Abstract
Purpose
To assess local control, event-free survival (EFS), and overall survival (OS) rates in 71 patients with localized, completely resected (Group I) alveolar rhabdomyosarcoma (ALV RMS) and their relation to radiation therapy (RT) on IRSG Protocols -III and -IV, 1984-1997.
Methods
Chart review and standard statistical procedures.
Patients and Tumors
Patients were 1 to 18 years at diagnosis (median, 6 years). Primary tumor sites were extremity/trunk (N=54), head/neck (N=9), genitourinary tract (N=7), and perineum (N=1). Thirty patients received VA±C with RT; 41 received VA±C alone. RT was assigned, not randomized.
Results
54 patients had Stage 1 (favorable site, any size) or Stage 2 (unfavorable site, ≤5 cm) tumors. Eight-year EFS was 90%, with 100% local control for 17 patients given RT. Eight-year EFS was 88%, with 92% local control for 37 patients without RT; P=0.52 for EFS comparisons, 0.3 for local control comparisons. In 17 Stage 3 patients (unfavorable site, tumors >5 cm, N0), 8-year EFS was 84% with 100% local control in 13 patients given RT; 8-year EFS was only 25% and local control 50% in 4 patients without RT. Local recurrence was the most common site of first failure in non-irradiated patients.
Conclusion
Patients with Stages 1-2 ALV RMS had slightly but statistically insignificantly improved local control, EFS, and OS rates when local RT was given. The need for local RT in Stages 1-2 patients deserves evaluation in a randomized study. Local control, EFS, and OS rates were significantly improved in Stage 3 patients receiving local RT.
Keywords: Soft Tissue Sarcoma, Rhabdomyosarcoma, Pediatric Oncology
INTRODUCTION
The Intergroup Rhabdomyosarcoma Study Group (IRSG) was formed in 1972 to elucidate the biology and optimize therapy for patients <21 years with newly diagnosed rhabdomyosarcoma (RMS) and undifferentiated soft-tissue sarcoma (UDS). Sequential protocols were developed, designated as IRS-I through IRS-IV [1-4]
In 1999, Wolden et al. reported outcome of 396 patients with localized, completely resected tumors treated on the IRS-I through -III protocols, including 273 patients with embryonal (E) RMS, 91 with ALV RMS, and 32 with UDS.[5] Failure-free survival for patients with localized, completely resected ALV RMS or UDS was improved with planned post-operative RT and chemotherapy.[5]
This report combines the Group I patients with ALV RMS from IRS-III and IRS-IV, to ascertain local control, event-free and overall survival, according to whether the patients received or did not receive RT to the local tumor site. As the goals of IRSG studies are to improve outcome, maintain form and function, minimize morbidity and improve quality of life, we wondered whether a subset of patients could be identified in whom local RT to the primary site might be safely omitted, without compromising outcome. Primary RT in Group I patients did not improve outcome.[5] Thus, in this report on patients with localized ALV RMS, we have omitted the patients with UDS as we now realize they represent a heterogeneous histiotype, with variable biology, and have a disease distinct from ALV RMS.
METHODS
Grouping and Staging Systems in IRS-III and IRS-IV
The details of the Surgico-pathological Grouping system and the TNM Staging system appear in Tables 1 and 2 of Reference 4. Group I patients have localized, completely resected tumors without regional lymph nodal involvement. Group I can include TNM Stages 1, 2, and 3 based on tumor site, invasiveness, and size. Stage 1 tumors arise in favorable sites: orbit, head and neck, (but not a non-orbital parameningeal site), vulva, vagina, cervix, uterus, or paratesticular region (but not the bladder/prostate or kidney), or the biliary tract, regardless of tumor diameter or invasiveness. Stage 2 tumors arise in unfavorable sites: non-orbital cranial parameningeal, bladder/prostate/kidney, extremity, trunk, retroperitoneum/pelvis, perineum, and other locations; they are ≤5 cm, invasive or non-invasive, without regional node involvement. Stage 3 tumors arise in unfavorable sites, are >5 cm, invasive or non-invasive, or can be of any size with involved regional lymph nodes. However, Group I patients by definition have no clinical or pathological regional lymph node involvement at diagnosis. Patients with metastatic disease, Stage 4, are not included in this analysis.
