Abstract
Lead(II)-induced cleavage can be used as a tool to probe conformational changes in RNA. In this report, we have investigated the conformation of M1 RNA, the catalytic subunit of Escherichia coli RNase P, by studying the lead(II)-induced cleavage pattern in the presence of various divalent metal ions. Our data suggest that the overall conformation of M1 RNA is very similar in the presence of Mg2+, Mn2+, Ca2+, Sr2+ and Ba2+, while it is changed compared to the Mg2+-induced conformation in the presence of other divalent metal ions, Cd2+ for example. We also observed that correct folding of some M1 RNA domains is promoted by Pb2+, while folding of other domain(s) requires the additional presence of other divalent metal ions, cobalt(III) hexamine or spermidine. Based on the suppression of Pb2+ cleavage at increasing concentrations of various divalent metal ions, our findings suggest that different divalent metal ions bind with different affinities to M1 RNA as well as to an RNase P hairpin–loop substrate and yeast tRNAPhe. We suggest that this approach can be used to obtain information about the relative binding strength for different divalent metal ions to RNA in general, as well as to specific RNA divalent metal ion binding sites. Of those studied in this report, Mn2+ is generally among the strongest RNA binders.
INTRODUCTION
Folding of RNA is facilitated by the presence of metal ions. It has been demonstrated that several divalent metal ions can promote the folding of RNA molecules required for catalytic activity (1). Our studies focus on the mechanism of cleavage by RNase P, an endoribonuclease in which the catalytic activity resides in the RNA subunit, RNase P RNA (2). At physiological pH the reaction catalyzed by Escherichia coli RNase P RNA, M1 RNA, can proceed in the presence of Mg2+, Mn2+ and Ca2+, and among these, Mg2+ promotes cleavage most efficiently (3–10). These findings suggest that M1 RNA is folded in a similar way in the presence of each of these three divalent metal ions. Cross-linking analysis of M1 RNA and its substrate, and lead(II)-induced cleavage studies of M1 RNA, also indicate that the overall conformation of M1 RNA is not significantly altered due to replacement of Mg2+ with either Ca2+ or Mn2+ (6,11). To investigate whether the structure of M1 RNA does change as result of replacing Mg2+ with other divalent metal ions, we decided to study Pb2+ cleavage of M1 RNA in the presence of different concentrations of Mg2+, Ca2+ and Mn2+, as well as in the presence of other divalent metal ions that do not promote M1 RNA cleavage by themselves. Our data suggest that: (i) M1 RNA is similarly folded in the presence of Mg2+, Mn2+, Ca2+, Sr2+ and Ba2+, while it is changed compared to the Mg2+-induced conformation in the presence of other divalent metal ions, for example Cd2+; (ii) folding of some M1 RNA domains occurs in the presence of Pb2+ only, while folding of other domain(s) requires the additional presence of other divalent metal ions, cobalt(III) hexamine or spermidine; and (iii) different divalent metal ions bind with different affinities to M1 RNA, where Mn2+ is in general among the strongest RNA binders. Our findings are discussed in relation to the hypothesis that there are two categories of divalent metal ions, structural and catalytic, in the M1 RNA-catalyzed reaction.
MATERIALS AND METHODS
Preparation of RNA and labeling procedures
The M1 RNA gene construct behind the T7 promoter, used to generate M1 RNA according to Milligan et al. (12), has been described elsewhere (13). The pATSerCGSyn was chemically synthesized and purchased from Xeragon AG (Zürich, Switzerland) whereas yeast tRNAPhe was purchased from Sigma (Stockholm, Sweden).
The different RNA molecules were labeled at the 3′-end with [32P]pCp according to standard procedures. The labeled RNA were subsequently purified on denaturing polyacrylamide gels where the percentage of polyacrylamide used was dependent on the size of the RNA. The various RNA molecules of correct size were eluted as previously described (8).
