Abstract
Purposes
Compare results of clinical/radiographic studies before second-look procedures (SLP) with SLP specimens from patients with gross residual sarcoma at diagnosis, and relate tumor viability to outcome.
Patients
73 patients underwent SLP before completing chemotherapy, with (n=59) or without (n=14) radiotherapy. Tumor sites were bladder/prostate (n=27), head/orbit/parameningeal (n=22), extremity/trunk (n=14), and retroperitoneum/pelvis (n=10).
Results
1/14 patients (7%) with clinical/radiographic complete response (CR) had viable tumor. 35/59 patients (59%) without CR had viable tumor. Five-year failure-free survival (FFS) rates were 81% in 37 patients without viable tumor and 53% in 36 patients with viable tumor (Cox proportional hazards adjusted P=0.05). Five-year FFS rates were 67% in 15 patients with clear margins and 43% in 21 patients with tumor-involved margins (n=18) or viable gross tumor (n=3) (Cox proportional hazards adjusted P=0.04). Five-year survival was 78–79% among 73 patients with and 333 patients without SLP during treatment.
Conclusions
SLP can show whether viable tumor is present and may be beneficial in selected patients with rhabdomyosarcoma. Disappearance of tumor (CR) usually correlated with no viable tumor at SLP. But 41% of patients without CR had no viable tumor. Those without viable tumor had increased FFS, but not survival, compared to those with viable tumor.
Keywords: Second-Look Procedures, IRS-IV Protocol, Childhood Rhabdomyosarcoma
INTRODUCTION
The Intergroup Rhabdomyosarcoma Study Group (IRSG) was founded in 1972 to elucidate biology and improve treatment results for patients <21 years with newly diagnosed rhabdomyosarcoma (RMS) and undifferentiated soft-tissue sarcoma (UDS). Sequential protocols were developed, designated as IRS-I through IRS-IV. [1–4]
The goals of this report are (1) to compare the results of treatment response assessment by radiographic imaging and clinical examinations prior to a second-look procedure (SLP) with the results of pathologic examination of tissue obtained at SLP, performed prior to completing IRS-IV therapy; (2) to ascertain whether there was a relationship between tumor viability status and patients’ outcome, and (3) to compare patients having an SLP with the larger group of patients not having an SLP during their IRS-IV treatment program.
PATIENTS AND METHODS
Patients
Eligible for entry on the IRS-IV protocol were previously untreated, newly diagnosed patients <21 years with RMS or UDS of soft tissue, who were enrolled after informed consent was obtained and began to receive chemotherapy within 42 days after diagnosis. The IRS-IV trial was planned to improve failure-free survival (FFS) rates, relative to IRS-III, by adopting two new strategies, each assessed in a randomized fashion. The FFS rates of three combinations of chemotherapeutic agents (vincristine, actinomycin D, and cyclophosphamide (VAC); VA plus ifosfamide (VAI); and VI plus etoposide (VIE) were compared. Also, conventional, once-daily radiation therapy (RT) was compared to hyperfractionated, twice-daily RT for patients with localized, gross residual sarcoma.[5] The results showed no significant difference in FFS rates among the chemotherapy combinations, nor between the RT regimens, and therefore the patients were considered together for the purpose of this analysis. Details of the treatments, presurgical tumor-node-metastasis (TNM) Staging system and Surgico-pathologic Grouping system have been reported.[4] The IRS-IV protocol stated that the purposes of second-look procedures (SLP) in patients enrolled with localized, gross residual sarcoma were to confirm clinical response, to evaluate pathologic response to therapy, and to remove residual tumor, if feasible, to achieve local tumor control. The protocol recommended that SLP be considered for patients who still had a persistent tumor mass at week 46 or 47, approximately four weeks after completing treatment. Chemotherapy was to end at approximately week 43, unless delayed for completion after resolution of prior toxicity.
Definitions
(1) We defined SLP as any procedure that was undertaken after initiation of chemotherapy but before completing it, and that produced suitable material for pathologic examination. Despite IRS-IV protocol recommendations, 73 patients underwent SLP before they completed chemotherapy. These included: patients who had a planned, delayed excision of primary tumor; patients whose treatment team decided to remove clinically/radiographically detectible tumor at some time prior to the end of the 43 weeks’ period of protocol chemotherapy; and patients taken off protocol prior to completion of protocol-guided treatment. Patients might or might not have received RT prior to the performance of the SLP. (2) We defined viable tumor as the presence of malignant, residual “small blue cell” RMS or UDS. Scattered, isolated, differentiated rhabdomyoblasts were not considered as evidence of viable tumor.[6]
Methods
Two pediatric oncologists (CA, BR), one pediatric orthopaedic surgical oncologist (KB), and one radiation oncologist (MW) reviewed the charts of patients with localized, gross residual tumors at selected sites (bladder/prostate-CA, head/orbit/parameningeal region-MW, trunk/extremity-KB, retroperitoneum/pelvis-BR) to ascertain the best response to treatment by radiologic imaging and/or clinical examinations performed prior to SLP. Responses were categorized as complete (CR, no residual mass detected), partial (PR, tumor 50% to 99% smaller), no response (NR), or locally progressive disease (PD). Imaging studies included one or more of the following: magnetic resonance imaging, computerized tomographic scans, and ultrasound examinations. No positron-emission tomographic (PET) scans were available.
