Abstract
GroEL recognizes proteins that are folding improperly or that have aggregation-prone intermediates. Here we have used as substrates for GroEL, wildtype (WT) coat protein of phage P22 and 3 coat proteins that carry single amino acid substitutions leading to a temperature-sensitive folding (tsf) phenotype. In vivo, WT coat protein does not require GroEL for proper folding, whereas GroEL is necessary for the folding of the tsf coat proteins; thus, the single amino acid substitutions cause coat protein to become a substrate for GroEL. The conformation of WT and tsf coat proteins when in a binary complex with GroEL was investigated using tryptophan fluorescence, quenching of fluorescence, and accessibility of the coat proteins to proteolysis. WT coat protein and the tsf coat protein mutants were each found to be in a different conformation when bound to GroEL. As an additional measure of the changes in the bound conformation, the affinity of binding of WT and tsf coat proteins to GroEL was determined using a fluorescence binding assay. The tsf coat proteins were bound more tightly by GroEL than WT coat protein. Therefore, even though the proteins are identical except for a single amino acid substitution, GroEL did not bind these substrate polypeptides in the same conformation within its central cavity. Therefore, GroEL is likely to bind coat protein in a conformation consistent with a late folding intermediate, with substantial secondary and tertiary structure formed.
INTRODUCTION
The primary amino acid sequence encodes for both the folding and the 3-dimensional structure of a protein (Anfinsen 1973). However, for many proteins folding in vivo requires facilitation by folding accessory proteins to avoid irreversible aggregation (Fenton and Horwich 1997). In bacteria, the major folding assistants are the complex of GroEL, an ATPase, and GroES. The crystal structures for both GroEL and the GroEL:GroES:ADP7 complex have been solved (Braig et al 1994; Xu et al 1997). GroEL is a homotetradecamer arranged in 2 heptameric rings constructed from 57-kDa monomeric units, and GroES consists of a single heptameric ring of 10-kDa subunits. The central cavity of GroEL is lined with hydrophobic residues that have been identified as the peptide binding site. When GroES and ATP bind to the ring of GroEL that contains the bound substrate polypeptide, there is a conformational change that buries the hydrophobic amino acids and exposes hydrophilic residues in the central cavity of GroEL (Xu et al 1997). However, the exact mechanism by which GroEL and GroES function in the folding of a substrate protein remains controversial and may be dependent on the polypeptide substrate (Fisher 1998).
The interactions between the bound polypeptide and GroEL has been the subject of several studies. Both hydrophobic and ionic interactions have been implicated in the binding of substrate polypeptides to GroEL (Zahn et al 1994; Perrett et al 1997; Brazil et al 1998). GroEL has been shown to bind to substrates in a conformation with ordered secondary structure or in an extended conformation (Landry and Gierasch 1991; Schmidt and Buchner 1992; Buckle et al 1997). Moreover, GroEL has been observed to bind to molten globule intermediates, as well as late folding intermediates (Martin et al 1991; Hayer-Hartl et al 1994; Katsumata et al 1996; Goldberg et al 1997).
In addition to the studies described above that investigated the conformation of a particular bound substrate polypeptide, comparisons between homologous proteins derived from different species or from different cellular compartments have been used in order to understand how GroEL recognizes and binds substrate polypeptides (Staniforth et al 1994; Mattingly et al 1995, 1998; Clark et al 1996; Ellis and Hartl 1996; Clark and Frieden 1997, 1999). The difficulty presented by these studies is that the proteins are only about 30–50% similar in primary sequence. Thus, variation seen in the interaction of the substrate polypeptide with GroEL, either through changes in the conformation of the bound substrate polypeptides or in the affinity of binding, may be due to these substantial differences in the primary sequence of the proteins investigated and not in the manner that GroEL has bound the polypeptides.
The binding by GroEL of substrate polypeptides with single amino acid substitutions has been compared to the binding of the corresponding wildtype (WT) polypeptide. GroEL has been shown to bind preferentially to substrates with a hydrophobic or positively charged amino acid introduced into the sequence from studies using mutants of barley chymotrypsin inhibitor as a substrate (Itzhaki et al 1995). Interaction with single point mutants of yeast citrate synthase indicated GroEL favored binding to mutants that were thermodynamically less stable than WT citrate synthase (Zahn et al 1996). Conversely, single amino acid substitutions in barnase did not significantly change the energetics of its interaction with GroEL; however, barnase does not form a stable complex with GroEL (Gray and Fersht 1993; Corrales and Fersht 1995). Thus, we can conclude from these studies that the interaction of a substrate polypeptide with GroEL may be particular both to the reaction conditions and to the substrate.
Here we have investigated the interaction of the coat protein of bacteriophage P22 and GroEL. Coat protein is a 47-kDa protein comprised of 430 amino acids (Eppler et al 1991). Eighteen amino acid substitutions at 17 sites throughout the sequence of P22 coat protein have been identified that cause production of phage to be temperature sensitive (Gordon and King 1993). In vivo, these temperature-sensitive folding (tsf) mutants exhibit a sharply reduced yield of soluble coat protein at the nonpermissive temperature. The newly synthesized tsf coat proteins are instead found in inclusion bodies (Gordon and King 1993). Overexpression of GroEL and GroES has been shown to prevent the aggregation of all 18 tsf mutants at nonpermissive temperatures, while having no effect on the folding or aggregation of WT coat protein (Gordon et al 1994; Nakonechny and Teschke 1998). The requirement for increased concentrations of GroEL to facilitate the folding of the tsf coat protein mutants in vivo would suggest that the single amino acid substitutions alter the folding of coat protein, thereby causing the tsf mutant proteins to become substrates for GroEL. In addition, phage with tsf coat protein substitutions when grown on cells containing mutated GroEL or GroES are unable to form viable progeny at significantly lower temperatures than when grown on cells with WT GroEL/S. Phage carrying WT coat protein are not affected by mutated GroEL or GroES (Nakonechny and Teschke 1998). Again, these data indicate that the tsf substitutions lead coat protein to become a substrate of GroEL/S.