Pb2+-induced cleavage of M1 RNA, pATSerCGSyn and yeast tRNAPhe
M1 RNA. Lead(II)-induced cleavage of 3′-end-labeled M1 RNA was performed essentially as described previously (8,14). Approximately 20 000–30 000 c.p.m. of labeled M1 RNA was mixed with ∼2.5 pmol of unlabeled M1 RNA and preincubated in 50 mM Tris–HCl pH 7.5, 100 mM NH4Cl and various concentrations of different metal ion concentrations as indicated. The RNA was heated for 2 min at 70°C, followed by incubation for 10 min at 37°C. In all cases we used freshly prepared MeCl2, except for barium where Ba(OAc)2 was used. Cleavage was initiated by the addition of freshly prepared Pb(OAc)2 to a final concentration of 0.5 mM (final reaction volume 10 µl). The reaction was allowed to proceed for 6 min at 37°C (7 min in the case of Co(NH3)63+ and spermidine; Fig. 3). To terminate the reaction, 2 vol stop solution (30 mM EDTA in 10 M urea supplemented with 0.1% bromophenol blue) was added. The cleavage products were separated on 8% denaturing polyacrylamide gels and detected using a PhosphorImager (Molecular Dynamics).
Figure 3.
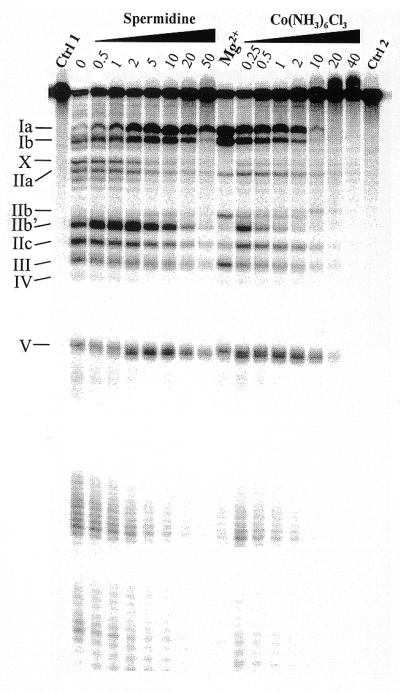
Lead(II)-induced cleavage patterns of M1 RNA in the presence of increasing concentrations (in mM) of the Mg(H2O)62+ analog Co(NH3)63+ and spermidine, as indicated. Cleavage was performed at the indicated concentrations and 37°C, as outlined in Materials and Methods and Figure 2 legend, with the exception that the time of incubation in the presence of Pb2+ was 7 instead of 6 min. Ctrl 1, incubation in the presence of 50 mM Tris–HCl pH 7.5, 100 mM NH4Cl, 10 mM Co(NH3)63+, 10 mM spermidine, 10 mM MgCl2 and without Pb2+; Ctrl 2, as Ctrl 1 but incubation in the presence of 50 mM Tris–HCl pH 7.5 and 100 mM NH4Cl (without Pb2+) only; Mg2+, Pb2+ cleavage in the presence of 50 mM Tris–HCl pH 7.5, 100 mM NH4Cl and 5 mM MgCl2. Roman numerals indicate the cleavage sites as shown in Figure 1.
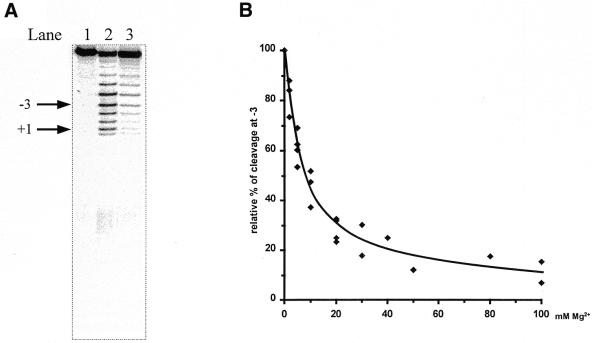
pATSerCGSyn. After gel purification ∼40 000 c.p.m. of 3′-end-labeled pATSerCGSyn was mixed with 20 pmol of unlabeled pATSerCGSyn in 50 mM Tris–HCl pH 7.2, 100 mM NH4Cl and different concentrations of divalent metal ions (0–50 mM). The mixture was heated and preincubated as described above. Lead(II)-induced cleavage was initiated by adding freshly prepared Pb(OAc)2 to a final concentration of 2 mM and terminated as described above. The cleavage products were separated on a 22% denaturing polyacrylamide gel followed by detection using a PhosphorImager. The frequency of cleavage as a function of divalent metal ion concentration was calculated (see Fig. 5 for which of the fragments we used for the calculations) where 100% cleavage represents cleavage in the presence of Pb2+ alone. The percentage of cleavage was plotted against the divalent metal ion concentration and from these plots apparent Ki values were calculated. The apparent Ki value was defined as the concentration of divalent metal ion resulting in 50% inhibition of Pb2+ cleavage.
