A crucial factor regulating the specific association of all molecules in solution is their desolvation and the modulation of this desolvation by cosolutes such as salts. Since the pioneering experiments of Hofmeister on the “salting out” of aqueous solutions of proteins[1], a deluge of research has allowed us to think of changes in preferential solvation as the main cause of shifted equilibria[2]. Whether preferentially excluded from or attracted to the surfaces of the associating molecules, these cosolutes can increase or decrease complex stability. However, information needed to determine the origin of Hofmeister effects involves more than equilibrium measurements. For this reason our newfound ability, reported here, to measure distinct changes in association (“on-rate”) versus dissociation (“off-rate”) kinetics, whose ratios are equilibrium constants, opens the possibility of instructive inquiry into the molecular basis of ionic action in all Hofmeister systems.
More than a decade ago it was demonstrated that a single protein nanopore could be used to detect exchange of neutral molecules between the pore and the bath[3]. Here we combine this “molecular Coulter counting” with osmotic stress to study the involvement of water restructuring in molecular complexation. In particular, we use an alpha-hemolysin (αHL) nanopore[4] as a sensor[5] to follow the effect of cosolutes, focusing on the effect of anions on a simple bimolecular complexation reaction at the single-molecule level. As a model of hydrophobic association, we examine the specific guest-host complexation of the cyclic carbohydrate γ-cyclodextrin (CD) with adamantane carboxylate (AD) in the presence of different salts (Figure 1). By monitoring transient obstructions of current through the nanopore by the CD-AD complex, we are able to resolve the complexation kinetics and their modification in the presence of the various salts. We find that the salts used here act to stabilize CD-AD complexes and that the binding free energy varies linearly with solution osmotic pressure. This tendency is sensitive to the type of salt; it tracks the Hofmeister ranking that typically reflects the exclusion tendency of different ions from non-polar surfaces.
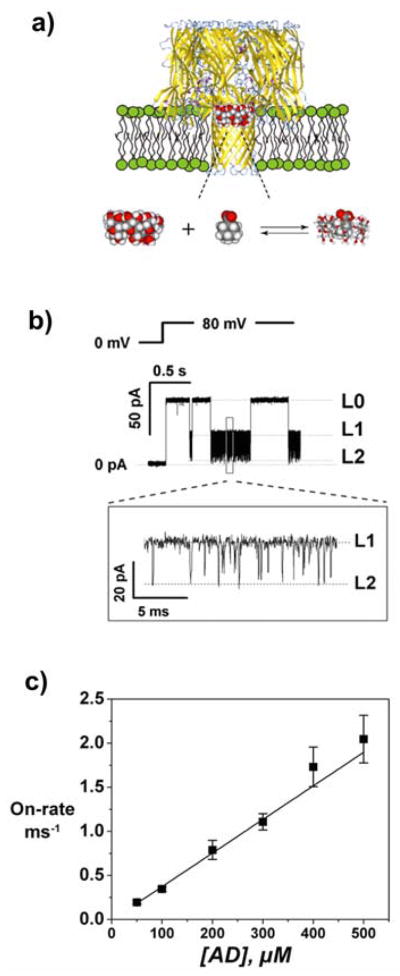
Figure 1.
Using an alpha-hemolysin (αHL) pore as a sensor[5] to probe cyclodextrin/adamantane carboxylate (CD-AD) complexation. a) Cartoon of the complexation process. b) Corresponding recording of single pore current. The lipid bilayer was bathed in 1.0 M NaCl solution; αHL was added to the cis-side of the bilayer, and CD (100 μM) and AD (200 μM) to the trans-side. In the absence of CD and AD, the single αHL pore is fully open (level L0); CD produces transient partial blockages of the pore from level L0 to level L1; AD does not affect the current through the fully open channel, but additionally blocks its CD-blocked state to level L2. c) On-rate of AD binding to the αHL-CD complex scales linearly with AD concentration in the bulk, suggesting bimolecular reaction kinetics of the CD-AD complexation within the αHL pore.
