Abstract
Our previous studies with the use of non-selective cyclooxygenase (COX) inhibitor, indomethacin, demonstrated that prostanoids produced during endotoxaemia increase inducible nitric oxide (NO) synthase (iNOS) protein expression and NO synthesis, and decrease cyctochrome P450 (CYP) 4A1 protein expression and CYP 4A activity. The results suggest that dual inhibition of iNOS and COX by indomethacin restores blood pressure presumably due to increased production of 20-hydroxyeicosatetraenoic acid (20-HETE) derived from CYP 4A in endotoxaemic rats. The present study examined whether increased levels of vasoconstrictor eicosanoids, 20-HETE, PGF2α and thromboxane A2 (TxA2), would contribute to the effect of selective COX-2 inhibition to prevent endotoxin-induced fall in blood pressure associated with an increase in the production of vasodilator prostanoids, PGI2 and PGE2 and NO synthesis. Mean arterial blood pressure fell by 31 mmHg and heart rate rose by 90 beats per min. in male Wistar rats treated with endotoxin (10 mg/kg, i.p.). The fall in mean arterial pressure and increase in heart rate were associated with increased levels of 6-keto-PGF1α, PGE2, TxB2, and nitrite in the serum, kidney, heart, thoracic aorta and/or superior mesenteric artery. Systemic and renal 20-HETE and PGF2α levels were also decreased in endotoxaemic rats. These effects of endotoxin were prevented by a selective COX-2 inhibitor, N-(2-cyclohexyloxy-4-nitrophenyl)-methansulphonamide (10 mg/kg, i.p.), given 1 hr after injection of endotoxin. These data suggest that an increase in 20-HETE and PGF2α levels associated with decreased production of PGI2, PGE2, and TxA2, and NO synthesis contributes to the effect of selective COX-2 inhibitor to prevent the hypotension during rat endotoxaemia.
The expression of inducible nitric oxide (NO) synthase (iNOS) is enhanced in many tissues in response to mediators released by endotoxin [1,2]. This leads to increased generation of NO, which contributes to fall in blood pressure, vascular hyporeactivity, multiple organ failure and the high mortality rate that are associated with septic shock [2–5]. Systemic blockade of iNOS opposes the fall in blood pressure in endotoxic shock [2,3,5]. This is not only due to withdrawal of vasodilator effects of NO, but also is associated with increased activity of vasoconstrictor pathways including the sympathetic nervous, renin-angiotensin, endothelin and 20-hydroxyeicosatetraenoic acid (20-HETE) systems [2,6].
20-HETE, an eicosanoid synthesized from arachidonic acid primarily by cytochrome P450 (CYP) isoforms of the 4A and 4F classes in the vasculature, is one of the primary eicosanoids produced in the microcirculation [6,7]. 20-HETE participates in the regulation of vascular tone by blocking the large conductance calcium-activated potassium channels and by a direct effect on L-type calcium channels in several vascular beds, including kidney, cerebral, aortic, mesenteric and coronary arteries [6,7]. It has been reported that 20-HETE-induced constriction is also dependent on the presence of endothelium [8–10] and is abolished by inhibition of cyclooxygenase (COX) with indomethacin [10,11] or diclophenac [9] and by the endoperoxide/thromboxane receptor antagonist, SQ-29548 [9,10]. It has also been demonstrated that prostaglandin analogues of 20-HETE, 20-OH-PGG2 and 20-OH-PGH2, produced by COX in vascular endothelial cells mediate the vasoconstrictor effects of 20-HETE [10,11]. As opposed to its vasoconstrictor effect, 20-HETE also produces vasodilation in the renal, coronary, pulmonary and basiler arteries [12–15]. These vasodilatory responses of 20-HETE have been attributed to NO release [15], conversion of 20-HETE to 20-OH-PGE2 and 20-OH-PGF2α by COX [10,13,16], and increased formation of PGE2 [13] and prostacyclin (PGI2) [12–14].
It has been reported that NO inhibits renal CYP ω-hydroxylase activity and the production of 20-HETE [17,18]. Moreover, a NO-induced fall in the endogenous production of 20-HETE has also been found to contribute to the cyclic GMP-independent vasodilator effects of NO in the renal and cerebral microcirculations [17,19]. We have previously demonstrated that the fall in mean arterial pressure in endotoxaemic rats is also associated with a decrease in the expression of CYP 4A1/A3 protein and CYP 4A activity in the kidney and increased levels of nitrite in serum, kidney, heart, thoracic aorta and superior mesenteric artery [20–24]. Furthermore, administration of a synthetic analogue of 20-HETE, N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine (5,14-HEDGE), prevented hypotension and vascular hypo-reactivity associated with the changes in systemic and tissue NO production as well as iNOS protein expression in cardiac, renal and vascular tissues of rats treated with endotoxin [25,26]. These findings suggest that NO-induced inhibition of 20-HETE production and removal of its influence on vascular tone contributes to the fall in blood pressure and vascular hyporeactivity in endotoxic shock.
