Abstract
Lower extremity ulcers are a recognized complication of rheumatoid arthritis (RA). Their prevalence has not been assessed since the advent of more aggressive disease modifying antirheumatic therapies. The purpose of this study was to establish the period prevalence of lower extremity ulcers in a modern-day unselected cohort of patients with RA, and to report the features associated with ulcer development and response to therapy. Between June 2007 and June 2010, 366 RA patients were evaluated at the Georgetown Division of Rheumatology. Data were collected and analyzed retrospectively on demographics, antibody and prothrombotic profile, comorbidities, disease activity, and outcomes. The period prevalence of ulcers in this cohort of 366 patients with RA followed over 3 years was 4.37%. Patients with ulcers were predominantly female (81.25%) and more commonly African American (56.2%). The mean disease duration at ulcer development was 25.9 years. All patients with ulcers had erosive disease and 63% were seropositive. Only five patients (31.25%) healed over a mean follow-up of 22.8 months. However, in this small sample, treatment with anti-tumor necrosis factor-α (anti-TNFα) therapy was associated with significantly higher likelihood of healing (p=0.039). In this modern-day cohort of patients with RA, we found a prevalence of lower extremity ulcers of 4.37% over 3 years. Only 31.25% of patients healed after a mean 22.8 months of follow-up. However, treatment with a biologic agent was associated with a significant increased likelihood of healing (RR 3.27, 95% CI 0.59–18.29, p=0.039).
Keywords: Anti-tumor necrosis factor-α, Disease modifying antirheumatic drug (DMARD), Leg ulcer, Rheumatoid arthritis, Vasculitis, Wound healing
Introduction
Lower extremity ulcers are a known complication of rheumatoid arthritis (RA). Their pathogenesis is multifactorial [1, 2], with vasculitis [3], Felty’s Syndrome [4], trauma related to deformity, neuropathy, venous insufficiency [5], and arterial disease [6] all reported to play a role, while historical cohorts report a point prevalence of leg ulceration in RA of approximately 8–9% [7–9]. A more recent postal survey administered to 1,130 RA patients in West Yorkshire, England revealed a point prevalence of foot ulceration in RA of only 3.39%. This lower prevalence is likely due to the inclusion of only foot (and exclusion of other lower limb) ulceration. However, the authors also postulate that improved treatment of RA may have contributed to the lower prevalence of ulceration in this cohort.
Ulceration in RA is associated with long-standing erosive and seropositive disease [3]. It has been shown that other extra-articular vasculitic manifestations of RA have significantly declined with the advent of biologic agents and a trend towards more aggressive disease modifying antirheumatic drug (DMARD) therapy in the 1990s [10]. The purpose of the current study was to establish the prevalence of lower extremity ulcers in a modern-day unselected cohort of patients with RA over a 3-year period, and to report the features associated with ulcer development and response to therapy.
Methods
The study was approved by the Georgetown University Hospital Biomedical institutional review board. Consecutive patients evaluated in the Division of Rheumatology between 1 June 2007 and 30 May 2010 and fulfilling the American College of Rheumatology (ACR) criteria for RA were retrospectively identified using an ICD-9 diagnosis code search of the electronic medical record (Centricity, GE). To establish the period prevalence of ulcers in our population, all charts were reviewed for the presence of lower extremity ulcers during the study period. Data were collected on demographics, antibody profile, comorbidities, inflammatory markers, radiographic features, biopsy findings, and prothrombotic profile.
All available biopsy samples were reviewed by a single investigator (VKS) to assess for evidence of vasculitis. All biopsies were performed during diagnostic clinical evaluation by an experienced plastic surgeon (CEA). Where possible, biopsies included a small piece of intact skin adjacent to the ulcer edge. Vasculitis was considered to be present if vessels distant from the ulcer bed demonstrated infiltration with polymorphonuclear neutrophils or mononuclear cells, or there was evidence of wall destruction or leukocytoclasis.
In our center, patients with lower extremity ulcers associated with autoimmune disease are referred to the Center for Wound Healing for evaluation by a plastic surgeon experienced in the management of complex wounds. Local and invasive wound care is performed according to standard protocol. All patients with ulcers are evaluated for diabetes, venous and arterial insufficiency.
To evaluate associations with rheumatoid arthritis disease activity, Disease Activity Score-28 (DAS-28) and wound surface area at the initial and most recent follow-up visits, along with medication exposures, were recorded. Data were analyzed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). Descriptive statistics was used for clinical characteristics, and chi-square test was used to analyze the outcome data. The p values were always two tailed, and a p<0.05 was considered significant.
