Abstract
Objective. Macrophage migration inhibitory factor (MIF) is considered as an important mediator in the pathogenesis of neoplasia. The aim of the present study was to evaluate whether MIF could be used as a marker for hepatocellular carcinoma (HCC) detection. Material and methods. Biodistribution and whole-body autoradiography studies of 131I-labeled anti-MIF monoclonal antibody (McAb) and 131I-labeled control IgG were performed. The HCC-bearing mice were injected with 3.7 MBq of each agent and killed at 24, 48, and 72 h postinjection (p.i.). The organs, blood, and HCC tissues were removed from model mice, weighed, and counted using a gamma-counter. The expression of MIF mRNA and protein within HCC tissues was confirmed by RT-PCR and immunohistochemistry. Results. HCCs in model mice could be adequately visualized at 24 h p.i. The target-to-non-target (T/NT) ratios were 6.72 ± 1.09 (24 h), 9.85 ± 0.81 (48 h), and 12.31 ± 0.57 (72 h) for 131I-labeled anti-MIF McAb group, whereas in the control group of 131I-IgG, T/NT ratios were 4.65 ± 0.63 (24 h), 6.12 ± 0.60 (48 h), and 8.23 ± 0.35 (72 h) (p < 0.05). MIF mRNA expression was twofold higher in the HCC tissues than in the healthy liver tissues. MIF protein expression was much higher in the HCC tissues than in controls. Conclusions. Our findings suggested that 131I-anti-MIF McAb could be rapidly and specifically localized in tumors. Thus, MIF could be used as a marker for HCC tumor detection.
Keywords: Anti-MIF McAb, immunohistochemistry, MIF, radioiodine, RT-PCR, tumor imaging
Introduction
Hepatocellular carcinoma (HCC) is one of the most frequently reported malignant tumors in contemporary medicine and is becoming increasingly significant in clinical research [1,2]. Detection of HCC at an earlier stage is very important for patient's prognosis. For decades, α-fetoprotein (AFP) has been used as a serum marker for HCC; however, it is insufficient for HCC screening. The ability of abdominal imaging to detect HCCs has improved dramatically over the last few decades. Although ultrasonography (US) and computed tomography (CT) usually accurately depict HCC [3], attempts to improve the methods of staging and follow-up for patients with known HCC have led to the evaluation of nuclear imaging systems in HCC [4]. Therefore, efforts are being made to identify alternative tracers for screening and staging HCCs in patients at risk.
Macrophage migration inhibitory factor (MIF) has been considered as the most interesting factor because of its diverse functions [5]. Originally described as a product of activated T cells that inhibits the migration of macrophages, MIF was subsequently found to be involved in the development of pathological changes associated with several acute and chronic inflammatory disease processes, such as acute respiratory distress syndrome [6], septic shock [7], rheumatoid arthritis [8], atherosclerosis [9], glomerulonephritis [10], and allograft rejection [11]. Recent studies have shown that MIF plays an important role in the tumor formation [12].
MIF has been shown to promote cell proliferation and tumor angiogenesis [13], and over-expression of MIF has been reported in various types of cancers, including prostate tumors, breast cancer, colon carcinomas, HCCs, and glioblastoma. In the current study, we labeled anti-MIF monoclonal antibody (McAb) with radioiodine Na131I in order to determine the role of MIF in HCC progression. In addition, we investigated its biodistribution and imaging characteristics in vivo using an HCC tumor-bearing model in mice. The results of our study indicated that MIF could be used as a supplementary biomarker for the detection of HCCs.
Material and methods
Radioiodination of anti-MIF McAb
All commercially available chemicals were of analytic grade and anti-MIF McAb (R&D Systems), and IgG (ZSGB-BIO) were of pharmaceutical grade. Anti-MIF McAb and control IgG were iodinated with Na131I (specific activity 37 MBq/mg, China Institute of Atomic Energy) using the Iodogen technique. Radioiodinated anti-MIF McAb and control IgG were separated from free iodine using size exclusion columns (Sephadex G-25, Pharmacia). The specific activity of radioiodinated anti-MIF McAb is 28.56 GBq/μmol, and that of radioiodinated IgG is 27.63 GBq/μmol. The radiochemical purity of radioiodinated anti-MIF McAb is >95% (as revealed by paper chromatography), and that of radioiodinated IgG is >95% (paper chromatography). The immunological activities of 131I-labeled anti-MIF McAb were confirmed by ELISA (enzyme-linked immunosorbent assay) (data not shown).