Figure 5.
(A) Lead(II)-induced cleavage of 3′-labeled pATSerCGSyn in the absence and presence of Pb(OAc)2, as indicated. The arrows indicate the fragment (–3) used to calculate the apparent Ki values given in Table 1 and the RNase P cleavage site at position +1. The sizes of the fragments were determined by parallel runs of pATSerCGSyn cleaved with M1 RNA, which cleaves this substrate at the +1 position (see also 11). Pb2+ cleavage was performed at 37°C for 6 min as described in Materials and Methods. Lane 1, no Pb(OAc)2 added; lane 2, 2 mM (final concentration) of Pb(OAc)2 added (no other divalent metal ion added); lane 3, 2 mM Pb(OAc)2 and 50 mM MgCl2 (final concentrations) added. (B) Relative percentage of Pb2+ cleavage plotted as a function of increasing concentration of MgCl2 using fragment –3, as indicated in (A). The apparent Ki value was determined as the concentration of MgCl2 resulting in 50% inhibition. The apparent Ki values are summarized in Table 1.
Yeast tRNAPhe. Pb2+ cleavage of yeast tRNAPhe was essentially performed as described by Behlen et al. (15). Gel purified 3′-labeled yeast tRNAPhe (15 000 c.p.m.) was mixed with 100 ng of unlabeled yeast tRNAPhe in 50 mM Tris–HCl pH 7.2, 15 mM NH4Cl, 1.5 mM spermidine and different concentrations (0–100 mM) of divalent metal ions (Mg2+, Ca2+, Sr2+, Mn2+ or Zn2+). The mixture was heated at 70°C for 1 min followed by incubation at 25°C for 5 min. Lead(II)-induced cleavage was initiated by the addition of freshly prepared Pb(OAc)2 to a final concentration of 0.5 mM. The reaction was terminated after incubation for an additional 3 min at 25°C by the addition of stop solution (25 mM EDTA, 7 M urea and 0.1% bromophenol blue). The products were separated on a 10% denaturing polyacrylamide gel and apparent Ki values were calculated as described above for pATSerCGSyn.
RESULTS
Conformational changes of M1 RNA as a function of increasing Mg2+ concentration
Lead(II)-induced cleavage has previously been used to monitor structural changes in M1 RNA as a result of base substitutions, deletions or interaction with the substrate (14,16–19). In the first set of experiments we investigated the Pb2+ cleavage pattern of M1 RNA as a function of increasing Mg2+ concentration to probe the conformation of M1 RNA at different Mg2+ concentrations. From the data summarized in Figure 1 and shown in Figure 2, we conclude that M1 RNA undergoes conformational changes with increasing Mg2+ concentration and that M1 RNA is completely folded at 5–10 mM Mg2+, in keeping with previous findings (20–22). Also, consistent with previous reports (14) we observed that higher concentrations of Mg2+ suppressed the lead(II) cleavage pattern, indicating that Mg2+ binds to the same or overlapping sites as Pb2+. Irrespective of Mg2+, Pb2+ cleavage at positions IIc, III, IV and V was detected (for numbering of cleavage sites see Fig. 1). This suggests that the folding of these regions of M1 RNA, including the P15 internal loop, is promoted by Pb2+ alone, in keeping with our previous data (23). In contrast, Pb2+ cleavage at positions Ia, Ib and IIb was not observed or was significantly reduced (Ib) in the absence of Mg2+. Note that band IIb appears with a simultaneous reduction of cleavage at site IIb′. Also, a band (X) migrating slightly slower than IIa was only observed in the presence of Pb2+ and low Mg2+ concentrations. Hence, proper folding of this part of M1 RNA that results in efficient Pb2+ cleavage at these positions (Ia, Ib and IIb) depends on the presence of Mg2+. In conclusion, an increase in the concentration of Mg2+ resulted in conformational changes of M1 RNA. These findings are in agreement with the notion that the folding of M1 RNA proceeds via intermediates (20–22,24).