Remarkably, both on-rates and off-rates are affected. While chloride and bromide salts mainly impact the off-rates, sulfate dominates the equilibrium shift by changing the on-rate. This is a first indication that the mechanisms by which different salts modulate macromolecular association are quite distinct, providing valuable clues to the role of solvation as it modifies macromolecular interactions in crowded cellular environments[6].
Cyclodextrin molecules are shaped as truncated hollow cones with a non-polar hydrophobic inner cavity. The ability to incorporate hydrophobic “guest” molecules[7,8] not only makes cyclodextrins important in the pharmaceutical and food industries[8] but also provides a convenient model for specific hydrophobic interactions[9,10].
Recent thermodynamic studies of CD-AD complexation in bulk aqueous environments have shown that association involves the release of water from the interacting surfaces[10,11]. Watching complexation as a dynamic process now allows us to assess the effect of salt on the kinetics of complex formation. To probe the association reaction of γ-cyclodextrin (eight glucose units) with AD, we first reconstitute a single αHL channel[4] into a planar lipid bilayer[12]. Using the method described by Gu and colleagues,[5] we then monitor the changes in the channel ion current in the presence of CD and AD. Figures 1a and 1b illustrate the CD-AD complexation as detected with the wild-type αHL channel.
We determine the equilibrium binding constant of γCD with the channel as ~1.5×104 M−1, about 100 times greater than that reported for β-cyclodextrin (composed of seven glucose units)[5]. Because γCD is bound to the channel for longer periods, this molecule allows us to easily track multiple events of CD-AD complexation. The residual channel conductance (L1 in Figure 1b) is close to 0.4 nS, and is sensitive to the presence of AD which produces additional blockages to almost total current occlusion (L2 level). The unblocked state of the channel (level L0) is insensitive to the presence of AD. The on-rate for blockages, observed at level L1, scales linearly with AD concentration in the bulk, suggesting bimolecular reaction kinetics (Figure 1c).
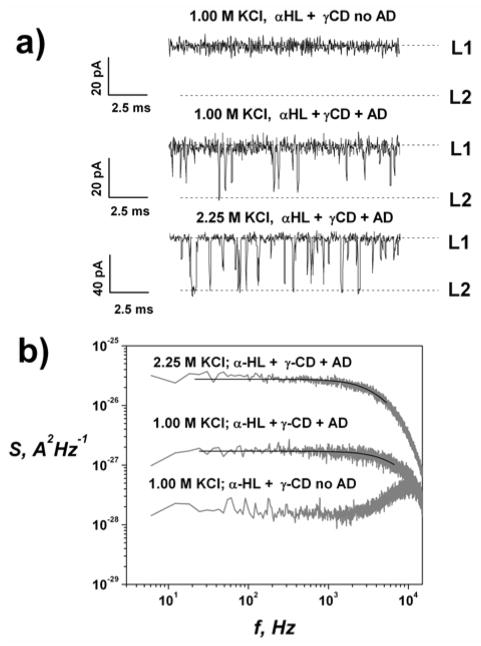
Figure 2a gives current traces of the CD-blocked state of the channel without AD and in its presence at two different KCl concentrations. Rate constants for CD-AD complexation are derived from the mean unblocked time (τo) and mean residence time (τr) obtained by noise analysis[13] (Figure 2b). The equilibrium binding constant (Keq) was directly determined from the AD-induced reduction of the current of the CD-blocked state by Keq = (1 − p)/(p[AD]), where p = 〈I〉/I max is the probability of finding the αHL-CD complex in the AD-unblocked state, 〈I〉 is the average (over all points) current through the CD-blocked state of the is the current αHL channel in the presence of AD, and Imax through the CD-blocked state in the AD-free solution. Power spectra were well fit by a Lorentzian function (Figure 2b) suggesting bimolecular reaction kinetics of CD-AD complexation for all salt concentrations.