Treatment of Patients on IRS-III and IRS-IV Protocols
On IRS-III, Group I patients with UDS received non-intensive vincristine and actinomycin D (VA) chemotherapy, without RT. Patients with ARMS received intensified chemotherapy with VA plus cyclophosphamide (collectively called VAC), cisplatin, and doxorubicin. The protocol specified 41.4 Gray (Gy) of local RT, given in 1.8 Gy fractions, over 4 to 5 weeks to the pre-operative tumor bed plus a 2 cm margin, beginning at week 6.[5] The FFS rate for the 27 IRS-III patients with ALV tumors given local RT, 26 of whom also received intensified chemotherapy, was better than that for the 29 patients who did not receive RT, including 15 ALV patients who received intensified chemotherapy and 14 UDS patients who received only VA. The 10-year FFS rates were 95% versus 69% [P=0.01].[5] These results were not available when the IRS-IV protocol was implemented.
IRS-IV patients with Group I orbit-eyelid and paratesticular tumors received VA but no RT. Patients with Group I, Stages 1 and 2 tumors at other sites received VAC ± ifosfamide and etoposide, without RT, regardless of histologic subtype. Patients with Stage 3 Group I ALV RMS were assigned to the same chemotherapy regimens as Stage 1 and 2 patients, with 41.4 Gy local RT given also. Patients who underwent an amputation of the primary tumor were assigned to receive chemotherapy but no RT.[4]
Patients and Tumors
Patients eligible for entry on IRSG-III and -IV protocols were previously untreated, newly diagnosed, <21 years with RMS or soft tissue UDS. The protocols were approved by local Human Investigations Committees; informed consent was obtained from each participant or participant’s guardian. The tumors’ primary sites were reviewed by members of the IRSG Surgical Subcommittee. The IRSG central pathology reviewers utilized the criterion that 50% or more of the tumor tissue examined had an ALV RMS pattern, either classical or solid, in order to be classified as ALV.[6] Those with less than 50% ALV pattern were classified as ERMS. IRSG Pediatric Oncologists reviewed schedules and doses of the components of the VAC chemotherapy program, as well as compliance with protocol guidelines’ stipulations regarding timing of therapy. IRSG Radiation Oncologists reviewed the RT dose, volume, and timing as well as compliance with the protocol guidelines. All records were then re-reviewed by 4 of the authors (RBR, SSD, SLW, and JRA) to confirm site of primary tumor, site of relapse, RT and chemotherapy details, so as to correlate local control, EFS, and overall survival rates with the available information.
Statistical Analysis
Local and local-regional control was defined as lack of tumor recurrence at the primary site and/or regional lymph nodes. Event-free survival (EFS) was defined as the time from study enrollment to disease recurrence or death. Overall survival was defined as the time from enrollment to death from any cause. EFS and survival durations for patients who did not experience an event were censored at the patient’s last date of contact. The Kaplan-Meier method was used to estimate the EFS and survival distributions. [7] The local control data do not represent actuarial projections. P values of <0.05 were considered statistically significant, unless otherwise indicated for multiple subgroup comparisons. Analyses were based on data available by February, 2007.
RESULTS
Patients
Seventy-one patients with Group I ALV histology tumors were treated on IRS-III (N=39) and IRS-IV (N=32); 40 males and 31 females (1.29:1). Their ages at diagnosis ranged from 1 to 18 years (median, 6 years).
Tumors
Pathology. All patients were diagnosed with ALV RMS by central pathology review. Fifty-nine patients had 100% ALV tumors, of which 13 (22%) were the solid variant, and the others typical alveolar tumors with empty spaces and prominent blood vessels [6]; one patient had an ALV RMS without a known percentage. Ten patients had mixed ALV (50-90%) and ERMS. One patient had ALV RMS with neural elements (ALV ectomesenchymoma). Tumor Sites. Twenty-eight patients had tumor sites in the lower extremity, while 16 had tumor in the upper extremity. Ten patients had a truncal tumor, and nine had a tumor in the head and neck region. Seven patients had a genitourinary tract tumor: 6 in paratesticular structures and 1 in the vulva. One patient had a perineal tumor. Tumor Stage. Fourteen patients had Stage 1, 40 had Stage 2, and 17 had Stage 3 tumors. The numbers of patients in each Stage were similar in IRS-III and IRS-IV. Tumor Size. Tumor diameter, available in 70 patients, ranged from 0.9 cm to 20 cm in greatest dimension (median, ~4 cm). Tumor Invasiveness. Sixty-two patients had non-invasive (T1) tumors, 6 were invasive (T2), and 3 were unknown.