Results
In the 3 years of this study, 366 RA patients were evaluated, and 16 had active leg ulcers giving a prevalence of 4.37% over 3 years. Patients with ulcers were predominantly female (81.25%). In the ulcer group, 56.25% were African American compared to only 21% of the RA population without ulcers. The mean age at first ulcer was 64.8±3.5 years. The mean disease duration at the time of ulcer development was 25.9±4.9 years. In three patients, a formal diagnosis of RA had not been made prior to development of ulcers; however, on rheumatologic evaluation, they met the ACR criteria for RA and in retrospect, all of these patients had had joint symptoms consistent with RA for some years prior to ulcer development.
All 16 patients with ulcers in this cohort had radiographic evidence of erosive disease, and 63% were rheumatoid factor or anti-cyclic citrullinated peptide positive. At the initial visit with an ulcer, less than half of the patients were in clinical remission based on DAS-28 score <3.2.
Comorbid conditions
Of the 16 patients with ulcers, two had concomitant well-controlled diabetes. Patients were evaluated for vascular disease. Venous insufficiency was seen in two patients and arterial disease was identified in two other patients.
Ulcer features
Biopsy specimens were available to review in 12 of the 16 patients with ulcers. Only three had biopsy evidence of vasculitis (Fig. 1). In five, the biopsy was inconclusive, three patients had gangrene, and one patient had cholesterol emboli syndrome (reported elsewhere [11]).
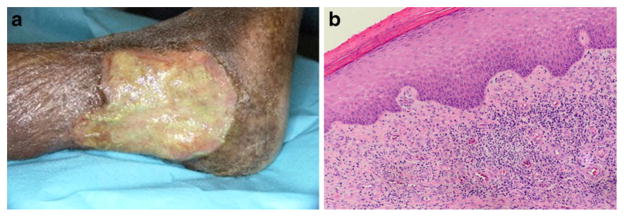
Fig. 1.
a Photograph of a rheumatoid arthritis-associated leg ulcer. b Hematoxylin and eosin stained biopsy tissue from the same patient demonstrating leukocytoclastic vasculitis in tissue adjacent to the ulcer border with an area of intact epidermis
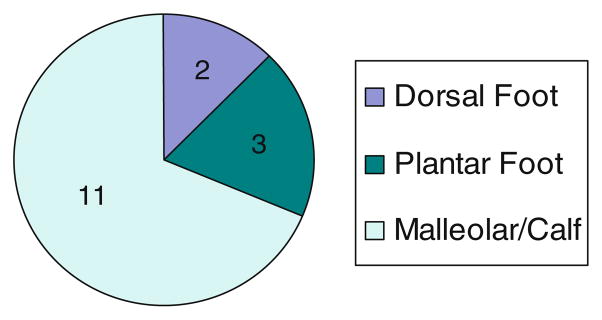
Ulcer size did not correlate with biopsy features or outcome. Ulcers were bilateral in 43.75%. The distribution of ulcers is shown in Fig. 2. Vasculitis was not seen in the patients with ulceration only on the feet. In contrast, 3 of the 11 patients with lesions in the malleolar or calf region had biopsy evidence of vasculitis.
Fig. 2.
Ulcer distribution
Prothrombotic evaluation
Antiphospholipid profile in this cohort of RA patients with ulcers was similar to that in the general population. Three patients had weakly positive lupus anticoagulant titers (ratio 1.2–1.4), and two patients with low titer antiphospholipid antibodies (one with anti-cardiolipin IgA antibody of 23 units/mL, and one with beta-2 glycoprotein 1 IgA of 16 units/mL).
Frequency of genetic prothrombotic states was similar to that reported in the general population. None of the patients had the factor V Leiden mutation, MTHFR C677T heterozygous mutation was found in three patients, four of the patients were heterozygous for the PAI-1 mutation, and one was homozygous for this mutation.