Figure 2.
a) Effect of KCl concentration on CD-AD complexation in the αHL pore at −80 mV applied voltage. b) Power spectral densities of the AD-induced fluctuations in the αHL-CD complex. Solid lines are Lorenzian fits to the spectra. In the absence of AD the Lorenzian component is not seen (lowest spectrum).
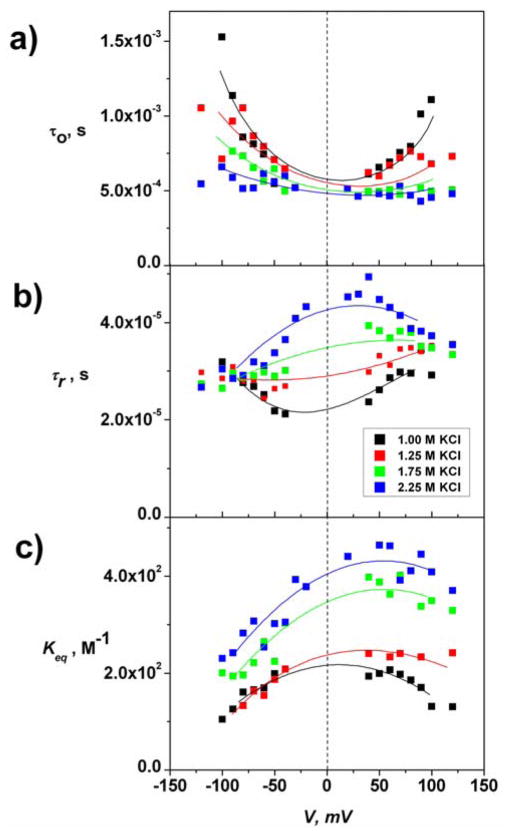
To eliminate the strong effect of electroosmotic water flow through the channel when voltage is applied[14], we considered the binding in the limit of V → 0 by interpolating values of τo, τr, and Keq, measured over a wide voltage range (Figure 3). We find that the CD-AD binding constant Keq (in 1.0 M KCl) is close to 2×102 M−1, with a characteristic on-rate of ~ 8.5×106 s−1 M−1 and residence AD time inside CD of ~ 2×10−5 s. In contrast, in bulk aqueous solutions Keq as measured by Isothermal Titration Calorimetry (ITC) is ~ 3.5×102 M−1 (present work and ref. [10a]). This difference is likely due to altered molecular solvation in the bulk vs. that in the pore. In addition, CD binding to the channel may impose specific steric constraints on the complexation geometry as well as possible distortion of CD conformation.
Figure 3.
Kinetic and equilibrium parameters of CD-AD complexation at different KCl concentrations. (a) Unblocked time (τo) for αHL-CD, (b) AD residence time (τr.) within CD-AD complexes in the αHL pore, (c) CD-AD association constant, Keq. At zero voltage a 2.25 increase in KCl concentration produces an approximately two-fold increase in the stability of the CD-AD inclusion complex, as τr increases from ~2×10−5 s to ~4.5×10−5 s, while τo, in the limit of small voltages stays almost unaffected, decreasing from ~5.7×10−4 s to ~4.9×10−4 s (Figure 2B). The change in residence time accounts for almost all the increase in the CD-AD equilibrium binding constant.
To assess the impact of salt on association, we follow CD-AD binding as we vary the concentration of salts in the bath solution (Figure 3). Upon increase of the KCl concentration from 1.00 M to 2.25 M we observe changes in both unblocked and residence time.
The influence of KBr and Na2SO4 salts on the CD-AD interaction was similarly evaluated. For these measurements, we reconstituted the αHL channel in the membrane at 1.0 M KCl (or NaCl) concentration and injected aliquots of concentrated KBr or Na2SO4 salts keeping AD and CD concentrations fixed.