The phenotype of the tsf coat protein mutants can be replicated in in vitro folding experiments (Teschke 1999). An assembly-competent state of the tsf coat protein is attained when the purified proteins are refolded from denaturant at temperatures from 4 to 20°C. However, if the proteins are refolded at temperatures from 30 to 36°C, the tsf coat proteins aggregate. The tsf coat proteins that are first folded at low temperature and then shifted to high temperature also aggregate. WT coat protein is resistant to aggregation during folding, or once folded, at all temperatures tested. The secondary and tertiary structures of the tsf mutant proteins are altered as compared to WT coat protein. In addition, the tsf coat proteins bind more of the hydrophobic dye, bisANS, than does WT coat protein (Teschke 1999). Thus, the single amino acid substitutions that give rise to the tsf phenotype have substantial effects on the folded state of coat protein.
Recent experiments have shown that the tsf coat proteins fold from denaturant at the same rate as WT coat protein; rather, the unfolding rate was changed by the tsf amino acid substitutions (C.M.T., unpublished results). Thus, the binding of coat protein to GroEL is unlikely to be controlled by the kinetics of folding. Because the single amino acid substitutions of the tsf coat proteins cause them to fold into different final conformations in vitro without affecting the rate of folding, we investigated whether the conformation of WT and tsf coat proteins bound by GroEL would be the same, indicating that the interaction occurred by binding an early folding intermediate or reflect the differences that were observed in the folded state that would suggest that GroEL binds coat protein as a late folding intermediate.
MATERIALS AND METHODS
Chemicals
Ultrapure guanidine hydrochloride (GuHCl) and urea were purchased from ICN (Costa Mesa, CA, USA). BisANS was purchased from Molecular Probes (Eugene, OR, USA). Lyophilized proteases on beads were obtained from Sigma (St. Louis, MO, USA). All other chemicals were reagent grade from common sources.
Buffers
The buffer used in all of the experiments was phosphate buffer (20 mM NaPO4, pH 7.6, 10 mM KCl). Tris-based buffers were used during purification where noted.
Purification of coat proteins
The tsf coat proteins used were obtained from empty procapsid shell stocks that were prepared as previously described (Prevelige et al 1988; Galisteo et al 1995; Teschke 1999).
Purification of GroEL/S
Escherichia coli XL1-Blue cells from Stratagene (La Jolla, CA, USA) containing the plasmid pSigE that carries the GroEL/S operon in pSE380 (Stratagene) were grown at 37°C to a concentration of 4 × 108 cells/mL. GroEL/S overproduction was induced by the addition of isopropyl-d-thiogalactopyranoside to a final concentration of 0.6 mM. After incubation overnight, the cultures were spun briefly in a GSA rotor at 10 000 rpm to pellet the cells. The cells were washed twice in 0.1 M Tris base, pH 7.6, with HCl and then suspended at a concentration of 2.5 × 1010 cells/mL in the same buffer with 0.3 M sucrose and 12.5 mM ethylenediaminetetraacetic acid (EDTA). Spheroplasts were produced by the addition of lysozyme to a final concentration of 200 μg/mL, then incubated on ice for 30 minutes with periodic gentle shaking. The spheroplasts were stabilized by the addition of MgCl2 to a final concentration of 15 mM, pelleted, suspended in phosphate buffer with 1 mM phenylmethylsulfonylfluoride, and frozen overnight at −80°C (Hazelbauer and Harayama 1979). The thawed, lysed spheroplasts were incubated at 37°C for 30 minutes with 20 μg/mL RNase followed by incubation in 5 mM MgCl2 and 20 μg/mL DNase for an additional 30 minutes. The processed lysates were centrifuged to remove debris and stored at −80°C.
After lysis, the GroEL purification was done similarly to the protocols described by Staniforth et al (1994) and Clark et al (1996). Briefly, the cell lysate was run over a Q-Sepharose fast flow ion-exchange column (Pharmacia LKB, Piscataway, NJ, USA), equilibrated with 50 mM Tris base, 25 mM NaCl, 2 mM EDTA, pH 7.6, with HCl. Proteins were eluted from the column using a linear 0–0.75 M NaCl gradient in the same buffer. Fractions containing GroEL were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), pooled, and precipitated with 70% (NH4)2SO4 at 4°C. The precipitant was suspended in a small volume of the column buffer containing 10 mM MgCl2 and 1 mM ATP, run over a Sephacryl S-300 column (Pharmacia LKB), and fractions containing GroEL were pooled and precipitated with 70% (NH4)2SO4 at 4°C. The precipitant was suspended in phosphate buffer and dialyzed against phosphate buffer containing 0.5 mg/mL acid-washed charcoal to remove nucleotides and residual (NH4)2SO4. To eliminate contaminating peptides, the GroEL was run over a Reactive Red 120 agarose affinity column (Pharmacia LKB) equilibrated with phosphate buffer. Fractions containing GroEL were identified, pooled, and concentrated in a CentriPrep50 from Millipore (Bedford, MA, USA). Glycerol was added to the buffer to a final concentration of 10%, and aliquots were stored at −80°C. Just prior to use, aliquots were thawed and the GroEL concentration was determined using the bicinchoninic acid protein assay from Pierce (New York, NY, USA).
Refolding of coat protein
Empty procapsid shells were unfolded by incubation in 6.75 M urea. After the shells were incubated for 30 minutes at room temperature, a time sufficient for complete dissociation of the shells and unfolding of the coat protein subunits (data not shown), refolding was initiated by rapidly diluting the urea 20-fold with phosphate buffer. The unfolded coat protein concentrations were 7.5 mg/mL or 2 mg/mL, and the final concentrations of refolded coat protein were 0.735 mg/mL or 0.1 mg/mL in 0.338 M urea.