Preparation of tumor-bearing animal model
Cell culture and all animal experiments were performed in accordance with the principles of the institutional, national, and international guidelines for humane use of animals for research. The HCC tumor models were established by subcutaneous injection of 5 × 106 H22 tumor cells into the front flank of female severe combined immunodeficient (SCID) mice (20–25 g, 6–8 weeks, Animal Center of Shandong University). Thyroid uptake was blocked by the addition of potassium iodide to the drinking water (0.1%) starting from 1 day before the experiments. When the tumor volume reached 500–600 mm3 (4 weeks after inoculation), the tumor-bearing nude mice were used for biodistribution and gamma imaging studies.
Biodistribution of 131I-anti-MIF McAb
The HCC tumor-bearing mice (24 mice/group) were injected with 3.7 MBq 131I-anti-MIF McAb or 131I-IgG in 0.2 ml of PBS via the tail vein to evaluate the distribution in major organs of mice. The mice were sacrificed at 24, 48, and 72 h post-injection (p.i.). Blood, heart, liver, spleen, kidney, brain, lung, intestine, muscle, bone, and tumor were harvested, weighed, and measured for radioactivity in a gamma-counter (1480 Wizard 3, PerkinElmer Life Sciences, Boston, MA, USA). Organ uptake was calculated as the percentage of injected dose per gram of tissue (%ID/g). Values were expressed as mean ± SD (n = 8/group).
Whole-body autoradiography
For whole-body autoradiography imaging, the HCC tumor-bearing mice were anesthetized with an intra-peritoneal injection of sodium pentobarbital at a dose of 45 mg/kg. Each animal was administered with 3.7 MBq of the 131I-anti-MIF McAb or 131I-IgG in 0.2 ml of PBS (n = 4/group). Mice were placed on their back on a storage phosphor screen plate in subdued light. Each mouse was exposed to the plate for 25 min. When the exposure needed to be stopped, the plate was immediately covered with an opaque plastic sheet, transferred to a scanner, and scanned by using typhoon trio + (laser red, 633 nm; pixel size, 200 mm; phosphor mode: best sensitivity). Serial images were acquired at 24, 48, and 72 h p.i.
RT-PCR
The expression of MIF mRNA was evaluated by RT-PCR. Total RNA was isolated from HCC tissues and healthy liver tissues using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), and DNA contaminant was removed by treatment with DNase I (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. Total RNA (5 μg) was reverse transcribed by using Revert Aid™ First Strand cDNA Synthesis Kits (Fermentas), cDNAs amplified with murine MIF specific primer using TaKaRa PCR Amplification Kit (TaKaRa). The primers were designed as follows: MIF (368 bp), sense primer, 5′-CCATGCC-TATGTTCATCGTG-3′; and antisense primer, 5′-GAACAGCGGTGCAGGTAAGTG-3′. Cycling conditions for amplification were as follows: 4 min denaturation step at 94°C, followed by 35 cycles of 30 s at 94°C, 1 min at 55°C, and 1 s at 72°C. PCR products were analyzed on 1.5% agarose gels and stained with ethidium bromide. All the experiments were performed in duplicate, and they were repeated at least three times. GAPDH mRNA expression was used as a loading control.
Histological and immunohistochemical analyses
HCC tissues and healthy liver tissues were fixed in 10% PBS-buffered formalin, and paraffin sections were stained with H&E and examined by light microscopy to assess the histological changes. Immunohistochemical analysis was performed using a SP-9002 Histostain™-Plus kit (ZSGB-BIO) according to the manufacturer's protocol. The sections were microscopically examined and the positively stained fields were observed. Ten fields per each section were observed.