For all three salts we observed an increase in binding constant, while the relative changes in τo and τr were different. Thermodynamically, the effect of salt on binding constants can be translated into a difference in the number of solute-excluding water molecules that are associated with complex formation. In all cases studied here, the changes in binding free energy of CD-AD complex formation ( δΔG = ΔG0 − RT Δ ln Keq) scale linearly with solution osmolarity, indicating a constant number of solute-excluding water molecules released from the interacting surfaces of CD and AD (or, equivalently, indicating a number of preferentially excluded solutes that varies linearly with solute concentration). This linearity allows us to characterize the CD-AD complexation reaction with a constant preferential hydration coefficient Δnwater, defined by:
| (1) |
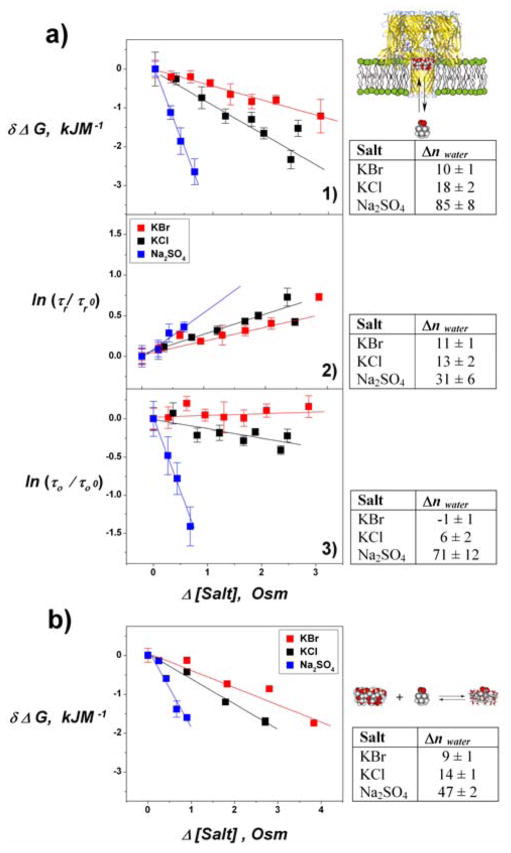
where μwater is water’s chemical potential, T the absolute temperature, R the ideal gas constant, 55.6 the number of moles of water in 1 Kg at normal conditions, and the solute ms osmolal concentration[10]. To compare complexation in the bulk with complexation inside the αHL channel, we measured δΔG in aqueous solutions using ITC in the presence of the same set of salts. Changes in δΔG in the bulk also show a linear dependence in solution osmolality. Figure 4 compares δΔG derived from ITC and channel experiments. For both channel (Figure 4a) and ITC (Figure 4b) measurements, CD-AD complexation is most affected by sulfate. Chloride has an intermediate effect, while bromide has the smallest. In contrast, substitution of potassium with sodium did not measurably influence CD-AD association; with both KCl and NaCl we obtained similar values of δΔG (data not shown). Using Eq. (1) we estimate the corresponding value of Δnwater in the channel and bulk (tables in Figure 4). For all the probed salts, the number of excluding water molecules follows the Hofmeister ranking for the anions (SO42− > Cl− > Br−)[1, 10b, 15]. We note that the preferential hydration is often, but not always, similar for complexation in bulk solution.
Figure 4.
Equilibrium and kinetic parameters for CD-AD complexation measured with αHL pore (a) and with Isothermal Titration Calorimetry, ITC (b). Free energy of CD-AD complexation δΔG, vs. changes in salt concentration (1) and kinetic parameters for binding: AD residence time (2) and unblocked time (3) of the αHL-CD complex. Using Eq. (1), the slope of these plots translates into changes in preferential hydration Δnwater upon CD-AD association shown in the corresponding tables. In deriving Δnwater in the tables of panels 2 and 3, we have assumed that the shift in equilibrium is solely due to the change in a given kinetic parameter inside the αHL ion channel.
We find that δΔG in the channel differs somewhat from that in the bulk. Interestingly, however, for all salts, the off-rates decreased with a slope that correlates well with change in δΔG in the bulk (table in Figure 4a, panel (2)). In fact, the ratio of slopes calculated from Figure 4a, panel (2) give 1 : 1.3 : 3 while ITC in the bulk gives 1: 1.5 : 4.5 for the Br−, Cl−, and SO42− (Figure 4b). This may come as no surprise, because intermediate states involved in complex dissociation must include steps that require the entry of water between the interacting hydrophobic surfaces. Salt, then, changes the cost of water penetration, making it more unfavorable for a water molecule to leave the bulk. Similar correlations were found in experiments with endonuclease and DNA[16].