Formation of GroEL coat protein complexes
Aliquots of unfolded coat protein were rapidly diluted with phosphate buffer containing GroEL at various molar ratios from 1:4 to 4:1 GroEL 14-mer:coat protein as detailed in each experiment.
Sucrose gradients
Refolded coat protein at a concentration of 100 μg/mL alone or with a 1:1 molar ratio of GroEL 14-mer was run on a 2-mL, 5–20% linear sucrose gradient that was constructed using a Gradient Master model 106 from Biocomp Instruments (Fredericton, NB, Canada). The gradients were centrifuged using an RP55-S rotor in a Sorvall RC M120EX for 90 minutes at 23°C at 166 991 × g (Sorvall, Newton, CT, USA). Aliquots of 200 μL fractionated from the top with a positive displacement pipetman were run on a 9% SDS polyacrylamide gel. The bands were visualized with Coomassie blue and the coat protein bands were quantified using the Kodak Digital Science Electrophoresis Documentation and Analysis System.
Tryptophan fluorescence
Fluorescence spectra of refolded coat protein complexed with GroEL were taken with an SLM Aminco-Bowman 2 spectrofluorometer thermostatted at 23°C. The excitation wavelength was set at 295 nm, the emission was monitored from 310 nm to 400 nm, and the band passes were set to 4 nm. Data were corrected for tyrosine fluorescence from GroEL. Below 0.3 μM of GroEL 14-mer, no light scattering was observed. There was a linear relationship between GroEL concentration and light scattering when the GroEL 14-mer concentrations were equal to or above 0.3 μM. Therefore, data collected using GroEL 14-mer concentrations above 0.3 μM were corrected for changes in intensity caused by light scattering.
Fluorescence quenching experiments
GroEL:coat protein complexes were diluted 1:10 with phosphate buffer containing up to 0.3 M acrylamide. Samples were incubated for 15 minutes, and fluorescence was measured as described above. The fluorescence intensity corrected for inner filter effect due to the acrylamide was approximated from the equation (Fcorr = Fobsantilog[OD295 + OD340/2]). The quenching data were analyzed using the Stern-Volmer equation using the plot F0/F vs Q where F0 is the fluorescence in the absence of quencher, and F is the fluorescence of the sample in the presence of the quencher, Q (Lehrer 1971; Lakowicz 1983).
Analysis of binding
Coat protein binding to GroEL was analyzed using a double titration method with the fluorescence excitation wavelength at 295 nm and the emission wavelength at 340 nm (Secnik et al 1992; Shi et al 1994; Teschke 1999). Titrations were performed in phosphate buffer at 23°C. Enhancement of coat protein fluorescence was quantified by first subtracting fluorescence of GroEL and correcting for light scattering and inner filter effect as described above. In the first titration the concentration of coat protein was held constant and the GroEL concentration was varied. However, because in vivo coat protein is the ligand, the experiment was repeated with the GroEL concentration held constant at 0.1 μM and the concentration of coat protein varied from 0 to 0.2 μM. Because the coat protein binding to GroEL showed positive cooperativity in the second titration, the data were analyzed with the Hill equation, log F/Fmax = n log[coat protein] − log Kd (Freidfelder 1982).
RESULTS
GroEL binds a wide variety of protein substrates (Fenton and Horwich 1997). Here we have investigated the interaction of GroEL with coat protein of phage P22. Coat protein provides a unique opportunity to investigate how GroEL binds to substrate polypeptides because single amino acid substitutions lead coat protein to become substrates for GroEL (Gordon et al 1994; Nakonechny and Teschke 1998). Thus, we have compared the binding by GroEL of WT coat protein, which is not a substrate for GroEL in vivo, to tsf mutant coat proteins.
WT and tsf coat proteins form complexes with GroEL
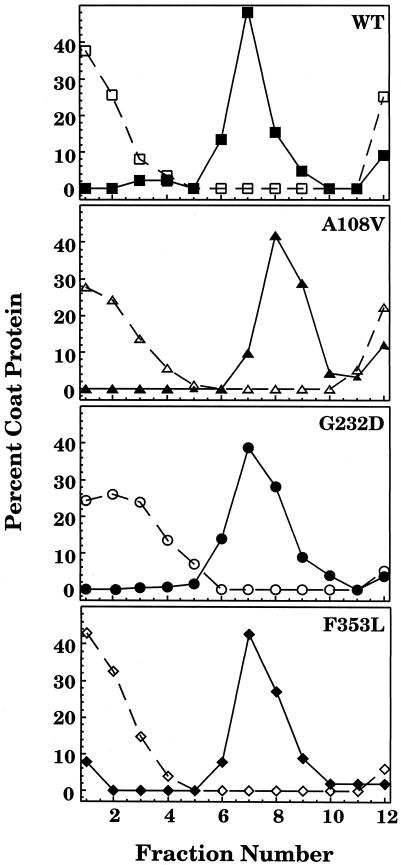
To demonstrate that coat protein can form complexes with GroEL in vitro, WT and tsf mutant coat proteins were refolded from the urea-denatured state by rapid dilution either with buffer alone or with buffer that contained GroEL. The protein solutions were sedimented through 5–20% sucrose gradients, fractionated, and analyzed by SDS-PAGE. The coat proteins folded with buffer alone remained at the top of the sucrose gradient in fractions 1–4 (Fig 1). GroEL sedimented further down the gradient, in fractions 6–9 (data not shown). When the WT and tsf coat proteins were refolded in the presence of a 1:1 molar ratio of GroEL 14-mer and sedimented through a sucrose gradient, there was little refolded coat protein remaining at the top of the gradient; rather the coat protein appeared in the same fractions as GroEL. Thus, most of the coat protein was associated with GroEL. These results indicated that a 1:1 molar ratio of GroEL 14-mer to coat protein monomer was sufficient to bind WT and tsf coat proteins.