Increased production of prostanoids by inducible COX (COX-2) has also been shown to contribute to systemic hypotension and related organ damage and decreased survival in animals and humans with sepsis [27]. Non-selective COX inhibitors, such as indomethacin, prevent [28] or do not improve [29] the lethal effects of endotoxin in animal models of sepsis. The beneficial effects of indomethacin are correlated with decreased levels of nitrite and prostanoids in biological fluids from endotoxaemic rats [30]. Indomethacin has also shown to abolish or significantly attenuate the decrease in blood pressure [31] or have no significant effect on blood pressure in endotoxaemic rats [32]. Our previous studies with the use of indomethacin demonstrated that prostanoids produced during endotoxaemia increase iNOS protein expression and NO synthesis, and decrease CYP 4A1 protein expression and CYP 4A activity. The results suggest that dual inhibition of iNOS and COX by indomethacin restores mean arterial pressure presumably due to increased production of 20-HETE derived from CYP 4A in endotoxaemic rats [24].
The present study was conducted to determine whether increased levels of 20-HETE and vasoconstrictor prostanoids, PGF2α and thromboxane A2 (TxA2), would contribute to the effect of selective COX-2 inhibitor, N-(2-cyclohexyloxy-4-nitrophenyl)-methansulphonamide (NS-398), to prevent endotoxin-induced fall in blood pressure associated with an increase in the production of vasodilator prostanoids, PGI2 and PGE2, and NO synthesis. Preliminary results have been presented in abstract form [33,34].
Materials and Methods
Endotoxic shock model
Experiments were performed on male Wistar rats (n = 51) (Research Center of Experimental Animals, Mersin University, Mersin, Turkey) weighing 250 to 300 g that were fed a standard chow. The rats were housed in an animal facility with a 12-hr light: dark cycle. All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Ethics Committee of Mersin University School of Medicine. Endotoxic shock was induced as previously described by Tunctan et al. [20]. Briefly, conscious rats received a 10 mg/kg (i.p.; sublethal dose) [20,35] injection of endotoxin (Escherichia coli lipopolysaccharide, O111:B4; Sigma Chemical Co., St. Louis, MO, USA) or an equivalent volume of saline (4 ml/kg, i.p.) at time 0. Mean arterial pressure and heart rate were measured using a tail-cuff device (MAY 9610 Indirect Blood Pressure Recorder System, Commat Ltd., Ankara, Turkey) during a control period at time 0 and 1, 2, 3 and 4 hr later. Separate groups of endotoxin-treated rats were given a selective COX-2 inhibitor, N-(2-cyclohexyloxy-4-nitrophenyl)-methansulphonamide (NS-398; Sigma Chemical, St. Louis, MO, USA) (10 mg/kg, i.p.) [34] 1 hr after injection of saline or endotoxin. The rats were euthanized 4 hr after the administration of endotoxin, and a blood sample, kidney, heart, thoracic aorta and superior mesenteric artery were collected for the measurement of 6-keto-PGF1α, PGE2, PGF2α, TxB2, nitrite and 20-HETE levels. Sera were obtained from blood samples by centrifugation at 23,910 x g for 15 min. at 4°C and stored at −20°C until analysed for the measurement of eicosanoid and nitrite levels. The tissues were homogenized in 1 ml of an ice-cold 20 mM HEPES buffer (pH 7.5) containing 20 mM β-glycerophosphate, 20 mM sodium pyrophosphate, 0.2 mM sodium orthovanadate, 2 mM EDTA, 20 mM sodium fluoride, 10 mM benzamidine 10, 1 mM dithiothreitol, 20 mM leupeptin and 10 mM aprotinin [20]. An aliquot of the supernatant was analysed for the measurement of total protein using Coomassie blue method [20]. Nitrite (stable product of NO) levels in the sera and tissue homogenates were measured by using the diazotization method based on the Griess reaction as an index for NOS activity [20, 36]. Briefly, samples (50 μl) were pipetted into 96-well microtitre plates and an equal volume of Griess reagent (1% sulphanylamide (25 μl) and 0.1% N-1-naphtylethylenediamine dihydrochloride (25 μl) in 2.5% ortophosphoric acid) was added to each well. After incubation for 10 min. at room temperature, absorbance was measured at 550 nm with a microplate reader. Standard curves were also constructed using sodium nitrite concentrations ranging from 0.25–50 μM. Serum and tissue prostanoid and 20-HETE concentrations were measured as indexes for COX and CYP 4A activity by ELISA according to the manufacturer’s instructions in the 6-keto-PGF1α, PGE2, PGF2α and TxB2 (Cayman Chemical Co., Ann Arbor, MI, USA), and 20-HETE (Detroit R&D, Inc., Detroit, MI, USA) assay kits, respectively.