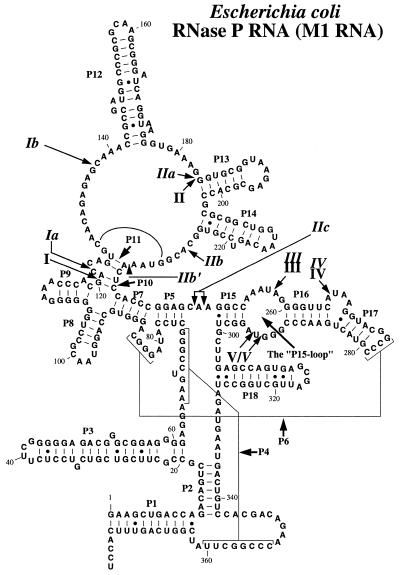
Figure 1.
A secondary structure model of M1 RNA according to Haas et al. (42). The Pb2+ cleavage sites are indicated by arrows and italic roman numerals (14,18), whereas the roman numerals indicate the Mg2+-induced cleavage sites according to Kazakov and Altman (3).
Figure 2.
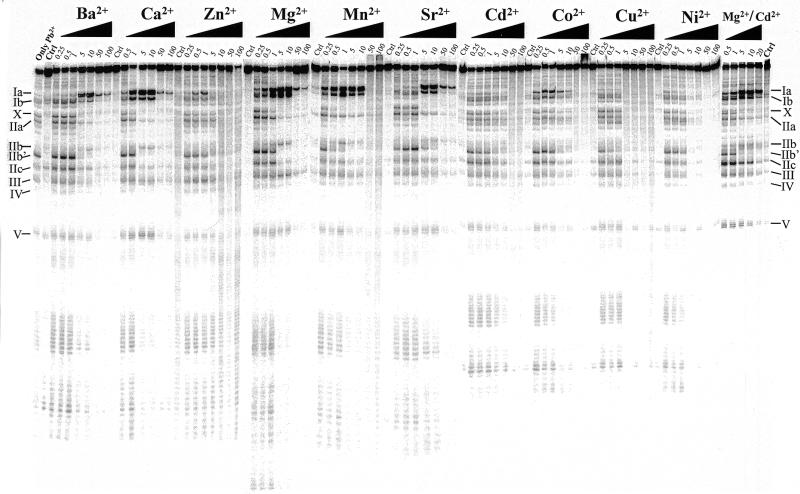
Lead(II)-induced cleavage patterns of M1 RNA in the presence of increasing concentrations (in mM) of various divalent metal ions, as indicated. Cleavage was performed at the indicated concentrations and 37°C, as outlined in Materials and Methods. Only Pb2+, incubation in the presence of 0.5 mM Pb2+ only; Ctrl, incubation in the presence of the divalent metal ion, 10 mM (final concentration), as indicated in the absence of Pb2+. Pb2+ cleavage in the presence of Mg2+:Cd2+ was performed under the following conditions: 0.2 M Mg2+:Cd2+ stock solutions with 5:3 Mg2+:Cd2+ ratio (or 5:1) were prepared and these were diluted to give the final concentration in mM as indicated (data not shown for cleavage performed using the latter conditions since the Pb2+ cleavage patterns were identical irrespective of Mg2+:Cd2+ ratio). The roman numerals refer to the sites of cleavage in M1 RNA as shown in Figure 1.
Overall conformation of M1 RNA in the presence of other divalent metal ions
Cleavage by M1 RNA can proceed in the presence of other divalent metal ions (see above). Therefore, we next analyzed the conformation of M1 RNA as a function of increasing concentrations of other divalent metal ions, as indicated in Figure 2. Here we observed that the lead(II)-induced cleavage pattern in the presence of Ca2+, Mn2+, Sr2+ or Ba2+ was very similar to the pattern observed in the presence of Mg2+. The Pb2+ cleavage pattern generated in the presence of 5 mM Mn2+ is in keeping with our previous data (11). These data suggest that the global conformation of M1 RNA in the presence of each of these divalent metal ions is very similar to the structure of M1 RNA formed in the presence of Mg2+. However, a higher concentration of Sr2+ or Ba2+ relative to Mg2+ was required for proper folding of M1 RNA, while a lower concentration of Mn2+ was required. In the case of Ca2+, approximately equal concentration, as in the case of Mg2+, was required. Furthermore, we observed that lower and higher concentrations of Mn2+ and Sr2+ or Ba2+, respectively, relative to Mg2+, were needed to reduce the site-specific Pb2+ cleavage (Fig. 2). These data suggest that Mn2+ has a higher overall affinity for M1 RNA than the other divalent metal ions. Based on these findings it is possible to rank these divalent metal ions with respect to affinity to M1 RNA: Mn2+ > Mg2+ ≈ Ca2+ > Sr2+ ≈ Ba2+, where Mn2+ has the highest affinity.