Statistical analysis
The data were analyzed using SPSS 11.0 software. Statistical analysis was performed using the unpaired Student's t-test. Difference was considered statistically significant when p was <0.05, and two-sided. All data were expressed as the means ± standard deviation (SD).
Result
Accumulation in the HCC tissues
The concentration of 131I-anti-MIF McAb or 131I-IgG in the HCC tissues was expressed as percentage of the initial dose (%ID/g). As shown in Figure 1A, both the radiopharmaceuticals significantly localized at sites of HCC tissues at 24 h p.i. The uptake of 131I-anti-MIF McAb in HCC tumors was 0.214 ± 0.021%ID/g at 24 h p.i. and retained to 0.086 ± 0.0013%ID/g at 72 h p.i. (n = 8/group). The uptake of 131I-anti-MIF McAb was significantly higher than that of 131I-IgG (p < 0.05). Target-to-non-target (T/NT) ratio for the 131I-anti-MIF McAb group was 6.72 at 24 h and increased continuously to 12.32 at 72 h. On the other hand, the T/NT ratio for the 131I-IgG group was 4.65 at 48 h and reached 8.23 at 72 h. 131I-anti-MIF McAb group showed the highest uptake (p < 0.05, see Figure 1B). Target-to-blood (T/B) ratios for the 131I-anti-MIF McAb group were 1.39 ± 0.21, 2.45 ± 0.13, and 2.79 ± 0.21 at 24, 48 and 72 h p.i., respectively. The T/B ratios for the 131I-anti-MIF McAb group were significantly higher than that of 131I-IgG group (p < 0.05, see Figure 1C).
Figure 1.
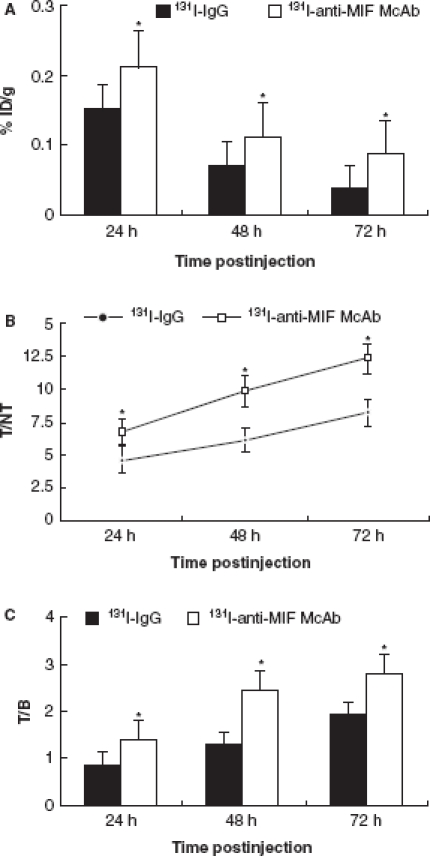
(A) Accumulation of 131I-anti-MIF McAb and 131I-IgG in the hepatocellular carcinoma (HCC) tissues (%ID/g). Values are means ± SD, n = 8 in all groups; *p < 0.05 vs. 131I-IgG. (B) Change of T/NT in the HCC tissues of 131I-anti-MIF and 131I-IgG McAb groups. Values are means ± SD, n = 8 in all groups; *p < 0.05 vs. 131I-IgG. (C) Change of T/B of 131I-anti-MIF McAb and 131I-IgG in HCC tissues. Values are means ± SD, n = 8 in all groups; *p < 0.05 vs. 131I-IgG.
Taken together, the data indicated that the 131I-labeled anti-MIF McAb was more specific and suitable for targeting HCC than the 131I-labeled IgG antibody.