Statistical analysis
All data were expressed as means ± S.E.M. Data were analysed by one-way ANOVA followed by Student-Newman-Keuls test for multiple comparisons, Kruskal-Wallis test followed by Dunn’s test for multiple comparisons and Student’s t or Mann-Whitney U tests when appropriate. A P value < 0.05 was considered to be statistically significant.
Results
Effect of selective COX-2 inhibition on the cardiovascular response to endotoxin
Endotoxin caused a gradual fall in mean arterial pressure (fig. 1A) and increase in heart rate (fig. 1B) over the 4-hr course of the experiment (p < 0.05). The changes in mean arterial pressure and heart rate reached a maximum 4 hr after the administration of endotoxin. Mean arterial pressure fell by 31 mmHg and heart rate rose by 90 bpm in rats treated with endotoxin. A selective COX-2 inhibitor, NS-398, prevented the fall in mean arterial pressure and the increase in heart rate in rats given endotoxin (p < 0.05). NS-398 had no effect on mean arterial pressure and heart rate when given to rats treated with vehicle (p > 0.05).
Fig. 1.
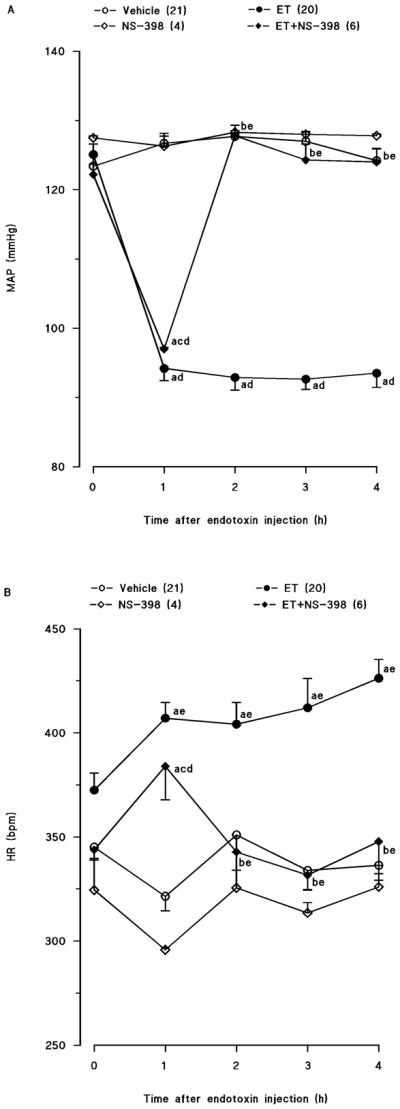
Time course of the effects of N-(2-cyclohexyloxy-4-nitrophenyl)-methansulphonamide (NS-398) on (A) mean arterial presure (MAP) and (B) heart rate (HR) following administration of saline (vehicle) (4 ml/kg, i.p.) or endotoxin (ET) (10 mg/kg, i.p.) to conscious rats. NS-398 (10 mg/kg, i.p.) was given 1 hr after administration of endotoxin. Data are expressed as means ± S.E.M. Number in parentheses indicate the number of animals studied per group. aSignificant difference from the corresponding value seen in rats treated with saline (vehicle) (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with vehicle and endotoxin (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with vehicle and NS-398 (p < 0.05). dSignificant difference from the time 0 h value within a group (p < 0.05). eSignificant difference from the time 1 hr value within a group (p < 0.05).