Much less intuitive is our finding that the reaction on-rates can also depend on the presence of cosolute (Figure 4a, panel (1)). The slight change in the on-rate induced by KBr might be well within the measurement error, but the data for KCl and, especially, for Na2SO4 demonstrate a clear increase in the on-rate with the increase of salt concentration. In the case of Na2SO4, this increase is so pronounced that it dominates the calculated Δnwater. Thus, based on our findings, we conclude that “salting out” is a result of either accelerated on-rates or slower off-rates, depending on the salt, and that either of these may, surprisingly, dominate the Hofmeister effect. The sulfate anion measurements demonstrate that not only the stability of the complex, as measured by the life time of the inclusion complex, but also the accessibility of the complexation site can be critically increased by preferentially excluded cosolutes.
Though the present study deals with a simple complexation reaction, our results are clearly relevant for more complicated processes, such as enzyme catalysis, where the role of solvation in the reaction rates is only recently starting to be recognized[6].
Experimental Section
Single-channel measurements
Bilayer lipid membranes were formed by the lipid monolayer opposition technique[12], from diphytanoylphosphatidylcholine (Avanti) on an aperture in a Teflon partition, dividing two (cis and trans) compartments of the chamber[13b]. Wild-type α-hemolysin (Calbiochem) from a diluted stock solution of 25 μg/ml containing 8 M urea solution was added to the cis-side of the chamber; γCD (100 μM) and AD (200 μM) were added to the trans-side. The sign of the applied voltage was given with respect to the trans-chamber. All the probing salt solutions were buffered with 10 mM Na phosphate buffer to pH 7. We reconstituted the αHL channel in the membrane at 1.0 M KCl (or NaCl) concentration and injected aliquots of concentrated KCl, KBr, or Na2SO4 salts from stock solutions that also contained identical molar fractions of γCD and AD. This enabled us to follow events of CD-AD complexation at the best possible resolution while still observing linearity in δΔG over a wide range of probing anion concentrations. All salts and were from Sigma; components of the Na phosphate buffer from Burdick and Jackson, CD and AD were from Fluka. All experiments were done at room temperature, T = 23.0(± 1.0) °C.
Conductance measurements were performed using an Axopatch 200B amplifier (Axon Instruments). The output signal was filtered by either a 100 kHz amplifier filter or by an in-line low-pass 8-pole Butterworth filter (Model 9002, Frequency Devices) at 15 kHz and saved with a sampling frequency of 50 kHz. Amplitude, life-time, and fluctuation analysis were performed using Clampfit 9.2 (Axon Instruments) and the in-house software.
Microcalorimetry
Isothermal titration calorimetric measurements were made at 25 °C using a VP-ITC microcalorimeter (MicroCal) as described in [10a]. In each experiment, 40 successive 10 μl injections of 40 mM γCD solutions were mixed into the thermostated cell containing 2 mM AD solution; solutions were stirred at 300 rpm. Injections lasted 10 s and were spaced at 3.5 min intervals. The heat release associated with AD and CD dilution was measured separately and did not substantially change the calculated thermodynamic properties. Solutions were in 10 mM Na phosphate buffer at pH 7. Both AD and CD solutions contained an additional salt, both with the same osmolality. Equilibrium constants were evaluated using standard MicroCal Origin software procedures. All fits to the data were consistent to with a 1:1 molar complexation ratio. Attempted fits to other possible complex ratios did not improve the overall fit, supporting the simplest assumption of 1:1 binding under all studied conditions. Presented results are averages of 2–4 repeats.
Acknowledgments
We are grateful to Donald Rau and Brian Todd for fruitful discussions and for reading the manuscript. This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Contributor Information
Daniel Harries, Email: daniel@huji.ac.il.