Imaging of HCC
The whole-body autoradiography images of the two radiotracers at 24, 48 and 72 h p.i. were shown in Figure 2. The HCC tumors of 131I-labeled anti-MIF McAb were clearly visible at 24 and 48 h p.i., with high contrast to the contralateral background. Comparative analysis of the scintigrams obtained at the three time points showed that the 131I-anti-MIF McAb group had remarkably clear images than those of the 131I-IgG group; this finding was in accordance with the high T/NT ratio (p < 0.05). These findings also indicate that the 131I-labeled anti-MIF McAb had higher specificity for targeting HCC than that of the 131I-labeled IgG antibody.
Figure 2.
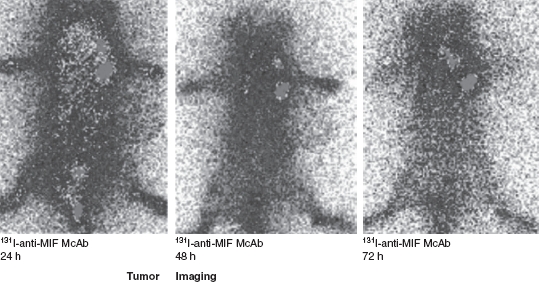
Images of the hepatocellular carcinoma (HCC) mice model. The 131I-anti-MIF McAb group had clear images in accordance with the high T/NT ratio.
MIF mRNA expression in HCC and healthy liver tissues
To confirm that the high intake of 131I-labeled anti-MIF McAb in HCC tissues was due to the high expression of MIF in locus, we analyzed the expression of MIF in HCC tissues and healthy liver tissues. As shown in Figure 3, there were little changes in MIF gene expression pattern in healthy liver tissues at all the three time points. However, MIF mRNA expression in HCC tissues was twofold higher than that in healthy liver tissues at 24 h p.i. (p < 0.05). The MIF mRNA levels in HCC tissues remained elevated up to 72 h p.i., in contrast to expression in healthy liver tissues (p < 0.05). MIF was secreted from many kind of cells within tumor tissues, including activated lymphocytes, macrophages, and tumor cells; these cells may produce high levels of MIF as is noted in HCC cases.
Figure 3.
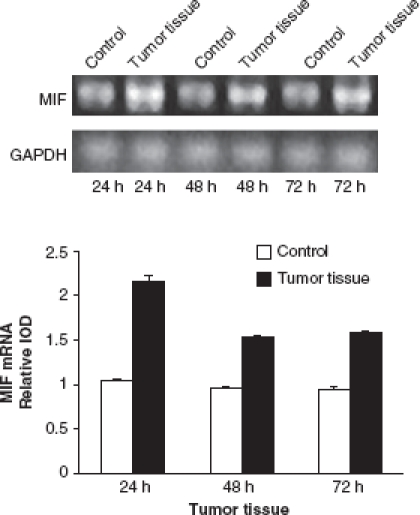
The expression of MIF mRNA in hepatocellular carcinoma (HCC) and healthy liver tissues. There were little changes in MIF gene expression in healthy liver tissues at the three time points. However, there was a twofold increase in MIF mRNA expression in HCC tissues at 24 h compared with healthy liver tissues (p < 0.05). The MIF mRNA levels in HCC tissues were keeping at a high level until 72 h compared with healthy liver tissues (p < 0.05). Semi-quantitative RT-PCR was performed in duplicate to minimize experimental error on the value calculated. All columnar values were expressed as means and standard deviations. A pattern of results was analyzed by repeating at least three times. p < 0.05 compared with the normal group.
MIF protein expression
Immunohistochemistry analysis showed that MIF was highly expressed in the HCC tissues (Figure 4). MIF expression significantly increased in the HCC specimens during the first 48 h p.i. (Figure 4A and B) and remained a high level until 72 h p.i. (Figure 4C). MIF protein expression was negative or weakly positive in healthy liver tissues (Figure 4D). We found a striking accumulation of macrophages in HCC tissues that may increase the expression of MIF. These results also showed that the high intake of 131I-labeled anti-MIF in locus was consistent with the high expression of MIF in HCC tissues.
Figure 4.