Effect of selective COX-2 inhibition on the endotoxin-induced increase in 6-keto-PGF1α, PGE2, PGF2α, TxB2, nitrite and 20-HETE levels
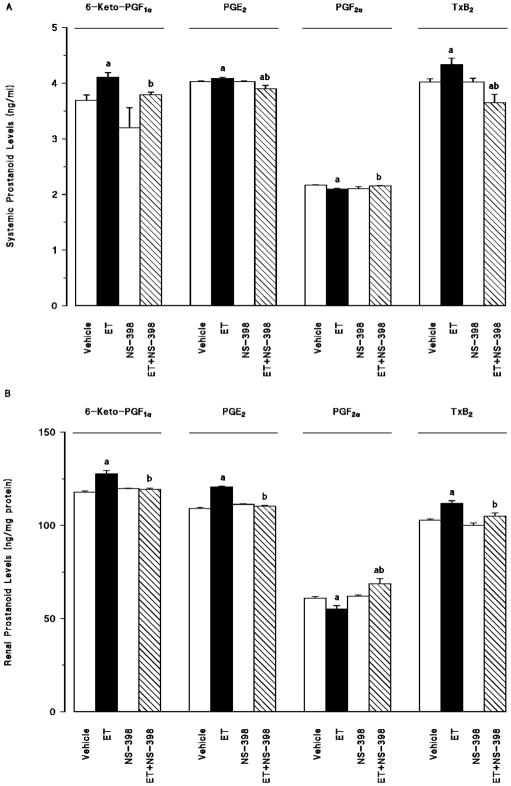
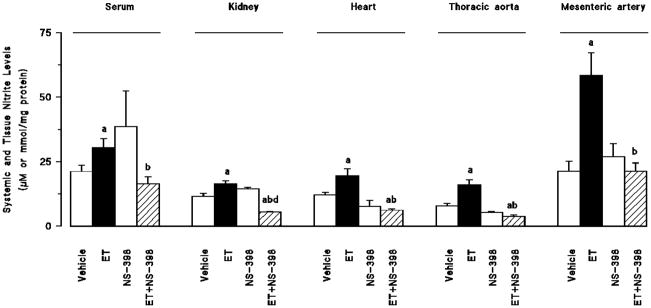
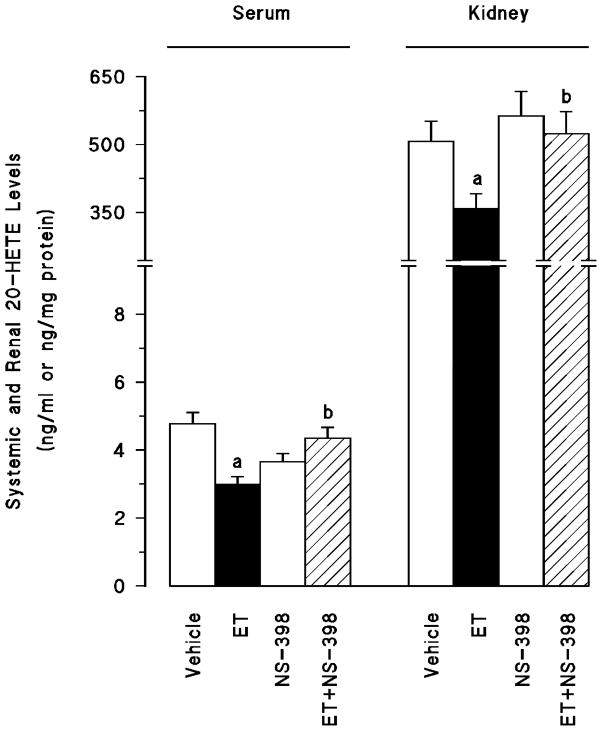
Endotoxin-induced fall in mean arterial pressure and increase in heart rate were associated with an increase in systemic (fig. 2a) and renal (fig. 2b) 6-keto-PGF1α, PGE2, and TxB2 levels as well as an increase in serum and tissue nitrite levels (fig. 3) (p < 0.05). Moreover, endotoxin decreased systemic and renal levels of PGF2α (fig. 2) and 20-HETE (fig. 4) (P < 0.05). Selective inhibition of COX-2 by NS-398 prevented the increase in serum and tissue levels of 6-keto-PGF1α, PGE2, TxB2 (fig. 2) and nitrite (fig. 3) as well as the decrease in systemic and renal PGF2α (fig. 2) and 20-HETE levels (fig. 4) in rats given endotoxin (p < 0.05). NS-398 had no effect on the systemic and tissue basal prostanoid, nitrite and 20-HETE levels when given to rats treated with vehicle (p > 0.05).
Fig. 2.
The effects of N-(2-cyclohexyloxy-4-nitrophenyl)-methansulphonamide (NS-398) on changes in (A) systemic and (B) renal prostanoid levels measured 4 hr after saline (vehicle) (4 ml/kg, i.p.) or endotoxin (ET) (10 mg/kg, i.p.) injection to Wistar rats. NS-398 (10 mg/kg, i.p.) given at 1 hr after saline or ET injection. Data are expressed as means ± S.E.M. of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (vehicle) (p < 0.05). bindicates a significant difference from the corresponding value seen in the rats treated with vehicle and endotoxin (p < 0.05).
Fig. 3.
The effects of N-(2-cyclohexyloxy-4-nitrophenyl)-methansulphonamide (NS-398) on changes in serum, kidney, heart, thoracic aorta and superior mesenteric artery nitrite levels measured 4 hr after saline (vehicle) (4 ml/kg, i.p.) or endotoxin (ET) (10 mg/kg, i.p.) injection to Wistar rats. NS-398 (10 mg/kg, i.p.) were given at 1 hr after or saline ET injection. Data are expressed as means ± S.E.M. of 5–15 animals. aSignificant difference from the corresponding value seen in rats treated with saline (vehicle) (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with vehicle and endotoxin (p < 0.05).
Fig. 4.