Results of pathologic examination of the SLP tissue were categorized at the registering institution as showing viable or non-viable tumor. The status of the tumor margins was noted in those with viable tumor (involved vs. not involved); patients with no viable tumor were considered to have negative margins. The tumors’ primary sites were reviewed by the above oncologists and by members of the Surgical Subcommittee of the IRSG (now designated the Soft Tissue Sarcoma Committee of the Children’s Oncology Group) and categorized in one of the four primary site groups. Institutional pathologists assessed tumor viability and margin status, and members of the IRSG Surgical and Pathology Subcommittees reviewed the operative and pathologic notes. Patients who lacked essential information in these categories were excluded from this report. The clinical characteristics and outcomes of the patients who underwent SLP were then compared to characteristics and outcomes of other patients in the four primary site groups with localized, gross residual sarcoma at diagnosis, who completed the first six weeks of therapy but did not undergo SLP prior to completing protocol-directed treatment.
Statistical Analysis
Failure-free survival (FFS) was defined as the time from study enrollment to disease progression or death. Overall survival (OS) was defined as the time from enrollment to death from any cause. The time of SLP was used as the initial time point for comparing FFS and OS distributions, rather than the date of enrollment, to avoid potential bias when comparing outcomes based on patient and disease characteristics, such as SLP findings, that were assessed following initiation of treatment.[7] Comparisons of FFS and OS distributions according to tumor viability and margin status were also performed using the date of enrollment as the initial time point for calculating survival, but the results were similar and are not presented here. FFS and OS for patients who had not experienced an event were censored at each patient’s last date of contact. The Kaplan-Meier method was used to estimate the FFS and OS distributions.[8] Differences between survival curves, defined by whether an SLP was performed, whether residual viable tumor was present, and if appropriate, whether the margins of the resected specimen showed involvement by viable tumor, were analyzed by the log-rank test.[9] The distributions of categorical patient characteristics were compared using a Chi-square test. A retrospective cohort design, wherein subjects were sampled based on whether they did (n=73) or did not (n=333) have an SLP during IRS-IV treatment, was used to identify the demographic and clinical factors associated with SLP. A Cox proportional hazards regression model was used to adjust FFS and OS comparisons between patient groups for possible confounding factors.[10] Because there were multiple factors which might contribute to differences in FFS and OS, a fully adjusted model incorporating patient age, TNM stage (1, 2 vs. 3), primary site (favorable vs. unfavorable), regional lymph nodal status (clinically involved by tumor, or not), tumors’ widest diameter at study entry, tumor invasiveness, and histology (embryonal vs. not embryonal) was used to assess significance of differences. Simpler models, adjusting only for factors associated with FFS or OS, were also fit. Results were similar when compared using the fully-adjusted vs. the simpler models, but the fully-adjusted model comparisons are quoted below. P values of less than 0.05 were considered statistically significant, unless otherwise indicated for multiple subgroup comparisons. Analyses were based on data available by July, 2005.
Follow-up
The median follow-up interval was 9.3 years (range, 2.8 to 12.9 years) for surviving SLP patients, and was 8.2 years (range, 0.8 to 13.2 years) for the surviving non-SLP patients.
RESULTS
Seventy-nine of the 499 eligible patients (16%) with gross residual, localized RMS or UDS arising in the bladder/prostate region, head/orbit/parameningeal region, extremity/trunk, or retroperitoneum/pelvis underwent SLP after beginning chemotherapy but before completing it. Seventy-three patients who had their SLP performed prior to completing chemotherapy, and had sufficient data regarding tumor response, tumor viability, and surgical margins, are the subjects of this report. We excluded 6 patients from this analysis: 4 patients had incomplete information regarding response, tumor viability, surgical margins, and type of SLP (n=1 each); and 2 other patients had multiple missing items. Types of SLP were categorized as biopsy, excision, or more specifically as indicated.
Types of Second-Look Procedure by Primary Site Group
Bladder/Prostate (n=27). In patients with these tumors, cystoscopic biopsies were often performed at multiple intervals.[11] In that case, if an open surgical procedure had been performed prior to completing chemotherapy, the results of that procedure were considered the important SLP to report due to the larger amount of tissue removed, rather than results of previous cystoscopic biopsies. If no open procedure had been performed, the first biopsy showing the best response (CR, PR, or NR) achieved prior to completing chemotherapy was the procedure reported, provided that that response was maintained on subsequent biopsies, if performed, in the absence of definite relapse. SLPs in bladder/prostate patients consisted of biopsy (n=14), tumor excision (n=5), partial cystectomy (n=2), exenteration (n=2); plus total cystectomy, laparotomy and biopsy, prostatectomy, and transurethral resection (n=1 each). Median time to SLP after study entry was 24 weeks (range, 4–55 weeks; the last was delayed to allow recovery from previous toxicity). Head/Orbit/Parameningeal (n=22). Types of SLP in these patients included biopsy (n=15) and tumor excision (n=7). Median time to SLP was 22 weeks (range, 7–37 weeks). Extremity/Trunk (n=14). Types of SLP in these patients consisted of tumor excision (n=12) and exploration and biopsy (n=2). Median time to SLP was 10 weeks (range, 9–28 weeks). Retroperitoneum/Pelvis (n=10). SLPs in these patients included tumor excision (n=7), laparotomy and biopsy (n=2) and transrectal biopsy (n=1). Median time to SLP was 20 weeks (range, 10–42 weeks).