We also studied lead(II)-induced cleavage in the presence of Co2+, Ni2+, Cu2+, Zn2+ and Cd2+. None of these divalent metal ions promote cleavage by themselves, rather, addition of any one of these metal ions is inhibitory (21; data not shown). From the data shown in Figure 2 it is clear that cleavage by Pb2+ in the presence of Ni2+, Cu2+ and Cd2+ resulted in a different pattern compared to the ‘Mg2+-induced’ cleavage pattern, in particular with respect to cleavage at sites Ia, Ib and IIb. In contrast, both Co2+ and Zn2+ promoted Pb2+ cleavage at sites Ia and Ib at low concentrations, while cleavage at site IIb was absent (Zn2+) or very weak (Co2+). These findings suggest that the conformation of the region(s) of M1 RNA involving sites Ia, Ib and IIb is different in the presence of in particular Ni2+, Cu2+ and Cd2+ relative to the conformation generated in the presence of Mg2+. We emphasize that none of these divalent metal ions (or combinations) by themselves induced detectable cleavage of M1 RNA under these conditions (Fig. 2).
From Figure 2 it is apparent that replacement of Mg2+ with Cd2+ resulted in a change in the conformation of M1 RNA. Previously it was shown that M1 RNA is catalytically active under reaction conditions where both Mg2+ and Cd2+ are present in a 5:1 ratio (9). Thus, we decided to study Pb2+ cleavage of M1 RNA in solutions with different ratios of Mg2+ and Cd2+ to investigate whether the structure of M1 RNA was changed compared to the structures observed in the presence of either of these divalent metal ions alone. As can be seen in Figure 2 the observed lead(II)-induced cleavage pattern was not significantly changed under conditions where the Mg2+:Cd2+ ratio was 5:1 or 5:3 compared to the pattern seen in the presence of only Mg2+ (data shown for Mg2+:Cd2+ ratio = 5:3 only). Taken together, this indicates that the conformation of M1 RNA was restored as a result of addition of Mg2+, giving a rationale to why M1 RNA is catalytically active in a solution of Mg2+ and Cd2+ but not in the presence of Cd2+ alone (or very low activity; 9).
M1 RNA structure in the presence of cobalt(III) hexamine and spermidine
Our data so far suggest that some, but not all, divalent metal ions can promote a ‘Mg2+-like’ conformation of M1 RNA as judged from the Pb2+ cleavage patterns. Cobalt(III) hexamine is considered to be an analog of fully solvated magnesium (25 and references therein). To investigate whether Pb2+ cleavage at particular sites Ia and Ib is promoted in the presence of this analog, we analyzed the Pb2+ cleavage pattern as a function of increasing concentration of Co(NH3)63+. As shown in Figure 3, we observed that cleavage at sites Ia, Ib and IIb (to a lesser extent) were also promoted in the presence of this Mg(H2O)62+ analog. At concentrations ≥1 mM the Pb2+ cleavage of M1 RNA was suppressed in a similar manner to that described above. These findings suggest that inner sphere metal ion coordination is not required to adopt a conformation of the region near sites Ia, Ib and IIb such that Pb2+ generates efficient cleavage at these positions.
It is known from previous studies that addition of spermidine to the M1 RNA cleavage reaction lowers the concentration requirements for Mg2+ (21,26). We therefore studied the influence of spermidine on the lead(II)-induced cleavage pattern. As observed in the case of addition of cobalt(III) hexamine, Pb2+ cleavage at sites Ia and Ib was promoted in the presence of spermidine, while cleavage in the IIa region and at IIb showed differences compared to Pb2+ cleavage in the presence of Mg2+. Given that cleavage by M1 RNA cannot be promoted by spermidine alone, this might suggest that a divalent metal ion-induced folding of these regions is necessary in order to adopt a catalytically active conformation of M1 RNA.