The effects of N-(2-cyclohexyloxy-4-nitrophenyl)-methansulphonamide (NS-398) on changes in systemic and renal 20-hydroxyeicosatetraenoic acid (20-HETE) levels measured 4 hr after saline (vehicle) (4 ml/kg, i.p.) or endotoxin (ET) (10 mg/kg, i.p.) injection to Wistar rats. NS-398 (10 mg/kg, i.p.) given at 1 hr after or saline ET injection. Data are expressed as means ± S.E.M. of 4–8 animals. aSignificant difference from the corresponding value seen in rats treated with saline (vehicle) (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with vehicle and endotoxin (p < 0.05).
Discussion
This is the first study to provide an evidence that COX-2-derived vasodilator prostaglandins, PGI2 and PGE2, as well as a vasoconstrictor prostanoid, TxA2, produced during endotoxaemia increase NO synthesis and decrease CYP 4A activity. Moreover, the results of the present study demonstrate that dual inhibition of PGI2, PGE2, and TxA2 synthesis and NO production by a selective COX-2 inhibitor restores mean arterial pressure and heart rate due to increased systemic and renal levels of 20-HETE and PGF2α.
There are several reports suggesting a direct link between arachidonic acid metabolites and NO [6,7,38,39]. The constitutive isoforms of COX and NOS enzymes play an important role in the regulation of several physiological states. On the other hand, under inflammatory conditions such as endotoxic shock, the inducible isoforms of these enzymes are induced in a variety of cells resulting in the production of large amounts prostanoids and NO. Increasing evidence suggests that there is considerable cross-talk between COX, NOS and CYP 4A enzymes. Indeed, arachidonic acid and its metabolites generated by COX isoforms have been shown to interfere with NO biosynthesis [38,39]. NO has also been demonstrated to activate COX enzymes, an event leading to overt production of prostanoids [38,39]. Moreover, it has been reported that NO inhibits renal CYP ω-hydroxylase activity and the production of 20-HETE [6,7]. Therefore, these data suggest that increased production of COX-2-derived prostanoids and NO might contribute to the endotoxin-induced decrease in CYP 4A activity during rat endotoxaemia.
Our previous findings with a selective iNOS inhibitor, 1,3-PBIT, demonstrate that activation of mitogen-activated protein kinase kinase-1/extracellular signal-regulated kinase-1/2/iNOS/soluble guanylyl cyclase/protein kinase G pathway contributes to the fall in mean arterial pressure and vascular reactivity in endotoxaemic rats, and the endotoxaemia-induced increase in iNOS-derived NO production suppresses renal CYP 4A protein expression and activity [22,40]. We have also previously demonstrated that the fall in mean arterial pressure in endotoxaemic rats is associated with a decrease in the expression of CYP 4A1/A3 protein in the kidney and increased levels of nitrite in the serum, kidney, heart, thoracic aorta and superior mesenteric artery [20–24]. Furthermore, administration of a synthetic analogue of 20-HETE, 5,14-HEDGE, prevented the hypotension and vascular hypo-reactivity associated with the changes in systemic and tissue NO production as well as endotoxin-induced cardiac, renal and vascular iNOS protein expression and endotoxin-induced decrease in eNOS protein levels in rats treated with endotoxin [25,26]. These findings suggest that iNOS-derived NO-induced inhibition of the formation of an arachidonic acid metabolite most likely 20-HETE, and removal of its influence on vascular tone contributes to the fall in blood pressure and vascular hyporeactivity in endotoxic shock. We have recently demonstrated that prostanoids produced during endotoxaemia increase iNOS protein expression and NO synthesis, and decrease CYP 4A1 protein expression and CYP 4A activity [24]. Moreover, dual inhibition of iNOS or COX by indomethacin restored renal CYP 4A protein level and CYP 4A activity and mean arterial pressure in endotoxin-treated rats, suggesting that the effects of non-selective inhibition of COX might be due to increased production of arachidonic acid metabolites derived via CYP 4A. In the present study, endotoxin-induced fall in mean arterial pressure and rise in heart rate were associated with an increase in 6-keto-PGF1α (an index for PGI2 production), PGE2, TxB2 (an index for TxA2 production) and nitrite (as an index for NO production) levels in the serum, kidney, heart, thoracic aorta and superior mesenteric artery, and a decrease in systemic and renal 20-HETE and PGF2α levels. These effects of endotoxin were prevented by a selective COX-2 inhibitor, NS-398. These results suggest that decreased production of PGI2, PGE2, and TxA2, and NO associated with increased levels of 20-HETE and PGF2α, contributes to the effect of selective COX-2 inhibitor to prevent the endotoxin-induced decrease in mean arterial pressure and increase in heart rate in endotoxaemic rats. Further characterization of the molecular mechanisms of interactions between COX, NOS and CYP 4A enzymes will provide the framework for extension of this work into understanding the role of arachidonic acid products and NO on the decrease in blood pressure during endotoxaemia.