Sergey M. Bezrukov, Email: bezrukos@mail.nih.gov.
References
- 1.Hofmeister F. Arch Exp Pathol Pharmakol. 1887;24:247–260. [Google Scholar]
- 2.a) Wyman J., Jr Adv Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]; b) Timasheff SN. Annu Rev Biophys Biomol Struct. 1993;22:67–97. doi: 10.1146/annurev.bb.22.060193.000435. [DOI] [PubMed] [Google Scholar]; c) Parsegian VA. Int Rev Cytol. 2002;215:1–31. doi: 10.1016/s0074-7696(02)15003-0. [DOI] [PubMed] [Google Scholar]; d) Fenimore PW, Frauenfelder H, McMahon BH, Young RD. Proc Natl Acad Sci USA. 2004;101:14408–14413. doi: 10.1073/pnas.0405573101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezrukov SM, Vodyanoy I, Parsegian VA. Nature. 1994;370:279–281. doi: 10.1038/370279a0. [DOI] [PubMed] [Google Scholar]
- 4.Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 5.Gu LQ, Braha O, Conlan S, Cheley S, Bayley H. Nature. 1999;398:686–690. doi: 10.1038/19491. [DOI] [PubMed] [Google Scholar]
- 6.a) Henzler-Wildman K, Kern D. Nature. 2007;450:964–972. doi: 10.1038/nature06522. [DOI] [PubMed] [Google Scholar]; b) Frauenfelder H. Nature Chem Biol. 2008;4:21–22. doi: 10.1038/nchembio0108-21. [DOI] [PubMed] [Google Scholar]
- 7.Rekharsky MV, Inoue Y. Chem Rev. 1998;98:1875–1918. doi: 10.1021/cr970015o. [DOI] [PubMed] [Google Scholar]
- 8.a) Saenger W. Angew Chem. 1980;92:343. [Google Scholar]; Angew Chem, Int Ed. 1980;19:344–362. [Google Scholar]; b) Davis ME, Brewster ME. Nature Rev Drug Discov. 2004;12:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 9.a) Cromwell WC, Bystrom K, Eftink MR. J Phys Chem. 1985;89:326–332. [Google Scholar]; b) Ross PD, Rekharsky MV. Biophys J. 1996;71:2144–2154. doi: 10.1016/S0006-3495(96)79415-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Becheri A, Nostro PL, Ninham BW, Baglioni PJ. Phys Chem B. 2003;107:3979–3987. [Google Scholar]
- 10.a) Harries D, Rau DC, Parsegian VA. J Am Chem Soc. 2005;127:2184–2190. doi: 10.1021/ja045541t. [DOI] [PubMed] [Google Scholar]; (b) Harries D, Rösgen J. Methods Cell Biol. 2008;84:679–735. doi: 10.1016/S0091-679X(07)84022-2. [DOI] [PubMed] [Google Scholar]
- 11.Taulier N, Chalikian TV. J Phys Chem B. 2006;110:12222–12224. doi: 10.1021/jp062467n. [DOI] [PubMed] [Google Scholar]
- 12.Montal M, Mueller P. Proc Natl Acad Sci USA. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) DeFelice LJ. Introduction to Membrane Noise. Plenum Press; New York: 1981. [Google Scholar]; b) Kullman L, Winterhalter M, Bezrukov SM. Biophys J. 2002;82:803–812. doi: 10.1016/S0006-3495(02)75442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu LQ, Cheley S, Bayley H. Proc Natl Acad Sci USA. 2003;100:15498–15503. doi: 10.1073/pnas.2531778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins K, Washabaugh M. Q Rev Biophys. 1985;18:323–422. doi: 10.1017/s0033583500005369. [DOI] [PubMed] [Google Scholar]
- 16.Sidorova NY, Rau DC. J Mol Biol. 2001;310:801–816. doi: 10.1006/jmbi.2001.4781. [DOI] [PubMed] [Google Scholar]