Regional Lymph Node Status
Thirty-four patients had no evidence of involved regional nodes and were considered clinically N0 (no nodal tumor). Node biopsy or sampling was performed in 37 patients, and no tumor-involved nodes were found.
Treatment
Chemotherapy. Sixty-eight patients received vincristine (2 mg/M2/dose in IRS-III, 1.5 mg/M2/dose in IRS-IV, reduced because of neurotoxicity), actinomycin D, and cyclophosphamide (VAC). Two patients received only vincristine and actinomycin D, and an additional patient had insufficient chemotherapy information. On IRS-III, 37 patients also received cisplatin and doxorubicin. On IRS-IV, 19 patients received ifosfamide and nine received etoposide. Outcome was similar among the patients who did or did not receive the other drugs in addition to VAC.[3,4] Drug administration was modified as needed due to toxicity. Radiation Therapy. In IRS-III (N=39 patients), 24 patients received RT and 9 did not receive RT, because of prior surgical removal of an extremity tumor by amputation (N=4), ray resection (N=2), or by orchidectomy (N=3). The remaining 6 patients received no RT, but with no stated reason for omission. These patients continued on the protocol despite receiving no RT. In IRS-IV (N=32 patients), 26 patients received no RT: 6 Stage 1 patients, including 2 with orchidectomy; 19 Stage 2 patients, including 2 with amputation and 1 with ray resection; and 1 Stage 3 patient who was amputated. Six other Stage 3 patients with tumor sizes ranging from 6 to 20 cm (median, 6.75 cm) received appropriate RT. Thus overall, 30 patients were treated with RT and 41 were treated without RT, but the choice was not randomized.
Event-Free Survival (EFS) and Local Control (Table I)
Table I.
Local Control and Estimates of Event-Free Survival (EFS) and Overall Survival (OS) at 8 Years from Study Entry*
| CATEGORY | LOCAL CONTROL | EFS (95% CI) | OS (95% CI) |
|---|---|---|---|
| Stage 1, N=14 | 12/14, 86% | 86% (54%, 96%) | 93% (59%, 99%) |
| Stage 2, N=40 | 39/40, 98% | 92% (76%, 97%) | 97% (81%, 99%) |
| Stages 1+2 | 51/54, 94% | 90% (78%, 96%) | 96% (85%, 99%) |
| Stage 3, N=17 | 15/17, 88% | 70% (42%, 86%) | 69% (36%, 88%) |
| Patients Below Are Categorized by Stage and Whether RT Was Given | |||
| Stages 1+2, No RT, N=37 |
34/37, 92% | 88% (72%, 96%) | 94% (78%, 98%) |
| Stages 1+2, Given RT, N=17 |
100% | 93% (61%, 99%) | 100% |
| Stage 3, No RT, N=4 |
2/4, 50% | 25% (1%, 67%) | 38% (1%, 81%) |
| Stage 3, given RT, N=13 |
100% | 84% (49%, 96%) | 81% (41%, 95%) |
Local control analysis contains all patients in the study, including those in whom the primary site was amputated;
Abbreviations: RT, radiation therapy; CI, confidence interval
Stages 1 and 2 (N=54). The 8-year EFS rates were 86% for Stage 1 patients, 92% for Stage 2, and 70% for Stage 3. Patients with Stages 1 and 2 tumors had a 90% EFS rate at 8 years compared to 70% for those with Stage 3 tumors (P=0.015, Figure 1). The 8-year EFS rate was 93% for 17 patients in Stages 1 and 2 who received RT to the primary tumor; all achieved local control. The 8-year EFS rate was 88% and the local control rate was also 88% for 37 patients in Stages 1 and 2 who did not receive RT to the primary tumor.(Figure 2). The P value for comparing the EFS rates was 0.52 and for the local control rates was 0.30. Stage 3 (N=17). The 8-year EFS rate was 84% for 13 Stage 3 patients who received RT; all achieved local control. The 8-year EFS rate was only 25% in 4 patients who did not receive RT, and the local control rate was 50% (2 of 4 patients). The P value for comparing the EFS rates was 0.0029. The numbers were too small to generate a meaningful P value for comparing the local control rates in the Stage 3 patients. Table I also shows the appropriate 95% Confidence Interval figures for each category.