In conclusion, the present study indicates that a fall in the production of vasoconstrictor eicosanoids, 20-HETE and PGF2α, and an increase in the levels of a vasoconstrictor prostanoid, TxA2, and vasodilator arachidonic acid products, PGI2, PGE2, as well as NO synthesis contribute to the hypotension in rats treated with endotoxin. Our findings also demonstrate that increased production of 20-HETE and PGF2α associated with decreased levels of PGI2, PGE2 and TxA2, and NO synthesis contribute to the effect of selective COX-2 inhibitors. Impairment of cardiovascular and renal function is critically involved in the pathophysiological sequale in septic shock finally resulting in multiorgan failure and death; restoration of these impaired functions should improve therapeutic benefit. In light of the important role of prostanoids, NO and 20-HETE in endotoxin-induced hypotension in rats, the interaction of COX, NOS and CYP 4A pathways should be considered when developing new strategies for drug development in the treatment of endotoxic shock. More importantly, further studies with selective COX-2 inhibitors as well as arachidonic acid metabolites generated via CYP 4A including stable analogues of 20-HETE in models of endotoxaemia could provide a novel approach to treat hypotension in septic shock.
The present study is limited by the measurement of mean arterial pressure, heart rate, 6-keto-PGF1α, PGE2, PGF2α, TxA2, 20-HETE and nitrite levels in serum and tissues of control and endotoxaemic rats treated with NS-398 to determine whether an increase in 20-HETE and PGF2α levels contributes to the effect of selective COX-2 inhibitor to prevent endotoxin-induced fall in blood pressure associated with an increase in the production of PGI2, PGE2, and TxA2, and NO synthesis. One of the future perspectives for the continuation of the study is to do further experiments, that are currently being done in our laboratory, such as vascular and inflammatory response studies and measurement of COX-2, CYP 4A1 and iNOS mRNA, and protein levels as well as activities of these enzymes in the renal, cardiac and vascular tissues. Additional experiments could also be conducted to repeat the same experiment in animals lacking COX-2 in order to demonstrate the validity of the proposed hypothesis. The pertinence of acute endotoxaemia as a model for human sepsis has also been questioned. However, the cellular and molecular mechanisms of the effect of several COX-2 inhibitors or prostanoid receptor antagonists in preventing endotoxin-induced fall in blood pressure demonstrated in this model, have been generally confirmed in more sophisticated models [32–34, 37–39, 41–43].
Acknowledgments
This work was supported by USPHS NIH Grant HLBI-19134-33A1, the Research Foundation of Mersin University (Project Code No: BAP ECZF EMB (BT) 2006-3), NIH Grant GM31278 and the Robert A. Welch Foundation.
References
- 1.Kleinert H, Schwarz PM, Förstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol Chem. 2003;384:1343–64. doi: 10.1515/BC.2003.152. [DOI] [PubMed] [Google Scholar]
- 2.Tunctan B, Altug S. The use of nitric oxide synthase inhibitors in inflammatory diseases: a novel class of anti-inflammatory agents. Cur Med Chem Anti-Inflammatory & Anti-Allergy Agents. 2004;3:271–301. [Google Scholar]
- 3.Cauwels A. Nitric oxide in shock. Kidney Int. 2007;72:557–65. doi: 10.1038/sj.ki.5002340. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes D, Assreuy J. Nitric oxide and vascular reactivity in sepsis. Shock. 2008;30:10–3. doi: 10.1097/SHK.0b013e3181818518. [DOI] [PubMed] [Google Scholar]
- 5.Hauser B, Bracht H, Matejovic M, Radermacher P, Venkatesh B. Nitric oxide synthase inhibition in sepsis? Lessons learned from large-animal studies. Anesth Analg. 2005;101:488–98. doi: 10.1213/01.ANE.0000177117.80058.4D. [DOI] [PubMed] [Google Scholar]
- 6.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41:175–93. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 7.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 8.Escalante B, Omata K, Sessa W, Lee SG, Falck JR, Schwartzman ML. 20-hydroxyeicosatetraenoic acid is an endothelium-dependent vasoconstrictor in rabbit arteries. Eur J Pharmacol. 1993;235:1–7. doi: 10.1016/0014-2999(93)90812-v. [DOI] [PubMed] [Google Scholar]