Fig 1.
Sucrose gradient sedimentation of refolded coat protein and binary complexes. WT and tsf coat proteins refolded either alone with buffer (open symbols) or in the presence of a 1:1 molar ratio of GroEL 14-mer to coat protein (closed symbols) were sedimented through 5–20% sucrose gradients. Following fractionation and visualization on SDS gels, the coat protein bands were quantified. The data are expressed as a percentage of the total coat protein in the gradient. Fraction 1 is the top of the gradient
Conformation of WT and tsf coat proteins complexed with GroEL probed by fluorescence
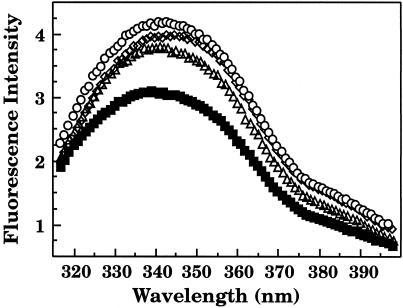
Fluorescence emission spectra of WT and tsf coat proteins bound to GroEL were compared to determine if there were changes in the environment of the 6 coat protein tryptophans (Eppler et al 1991). GroEL has no tryptophans to interfere in the analysis of the emission spectra of the coat protein:GroEL complexes (Hemmingsen et al 1988). WT coat protein in a complex with GroEL had significantly lower intensity as compared to the binary complex of each of the 3 tsf coat proteins (Fig 2). Consistent with our data, Galisteo et al (1995) reported that the refolded tsf mutants showed an increase in fluorescence intensity compared to refolded WT coat protein. They suggested that the tryptophans of WT coat protein were quenched in the folded state and therefore a change in the conformation of the folded tsf coat protein relieved this quenching (Galisteo et al 1995). In addition, the emission maximum of each mutant in the binary complex was shifted to longer wavelengths as compared to the binary complex of WT coat protein (Table 1). A red shift in fluorescence is generally due to an increase in the solvent exposure of tryptophans (Lakowicz 1983). The tsf coat protein mutants when folded alone in buffer also show a red shift in tryptophan fluorescence as compared to WT coat protein (Teschke 1999). These results suggest that the tryptophans of the tsf coat proteins remained more exposed to solvent than WT coat protein when bound by GroEL.
Fig 2.
Fluorescence emission spectra of coat protein:GroEL binary complexes. The fluorescence spectra were taken 45 minutes after dilution of the unfolded coat proteins with buffer that contained enough GroEL 14-mer for a 1:1 molar ratio with coat protein
Table 1.
Fluorescence emission maxima for coat protein in a binary complex with GroEL
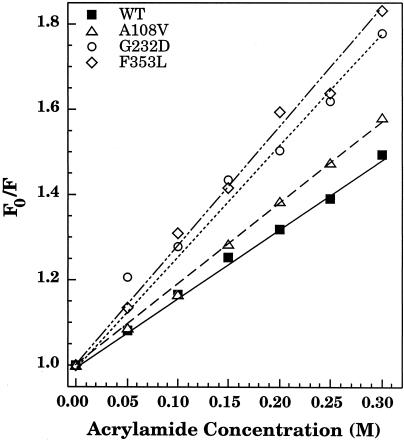
To confirm that the tryptophans of the tsf coat protein were indeed more solvent exposed than those of WT coat protein within the central cavity of GroEL, collisional quenching with acrylamide was used. Fluorescence of the coat protein:GroEL complexes was measured both before (F0) and after (F) the addition of acrylamide (Fig 3; Table 2). Stern-Volmer plots provide an indication of solvent accessibility where a steep slope indicates that the tryptophans are exposed to the acrylamide, while a shallow slope indicates that the tryptophans are protected from the acrylamide (Lakowicz 1983). The slope of the Stern-Volmer plot of the WT coat protein:GroEL complex was more shallow than each of the tsf coat protein:GroEL complexes. The tryptophans of G232D and F353L when in a complex with GroEL were substantially more solvent exposed than those of WT or A108V:GroEL complexes. Thus, these results were consistent with the results of the emission spectra and indicated that the tsf mutant coat proteins were bound in a different manner to GroEL than WT coat protein.
Fig 3.
Acrylamide quenching of tryptophan fluorescence. Initial fluorescence (F0) of the binary complexes of coat protein and GroEL 14-mer was measured. Following incubation for 15 minutes with acrylamide at concentrations from 0 to 0.3 M, the tryptophan fluorescence (F) was measured. The data are expressed as a ratio (F0/F) and are plotted against the acrylamide concentration
Table 2.