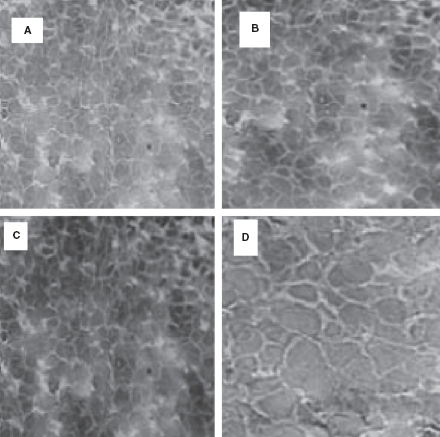
Expression of MIF in vivo. Expression of MIF protein was negative or weakly positive in healthy liver tissues (D). However, there was a significant increase in MIF expression in HCC specimens according to the time after injection in the first 48 h (A and B); and keeping the same level until 72 h (C).
Discussion
HCC is the fifth most common cancer worldwide; its incidence is very high in China where it is the second most common cancer. Because many patients are at increased risk of HCC, there is a need to develop reliable, sensitive, and specific imaging markers that will aid the clinician in early diagnosis. Several diagnostic modalities such as ultrasound, CT, MRI have been used to image HCCs; however, there is no optimal modality to detect this disease at an early stage. Nuclear medicine has been reported to play a complementary role in the evaluation of questionable lesions using high tumor to non-tumor contrast imaging techniques [14]. Finding an ideal radiopharmaceutical for the rapid, accurate, and unequivocal identification of HCCs is necessary in nuclear medicine imaging. Over the past decades, research efforts have been directed toward the development of monoclonal antibodies as radiophar-maceuticals for application in fields of radioimmunoimaging [15].
In the present study, overexpression of MIF mRNA was found in HCC tissues compared with non-tumor tissues and correlated with the expression of MIF shown by immunohistology. Consistently, serial images of whole-body autoradiography showed that the 131I-anti-MIF McAb group had much more clear images compared with the control 131I-IgG group. MIF is a cytokine produced by T lymphocytes, macrophages, and tumor cells, and its expression is associated with cell proliferation, cell cycle entry, angiogenesis, and tumorigenesis, resulting in antigen-specific immune responses between 131I-anti-MIF McAb and MIF, while uptake of 131I-IgG control group in tumor tissues is caused by nonspecific binding to Fc fragments of normal IgG. Although radiolabeled human polyclonal IgG had received considerable attention as a radiopharmaceutical for nuclear medicine imaging [16], it is far from the ideal tumor imaging reagent because of its lack of specificity. There are several novel findings in this study. First, this is the first report using radioiodinated anti-MIF McAb to demonstrate that the positive MIF expression in HCC tissues correlates with a significantly high MIF mRNA expression. Second, radioiodinated anti-MIF McAb was used as a prospective HCC imaging agent and provided clear image in tumor radioimmunoimaging.
MIF was originally described as a lymphokine involved in delayed hypersensitivity and various macrophage functions, including production of proinflammatory cytokines, glucocorticoid-induced immunomodulator, as well as induction of metallo-proteinase. Furthermore, MIF is suggested to play a tumorigenic role because of its ability to drive activation responses by sustaining ERK1 and ERK2 MAP-kinase activation and by suppressing p53-dependent growth arrest and apoptosis; these functions suggest that MIF promotes malignant progression of tumors. Several studies have documented that MIF is expressed in the sera and liver of patients with HCC. Intracellular MIF mRNA and protein were found to be overexpressed in HCC. Patients with high MIF-expressing tumors had a worse disease-free survival [17]. These findings indicated that the HCC-progressive effect of MIF may be attributable to its action in inducing tumor-associated angiogenesis, immunomodulation, and alterations in the tumor suppressive pathway. The potential of an anti-MIF therapeutic strategy [18,19] has been highlighted by the ability of a neutralizing anti-MIF McAb to attenuate pro-inflammatory cytokine production. Therefore, MIF is considered as a potential target protein in many pathophysiological states.
Our study showed that the 131I-anti-MIF McAb has a high target-to-background ratio and has relatively low level of accumulation in non-target tissues, that its expression is highly associated with the local expression of MIF, and that it is considerably better than nonspecific IgG for detecting HCCs by radioimmunoimaging. Hence, we propose that MIF may be a good marker for early HCC detection.