- 9.Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41:801–6. doi: 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- 10.Schwartzman ML, Falck JR, Yadagiri P, Escalante B. Metabolism of 20-hydroxyeicosatetraenoic acid by cyclooxygeanse: formation and identification of novel endothelium-dependent vasoconstrictor metabolites. J Biol Chem. 1989;264:11658–62. [PubMed] [Google Scholar]
- 11.Escalante B, Sessa WC, Falck JR, Yadagiri P, Schwartzman ML. Vasoactivity of 20-hydroxyeicosatetraenoic acid is dependent on metabolism by cyclooxygenase. J Pharmacol Exp Ther. 1989;248:229–32. [PubMed] [Google Scholar]
- 12.Carroll MA, Garcia MP, Falck JR, McGiff JC. Cyclooxygenase dependency of the renovascular actions of cytochrome P450-derived arachidonate metabolites. J Pharmacol Exp Ther. 1992;260:104–9. [PubMed] [Google Scholar]
- 13.Fang X, Faraci FM, Kaduce TL, Harmon S, Modrick ML, Hu S, et al. 20-Hydroxyeicosatetraenoic acid is a potent dilator of mouse basilar artery: role of cyclooxygenase. Am J Physiol Heart Circ Physiol. 2006;291:H2301–7. doi: 10.1152/ajpheart.00349.2006. [DOI] [PubMed] [Google Scholar]
- 14.Pratt PF, Falck JR, Reddy KM, Kurian JB, Campbell WB. 20-HETE relaxes bovine coronary arteries through the release of prostacyclin. Hypertension. 1998;31:237–41. doi: 10.1161/01.hyp.31.1.237. [DOI] [PubMed] [Google Scholar]
- 15.Yu M, McAndrew RP, Al-Saghir R, Maier KG, Medhora M, Roman RJ, et al. Nitric oxide contributes to 20-HETE-induced relaxation of pulmonary arteries. J Appl Physiol. 2002;93:1391–9. doi: 10.1152/japplphysiol.00247.2002. [DOI] [PubMed] [Google Scholar]
- 16.Carroll MA, Capparelli MF, Doumand AB, Cheng MK, Jiang H, McGiff JC. Renal vasoactive eicosanoids: interactions between cytochrome P450 and cyclooxygenase metabolites during salt depletion. Am J Hypertens. 2001;14:159A. [Google Scholar]
- 17.Alonso-Galicia M, Sun CW, Falck JR, Harder DR, Roman RJ. Contribution of 20-HETE to the vasodilator actions of nitric oxide in renal arteries. Am J Physiol. 1998;275:F370–8. doi: 10.1152/ajprenal.1998.275.3.F370. [DOI] [PubMed] [Google Scholar]
- 18.Wang MH, Wang J, Chang HH, Zand BA, Jiang M, Nasjletti A, et al. Regulation of renal CYP4A expression and 20-HETE synthesis by nitric oxide in pregnant rats. Am J Physiol. 2003;285:F295–302. doi: 10.1152/ajprenal.00065.2003. [DOI] [PubMed] [Google Scholar]
- 19.Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–5. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- 20.Tunctan B, Korkmaz B, Yildirim H, Tamer L, Atik U, Buharalioglu CK. Increased production of nitric oxide contributes to renal oxidative stress in endotoxemic rat. Am J Infect Dis. 2005;1:111–5. [Google Scholar]
- 21.Tunctan B, Korkmaz B, Yildirim H, Tamer L, Atik U, Buharalioglu CK. Reversal of endotoxin-induced hypotension by inhibition of inducible nitric oxide synthase activity is associated with improved oxidative status in rat heart, aorta and mesenteric artery. Turkish J Med Sci. 2006;36:71–80. [Google Scholar]
- 22.Tunctan B, Yaghini FA, Estes A, Malik KU. Inhibition by nitric oxide and cyclooxygenase of cytochrome P450 4A expression and activity contributes to endotoxin-induced hypotension in rats. Nitric Oxide: Biol Chem. 2006;14:51–7. doi: 10.1016/j.niox.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Tunctan B, Korkmaz B, Dogruer ZN, Tamer L, Atik U, Buharalioglu C. K. Inhibition of extracellular signal-regulated kinase (ERK1/2) activity reverses endotoxin-induced hypotension via decreased nitric oxide production in rats. Pharmacol Res. 2007;56:56–64. doi: 10.1016/j.phrs.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Tunctan B, Yaghini FA, Estes A, Malik KU. Prostaglandins inhibit cytochrome P450 4A activity and contribute to endotoxin-induced hypotension in rats via nitric oxide production. Arch Pharm Res. 2008;31:856–65. doi: 10.1007/s12272-001-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tunctan B, Korkmaz B, Buharalioglu CK, Sahan Firat S, Anjaiah S, Falck J, et al. A 20-HETE agonist, N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine, opposes the fall in blood pressure and vascular reactivity in endotoxin-treated rats. Shock. 2008;30:329–35. doi: 10.1097/SHK.0b013e31816471c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuez T, Korkmaz B, Buharalioglu CK, Sahan-Firat S, Falck J, Tunctan B, et al. A synthetic analogue of 20-HETE, 5,14-HEDGE, reverses endotoxin-induced hypotension via increased 20-HETE levels associated with decreased iNOS protein expression and vasodilator prostanoid production in rats. Basic Clin Pharmacol. 2009 doi: 10.1111/j.1742-7843.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsiotou AG, Sakorafas GH, Anagnostopoulos G, Bramis J. Septic shock; current pathogenetic concepts from a clinical perspective. Med Sci Monit. 2005;11:RA76–85. [PubMed] [Google Scholar]