Results of SLP by Primary Site Group
Table 1 shows information about primary sites, initial tumor size (widest diameter), clinical/radiographic response prior to SLP, tumor viability, timing of SLP as related to RT status, sites of first failure, and the overall survival status of the patients. Median tumor sizes ranged from 4 cm to 12 cm, with the smallest ones in the head/orbit/parameningeal patients and the largest in the retroperitoneal/pelvic patients. The majority of the bladder/prostate patients and head/orbit/parameningeal patients received RT prior to SLP, while less than half of the other patients were so treated. Rates of clinical/radiographic complete response prior to SLP were 10/27 (37%) in bladder/prostate patients, 3/22 (14%) in head/orbit/parameningeal patients, 1/14 (7%) in extremity/trunk patients, and 0 in 10 retroperitoneal/pelvic patients (0%). Sixty-seven percent of the patients with bladder/prostate and 64% of patients with head/orbit/parameningeal tumors had no viable tumor found at SLP, while only 20% to 21% of the patients with retroperitoneal/pelvic and extremity/trunk tumors had no viable tumor at SLP, respectively (P=0.005). The five-year survival rates were 89% in bladder/prostate patients, 91% in head/orbit/parameningeal patients, 60% in retroperitoneal/pelvic patients, and 48% in extremity patients (P=0.003).
TABLE 1.
SUMMARY OF TUMOR SIZE, BEST CLINICAL/RADIOGRAPHIC RESPONSE BEFORE SLP, RELATIONSHIP OF RADIOTHERAPY (RT) TO SLP, TUMOR VIABILITY, AND SURVIVAL BY PRIMARY SITE*
| Primary Site, Number of Patients | Tumor Size, Widest Diameter (cm) | Response Prior to SLP, Number (No.) | No. with No Viable Tumor at SLP, by Response | Timing of SLP Relative to RT, Number | Total of Site(s) of First Failure | 5-year Overall Survival Rate (95% Confidence Interval) |
|---|---|---|---|---|---|---|
| Bladder/Prostate, 27 | Median, 6.5; Range, 1–17 | CR, 10 PR, 14 NR, 3 |
CR, 9 PR, 8 NR, 1 Total, 67% |
Before, 5 After, 17 Never, 5 |
Local, 2; Local + Distant, 1; Distant, 1 | 89% (69% to 96%) |
| Head/Orbit/Parameningeal, 22 | Median, 4.5; Range, 1–10 | CR, 3 PR, 14 NR, 5 |
CR, 3 PR, 8 NR, 3 Total, 64% |
Before, 2 After, 18 Never, 2 |
Local, 3; Local + Distant, 1; Distant, 2 | 91% (68% to 98%) |
| Extremity/Trunk, 14 | Median, 7; Range, 3–15 | CR, 1 PR, 11 NR, 2 |
CR, 1 PR, 2 NR, 0 Total, 21% |
Before, 8 After, 2 Never, 4 |
Local, 4; Local + Distant, 1; Reg. LN, 2; Distant, 1 | 48% (21% to 71%) |
| Retroperito-neum/Pelvis, 10 | Median, 12.5 Range, 6–19 |
CR, 0 PR, 7 NR/PD, 3 |
CR, 0 PR, 2 NR/PD, 0 Total, 20% |
Before, 3 After, 4 Never, 3 |
Local, 4; Local + Distant, 1; Reg. LN, 1 | 60% (25% to 83%) |
Abbreviations: SLP=second-look procedures, CR=complete response, PR=partial response, NR=no response, PD= progressive local disease, RT=radiation therapy, No.=number, Reg. LN=regional lymph nodes
Timing of the SLP and Relationship to Treatment with RT
Most SLP procedures (64/73, 88%) were accomplished before week 36. The others were performed before (n=8) or after (n=1) 43 weeks; the last patient’s chemotherapy had been delayed to allow recovery from prior toxicity. Forty-one of 59 patients given RT (69%) had SLP after completing RT, and 18 had SLP prior to receiving RT. Fourteen patients received no RT during their treatment program, in violation of the protocol, but they remained on study to obtain follow-up information.