Outcomes
Over a mean follow-up of 22.8 months, 11 of the 16 patients achieved clinical remission of their arthritis. However, only five patients achieved ulcer healing (Table 1). While 13 patients were treated with non-biologic DMARD, only 5 of the 16 patients received treatment with biologic anti-tumor necrosis factor-α (anti-TNFα) agents. One of these patients required amputation due to development of cholesterol emboli and was excluded from further analysis. In the remaining patients, treatment with a biologic agent was associated with a significant increased likelihood of healing, p=0.039 (RR 3.27, 95% CI 0.59–18.29, Fig. 3), suggesting that patients with RA-associated ulcers benefit from addition of anti-TNFα agents to improve wound outcomes. The overall healing rate seen in this retrospective study was only 31.25% demonstrating how challenging these ulcers can be to treat, and reiterating the importance of further studies to provide evidence in the management of these lesions.
Table 1.
Outcomes and treatment of the 16 patients with active leg ulceration
| Patient no. | DAS-28 at ulcer development | DAS-28 at healing or most recent visit | Remission (+/−) | Medication
|
Ulcer outcome | Follow-up duration (months) | Total time to ulcer healing | Time to ulcer healing after starting DMARD or anti-TNF therapy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HCQ | Non-biol DMARD | Steroid | Anti-TNF | ||||||||
| 1 | 6.99 | 7.47 | − | + | + | + | − | Not healed | 20.4 | − | − |
| 2 | 1.47 | 1.44 | + | + | + | + | + | Not healed | 30.6 | − | No response |
| 3 | 2.02 | 1.79 | + | + | + | − | + | Healed | 22.3 | 22.3 | 4 |
| 4 | 7.52 | 2.86 | + | − | + | − | + | Healed | 16.3 | 16.3 | 4 |
| 5 | 5.89 | 2.73 | + | + | + | − | − | Not healed | 19.0 | − | − |
| 6 | 3.03 | 3.03 | + | + | + | + | + | Amputated | 9.8 | 9.8 | 1 |
| 7 | 4.07 | 2.30 | + | − | + | − | − | Not healed | 5.7 | − | − |
| 8 | 3.16 | 3.50 | − | − | + | + | − | Not healed | 1.5 | − | − |
| 9 | 7.53 | 7.75 | − | + | − | − | − | Not healed | 45.7 | − | − |
| 10 | 1.40 | 1.86 | + | + | + | − | + | Healed | 10.6 | 10.6 | 1 |
| 11 | 2.46 | 2.46 | + | − | − | − | − | Not healed | 2.0 | − | − |
| 12 | 2.91 | 2.55 | + | − | + | + | − | Healed | 21.1 | 21.1 | 3.5 |
| 13 | 2.80 | 1.59 | + | + | + | + | − | Healed | 116.3 | 116.3 | 116.3 |
| 14 | 3.62 | 3.62 | − | − | + | − | − | Died | 3.1 | − | − |
| 15 | 2.67 | 2.60 | + | − | − | + | − | Not healed | 17.0 | − | − |
| 16 | 7.99 | 7.99 | − | − | + | − | − | Not healed | 3.0 | − | − |
HCQ hydroxychloroquine, Non-biol DMARD non-biologic disease modifying antirheumatic drug, Anti-TNF anti-tumor necrosis factor-α agent
Fig. 3.
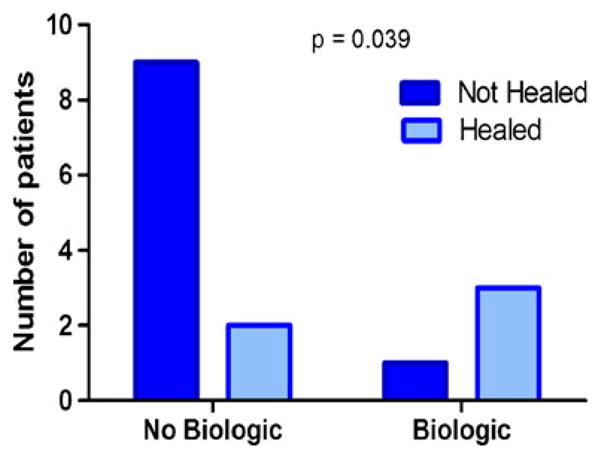
Patients treated with biologic agents were significantly more likely to have healed at the last follow-up than those who were not treated with biologic agents (chi-square test, p=0.039)
Discussion
The period prevalence of leg ulcers in this cohort of patients with RA was 4.37% and after a mean of 22.76 months of follow-up, only 31.25% had healed. These data indicate that although ulcer prevalence has improved since the advent of more effective therapies for RA, ulcers remain an important clinical problem.