Acrylamide quenching of coat protein in a binary complex with GroEL
Proteolysis of coat protein within the central cavity of GroEL
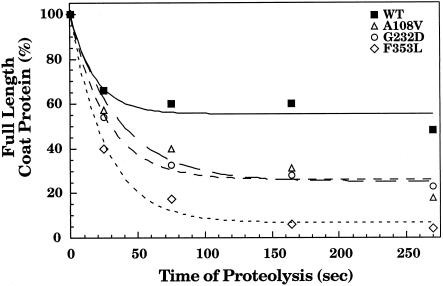
We wanted to examine the conformation of WT and tsf coat proteins within the central cavity of GroEL using a method other than fluorescence, thereby allowing an independent measure of any changes observed. Thus, the conformation of WT and tsf coat proteins in a complex with GroEL was probed with chymotrypsin attached to agarose beads. Chymotrypsin was chosen for this experiment because it is a fairly nonspecific protease and can be used as a general probe for regions of coat protein that are protease accessible. Chymotrypsin attached to agarose beads was used in this experiment because coat protein is rather sensitive to protease digestion and proteases attached to beads have lower activity than free proteases (Teschke 1999). In addition, a recent crystal structure of GroEL with bound substrate peptides showed that the peptide binding site is at the top of the central cavity of GroEL, not deep within the cavity, indicating that substrate polypeptides would be accessible to protease attached to beads (Chen and Sigler 1999). Following proteolysis for fixed periods of time, the remaining full-length coat protein was quantified, and the results were plotted against the time of proteolysis (Fig 4). WT coat protein in the binary complex with GroEL was most resistant to proteolysis with 60% full-length coat protein remaining after digestion with chymotrypsin for about 5 minutes. Conversely, the tsf mutants were substantially more sensitive to digestion by chymotrypsin. Full-length F353L within the central cavity of GroEL was nearly completely digested by around 5 minutes. The patterns of peptides generated by the proteolysis of WT and each tsf mutant were compared to determine if the proteins were bound differently by GroEL. Few differences were detected in the peptide patterns generated by proteolysis of WT and tsf coat proteins in a binary complex with GroEL (data not shown). In addition, the peptide patterns for the digestion of the folded WT and tsf coat proteins alone were similar to those generated when the binary complexes were digested, indicating that the same sites are accessible to protease when the coat proteins are bound to GroEL and when folded alone in buffer. Coat protein is cleaved near its center when digested with chymotrypsin (Lanman et al 1999). Those large peptides are then further digested by the protease. These results indicated that for each protein the regions that were accessible to the protease were similar, but that the mutant coat proteins were less tightly folded within the central cavity of GroEL than WT coat protein. The digested binary complexes were run through 5–20% sucrose gradients to determine if all of the peptides stayed tightly bound by GroEL. We found that all of the peptides generated from digestion with the protease remained in a complex with GroEL, regardless of whether the binary complex was formed with WT or tsf coat proteins. Thus, we can conclude that all regions of coat protein can interact with GroEL.
Fig 4.
Proteolytic susceptibility of refolded coat protein and binary complexes. The binary complexes of coat protein:GroEL 14-mer were treated with chymotrypsin attached to agarose beads at room temperature. The residual full-length coat protein was quantified and the fraction remaining at each time point was calculated. The lines drawn are to aid the eye
Binding affinities and stoichiometry of coat protein:GroEL complexes
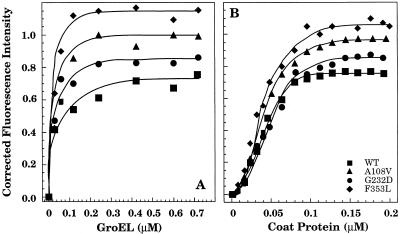
To determine the minimum number of coat proteins bound to GroEL and their binding affinities, titrations varying either the concentration of coat protein or GroEL were carried out, and the change in fluorescence intensity was measured. Irrespective of whether the concentration of GroEL or coat protein was held constant in the titration, the maximum fluorescence change occurred when the molar ratio of coat protein:GroEL 14-mer was approximately 1:1.
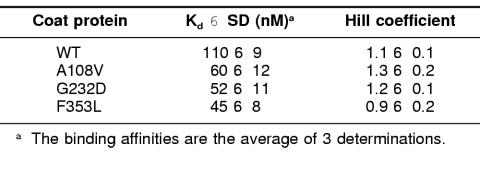
Binding of the coat proteins to GroEL was done 2 ways: either the coat protein concentration was held constant and the amount of GroEL 14-mer was varied or the concentration of GroEL was held constant while the concentration of coat protein was varied (Fig 5). The increase in fluorescence intensity that occurred when coat protein was bound by GroEL when plotted against the GroEL concentration gave the hyperbolic binding isotherm expected (Fig 5a). However, when the GroEL concentration was held constant and the concentration of coat protein was varied, a sigmoidal relationship in the binding isotherm was obtained (Fig 5b). To determine the number of coat protein binding sites on GroEL and their dissociation constants from a sigmoidal curve, the data were analyzed utilizing a Hill plot (Freidfelder 1982). From the double log transformation, the Kd is determined from the antilog of the y-intercept while the minimum number of binding sites comes from the slope of the line. In each case, GroEL binds a minimum of 1 coat polypeptide, which agrees with the titration varying GroEL concentration (Table 3). However, WT coat protein bound to GroEL with a dissociation constant higher than that of the tsf mutants (Table 3). The results from the Scatchard analysis of the titration where the concentration of coat protein was held constant and the concentration of GroEL varied were within the range of the Hill plot analysis (data not shown). The increased binding affinities of the tsf coat proteins for GroEL correlates well with the requirement for GroEL in vivo to fold the mutants properly and prevent aggregation.
Fig 5.
Binding of coat protein to GroEL. The increase in fluorescence of the WT and tsf binary complexes was followed at different ratios of coat protein to GroEL 14-mer. The corrected fluorescence intensity was plotted against the concentration of the protein being varied. (A) The concentration of coat protein was held constant at 0.2 μM while the concentration of GroEL was varied from 0 to 0.8 μM. (B) With the concentration of GroEL constant at 0.1 μM, the coat proteins were refolded at concentrations from 0 μM to 0.2 μM. The lines are drawn to aid the eye
Table 3.
Binding of coat protein to GroEL
DISCUSSION
Here we have investigated the conformation of WT coat protein and mutants of coat protein that require GroEL for proper folding at high temperatures, known as tsf mutants, when bound in the central cavity of GroEL. Because the tsf mutants do not fold substantially more slowly that WT coat protein in vitro (C.M.T., unpublished data), it seemed unlikely that the rate of folding was the determining factor by which coat protein was bound by GroEL. Thus, we thought that the conformation of the coat protein folding intermediates might instead be a deciding factor.