Results of Pre-SLP Imaging and Clinical Examinations and Correlation with Treatment and Pathologic Findings
Clinical/Radiographic Complete Response (CR). Fourteen patients had complete disappearance of tumor by radiographic imaging and/or clinical examinations before SLP. Of these, 13 patients (93%) had no viable tumor detected in the SLP specimen; 1 patient with a bladder tumor still had viable tumor. Less Than Clinical/Radiographic Complete Response. Fifty-nine patients had a residual mass, including two with retroperitoneal/pelvic tumors (n=1 each) who developed locally PD just before the SLP. Twenty-four of these 59 patients (41%) had no viable tumor detected. Mature rhabdomyoblasts were found in specimens obtained from six patients with bladder/prostate tumors, but were not considered viable tumor, in view of reports that they are differentiated cells and are usually not frankly malignant.[6] One of these six patients developed biopsy-proven recurrent sarcoma in the epigastric region 3 years after diagnosis of a localized prostate tumor and died ten months later. Another patient developed local (prostate) and distant (lung) disease 2.3 years after diagnosis and died approximately 5 months later. The other four patients with mature rhabdomyoblasts were continuously relapse-free and alive at 5 to 11 years after beginning treatment. The remaining 35 patients (59%) with less than CR had viable tumor in the specimen. These included patients with bladder/prostate, head/orbit/parameningeal, and retroperitoneal/pelvic primaries (n=8 each) plus 11 with extremity/trunk primaries. The rates of patients with viable tumor at SLP by primary site were 9/27 (33%) with bladder/prostate lesions, 8/22 (36%) with head/orbit/parameningeal lesions, 11/14 (79%) with extremity/trunk lesions, and 8/10 (80%) with retroperitoneum/pelvis lesions.
Patients Without Viable Tumor
Thirty-seven patients had no viable tumor in the SLP specimens, including 27 of the 37 patients (73%) who received RT prior to SLP and 10 who received no RT prior to SLP.
Patients With Viable Tumor
Thirty-six patients had viable tumor, including one with no detectible mass before SLP (but with tumor detected in the SLP specimen), and 35 patients with residual masses at the time of the SLP, including 2 with local progression before RT. Thirteen patients (36%) received RT before the SLP, 14 (39%) received RT after the SLP, and 9 (25%) received no RT during their treatment program.
Considering only the 59 irradiated patients, the likelihood of finding viable tumor at SLP was 13 of 41 (32%) in patients given RT before SLP, compared to 14 of 18 (78%) in patients who received RT after SLP (P=0.001).
Surgical Margins
Of the 36 patients with viable tumor at SLP, 15 patients had complete excisions with negative margins, 18 had grossly complete excisions with microscopic tumor-involved margins, and 3 were left with gross residual tumor. Patients with no viable tumor were considered to have negative margins.
Failure-Free Survival (FFS) and Overall Survival (OS) Rates
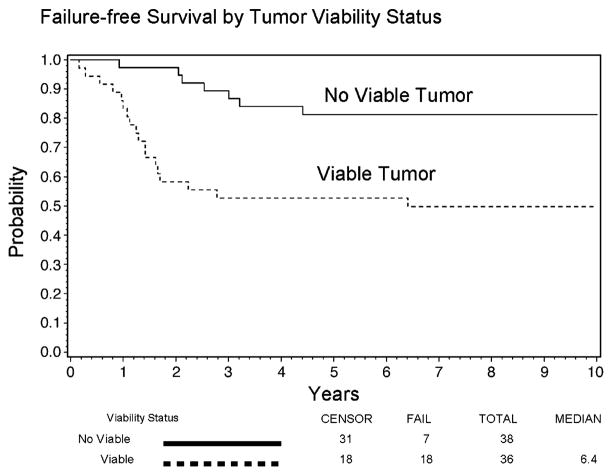
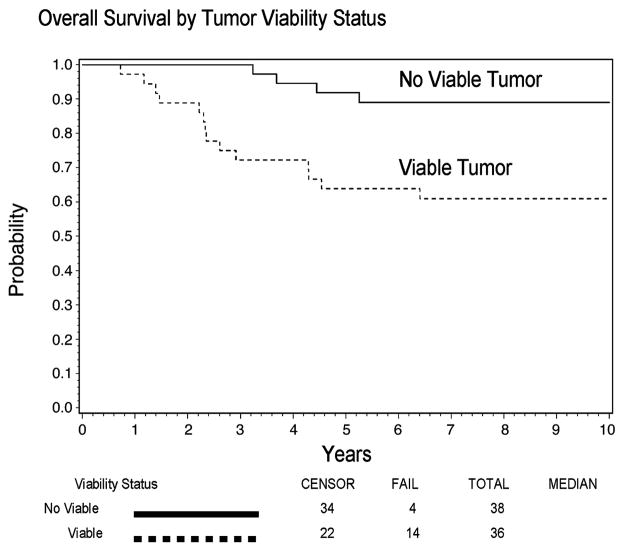
The respective five-year FFS rates among patients without viable tumor at SLP compared to those with viable tumor were 81% and 53%, and the corresponding five-year OS rates were 89% and 67%. Over the entire follow-up period, after adjusting for age, stage, primary site, tumor size, tumor invasiveness, regional lymph node status, and histology, failure-free survival was significantly prolonged in patients with no viable tumor (HR=0.38 for no viable vs. viable tumor, P=0.05, 95% Confidence Interval [CI] = 0.15 to 0.99). Overall survival was prolonged, although not significantly (HR=0.42 for no viable vs. viable, P=0.2, 95% CI = 0.12 to 1.41), based on comparisons adjusted for age, stage, primary site, tumor size, tumor invasiveness, regional lymph node involvement, and histology. Figures 1 and 2 show the FFS and OS curves graphically. Only one death occurred more than six years after study enrollment. Five-year FFS rates of the 36 patients with viable tumor, according to status of the tumor margins after SLP, were 39% for 18 subjects with microresidual, tumor-involved margins, 67% (2 of only 3 patients) for subjects with gross residual tumor, and 67% for 15 subjects with complete tumor removal. The FFS rates were lower among subjects with positive margins or gross residual tumor (n=21) compared to subjects with microscopically complete tumor removal (n=15) after adjusting for age, stage, primary site, tumor size, tumor invasiveness, regional lymph node involvement, and histology (HR=3.95 for positive margins and gross residual tumor vs. complete tumor removal, P=0.04, 95% CI = 1.04 to 15.05).