- 28.Ashorobi RB, Williams PA. Indomethacin and alpha-tocopherol enhanced survival in endotoxic rats. Cent Afr J Med. 1995;41:216–19. [PubMed] [Google Scholar]
- 29.Tunctan B, Altug S, Uludag O, Abacioglu N. Time-dependent variations in serum nitrite, 6-keto-prostaglandin F1α and thromboxane B2 levels induced by lipopolysaccharide in mice. Biol Rhythm Res. 2000;1:499–514. [Google Scholar]
- 30.Futaki N, Takahashi S, Kitagawa T, Yamakawa Y, Tanaka M, Higuchi S. Selective inhibition of cyclooxygenase-2 by NS-398 in endotoxin shock rats in vivo. Inflam Res. 1997;46:496–502. doi: 10.1007/s000110050232. [DOI] [PubMed] [Google Scholar]
- 31.Fatehi-Hassanabad Z, Muller M, Andriantsitohaina R, Furman BL, Parratt JR, Stoclet JC. Influence of indomethacin on the haemodynamic effects of lipopolysaccharide in rats. Fundam Clin Pharmacol. 1996;10:258–63. doi: 10.1111/j.1472-8206.1996.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 32.Vayssettes-Courchay C, Bouysset F, Verbeuren TJ. Involvement of COX and NOS induction in the sympatho-activation during sepsis. Auton Neurosci. 2002;98:33–6. doi: 10.1016/s1566-0702(02)00027-9. [DOI] [PubMed] [Google Scholar]
- 33.Tunctan B, Korkmaz B, Cuez T, Buharalioglu CK, Sahan-Firat S, Falck J, et al. Interactions between cytochrome P4504A, cyclooxygenase and nitric oxide synthase during endotoxemia: therapeutic implications for inflammatory diseases. EHRLICH II - 2nd World Conference on Magic Bullets, Celebrating the 100th Anniversary of the Nobel Prize Award to Paul Ehrlich; Nurnberg, Germany. October 3–5; 2008. p. A-330. Abstract Book. [Google Scholar]
- 34.Tunctan B, Korkmaz B, Cuez T, Sahan-Firat S, Yildirim H, Tamer L, et al. Increased production of 20-HETE contributes to the effects of COX inhibitors to prevent the decrease in lipid peroxidation and increase in catalase activity during endotoxemia. FASEB J. 2009;23:937.13. [Google Scholar]
- 35.Luss H, Watkins SC, Freeswick PD, Imro AK, Nussler AK, Billiar TR, et al. Characterization of inducible nitric oxide synthase expression in endotoxemic rat cardiac myocytes in vivo and following cytokine exposure in vitro. J Mol Cell Cardiol. 1995;27:2015–29. doi: 10.1016/0022-2828(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 36.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 37.Tunctan B, Altug S, Uludag O, Demirkay B, Abacioglu N. Effects of cyclooxygenase inhibitors on nitric oxide production and survival in a mice model of sepsis. Pharmacol Res. 2003;48:37–48. [PubMed] [Google Scholar]
- 38.Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–52. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- 39.Cuzzocrea S, Salvemini D. Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney Int. 2007;71:290–7. doi: 10.1038/sj.ki.5002058. [DOI] [PubMed] [Google Scholar]
- 40.Korkmaz B, Buharalioglu K, Demiryürek TA, Sahan-Firat S, Cuez T, Tunctan B. Activation of MEK1/ERK1/2/iNOS/sGC/PKG pathway contributes to the fall in blood pressure and vascular reactivity in endotoxemic rats. FASEB J. 2009;23:932.5. [Google Scholar]
- 41.Höcherl K, Dreher F, Kurtz A, Bucher M. Cyclooxygenase-2 inhibition attenuates lipopolysaccharide-induced cardiovascular failure. Hypertension. 2002;40:947–53. doi: 10.1161/01.hyp.0000041221.13644.b9. [DOI] [PubMed] [Google Scholar]
- 42.Höcherl K, Schmidt C, Kurt B, Bucher M. Activation of the PGI(2)/IP system contributes to the development of circulatory failure in a rat model of endotoxic shock. Hypertension. 2008;52:330–5. doi: 10.1161/HYPERTENSIONAHA.108.112029. [DOI] [PubMed] [Google Scholar]
- 43.Mittra S, Hyvelin JM, Shan Q, Tang F, Bourreau JP. Role of cyclooxygenase in ventricular effects of adrenomedullin: is adrenomedullin a double-edged sword in sepsis? Am J Physiol Heart Circ Physiol. 2004;286:H1034–42. doi: 10.1152/ajpheart.00337.2003. [DOI] [PubMed] [Google Scholar]