were not affected by treatment with an aldehyde dehydrogenase inhibitor. (B) Plasma alcohol levels were unaffected by aldehyde dehydrogenase inhibition while (C) plasma acetaldehyde levels were increased. *P < 0.001 compared to controls, n=6 in duplicate.
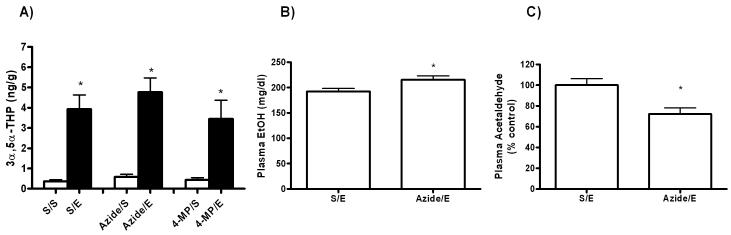
Next, we inhibited alcohol dehydrogenase and the catalase enzyme, which convert ethanol to acetaldehyde in the liver and brain, respectively. Inhibition of alcohol dehydrogenase with the competitive inhibitor 4-methylpyrazole (200 mg/kg) had no effect on 3α,5α-THP levels compared to ethanol alone (Figure 4A). However, there was no detectable change in plasma acetaldehyde concentrations, even though ethanol levels were increased from 195 mg/dl to 246 mg/dl and still elevated after 6 hours (data not shown). Furthermore, sodium azide (10mg/kg) had no effect on ethanol-induced increases in. 3α,5α-THP levels (Fig. 4A). In addition, sodium azide pretreatment did not significantly affect steroid levels compared to saline pretreated controls. Plasma ethanol concentrations were slightly increased and plasma acetaldehyde levels were decreased by 27.8 ± 8.8% following catalase inhibition when compared to ethanol alone (Fig. 4B and 4C).
Fig. 4. Effects of pretreatment with 4-methylpyrazole and sodium azide on ethanol-induced 3α,5α-THP elevation.
Animals were administered 4-methylpyrazole (200mg/kg, i.p.) or sodium azide (10 mg/kg, i.p.) prior to an acute injection of ethanol or saline and steroids were measured 45 minutes following injections. (A) 3α,5α-THP levels were increased in the rat cerebral cortex after acute ethanol (2 g/kg, i.p.) administration and were not affected by treatment with either inhibitor. (B) Plasma alcohol levels were increased in animals receiving the catalase inhibitor and (C) plasma acetaldehyde concentrations were decreased when catalase was inhibited. *P < 0.05 compared to controls, n=6 in duplicate.
DISCUSSION
The current study was performed to address a previously unexplored issue of whether acetaldehyde is involved in ethanol-induced increases in neuroactive steroids. Systemic administration of 2 g/kg ethanol elicits an increase in neuroactive steroids that is not altered by manipulation of ethanol metabolism. While high concentrations of acetaldehyde can stimulate increases in neuroactive steroids, the administration of acetaldehyde at doses eliciting concentrations similar to those produced after ethanol administration does not increase neuroactive steroid levels. Taken together, these results indicate that ethanol is primarily responsible for ethanol-induced increases in neuroactive steroids.
The most important factor in determining whether acetaldehyde contributes to ethanol-induced increases in neuroactive steroids is to establish what concentration of acetaldehyde exists in the blood following ethanol administration. Acetaldehyde levels remained relatively steady across time and were low due to the high level of ALDH activity. Since acetaldehyde is toxic, the body is very efficient at breaking it down and its clearance is much larger than that of ethanol (Fujimiya et al., 2002). Next, the effect of acetaldehyde administration at doses that produce similar concentrations was evaluated for their affect on neuroactive steroid levels. It is important to keep in mind that the acetaldehyde levels decline more rapidly following acetaldehyde vs ethanol administration since it is not continuously formed and metabolized. Pretreatment with cyanamide stabilized acetaldehyde levels across time to allow evaluation of acetaldehyde effects on 3α,5α-THP levels.
In the present study, plasma acetaldehyde concentrations were critical in determining the role of acetaldehyde in ethanol-induced increases in neuroactive steroids. We used a dose of ethanol (2g/kg) that produces peak increases in 3α,5α-THP levels. When plasma acetaldehyde concentrations following systemic acetaldehyde administration were lower than or comparable to levels observed following ethanol administration, no increase in 3α,5α-THP levels was observed. However, when plasma acetaldehyde concentrations were greater than levels following ethanol administration an increase in 3α,5α-THP levels was detected.