Figure 1.
Failure-Free Survival of 73 IRS-IV Patients Who Underwent Second-Look Procedures Prior to Completion of Treatment, by Tumor Viability Status
Figure 2.
Overall Survival of 73 IRS-IV Patients Who Underwent Second-Look Procedures Prior to Completion of Treatment, by Tumor Viability Status
Local Control
Table 2 shows local control rates related to tumor viability at SLP and whether RT was administered during the treatment period. The variable timing of RT delivery and differing patient and disease factors could confound comparisons of local failure rates among the RT delivery groups (RT given before or after SLP, or no RT at all). Therefore, no formal statistical comparison was made beyond a descriptive summary. Patients with no viable tumor had the best local control rates, with or without RT. Patients with viable tumor had inferior local control rates, especially when RT was never given.
TABLE 2.
LOCAL CONTROL RATES BY TUMOR VIABILITY AND RT STATUS*
| Viability | RT Not Given. Number of Patients With Local Control, % | RT Given. Number of Patients With Local Control, % |
|---|---|---|
| No Viable Tumor, n=37 | 5/5, 100 % | 29/32, 91% |
| Viable Tumor, n=36 | 3/9, 33% | 19/27, 70% |
| Column Totals | 8/14, 57% | 48/59, 81% |
Abbreviation: RT=radiation therapy
Among the 14 patients who never received irradiation during their IRS-IV treatment program, none of the 5 with no viable tumor at SLP developed recurrent disease. Of 3 with viable tumor but negative margins, one relapsed locally and died. Each of the 6 with viable tumor and positive margins relapsed: 4 locally (1 survived) and 2 distantly (1 had relapsed locally simultaneously; both died).
Types of Recurrence and Relation to RT and Tumor Viability, Table 3
TABLE 3.
SITES OF RECURRENCE AND SURVIVAL IN 24 PATIENTS, AS RELATED TO RADIATION THERAPY (RT) AND TUMOR VIABILITY AT SLP*
| Recurrence Sites, Number of Patients | Patients with Viable Tumor at SLP, n=17 | Number of Patients Alive, Time After Study Entry | Patients with No Viable Tumor at SLP, n=7 | Number of Patients Alive, Time After Study Entry |
|---|---|---|---|---|
| Local, 13 RT, 8; no RT, 5 | 11 | 3, at 11.41, 8.21, and 7.20 Years | 2 | 2, at 11.31 and 6.41 Years |
| Local + Distant, 4 RT, 3; no RT, 1 | 3 | 0 | 1 | 0 |
| Regional LN, 3* RT, 3; no RT, 0 | 2 | 1, at 11.82 Years | 1 | 0 |
| Distant, 4 RT, 3; no RT, 1 | 1 | 0 | 3 | 1, at 10.41 Years |
Abbreviations: SLP=second-look procedures, LN=regional lymph node recurrence;
1 superscript=received RT before SLP,
2 superscript=received RT after SLP,
0 superscript=never received RT
Twenty-four patients developed recurrent disease, including 2 bladder/prostate patients with CR before SLP and no viable tumor, 17 with PR, 3 with NR, and 2 with local PD. Seventeen patients (71%) had received RT and 7 had not. Seventeen patients had viable tumor and 7 had no viable tumor. Seventeen of the 24 patients (71%) died. The seven remaining patients were alive at 6 to 11 years after study entry, including 5 of 13 with isolated local recurrence, 1 of 3 with regional lymph node recurrence and 1 of 8 with distant metastases. Six of the 7 survivors had received RT before (n=5) or after (n=1) the SLP. None of the 4 patients with simultaneous local and distant metastases survived. The cumulative incidence rates of failure at 5 years from the date of SLP were: any local recurrence, 23%; regional lymph-node recurrence, 4%; and distant metastases, 11%. The local failure rates were 39% in the 36 patients with viable tumor and 8.5% in the 37 patients without viable tumor (P=0.001). Regional and distant failure rates were 5.5% and 10.5% in those with viable tumor and 3% and 11%, respectively, in patients without viable tumor (P=0.4 and 0.7, respectively).