Relative RNA binding affinities for different divalent metal ions
As discussed above, Mn2+ ions bind more strongly to M1 RNA than Mg2+, while Sr2+ binds with a lower affinity. To investigate whether this is a general behavior of these divalent metal ions relative to RNA, we studied Pb2+ cleavage of two other RNA molecules, pATSerCGSyn and yeast tRNAPhe (Fig. 4). The former is a well-characterized model substrate for RNase P (11,23) whereas lead(II)-induced cleavage and the structure of the latter is well-documented (27 and references therein). First, we demonstrated that pATSerCGSyn is indeed cleaved in the presence of Pb2+ (Fig. 5). As shown in Figure 5, cleavage occurred at several positions in the 5′ leader. Secondly, we added increasing concentrations of indicated divalent metal ions (Fig. 5; Table 1), which resulted in suppression of Pb2+-induced cleavage in a concentration-dependent manner (Fig. 5; data shown for Mg2+ only). Apparent Ki values for Mg2+, Mn2+, Ca2+, Sr2+, Zn2+ and Co(NH3)63+ were calculated and the result showed that Mn2+ (and Zn2+) suppressed Pb2+ cleavage ∼3- and 5-fold more efficiently compared to Mg2+ and Sr2+, respectively (Table 1). The same tendency was observed when we studied suppression of Pb2+-induced cleavage of yeast tRNAPhe, with the exception that a significantly higher apparent Ki value was observed for Zn2+. These findings, together with the data discussed above, clearly suggest that different divalent metal ions bind to RNA, generally with different strength, and that Mn2+ is among the strongest binders studied in this report. Furthermore, our Pb2+ cleavage studies using pATSerCGSyn suggest that different divalent metal ions can bind in the vicinity of the RNase P cleavage site. This is in keeping with previous reports where Mg2+ cleavage of a similar hairpin–loop RNA structure was studied (28,29). In addition, data obtained from the addition of Co(NH3)63+ suggest that inner sphere coordination is not required to give suppression of Pb2+ cleavage in the 5′ leader.
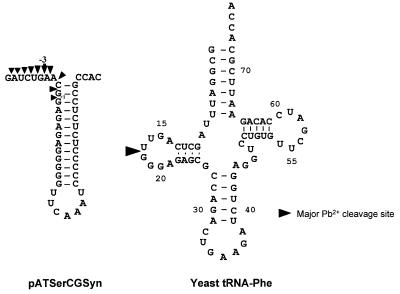
Figure 4.
The secondary structures of pATSerCGSyn and yeast tRNAPhe. The lead(II)-induced and RNase P cleavage sites are indicated by arrows. The number –3 refers to the cleavage fragment we used to calculate the apparent Ki values.
Table 1. Apparent Ki values for inhibition of Pb2+-induced cleavage of pATSerCGSyn and yeast tRNAPhe.
| Divalent Me2+ |
Apparent Ki/mM pATSerCGSyn |
Apparent Ki/mM
yeast tRNAPhe |
Ionic radius (Å)a |
Coordination numbera |
Hardness absolutea |
Exchange rates for H2O (s–1)a |
| Mg2+ | 9.3 ± 2.2 | 13 ± 1 | 0.72 | 6 | 47.59 | 105 |
| Ca2+ | 6.7 ± 3.1 | 17 ± 1.6 | 1.12 | 8 | 19.52 | 108 |
| Sr2+ | 16 ± 3.0 | 41 ± 0.1 | 1.18 | 6 | 27.30 | 108–109 |
| Mn2+ | 3.4 ± 0.75 | 5.6 ± 0.4 | 0.67 | 6 | 9.02 | 107 |
| Zn2+ | 2.8 ± 0.8 | 51 ± 0.8 | 0.74 | 6 | 10.88 | 107 |
| Co(NH3)63+ | 1.0 ± 0.6 | ND | 0.61 | 6 | 8.90 | 1 |
The Ki values were determined as outlined in Materials and Methods and each number is an average of several independent experiments.
ND, not determined.
aNumbers taken from Feig and Uhlenbeck (27) and Cowan (43).