Previous studies with acetaldehyde administration involve various routes of administration as well as a variety of strains and species making it difficult to reliably compare results across studies. However, in studies with systemic acetaldehyde administration, doses of at least 100 mg/kg were required to observe locomotor depression (Tambour et al., 2006) and sedative and hypnotic effects (Quertemont et al., 2004). This is the same dose required to elevate 3α,5α-THP levels in our study.
Indeed, we found that 100 mg/kg was the lowest acetaldehyde dose, without ALDH inhibition, that increased 3α,5α-THP levels, despite producing low acetaldehyde levels (approx 2.5 μM) compared to ethanol (7 μM). Since acetaldehyde is rapidly metabolized, its concentration would be expected to be low after 45 minutes without ALDH inhibition. Furthermore, when acetaldehyde levels were increased through ALDH inhibition, lower doses of acetaldehyde were capable of increasing 3α,5α-THP levels and produced plasma acetaldehyde concentrations greater than those seen after ethanol administration. This strongly suggests the importance of acetaldehyde concentration in producing or contributing to behavioral effects of ethanol.
When ethanol was administered to the rats, inhibition of ALDH increased acetaldehyde levels, but did not have any increased effect on 3α,5α-THP levels in the cortex when compared to animals treated with ethanol alone. However, the present results cannot rule out the possibility that acetaldehyde may contribute to the elevation of neuroactive steroids via synergistic actions with sub-maximal doses of ethanol. It is possible that very high concentrations of acetaldehyde alone are required in order to elicit increases in neuroactive steroids whereas lower doses, in conjunction with ethanol, may contribute to increased 3α,5α-THP levels. Indeed, acetaldehyde has been shown to activate the hypothalamic-pituitary-adrenal (HPA) axis (Kinoshita et al., 2001), which is involved in neuroactive steroid synthesis. Furthermore, acetaldehyde is metabolized to acetate, which has also been shown to have CNS effects, although these appear to involve locomotor actions mediated by adenosine receptors (Carmichael et al., 1991).
The primary enzyme responsible for ethanol metabolism is alcohol dehydrogenase in the liver. In addition to alcohol dehydrogenase, CYP2E1 and catalase are also involved in ethanol metabolism. CYP2E1 is an inducible enzyme (Lieber and DeCarli, 1970) that plays a more important role in dependent individuals than after acute ethanol exposure. The catalase enzyme provides another pathway through which ethanol can be metabolized (Aragon et al., 1992) and plays a significant role in ethanol metabolism in the brain. Inhibition of catalase activity would be expected to have a marked effect on brain acetaldehyde concentrations, however, catalase inhibition did not affect cerebral cortical 3α,5α-THP levels in these experiments. Inhibition of alcohol dehydrogenase also failed to elicit any changes in 3α,5α-THP levels. However, inhibition of alcohol dehydrogenase activity is not as favorable for determining acetaldehyde’s role in ethanol’s effects because brain alcohol dehydrogenase activity is very low (Beisswenger et al., 1985).
The lack of effect of 4-methylpyrazole on acetaldehyde levels raises some concern. However, others have reported that inhibition of alcohol dehydrogenase fails to alter plasma acetaldehyde levels in the absence of an ALDH inhibitor (Quertemont and Didone, 2006). Since ethanol levels were increased up to 6 hrs following 4-methylpyrazole administration, we presume that alcohol dehydrogenase was inhibited. Changes in acetaldehyde levels may approach the limit of detection following 4-methylpyrazole administration. However, we are able to detect dose-dependent acetaldehyde levels following acetaldehyde administration as well as the expected increases when ALDH is inhibited. Hence, it is reasonable to conclude that ethanol-induced increases in 3α,5α-THP levels are independent of acetaldehyde. The lack of effect of 4-methylpyrazole on ethanol-induced increases in 3α,5α-THP levels also suggests that NADH/NAD+ redox changes secondary to ethanol metabolism are not involved in this effect of ethanol. To date, there are not consistent results for alterations in alcohol dehydrogenase activity affecting ethanol consumption patterns, suggesting that ALDH activity and acetaldehyde levels are important factors in regulating drinking behaviors.
Acetaldehyde has been implicated in some of the behavioral effects of ethanol although the precise role and mechanism of action remain unclear. Importantly, direct administration of acetaldehyde results in some of the same behavioral effects as ethanol providing a basis for the idea that acetaldehyde contributes to behavioral effects following ethanol administration. However, many experiments used higher concentrations of acetaldehyde then what would be expected from endogenous ethanol metabolism. Therefore, experiments conducted here focused on the effects of physiologically relevant acetaldehyde concentrations on 3α,5α-THP levels. Interestingly, when plasma acetaldehyde concentrations were comparable to levels found after ethanol administration there was no increase in cerebral cortical 3α,5α-THP levels suggesting that acetaldehyde is not responsible for ethanol-induced increases in neuroactive steroids.