Comparison of Primary Tumor Sites Among Patients Who Did or Did Not Undergo SLP Prior to Completing IRS-IV Treatment, Table 4
TABLE 4.
IRS-IV PATIENT COMPARISONS BY PRIMARY SITES*
| Primary Sites | 73 SLP Patients, Number (% Column Total) | 333 Non-SLP Patients, Number (% Column Total) | P Value for Differences Between SLP and non-SLP Groups of Patients |
|---|---|---|---|
| Bladder/Prostate | 27 (37%) | 31 (9%) | <0.001 |
| Head/Orbit/Parameningeal | 22 (30%) | 236 (71%) | <0.001 |
| Extremity/Trunk | 14 (19%) | 38 (11%) | 0.07 |
| Retroperitoneum/Pelvis | 10 (14%) | 28 (8%) | 0.2 |
Abbreviation: SLP=second-look procedures
There was a significant difference in the primary site distributions among the 73 SLP patients and 333 non-SLP patients (P<0.001, overall Chi-square test). Pair-wise testing at a 0.01 alpha level, to account for the multiple comparisons, suggested that there was no significant difference in the proportions of patients with retroperitoneum/pelvis primaries in the SLP and non-SLP groups, but the SLP patients had a marginally greater proportion of extremity/trunk primaries, a significantly smaller proportion of head/orbit/parameningeal primaries, and a significantly larger proportion of bladder/prostate primaries than the non-SLP patients. Thus the SLP patients had a significantly greater proportion of tumors arising at unfavorable sites compared to the non-SLP patients.
Stage, Tumor Size, Histology, Invasiveness, Regional Lymph Node Status, and Patients’ Age
The SLP patients had significantly more Stage 3 tumors (unfavorable site and tumor size >5cm and/or regional lymph node tumor involvement) than the non-SLP patients (P=0.005). Therefore, SLP patients were considered likely to have a worse prognosis than non-SLP patients. However, similar distributions were noted for the percentage of patients with embryonal RMS (SLP, 64% vs. non-SLP, 66%), percentage with non-invasive tumors (SLP, 29% vs. non-SLP, 33%), percentage with regional lymph node involvement (SLP, 18% vs. non-SLP, 20%), and age 1–9 years at diagnosis (SLP, 67% vs. non-SLP, 68%); P values were between 0.5 and 0.9 for these comparisons.
Failure-Free Survival (FFS) and Overall Survival (OS) Rates Among Patients Who Did or Did Not Undergo SLP
The FFS and OS distributions did not differ significantly between the groups of patients before or after (P=0.5 to 0.6 respectively) adjusting for age, stage, primary site, tumor size, tumor invasiveness, regional lymph node status, and histology. The FFS rates at 5 years were 67% for the SLP patients and 74% for the non-SLP patients (unadjusted hazard ratio (HR) = 1.18 for the SLP cohort vs. the non-SLP cohort, P=0.5, 95% CI = 0.76 to 1.86; adjusted HR = 1.12 for the SLP cohort vs. the non-SLP cohort, P=0.6, 95% CI = 0.70 to 1.79). The 5-year OS rates were 79% for the SLP patients and 78% for the non-SLP patients (unadjusted HR = 0.89 for SLP vs. non-SLP, P=0.6, 95% CI = 0.52 to 1.49; adjusted HR = 0.83 for SLP vs. non-SLP, P=0.5, 95% CI = 0.48 to 1.42).
DISCUSSION
This experience indicates that results of clinical/radiologic studies can be misleading with regard to presence of viable tumor, especially in patients who still have a residual mass after some period of treatment. Nearly half of the IRS-IV patients with residual tumors had no viable tumor found in the SLP specimen. It is important that patients with no viable tumor found at SLP had better failure-free survival rates than the group with viable tumor at SLP. Viable tumor was found most often in patients with extremity/trunk and retroperitoneum/pelvis sarcomas. In addition, patients with viable sarcoma had improved failure-free survival rates when residual viable tumor was removed completely at SLP, compared to patients left with microscopic or gross tumor after SLP (P=0.04). However, the similar overall survival rates among the 73 SLP patients compared to the 333 non-SLP patients indicate that performing SLP prior to completion of therapy did not improve patients’ survival rate.