GroEL binds coat protein as a late folding intermediate
In vitro, Teschke (1999) showed that the tryptophans of the refolded tsf mutants are more solvent exposed than those of WT coat protein. The tsf coat proteins were found to be more susceptible to proteolysis and bind the hydrophobic dye, bisANS, more tightly than the refolded WT coat protein. In addition, changes in the circular dichroism spectra indicated changes in the secondary structures. Although the tsf mutants had these alterations in conformation, the refolded tsf coat proteins are assembly competent when refolded at low temperatures, albeit with assembly kinetics slower than that of WT coat protein. However, the tsf coat proteins were aggregation prone when folded at high temperatures. Even when refolded at low temperatures and shifted to high temperatures, the tsf coat proteins aggregated. Thus, the tsf mutants are conformationally different than refolded WT coat protein that was aggregation resistant at all tested temperatures. Therefore, we tested whether the tsf coat proteins would display similar alterations in conformation when in binary complexes with GroEL. If similar conformational differences were observed when WT and tsf coat proteins were bound by GroEL, this would suggest that GroEL bound the coat proteins in a conformation resembling a late folding intermediate. If all the proteins were bound in a similar conformation, then the coat proteins were likely to be bound early in their folding, before conformational differences would be established.
When we investigated the conformation of WT and tsf coat proteins bound by GroEL, we observed differences in the bound conformation. The tryptophans of the tsf coat proteins were more solvent exposed than those of WT coat protein. Additionally, the tsf coat proteins were less protease resistant than WT coat protein when bound by GroEL. These results were similar to the differences that we observed when WT and the tsf coat proteins were folded in vitro in the absence of GroEL. Thus, it seems likely that coat protein is bound by GroEL as a late folding intermediate whose conformation is similar to that of the folded species. The binding of late folding intermediates by GroEL has been observed for several proteins and may therefore represent a common mode of binding of substrate polypeptides (Martin et al 1991; Hayer-Hartl et al 1994; Katsumata et al 1996; Goldberg et al 1997).
The nature of the interaction of coat protein with GroEL
The tsf amino acid substitutions lead to increased affinities of binding by GroEL as compared to the binding of WT coat protein, which suggests that the tsf mutants preferentially bind to GroEL, perhaps to avoid aggregation. This is consistent with previous studies that also showed that single amino acid substitutions changed the strength of the interaction of the substrate polypeptide with GroEL (Itzhaki et al 1995; Zahn et al 1996). The significance of hydrophobic interactions for polypeptide binding by GroEL has been an area of great interest (Landry and Gierasch 1991; Landry et al 1992; Dessauer and Bartlett 1994; Fenton et al 1994; de Crouy-Chanel et al 1995; Lin et al 1995). Itzhaki et al (1995) carried out an extensive survey characterizing the interaction of WT and 32 representative single amino acid substitutions in barley chymotrypsin inhibitor 2 and GroEL and found that the binding of a substrate polypeptide to GroEL was favored by increased hydrophobicity or by the addition of positively charged side chains, while negatively charged substitutions impeded binding. However, the results with the coat protein mutants used here would disagree with the conclusions of Itzhaki et al (1995). All of the tsf coat proteins showed an increase in the affinity of binding by GroEL; however, only 1 of the mutants, A108V, had a substitution that would increase the hydrophobicity of coat protein. The other 2 mutants, F353L and G232D, have substitutions that decrease the hydrophobicity of coat protein. However, hydrophobic interactions are likely to be important in binding of coat protein by GroEL because the mutant coat proteins have more exposed hydrophobic surface area (Teschke 1999). Recently, it has been suggested that GroEL binds to exposed hydrophobic surfaces on aggregation-prone folding intermediates and not to a specific amino acid sequence (Clark et al 1996; Goldberg et al 1997).
Affinity of binding of WT and tsf coat proteins by GroEL
The shape of the binding isotherm when the concentration of GroEL was held constant and the concentration of coat protein was varied showed an intriguing sigmoidal shape, indicative of positive cooperativity. Coat protein was likely responsible for the sigmoidal curve because the titration of coat protein with varying levels of GroEL was not similarly sigmoidal. The sigmoidal relationship was not a result of the binding of multiple coat proteins to GroEL because the change in the fluorescence of the WT and tsf coat proteins complexed to GroEL reached a plateau at a 1:1 molar ratio.
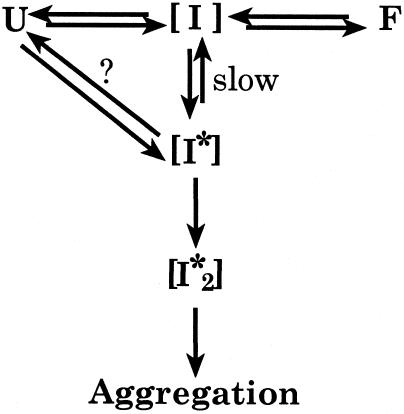
We would propose that the sigmoidal shape of the binding isotherm is due to the presence of 2 intermediates in the folding of coat protein, [I] and [I*]. The intermediate [I] is normally present in the folding of coat protein (Fig 6). The intermediate [I*], on the other hand, is either on the off-pathway reaction that leads to aggregation via [I*2], or it may be an alternate intermediate on the folding pathway, indicated by the equilibrium with the question mark in Figure 6. If [I*] only slowly converts to [I], then its concentration will increase as the concentration of folding coat protein increases. As the concentration of [I*] increases, the [I*2] concentration will also increase, which is expected because aggregation is concentration dependent. If the GroEL binds to [I*] with greater affinity than to [I], and [I*] accumulates as the coat protein concentration in the reaction is increased, then the binding curve would be expected to have the sigmoidal shape that we have observed.
Fig 6.