While these studies were performed in a rat model, the results may be applicable to humans. Adolescent males and females seen in the emergency room for alcohol intoxication had substantial increases in plasma levels of the neuroactive steroid 3α,5α-THP (Torres and Ortega, 2003; Torres and Ortega, 2004). Furthermore, various subjective effects of ethanol are diminished by prior administration of the neurosteroid biosynthesis inhibitor finasteride (Pierucci-Lagha et al., 2005). In contrast, laboratory administration of low or moderate ethanol doses had no effect on plasma 3α,5α-THP levels (Holdstock et al., 2006) or decreased 3α,5α-THP levels (Nyberg et al., 2005; Pierucci-Lagha et al., 2006). Though an explanation for these conflicting results is unresolved, the potential role of neurosteroids in human alcohol sensitivity has not been ruled out.
The present results support the theory that acetaldehyde modulates some of ethanol’s effects rather than mediating them. However, the finding that high acetaldehyde concentrations can increase neuroactive steroid levels leaves open the possibility that acetaldehyde could contribute to neurosteroid elevations after large quantities of ethanol consumption. Indeed, the extent of acetaldehyde’s effects would vary between individuals depending upon the respective activities of their alcohol metabolizing enzymes and the amount of ethanol consumed. For example, human studies have noted that Native American populations, which have high rates of alcohol abuse and dependence, have polymorphisms in alcohol metabolizing enzymes that may account for drinking behaviors (Wall et al., 2003). Other studies have suggested that high peripheral acetaldehyde concentrations are aversive and individuals with mutations in their ALDH2 gene metabolize acetaldehyde less rapidly and subsequently have a lower risk of developing alcoholism (for review Quertemont, 2004). In addition, animals with high ALDH activity have less acetaldehyde in their blood and tend to drink more ethanol (Quintanilla et al., 2005) while animals administered an adenoviral vector containing an ALDH2 antisense gene have reduced ALDH2 activity and reduced ethanol consumption (Ocaranza et al., 2008). Furthermore, ALDH2 knockout mice have increased blood acetaldehyde concentrations and drink less (Isse et al., 2005; Isse et al., 2002).
While speculative, it is intriguing to suggest a relationship between acetaldehyde and neuroactive steroids in risk for alcoholism. The production of neurosteroids is associated with an increased sensitivity to ethanol in rodents (for review Morrow et al., 2006) and possibly humans (Pierucci-Lagha et al., 2005). In the present study, high concentrations of acetaldehyde, which are associated with reduced drinking and risk for alcoholism, also increased neuroactive steroids. This effect may contribute to increased ethanol sensitivity and the decreased the risk for alcoholism. Therefore, while physiological concentrations of acetaldehyde do not appear to be responsible for ethanol-induced increases in neuroactive steroids in rats, the potential role of acetaldehyde cannot be excluded from contributing to ethanol actions. Further studies will be necessary to clarify the importance of acetaldehyde in the effects of ethanol.
Acknowledgement
This work was supported by NIH grants R37-AA10564 (ALM) and T32-AA007573.