The unfavorable outlook for patients with residual viable tumor and positive margins has been noted previously. The IRS-III protocol (1984–1991) recommended performing an SLP in patients with localized, gross residual sarcoma (except those with orbit and head tumors) whose mass had not disappeared after 20 weeks of chemotherapy along with RT, which was given to most patients during weeks 9 to 14.[3] A preliminary review of 109 IRS-III patients who underwent SLP around week 20 was presented in 1991.[12] Eighty-eight percent of patients in clinical/radiographic CR had no detectible tumor; their 3-year survival rate was 85%. Seventy-five percent of the partial responders (defined as ≥50% tumor shrinkage) were also free of detectible tumor, and 28% of the remainder were rendered tumor-free surgically; their 3-year survival rate was 83%. However, the 3-year survival rate of 14 patients with viable tumor and positive margins was only 62% (no P value was provided).[12]
Several papers from European oncologists discuss SLP and prediction of outcome in young patients with soft-tissue sarcoma according to response during treatment. None addresses tumor viability as a prognostic factor specifically, however. An investigation of SLP in 87 patients with localized, gross residual RMS enrolled on the German CWS-81 Protocol was published in 1989.[13] Response was assessed by CT scans and/or ultrasonography at week 7–9 and by biopsy or resection at week 16 following chemotherapy. RT was administered later according to the amount of residual tumor. Twenty-three patients (26%) had a clinical complete response (CCR) at week 7 to 9, and 25 (29%) had a good partial response (PR), defined as ≥67% shrinkage. Event-free survival rates were 95% in patients with CCR at week 7–9 and 61% in those with PR ≥67%. The 48 patients with CCR or good PR had a 77% EFS rate at five years, compared to an EFS rate of 31% in 26 with <67% shrinkage at 7 to 9 weeks (P=0.006).
The International Society of Pediatric Oncology (SIOP) MMT-84 study reported 38 patients with localized, gross residual RMS at sites other than the cranial parameningeal region in 1994.[14] The patients underwent biopsy for confirmation of their CCR after treatment with VAI chemotherapy for 3 to 6 courses. Thirty-six patients (95%) had biopsies showing no tumor cells. However, probably because RT was not routinely administered thereafter, 18 patients (50%) developed a local recurrence. The local recurrence rate was 48% for a similar group of patients who were neither biopsied nor radiated.[14]
In 2003, 125 patients with gross residual intraabdominal RMS were reported.[15] Progression-free survival at 10 years was 73% in 15 patients who had complete excision with RT, compared to 40% to 48% in patients with complete excision but no RT (n=14), or incomplete or no excision with or without RT (n=99). Similar findings were published from Italy in 2008. Eight of 27 patients (30%) who underwent SLP at a variety of sites but were never radiated had a local relapse, compared to only 1 of 12 (8.3%) who received RT before or after the SLP (P=0.14).[16] These data are similar to ours in showing the necessity of administering radiation therapy to achieve optimal local control in patients with localized, gross residual sarcoma.
Other information from IRS-IV has suggested that a residual mass after completion of therapy in patients who entered the protocol with localized, gross residual disease did not convey a significant risk of later treatment failure. Of 419 patients eligible for evaluation, 341 (81%) achieved a complete response, and 78 achieved a partial or no response by the end of protocol therapy.[17] The failure-free survival rates were similar, 80% vs. 78%, in those with CR vs. PR/NR, P=0.4. Overall survival and local, regional, and distant recurrence rates were also similar in the two groups. The authors found viable tumor in only 50% (n=8) of the 16 informative patients who underwent biopsy or resection of residual masses after completing treatment. The numbers were small, but there was no evidence that removing the residual mass improved outcome. No statement was made regarding tumor viability in the 30 complete responders who had an SLP.[17] They and we agree that it is difficult to recommend performing an SLP in patients whose mass disappears prior to or after completing therapy, because the great majority of those patients will have achieved a pathologic complete response.
The successor IRSG-V/Children’s Oncology Group Soft Tissue Committee protocols D9602 (for low-risk patients) and D9803 (for intermediate-risk patients) advocated considering to perform an SLP at approximately week 12 after beginning chemotherapy in patients with localized, gross residual sarcoma, in an attempt to reduce the dose of subsequent RT to the primary site. Analysis of those data should provide more information about the usefulness of SLP to help define the appropriate RT dose for selected patients with localized, gross residual RMS and UDS.
There are several caveats in interpreting the data presented here. This report is a retrospective review of relatively small numbers of selected patients, and there may be variables other than those considered which could have influenced outcomes. Criteria for choosing to perform an SLP were not stated in the IRS-IV protocol, and may have varied among different teams of oncologists. Only a randomized study, difficult to perform, could yield definitive data about this point. Thus the ultimate benefit to patients of having an SLP is still somewhat unclear. Based on these data, SLP may be considered more appropriate for patients who have residual extremity/trunk and retroperitoneum/pelvis tumors rather than head/orbit/parameningeal and bladder/prostate tumors, because in this series, the former were more likely to have viable tumor in the residual mass. Future studies of similar patients should include performing serial positron-emission tomographic (PET) scans, before and after initiating treatment, to assess viability of residual masses and to correlate scan results with pathologic examination of specimens obtained at SLP.
Acknowledgments
FUNDING SOURCE
Supported in part by Grants CA-24507, CA-29511, CA-72989, and CA-98543 from the National Cancer Institute, Bethesda, Maryland, USA. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm
The National Cancer Institute staff reviewed and approved the Intergroup Rhabdomyosarcoma Study Group Protocol IRS-IV.