Figure 1.
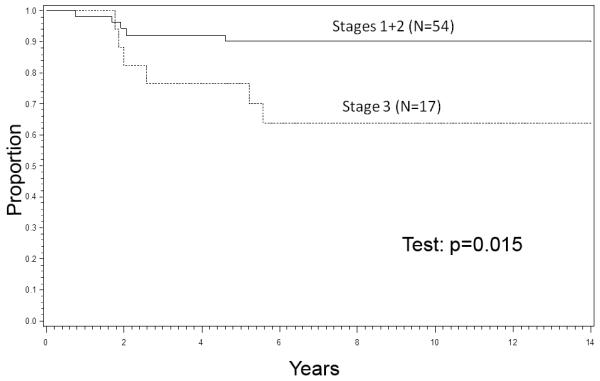
Event-Free Survival (EFS), Stages 1+2 vs. 3
Figure 2.
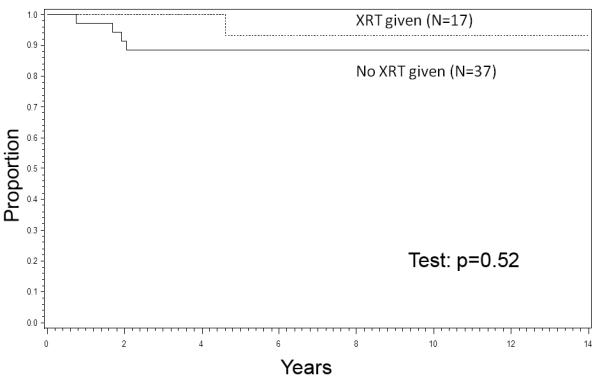
EFS by RT, Stages 1+2
Overall Survival (OS) Rates (Table I)
Eight-year OS rates were 93% for Stage 1 patients, 97% for Stage 2, and 69% for Stage 3. The survival rate of patients with Stages 1 and 2 disease exceeded that of patients with Stage 3 disease (P=0.01, Figure 3). Stages 1 and 2 (N=54). The 8-year OS rates were 100% for 17 patients with Stages 1 and 2 tumors given primary RT and 94% for 37 patients with Stages 1 and 2 disease not given RT (P=0.34). Stage 3 (N=17). The 8-year OS rate was 81% for 13 Stage 3 patients given RT, but only 38% (95% CI: 1%, 81%) for 4 patients not given primary RT (P=0.14).
Figure 3.
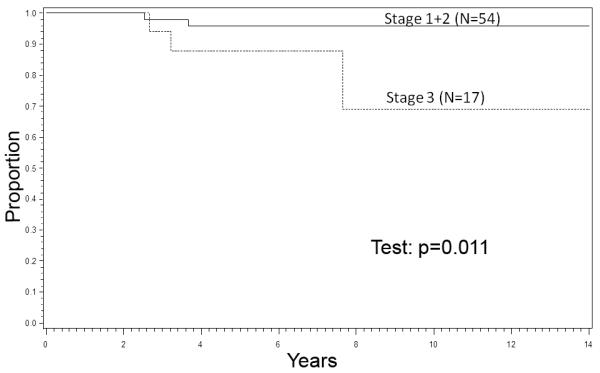
Survival, Stages 1+2 vs. 3
Patterns of Failure and Outcome (Table II)
Table II.