A model for the complex formation of WT and tsf coat proteins with GroEL. The sigmoidal relationship for coat protein binding to GroEL can be explained by the presence of 2 intermediates in the folding of coat protein, [I] and [I*]. The intermediate [I] is present in the on-pathway folding of coat protein, while [I*] is either an intermediate found off-pathway leading toward aggregation or may be an intermediate on the folding pathway, indicated by the presence of the question mark. If GroEL binds to [I*] with higher affinity than to [I], and the concentration of [I*] increases as the coat protein concentration is increased, then a sigmoidal shape to the curve could be expected
In conclusion, we have formed binary complexes of coat protein with GroEL and found that the conformation of the bound coat protein is consistent with GroEL binding coat protein as a late folding intermediate. Investigations of the interactions of other coat protein mutants, such as the global suppressors of the tsf substitutions, will clarify the role of the conformation of coat protein folding intermediates in the interaction with GroEL.
Acknowledgments
This work was supported by NIH grant GM53567. We thank Lili Aramli and Carole Capen for their thoughtful comments and critical review of this manuscript.
REFERENCES
- Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- Braig K, Otwinowski Z, Hegde R, et al. The crystal structure of the bacterial chaperonin GroEL at 2.8 Å. Nature. 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- Brazil BT, Ybarra J, Horowitz PM. Divalent cations can induce the exposure of GroEL hydrophobic surfaces and strengthen GroEL hydrophobic binding interactions. J Biol Chem. 1998;273:3257–3263. doi: 10.1074/jbc.273.6.3257. [DOI] [PubMed] [Google Scholar]
- Buckle AM, Zahn R, Fersht AR. A structural model for GroEL—polypeptide recognition. Proc Natl Acad Sci USA. 1997;94:3571–3575. doi: 10.1073/pnas.94.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Sigler PB. The crystal structure of a GroEL/peptide complex: plasticity as a basis for substrate diversity. Cell. 1999;99:757–768. doi: 10.1016/s0092-8674(00)81673-6. [DOI] [PubMed] [Google Scholar]
- Clark AC, Frieden C. GroEL-mediated folding of structurally homologous dihydrofolate reductases. J Mol Biol. 1997;268:512–525. doi: 10.1006/jmbi.1997.0969. [DOI] [PubMed] [Google Scholar]
- Clark AC, Frieden C. The chaperonin GroEL binds to late-folding non-native conformations present in native Escherichia coli and murine dihydrofolate reductases. J Mol Biol. 1999;285:1777–1788. doi: 10.1006/jmbi.1998.2403. [DOI] [PubMed] [Google Scholar]
- Clark AC, Hugo E, Frieden C. Determination of regions in the dihydrofolate reductase structure that interact with the molecular chaperonin GroEL. Biochemistry. 1996;35:5893–5901. doi: 10.1021/bi953051v. [DOI] [PubMed] [Google Scholar]
- Corrales FJ, Fersht AR. The folding of GroEL-bound barnase as a model for chaperonin-mediated protein folding. Proc Natl Acad Sci USA. 1995;92:5326–5330. doi: 10.1073/pnas.92.12.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crouy-Chanel A, El Yaagoubi A, Kohiyama M, et al. Reversal by GroES of the GroEL preference from hydrophobic amino acids toward hydrophilic amino acids. J Biol Chem. 1995;270:10571–10575. doi: 10.1074/jbc.270.18.10571. [DOI] [PubMed] [Google Scholar]
- Dessauer C, Bartlett S. Identification of a chaperonin binding site in a chloroplast precursor protein. J Biol Chem. 1994;269:19766–19776. [PubMed] [Google Scholar]
- Ellis RJ, Hartl FU. Protein folding in the cell: competing models of chaperonin function. FASEB J. 1996;10:20–26. doi: 10.1096/fasebj.10.1.8566542. [DOI] [PubMed] [Google Scholar]
- Eppler K, Wykoff E, Goates J, et al. Nucleotide sequence of the bacteriophage P22 genes required for DNA packaging. Virology. 1991;183:519–538. doi: 10.1016/0042-6822(91)90981-g. [DOI] [PubMed] [Google Scholar]
- Fenton WA, Horwich AL. GroEL-mediated protein folding. Protein Sci. 1997;6:743–760. doi: 10.1002/pro.5560060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton WA, Kashl Y, Furtak K, et al. Residues in chaperonin GroEL required for polypeptide binding and release. Nature. 1994;371:614–619. doi: 10.1038/371614a0. [DOI] [PubMed] [Google Scholar]
- Fisher MT. GroE chaperonin-assisted folding and assembly of dodecameric glutamine synthetase. Biochemistry (Moscow) 1998;63:382–398. [PubMed] [Google Scholar]
- Freidfelder D. 1982 Physical Biochemistry Applications to Biochemistry and Molecular Biology. W.H. Freeman and Co., San Francisco. [Google Scholar]
- Galisteo ML, Gordon CL, King J. Stability of wild-type and temperature-sensitive protein subunits of the phage P22 capsid. J Biol Chem. 1995;270:16595–16601. doi: 10.1074/jbc.270.28.16595. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Zhang J, Sondek S, et al. Native-like structure of a protein-folding intermediate bound to the chaperonin GroEL. Proc Natl Acad Sci USA. 1997;94:1080–1085. doi: 10.1073/pnas.94.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CL, King J. Temperature-sensitive mutations in the phage P22 coat protein which interfere with polypeptide chain folding. J Biol Chem. 1993;268:9358–9368. [PubMed] [Google Scholar]
- Gordon CL, Sather SK, Casjens S, et al. Selective in vivo rescue by GroEL/ES of termolabile folding intermediates to phage P22 structural proteins. J Biol Chem. 1994;269:27941–27951. [PubMed] [Google Scholar]