Similar to other investigators [1, 3], we found that even in a center experienced in the management of autoimmune ulcers, pathologic features of vasculitis were not always evident on tissue biopsy of RA ulcers. However, this cohort of RA patients with ulcers all had radiographic evidence of erosive disease, and 63% were seropositive, suggesting that extra-articular rheumatoid disease contributes to the development of these lesions. In conjunction with the Center for Wound healing, we adopt a multidisciplinary approach to the management of complex wounds. This includes comprehensive evaluation for venous and arterial disease and aggressive management of diabetes. While we found four of the 16 patients (25%) with ulcers had concomitant venous or arterial disease, these ulcers did not heal in response to vascular intervention alone. Similarly, both patients with diabetes had well-controlled hemoglobin A1c levels (6.2% and 6.9%), so the ulcers were not thought to be due purely to diabetes.
Lower extremity ulcers are seen in other autoimmune diseases and have been reported to be associated with antiphospholipid antibodies and other prothrombotic states [12]. In our cohort of patients with scleroderma-associated leg ulcers, we found 50% with clinically significant titers of antiphospholipid antibodies [13]. In contrast, in this group of patients with RA-associated ulcers, none had significantly elevated antiphospholipid antibody titers and frequency of genetic prothrombotic states were similar to that reported in the general population.
At the time of presentation with leg ulceration, less than half of the patients in this study were in clinical remission from their RA based on DAS-28 score <3.2. Concern regarding infection risk often makes clinicians hesitant about aggressive immunosuppression in such patients. Indeed, until recently, active lower extremity ulceration was considered an absolute contraindication to treatment with anti-TNFα therapy in the United Kingdom [14]. The British biologics register has reported a significant increase in the rate of serious skin and soft tissue infections in patients treated with anti-TNF therapy [15], and reported an association between extra-articular manifestations of RA and increased risk of infection. In the current study, none of the 366 patients followed with RA developed ulceration resulting from infection related to anti-TNFα or other DMARD therapy. One patient with an established ulcer developed a wound infection while receiving anti-TNFα therapy, and as a result, the anti-TNFα therapy was discontinued. This relatively low incidence of infection may again be reflective of the multidisciplinary approach to wound care in our center and we recognize that it may not be reflective of the experiences of community-based practices.
One of the major limitations of this study is the small sample size. Our data show that RA-associated ulcers remain challenging to treat with less than one third healed after almost 2 years of follow-up. Based on data from the diabetic literature, in response to effective therapy, most leg ulcers will heal at a rate of 10% reduction in surface area per week [16]. Notably, in the cohort of RA patients with leg ulcers studied here, even the patients who ultimately healed had mean a time to healing of 32.7 months. While the sample size was small, we did find that ulcer healing was significantly more likely to occur in patients treated with biologic anti-TNFα agents, and that the time to wound healing once the anti-TNFα agent was started was comparable to that seen in diabetes (Table 1). These findings are similar to those reported by others who recommend anti-TNFα agents as an alternative to steroids and cyclophosphamide in rheumatoid leg ulcers [17–19]. A study comparing outcomes of ulcer patients with RA and other connective tissue diseases to those with ulcers from other causes is planned.
Conclusions
Even in the era of anti-TNFα and non-biologic DMARD therapy, the period prevalence of lower extremity ulcers in RA is 4.37% over 3 years. We found healing rates of only 31.25% in 22.6 months of follow-up. Although this was a small, retrospective study, there was a significantly improved rate of healing in patients treated with biologic anti-TNFα agents.
Acknowledgments
This work was supported by the Physician Scientist Development Award from the American College of Rheumatology Research and Education Foundation and by award numbers KL2RR031974 and UL1RR031975 from the National Center for Research Resources.
Footnotes
Disclosures None.