Patterns of Failure Among the Patients with Group I Alv Rms Who Did Not Have An Amputation
| Pt. No.; Age (years) + Gender |
Primary Site, Size (cm); Stage |
Regional Lymph Nodes at Diagnosis |
Local RT Dose, Gray |
Chemotherapy | Type of Failure; Time After Study Entry |
Survival Status; Time After Study Entry |
|---|---|---|---|---|---|---|
| 1; 12M | R Shoulder, 2; Stage 2 |
Clinical N0 |
0 | VA | Local; 1.7 years |
NED; 16.2 years+ |
| 2; 10M | L chest wall, 6.5; Stage 3 |
Clinical N0 |
0 | VAC, Dox, Cpt | Local; 1.8 years |
Dead, rafting accident; no tumor recurrence; 7.6 years |
| 3; 2M | R neck, 4; Stage 1 |
Pathologic N0 |
0 | VAC | Local; 0.7 year |
NED; 5.9 years+ |
| 4; 4M | L lip, 3; Stage 1 |
Clinical N0 |
0 | VAC, Etop, Ifos | Locoregional; 1.9 years |
Dead of tumor; 3.7 years |
| 5; 4F | R thigh, 3; Stage 2 |
Pathologic N0 |
41.4 | VAC, Dox, Cpt | Regional; 4.6 years |
NED; 9.6 years+ |
| 6; 1.4M |
R thigh, 6; Stage 3 |
Pathologic N0 |
36; Ir192 Brachy- therapy |
VAC | DM; 5.6 years |
Dead of tumor; 7.6 years |
| 7; 13F | L arm, 7.5; Stage 3 |
Clinical N0 |
41.6 | VAC, Dox, Cpt | DM; 2.6 years |
Dead after BMT; 3.2 years |
Abbreviations: No., number; L, left; R, right; NED, no evidence of disease; RT, radiotherapy; N0, no lymph node involvement; VAC, vincristine, actinomycin D, cyclophosphamide; Dox, doxorubicin; Cpt, cisplatin; Etop, etoposide (VP-16); Ifos, ifosfamide; + alive at last contact; DM, distant metastasis (including lymph nodes proximal to regional lymph nodes, if applicable); BMT, bone marrow transplantation
To examine the role of RT as relates to local control, and secondarily to EFS and OS, we excluded 14 patients who were not assessable for local control because of a prior surgical procedure which removed the target organ, and thus were not irradiated by protocol guidelines. These 14 patients (9 IRS-III, 5 IRS-IV) included 6 with an amputation, 3 with a ray resection and 5 with an orchidectomy. Thus, as shown in Table II, there were 7 non-amputated patients in whom the pattern of first failure as local, regional, or metastatic could be assessed. Of these 7 patients, 4 (57%) were not irradiated and suffered a local recurrence. The 3 other irradiated patients were controlled locally but suffered a regional or distant failure. Of 3 non-irradiated patients who relapsed locally but without simultaneous regional or distant metastases, 2 survived at 5.2 and 14.5 years after retrieval therapy. One patient with local recurrence was successfully retreated, but died more than 5 years later in an accident, without tumor. The patient with local-regional relapse died with tumor. The patient who developed regional nodal relapse at 4.6 years relapsed a second time 2 years later in a regional nodal area but was alive without disease 3 years later at last followup. Only one of the 5 patients who developed distant metastases survived.
Rates of relapse were 2/14 (14%) in Stage 1, 3/40 (7.5%) in Stage 2, and 5/17 (29%) in Stage 3 patients. The failure rates were 4 of 27 among those not given RT (15%) vs. 3 of 30 among those given primary RT (10%, P=0.46).
DISCUSSION
Throughout IRSG studies, patients with ALV RMS have had a worse outcome than those with ERMS.[1-4] Large contemporary pediatric oncology groups in Europe also reported significantly worse outcome among patients with ALV RMS compared to those with ERMS.[8-10]
The results of this retrospective review differ from those when UDS was included in the analysis. Local control and EFS rates were improved in irradiated patients with Stage 1 or 2 disease, as compared to non-irradiated patients, but lacked statistical significance, possibly due to small patient numbers. However, for Stage 3, Group I ALV patients, the improvement in local control and EFS with local radiation is quite apparent. The likelihood of retrieval after recurrence was greatly diminished in the Stage 3 patients who relapsed. Thus, although the numbers of these subgroups of patients is small, the outcome in Group I Stage 3 ALV RMS continues to be inferior to those with ERMS histology, warranting continual aggressive therapy including RT to the primary site. We undertook this retrospective analysis with the hope of identifying a subgroup, perhaps the Stage 1 and 2 patients, in whom we might safely omit RT from the recommended initial therapy program. The slightly lower local control, EFS and OS rates indicate the need for a randomized study of local RT in Stages 1 and 2 patients.
We emphasize that patients reported here represent a rare and heterogeneous group and do not include patients with known regional nodal involvement. The subset of 71 patients reported here constituted 39% of the 184 patients with ALV RMS, but only 9% of the total number of 798 patients.[4]
IRSG reports have consistently emphasized local control and EFS when evaluating local therapy, with less emphasis on OS, which incorporates retrieval therapy and mandates analysis of complications and toxicity of the total therapy. In this retrospective review local control and EFS were improved among irradiated patients as compared with non-randomized, non-irradiated patients. The differences in outcome were less impressive among those with Stage 1 and 2 tumors with and without RT, but inferior among the non-irradiated patients with stage 3 tumors, which were large, may have had undetected involved regional lymph nodes, and occurred in unfavorable sites.