- Gray TE, Fersht AR. Refolding of barnase in the presence of GroE. J Mol Biol. 1993;232:1197–1207. doi: 10.1006/jmbi.1993.1471. [DOI] [PubMed] [Google Scholar]
- Hayer-Hartl MK, Ewbank JJ, Creighton TE, et al. Conformation specificity of the chaperonin GroEL for the compact folding intermediates of alpha-lactalbumin. EMBO J. 1994;13:3192–3202. doi: 10.1002/j.1460-2075.1994.tb06618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer GL, Harayama S. Mutants in transmission of chemotactic signals from two independent receptors of. E. coli. Cell. 1979;16:617–625. doi: 10.1016/0092-8674(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Hemmingsen SM, Woolford C, van der Vies SM, et al. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988;333:330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Itzhaki LS, Otzen DE, Fersht A. Nature and consequences of GroEL–protein interactions. Biochemistry. 1995;34:14581–14587. doi: 10.1021/bi00044a037. [DOI] [PubMed] [Google Scholar]
- Katsumata K, Okazaki A, Tsurupa GP, et al. Dominant forces in the recognition of a transient folding intermediate of alpha-lactalbumin by GroEL. J Mol Biol. 1996;264:643–649. doi: 10.1006/jmbi.1996.0666. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. 1983 Principles of Fluorescence Spectroscopy, vol 496. Plenum Press, New York. [Google Scholar]
- Landry SJ, Gierasch LM. The chaperonin GroEL binds a polypeptide in an alpha-helical conformation. Biochemistry. 1991;30:7359–7362. doi: 10.1021/bi00244a001. [DOI] [PubMed] [Google Scholar]
- Landry SJ, Jordan R, McMacken R, et al. Different conformations for the same polypeptide bound to chaperones DnaK and GroEL. Nature. 1992;355:455–457. doi: 10.1038/355455a0. [DOI] [PubMed] [Google Scholar]
- Lanman J, Tuma R, Prevelige PE Jr. Identification and characterization of the domain structure of bacteriophage P22 coat protein. Biochemistry. 1999;38:14614–14623. doi: 10.1021/bi9915420. [DOI] [PubMed] [Google Scholar]
- Lehrer SS. Solute perturbations of protein fluorescence. The quenching of the tryptophanyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry. 1971;10:3254–3263. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- Lin Z, Schwartz F, Eisenstein E. The hydrophobic nature of GroEL–substrate binding. J Biol Chem. 1995;270:1011–1014. doi: 10.1074/jbc.270.3.1011. [DOI] [PubMed] [Google Scholar]
- Martin J, Langer T, Boteva R, et al. Chaperonin-mediated protein folding at the surface of groEL through a ‘molten globule’-like intermediate. Nature. 1991;352:36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Mattingly JR Jr, Iriarte A, Martinez-Carrion M. Homologous proteins with different affinities for GroEL: the refolding of the aspartate aminotransferase isozymes at varying temperatures. J Biol Chem. 1995;270:1138–1148. doi: 10.1074/jbc.270.3.1138. [DOI] [PubMed] [Google Scholar]
- Mattingly JR Jr, Torella C, Iriarte A, et al. Conformation of aspartate aminotransferase isozymes folding under different conditions probed by limited proteolysis. J Biol Chem. 1998;273:23191–23202. doi: 10.1074/jbc.273.36.23191. [DOI] [PubMed] [Google Scholar]
- Nakonechny WS, Teschke CM. GroEL and GroES control of substrate flux in the in vivo folding pathway of phage P22 coat protein. J Biol Chem. 1998;273:27236–27244. doi: 10.1074/jbc.273.42.27236. [DOI] [PubMed] [Google Scholar]
- Perrett S, Zahn R, Stenberg G, et al. Importance of electrostatic interactions in the rapid binding of polypeptides to GroEL. J Mol Biol. 1997;269:892–901. doi: 10.1006/jmbi.1997.1081. [DOI] [PubMed] [Google Scholar]
- Prevelige PE Jr, Thomas D, King J. Scaffolding protein regulates the polymerization of P22 coat subunits into icosahedral shells in vitro. J Mol Biol. 1988;202:743–757. doi: 10.1016/0022-2836(88)90555-4. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Buchner J. Interaction of GroE with an all-beta-protein. J Biol Chem. 1992;267:16829–16833. [PubMed] [Google Scholar]
- Secnik J, Gelfand CA, Jentoft JE. Retroviral nucleocapsid protein specifically recognizes the base and the ribose of mononucleotides and mononucleotide components. Biochemistry. 1992;31:2982–2988. doi: 10.1021/bi00126a020. [DOI] [PubMed] [Google Scholar]
- Shi L, Palleros DR, Fink AL. Protein conformational changes induced by 1,1′-bis(4-anilino-5-naphthalenesulfonic acid): preferential binding to the molten globule of Dna K. Biochemistry. 1994;33:7536–7546. doi: 10.1021/bi00190a006. [DOI] [PubMed] [Google Scholar]
- Staniforth R, Cortes A, Burston S, et al. The stability and hydrophobicity of cytosolic and mitochondrial malate dehydrogenases and their relation to chaperonin-assisted folding. FEBS Lett. 1994;344:129–135. doi: 10.1016/0014-5793(94)00348-3. [DOI] [PubMed] [Google Scholar]
- Teschke CM. Aggregation and assembly of phage P22 temperature-sensitive coat protein mutants in vitro mimic the in vivo phenotype. Biochemistry. 1999;38:2873–2881. doi: 10.1021/bi982739f. [DOI] [PubMed] [Google Scholar]
- Xu Z, Horwich AL, Sigler PB. The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex. Nature. 1997;388:741–750. doi: 10.1038/41944. [DOI] [PubMed] [Google Scholar]
- Zahn R, Lindner P, Axmann S, et al. Effect of single point mutations in citrate synthase on binding to GroEL. FEBS Lett. 1996;380:152–156. doi: 10.1016/0014-5793(96)00013-0. [DOI] [PubMed] [Google Scholar]
- Zahn R, Spitzfaden C, Ottiger M, et al. Destabilization of the complete protein secondary structure on binding to the chaperone GroEL. Nature. 1994;368:261–265. doi: 10.1038/368261a0. [DOI] [PubMed] [Google Scholar]