REFERENCES
- Abe K, Yamaguchi S, Sugiura M, Saito H. The ethanol metabolite acetaldehyde inhibits the induction of long-term potentiation in the rat dentate gyrus in vivo. British Journal of Pharmacology. 1999;127(8):1805–1810. doi: 10.1038/sj.bjp.0702738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon CM, Rogan F, Amit Z. Ethanol metabolism in rat brain homogenates by a catalase-H2O2 system. Biochem.Pharmacol. 1992;44:93–98. doi: 10.1016/0006-2952(92)90042-h. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases “GABAergic” neurosteroids in alcohol-preferring rats. Eur J Pharmacol. 1999;384(2-3):R1–R2. doi: 10.1016/s0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- Beisswenger T, Holmquist B, Vallee B. chi-ADH is the sole alcohol dehydrogenase isozyme in mammalian brains: implications and inferences. Proc Natl Acad Sci U S A. 1985;24:8369–8373. doi: 10.1073/pnas.82.24.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6(7):565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Carmichael F, Israel Y, Crawford M, Minhas K, Saldivia V, Sandrin S, Campisi P, Orrgeo H. Central nervous system effects of acetate: contribution to the central effects of ethanol. Journal of Pharmacology & Experimental Therapeutics. 1991;259:403–408. [PubMed] [Google Scholar]
- Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, Biggio G. Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology. 2006;186:267–280. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behavioral Brain Research. 2007;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimiya T, Yamaoka K, Ohbora Y, Aki T, Shinagawa H. Michaelis-Menten elimination kinetics of acetaldehyde during ethanol oxidation. Alcoholism: Clinical and Experimental Research. 2002;26:49S–54S. doi: 10.1097/01.ALC.0000026976.05505.E2. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Bowen CA, Mirkis S, Purdy RH. Ethanol-like discriminative stimulus effects of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in female Macaca fascicularis monkeys. Psychopharmacology. 1996;124:340–346. doi: 10.1007/BF02247439. [DOI] [PubMed] [Google Scholar]
- Hirani K, Sharma AN, Jain NS, Ugale RR, Chopde CT. Evaluation of GABAergic neuroactive steroid 3alpha-hydroxy-5alpha-pregnane-20-one as a neurobiological substrate for the anti-anxiety effect of ethanol in rats. Psychopharmacology. 2005;180(2):267–278. doi: 10.1007/s00213-005-2169-7. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Nannini MA, Olive MF, Kelley SP, Mehmert KK. Allopregnanolone and pentobarbital infused into the nucleus accumbens substitute for the discriminative stimulus effects of ethanol. Alcohol Clin Exp Res. 2001;25(10):1441–7. doi: 10.1097/00000374-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Holdstock L, Penland SN, Morrow AL, De Wit H. Moderate doses of ethanol fail to increase plasma levels of neurosteroid 3α-hydroxy-5α-pregnan-20-one-like immunoreactivity in healthy men and women. Psychopharmacology. 2006;186:442–450. doi: 10.1007/s00213-005-0187-0. [DOI] [PubMed] [Google Scholar]
- Hu ZY, Bourreau E, Jung-Testas I, Robel P, Baulieu E-E. Neurosteroids: Oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:8215–8219. doi: 10.1073/pnas.84.23.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isse T, Matsuno K, Oyama T, Kitagawa K, Kawamoto T. Aldehyde Dehydrogenase 2 Gene Targeting Mouse Lacking Enzyme Activity Shows High Acetaldehyde Level in Blood, Brain, and Liver after Ethanol Gavages. Alcoholism: Clinical and Experimental Research. 2005;29:1959–1964. doi: 10.1097/01.alc.0000187161.07820.21. [DOI] [PubMed] [Google Scholar]
- Isse T, Oyama T, Kitagawa K, Matsuno K, Matsumoto A, Yoshida A, Nakayama K, Nakayama K, Kawamoto T. Diminished alcohol preference in transgenic mice lacking aldehyde dehydrogenase activity. Pharmacogenetics. 2002;12:621–626. doi: 10.1097/00008571-200211000-00006. [DOI] [PubMed] [Google Scholar]
- Jamal M, Ameno K, Wang W, Kumihashi M, Ameno S, Ikuo U, Shinji A, Ijiri I. Inhibition of acetaldehyde metabolism decreases acetylcholine release in medial frontal cortex of freely moving rats. Brain Research. 2005;1039:90–96. doi: 10.1016/j.brainres.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Janak PH, Redfern JEM, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–1112. [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in male and female rats. Alcoholism, Clinical and Experimental Research. 1998;22:2055–2061. [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, O’Buckley TK, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates ethanol-induced loss of righting reflex in rats. Brain Res. 2003;980:255–265. doi: 10.1016/s0006-8993(03)02978-0. [DOI] [PubMed] [Google Scholar]
- King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J Neurosci. 2002;22(24):10613–20. doi: 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Jessop DS, Finn DP, Coventry TL, Roberts DJ, Ameno K, Jiri I, Harbuz MS. Acetaldehyde, a metabolite of ethanol, activates the hypothalamic-pituitary-adrenal axis in the rat. alcohol and alcoholism. 2001;36:59–64. doi: 10.1093/alcalc/36.1.59. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. Hepatic Microsomal Ethanol-oxidizing System. Journal of Biological Chemistry. 1970;245:2505–2512. [PubMed] [Google Scholar]