There are important differences among these patients and those reported earlier by Wolden et al.[5] The earlier review included patients with undifferentiated sarcoma (UDS) along with the patients with ALV disease. The IRS-III “radiation-therapy” patient subset included 27 patients with ALV histology tumors (26 of whom received aggressive chemotherapy). The IRS-III “no-radiation-therapy” patient subset (N=29) included 15 patients with ALV histology tumors receiving aggressive chemotherapy along with 13 patients with UDS and one with an ALV histology tumor, all 14 of whom received non-aggressive chemotherapy with VA only. We now realize that patients with UDS have a distinct biology that differs from that of patients with ALV RMS, and believe that patients with UDS are more appropriately managed on non-RMS soft tissue sarcoma protocols.
Recent European trials include patients grouped differently from the patients reported here, and thus are not comparable.[10,11]
Thus, the ultimate need for primary RT in the initial treatment of children with completely resected, ≤5 cm ALV RMS tumors and no regional lymph node involvement (IRSG Group I, Stages 1 and 2) should continue to be investigated. At present we continue to recommend aggressive therapy for all patients with Group I ALV tumors, including intensive chemotherapy and primary RT. Ongoing analysis of subsequent patients who have undergone precise staging of the primary tumor and regional lymph nodes plus intensive therapy [12] may identify a subgroup in whom local RT can be safely omitted.
ACKNOWLEDGMENTS
Supported in part by Grants CA-24507, CA-29511, CA-72989, and CA-98543 from the National Cancer Institute, Bethesda, Maryland, USA. A complete listing of grant support for research conducted by CCG and POG before the COG grant of 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm
Footnotes
Presented at the 40th Annual Meeting of the International Society of Pediatric Oncology (SIOP) in Berlin, Germany, on Saturday, October 4, 2008
The authors report no conflicts of interest to disclose.
REFERENCES
- 1.Maurer HM, Beltangady M, Gehan EA, et al. The Intergroup Rhabdomyosarcoma Study-I: A final report. Cancer. 1988;61:209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Maurer HM, Gehan EA, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 4.Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 5.Wolden SL, Anderson JR, Crist WM, et al. Indications for radiotherapy and chemotherapy after complete resection in rhabdomyosarcomas: A report from the Intergroup Rhabdomyosarcoma Studies I to III. J Clin Oncol. 1999;17:3468–3475. doi: 10.1200/JCO.1999.17.11.3468. [DOI] [PubMed] [Google Scholar]
- 6.Newton WA, Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas: Pathologic aspects and proposal for a new classification – An Intergroup Rhabdomyosarcoma Study. Cancer. 1995;76:1073–1085. doi: 10.1002/1097-0142(19950915)76:6<1073::aid-cncr2820760624>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan GL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 8.Flamant F, Rodary C, Rey A, et al. Treatment of non-metastatic rhabdomyosarcomas in childhood and adolescence. Results of the second study of the International Society of Paediatric Oncology: MMT84. Eur J Cancer. 1998;34:1050–1062. doi: 10.1016/s0959-8049(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 9.Koscielniak E, Harms D, Henze G, et al. Results of treatment for soft tissue sarcoma in childhood and adolescence: A final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J Clin Oncol. 1999;17:3706–3719. doi: 10.1200/JCO.1999.17.12.3706. [DOI] [PubMed] [Google Scholar]
- 10.Stevens MCG, Rey A, Bouvet N, et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: Third study of the International Society of Paediatric Oncology—SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol. 2005;23:2618–2628. doi: 10.1200/JCO.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 11.Dantonello TM, Int-Veen C, Harms D, et al. Cooperative trial CWS-91 for localized soft tissue sarcoma in children, adolescents, and young adults. J Clin Oncol. 2009;27:1446–1455. doi: 10.1200/JCO.2007.15.0466. [DOI] [PubMed] [Google Scholar]
- 12.Arndt CAS, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate risk rhabdomyosarcoma: Children’s Oncology Group Study D9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]