Footnotes
This article is dedicated to the memories of Eugene Wiener, MD, and Stephen Qualman, MD, who devoted years of their lives to help children and adolescents with soft-tissue sarcoma.
Presented in part at the International Sarcoma Stuttgart Meeting in Stuttgart, Germany on June 15, 2005, the 37th Annual Meeting of the International Society of Pediatric Oncology in Vancouver, Canada, on September 22, 2005, and the Third International Tübingen-Symposium on Pediatric Solid Tumors in Tübingen, Germany on November 6, 2009
Authors’ contributions to this manuscript include the following. All authors listed were involved with concept development and study design. Drs. Maurer, Crist, and Meyer were sequential Chairs of the IRS Group and approved the study as it evolved over time. Drs. Qualman and Wiener were involved in initial stages of the project, but died before this revised draft was completed. Drs. Andrassy and Hayes-Jordan provided surgical input prior to and after Dr Wiener died. Drs. Arndt, Brown, Raney, and Wharam reviewed the primary data collected at the IRSG Statistical Office and prepared it for statistical analysis by Drs. Stoner and Anderson. All authors listed were involved with interpretation of the data, writing the report, and agreeing to submit the paper for publication.
The authors have no conflicts of interest to report. The funding came from the National Cancer Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maurer HM, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-I: A final report. Cancer. 1988;61:209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Maurer HM, Gehan EA, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Crist W, Gehan EA, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 4.Crist WM, Anderson JR, et al. Intergroup Rhabdomyosarcoma Study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson SS, Meza J, et al. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma – A report from the IRSG. Int J Radiat Oncol Biol Phys. 2001;51:718–728. doi: 10.1016/s0360-3016(01)01709-6. [DOI] [PubMed] [Google Scholar]
- 6.Arndt CAS, Hammond S, et al. Significance of persistent mature rhabdomyoblasts in bladder/prostate rhabdomyosarcoma. Results from IRS IV. J Pediatr Hematol Oncol. 2006;28:563–567. doi: 10.1097/01.mph.0000212978.21372.97. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JR. Commonly misused approaches in the analysis of cancer clinical trials. In: Crowley J, editor. Handbook of Statistics in Clinical Oncology. New York, NY: Marcel Dekker, Inc; 2001. pp. 491–502. [Google Scholar]
- 8.Kaplan GL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 9.Peto R, Pike MC, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II: analysis and examples. Br J Cancer. 1958;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox DR. Regression models and life-tables. J Roy Stat Soc, Series B. 1972;34:187–220. [Google Scholar]
- 11.Arndt C, Rodeberg D, et al. Does bladder preservation (as a surgical principle) lead to retaining bladder function in bladder/prostate rhabdomyosarcoma? Results from Intergroup Rhabdomyosarcoma Study IV. J Urol. 2004;171:2396–2403. doi: 10.1097/01.ju.0000127752.41749.a4. [DOI] [PubMed] [Google Scholar]
- 12.Wiener E, Lawrence W, et al. Survival is improved in Clinical Group III children with complete response (CR) established by second-look operations in the Intergroup Rhabdomyosarcoma Study (IRS) III. Med Pediatr Oncol. 1991;19:399. (Abstract 228) [Google Scholar]
- 13.Treuner J, Suder J, et al. The predictive value of initial cytostatic response in primary unresectable rhabdomyosarcoma in children. Acta Oncologica. 1989;28:67–72. doi: 10.3109/02841868909111184. [DOI] [PubMed] [Google Scholar]
- 14.Godzinski J, Flamant F, et al. Value of postchemotherapy bioptical verification of complete clinical remission in previously incompletely resected (Stage I and II pT3) malignant mesenchymal tumors in children: International Society of Pediatric Oncology 1984 Malignant Mesenchymal Tumors Study. Med Pediatr Oncol. 1994;22:22–26. doi: 10.1002/mpo.2950220105. [DOI] [PubMed] [Google Scholar]
- 15.Cecchetto G, Bisogno G, et al. Role of surgery for nonmetastatic abdominal rhabdomyosarcomas. A report from the Italian and German Soft Tissue Cooperative Groups studies. Cancer. 2003;97:1974–1980. doi: 10.1002/cncr.11285. [DOI] [PubMed] [Google Scholar]
- 16.Cecchetto G, Carretto E, et al. Complete second look operation and radiotherapy in locally advanced non-alveolar rhabdomyosarcoma in children: A report from the AEIOP Soft Tissue Sarcoma Committee. Pediatr Blood Cancer. 2008;51:593–597. doi: 10.1002/pbc.21702. [DOI] [PubMed] [Google Scholar]
- 17.Rodeberg DA, Stoner JA, et al. Prognostic significance of tumor response at the end of therapy in Group III rhabdomyosarcoma: A report from the Children’s Oncology Group. J Clin Oncol. 2009;27:3705–11. doi: 10.1200/JCO.2008.19.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]