Contributor Information
Victoria K. Shanmugam, Email: vks4@gunet.georgetown.edu, Division of Rheumatology, Immunology and Allergy, Georgetown University Hospital, 3800 Reservoir Road, NW Washington, DC 20007, USA
David M. DeMaria, Division of Rheumatology, Immunology and Allergy, Georgetown University Hospital, 3800 Reservoir Road, NW Washington, DC 20007, USA
Christopher E. Attinger, Center for Wound Healing, Georgetown University Hospital, 3800 Reservoir Road, NW Washington, DC 20007, USA
References
- 1.Pun YL, Barraclough DR, Muirden KD. Leg ulcers in rheumatoid arthritis. Med J Aust. 1990;153(10):585–587. doi: 10.5694/j.1326-5377.1990.tb126267.x. [DOI] [PubMed] [Google Scholar]
- 2.Oien RF, Hakansson A, Hansen BU. Leg ulcers in patients with rheumatoid arthritis—a prospective study of aetiology, wound healing and pain reduction after pinch grafting. Rheumatology. 2001;40(7):816–820. doi: 10.1093/rheumatology/40.7.816. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa JA. Are leg ulcers in rheumatoid arthritis due to vasculitis? Eur J Rheumatol Inflamm. 1983;6(3):288–290. [PubMed] [Google Scholar]
- 4.Cawley MID. Vasculitis and ulceration in rheumatic diseases of the foot. Baillières Clin Rheumatol. 1987;1(2):315–333. doi: 10.1016/s0950-3579(87)80006-7. [DOI] [PubMed] [Google Scholar]
- 5.McRorie ER, Ruckley CV, Nuki G. The relevance of large-vessel vascular disease and restricted ankle movement to the aetiology of leg ulceration in rheumatoid arthritis. Br J Rheumatol. 1998;37(12):1295–1298. doi: 10.1093/rheumatology/37.12.1295. [DOI] [PubMed] [Google Scholar]
- 6.Hafner J, Schneider E, Burg G, Cassina PC. Management of leg ulcers in patients with rheumatoid arthritis or systemic sclerosis: the importance of concomitant arterial and venous disease. J Vasc Surg. 2001;32(2):322–329. doi: 10.1067/mva.2000.106942. [DOI] [PubMed] [Google Scholar]
- 7.Thurtle O, Cawley M. The frequency of leg ulceration in rheumatoid arthritis: a survey. J Rheumatol. 1983;10(3):507–509. [PubMed] [Google Scholar]
- 8.Firth J, Hale C, Helliwell P, Hill J, Nelson EA. The prevalence of foot ulceration in patients with rheumatoid arthritis. Arthritis Care Res. 2008;59(2):200–205. doi: 10.1002/art.23335. [DOI] [PubMed] [Google Scholar]
- 9.McRorie E. The assessment and management of leg ulcers in rheumatoid arthritis. J Wound Care. 2000;9(6):289–292. doi: 10.12968/jowc.2000.9.6.25993. [DOI] [PubMed] [Google Scholar]
- 10.Watts RA, Mooney J, Lane SE, Scott DGI. Rheumatoid vasculitis: becoming extinct? Rheumatology. 2004;43(7):920–923. doi: 10.1093/rheumatology/keh210. [DOI] [PubMed] [Google Scholar]
- 11.Charabaty S, Shanmugam V. A 65-year-old man with longstanding seropositive rheumatoid arthritis and lower extremity ulceration. Arthritis Care Res. 2009;61(9):1275–1280. doi: 10.1002/art.24700. [DOI] [PubMed] [Google Scholar]
- 12.Shanmugam V, Steen V, Cupps T. Lower extremity ulcers in connective tissue disease. Isr Med Assoc J. 2008;10(7):534–536. [PubMed] [Google Scholar]
- 13.Shanmugam V, Price P, Attinger C, Steen V. Lower extremity ulcers in systemic sclerosis: features and response to therapy. Int J Rheumatol. 2010 doi: 10.1155/2010/747946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Collaborating Center for Chronic Conditions. Rheumatoid arthritis: national clinical guideline for management and treatment in adults. Royal College of Physicians; 2009. Feb, [PubMed] [Google Scholar]
- 15.Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DPM. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54(8):2368–2376. doi: 10.1002/art.21978. [DOI] [PubMed] [Google Scholar]
- 16.Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diab Care. 2003;26(6):1879–1882. doi: 10.2337/diacare.26.6.1879. [DOI] [PubMed] [Google Scholar]
- 17.Unger L, Kayser M, Nusslein HG. Successful treatment of severe rheumatoid vasculitis by infliximab. Ann Rheum Dis. 2003;62 (6):587–588. doi: 10.1136/ard.62.6.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews C, Fitzgerald O. Seropositive erosive rheumatoid arthritis (RA) Rheumatology. 2006;45(9):1100. doi: 10.1093/rheumatology/kel055. [DOI] [PubMed] [Google Scholar]
- 19.Hirche D, Rubbert A, Lunau L, Krieg T, Eming SA. Successful treatment of refractory rheumatoid arthritis-associated leg ulcerations with adalimumab. Br J Dermatol. 2005;152(5):1062–1064. doi: 10.1111/j.1365-2133.2005.06520.x. [DOI] [PubMed] [Google Scholar]