- Manrique H, Miquel M, Aragon CM. Brain catalase mediates potentiation of social recognition memory produced by ethanol in mice. Drug and Alcohol Dependence. 2005;79:343–350. doi: 10.1016/j.drugalcdep.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Morrow AL, Tokunaga S, McDaniel JR. Acute ethanol administration and acute allopregnanolone administration impair spatial memory in the Morris water task. Alcohol Clin Exp Res. 2002;26(11):1747–51. doi: 10.1097/01.ALC.0000037219.79257.17. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: A new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23(12):1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Myers WD, Gibson S, Ng KT, Singer G. Sex differences in acetaldehyde on body temperature and open-field performance in the rat. Drug Alcohol Depend. 1987;19:1–6. doi: 10.1016/0376-8716(87)90081-0. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Andersson A, Zingmark E, Wahlstrom G, Backstrom T, Sundstrom-Poromaa I. The effect of a low dose of alcohol on allopregnanolone serum concentrations across the menstrual cycle in women with severe premenstrual syndrome and controls. Psychoneuroendocrinology. 2005;30:892–901. doi: 10.1016/j.psyneuen.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Ocaranza P, Quintanilla M, Tampier L, Karahanian E, Sapag A, Israel Y. Gene Therapy Reduces Ethanol Intake in an Animal Model of Alcohol Dependence. Alcoholism: Clinical and Experimental Research. 2008;32:52–57. doi: 10.1111/j.1530-0277.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, Marx CE, Shampine LJ, Kranzler HR. Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of alcohol. Psychopharmacology. 2006;186:451–461. doi: 10.1007/s00213-005-0231-0. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30(6):1193–203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Puia G, Santi M, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Quertemont E. Genetic polymorphism in ethanol metabolism: acetaldehyde contribution to alcohol abuse and alcoholism. Molecular Psychiatry. 2004;9:570–581. doi: 10.1038/sj.mp.4001497. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Didone V. Role of acetaldehyde in mediating the pharmacological and behavioral effects of alcohol. Alcohol Res Health. 2006;29:258–265. [PMC free article] [PubMed] [Google Scholar]
- Quertemont E, Tambour S, Bernaerts P, Zimatkin SM, Tirelli E. Behavioral characterization of acetaldehyde in C57BL/6J mice: locomotor, hypnotic, anxiolytic and amnesic effects. Psychopharmacology. 2004;177:84–92. doi: 10.1007/s00213-004-1911-x. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Tambour S, Tirelli E. The role of acetaldehyde in the neurobehavioral effects of ethanol: A comprehensive review of animal studies. Progress in Neurobiology. 2005;75:247–274. doi: 10.1016/j.pneurobio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Quintanilla M, Tampier L, Sapag A, Israel Y. Polymorphisms in the mitochondrial aldehyde dehydrogenase gene (Aldh2) determine peak blood acetaldehyde levels and voluntary ethanol consumption in rats. Pharmacogenetics and Genomics. 2005;15:427–431. doi: 10.1097/01213011-200506000-00009. [DOI] [PubMed] [Google Scholar]
- Redila VA, Aliatas E, Smith BR, Amit Z. Effects of ethanol on an acetaldehyde drug discrimination with a conditioned taste aversion procedure. Alcohol. 2002;28:103–109. doi: 10.1016/s0741-8329(02)00270-7. [DOI] [PubMed] [Google Scholar]
- Shannon EE, Porcu P, Purdy RH, Grant KA. Characterization of the discriminative stimulus effects of neuroactive steroid pregnanolone in DBA/2J and C57BL/6J inbred mice. J Pharmacol Exp Ther. 2005;314:675–85. doi: 10.1124/jpet.104.082644. [DOI] [PubMed] [Google Scholar]
- Tambour S, Didone V, Tirelli E, Quertemont E. Locomotor effects of ethanol and acetaldehyde after peripheral and intraventricular injections in Swiss and C57BL/6J mice. Behavioral Brain Research. 2006;172:145–154. doi: 10.1016/j.bbr.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Tokunaga S, McDaniel JR, Morrow AL, Matthews DB. Effect of acute ethanol administration and acute allopregnanolone administration on spontaneous hippocampal pyramidal cell neural activity. Brain Res. 2003;967:273–280. doi: 10.1016/s0006-8993(02)04266-x. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology. 2003;28(6):1207–9. doi: 10.1038/sj.npp.1300170. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology. 2004;172(3):352–5. doi: 10.1007/s00213-003-1662-0. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20(5):1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall TL, Carr LG, Ehlers CL. Protective Associaton of Genetic Variation in Alcohol Dehydrogenase With Alcohol Dependence in Native American Mission Indians. American Journal of Psychiatry. 2003;160:41–46. doi: 10.1176/appi.ajp.160.1.41. [DOI] [PubMed] [Google Scholar]