Acknowledgments
This study was supported by a grant from the Natural Science Foundation of Shandong Province (No. Y2007C088) and a grant from the financial support from China Postdoctoral Science Foundation (No. 20090461227).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Nagasue N, Uchida M, Makino Y, Takemoto Y, Yamanoi A, Hayashi T, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105:488–94. doi: 10.1016/0016-5085(93)90724-q. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 4.Braga FJ, Flamen P, Mortelmans L, Stroobants S, Homans F, Maes A. Ga-67-positive and F-18 FDG-negative imaging in well-differentiated hepatocellular carcinoma. Clin Nucl Med. 2001;27:642–5. doi: 10.1097/00003072-200107000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Leu H, Kleemann R. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect. 2002;4:449–60. doi: 10.1016/s1286-4579(02)01560-5. [DOI] [PubMed] [Google Scholar]
- 6.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 7.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen J, Vasinrapee P, Cook R, Surprenant E, Spieth M, Wilson M. Hepatocellular carcinoma with intravascular extension: Ga-67 scintigraphic findings. Clin Nucl Med. 1998;23:171–82. doi: 10.1097/00003072-199803000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Morand EF, Leech M, Bernhagen J. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov. 2006;5:399–410. doi: 10.1038/nrd2029. [DOI] [PubMed] [Google Scholar]
- 10.Lan HY. Role of macrophage migration inhibition factor in kidney disease. Nephron Exp Nephrol. 2008;109 doi: 10.1159/000145463. [DOI] [PubMed] [Google Scholar]
- 11.Brown FG, Nikolic-Paterson DJ, Metz C, Bucala R, Atkins RC, Lan HY. Up-regulation of macrophage migration inhibitory factor in acute renal allograft rejection in the rat. Clin Exp Immunol. 1999;118:329–36. doi: 10.1046/j.1365-2249.1999.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lolis E, Bucala R. Macrophage migration inhibitory factor. Expert Opin Ther Targets. 2003;7:153–64. doi: 10.1517/14728222.7.2.153. [DOI] [PubMed] [Google Scholar]
- 13.Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R. An essential role for macrophage migration inhibitory factor in angiogenesis and the growth of a murine lymphoma. Mol Med. 1999;5:181–91. [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson P. Hepatocellular carcinoma: development and early detection. Cancer. 2008;4:128–31. doi: 10.1102/1470-7330.2008.9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.KhawliLA, Biela B, Hu P, Epstein AL. Comparison of recombinant derivatives Of chimericTNT-3 antibody for the radio-imaging of solid tumors. Hybrid Hybridomics. 2003;22:1–9. doi: 10.1089/153685903321538026. [DOI] [PubMed] [Google Scholar]
- 16.Datz FL, Anderson CE, Ahluwalia RJ. The efficacy of indium-111-polyclonal IgG for the detection of infection and inflammation. J Nucl Med. 1994;35:74–83. [PubMed] [Google Scholar]
- 17.Akbar SM, Abe M, Murakami H. Macrophage migration inhibitory factor in hepatocellular carcinoma and liver cirrhosis; relevance to pathogenesis. Cancer Lett. 2001;171:125–32. doi: 10.1016/s0304-3835(01)00606-1. [DOI] [PubMed] [Google Scholar]
- 18.Nishio Y, Nishihira J, Ishibashi T. Role of macrophage migration inhibitory factor (MIF) in peripheral nerve regeneration: anti-MIF antibody induces delay of nerve regeneration and the apoptosis of Schwann cells. Mol Med. 2002;8:509–20. [PMC free article] [PubMed] [Google Scholar]
- 19.Cvetkovic I, Stosic-Grujicic S. Neutralization of macrophage migration inhibitor factor-novel approach for the treatment of immunoinflammatory disorders. Int Immunopharmacol. 2006;6:1527–34. doi: 10.1016/j.intimp.2006.06.009. [DOI] [PubMed] [Google Scholar]