DISCUSSION
Lead(II)-induced cleavage is a way to probe conformational changes in an RNA as a result of point mutations, deletions and substrate interaction (14,16–19,30–32). In addition, Pb2+ cleavage has been used to monitor the structural requirements for binding of Pb2+ in yeast tRNAPhe that subsequently results in cleavage at a specific position (15,33). Here we demonstrated that it is possible to use this approach to monitor structural differences in E.coli RNase P RNA, M1 RNA, as a result of changes in divalent metal ion concentration and divalent metal ion replacements.
Kazakov and Altman (3) showed that Mg2+, Ca2+ and Ba2+ cleaved M1 RNA at approximately the same sites as Pb2+ [I (≈ Ia), II (= IIa), III–V for Mg2+ and Ca2+, while Ba2+ cleaves mostly at site I and to a small extent at the other positions; for details see Fig. 1]. This, together with our present data and data reported elsewhere (14), suggests that these metal ions bind to the same or to overlapping sites in M1 RNA, although with different affinities (this report). That various divalent metal ions bind to at least overlapping sites in solution is corroborated by our Pb2+ cleavage studies of an RNase P model substrate and of yeast tRNAPhe, as well as crystal structure studies of the latter (for a review see 27). In conclusion, our findings suggest that it is possible to obtain information about the relative binding strengths for different divalent metal ions to RNA generally, as well as to specific divalent metal binding sites, by studying their effect on Pb2+ cleavage of RNA molecules.
Our data showed that Mn2+ binds with a higher affinity compared to Mg2+ to three different RNA molecules studied in this report. From the data reported by Streicher et al. (31) it appears that this is also the case in the T4 phage-derived td intron system. These data are in keeping with an earlier finding that Mn2+ binds more strongly to DNA than Mg2+ does (34), as well as the finding that cleavage both by E.coli and Bacillus subtilis RNase P RNA requires lower concentration of Mn2+ than Mg2+ for optimal cleavage (4,35). The ionic radii of Mg2+ and Mn2+, as well as the coordination of these two divalent metal ions, are very similar (Table 1). However, a major difference is that Mn2+ binds nitrogen more frequently compared to Mg2+, while Mg2+ (and Ca2+) prefers oxygen (36). Therefore, it is plausible that the increased affinity of Mn2+ for RNA is due to the fact that Mn2+ coordinates more frequently by inner sphere binding to the bases. In fact, Mn2+ coordinates directly to N7 of G20 in the crystal structure of yeast tRNAPhe (37). The stronger binding of Mn2+ to RNA might consequently be a rationale to why addition of Mn2+ to the RNase P cleavage reaction resulted in an increased frequency of cleavage at an incorrect position (11).
By comparing the Pb2+ cleavage pattern generated in the presence of different divalent metal ions, we concluded that the conformation of M1 RNA is very similar in the presence of Mg2+, Mn2+, Ca2+, Sr2+ and Ba2+ (see above). Folding of M1 RNA in the presence of other divalent metal ions resulted in a changed overall conformation compared to the Mg2+ generated structure, in particular in regions near sites Ia, Ib and IIb. This is consistent with the notion that Mg2+, Mn2+ and Ca2+ alone promote catalysis (for references see above). In contrast, Sr2+ and Ba2+ do not result in cleavage at neutral pH and therefore these divalent metal ions are suggested to have a structural role under these conditions. This is in keeping with an earlier report that suggested that there are two classes of divalent metal ion binding sites in the M1 RNA-catalyzed reaction, catalytic and structural sites (21; see below). However, at higher pH and in the presence of ethanol, correct cleavage in the presence of either Sr2+ or Ba2+ alone has been observed (3), indicating that under specific conditions both these divalent metal ions can fulfil the role of catalytic metal ions. Furthermore, folding of the Pb2+ binding site that resulted in lead(II)-induced cleavage at the particular sites Ia and Ib was observed in the presence of Mg2+, Mn2+, Ca2+, Sr2+, Ba2+ (to a lower extent Zn2+ and Co2+), the Mg(H2O)62+ analog Co(NH3)63+ and spermidine. One interpretation of these findings is that the regions in the vicinity of sites Ia and Ib are mainly stabilized by positively charged ions, possibly by diffuse binding of fully hydrated metal ions (25). That efficient Pb2+-induced cleavage at sites Ia and Ib is dependent on a stabilized structure near site Ia is supported by our previous findings where we studied Pb2+ cleavage of M1 RNA variants carrying substitutions in the vicinity of site Ia (16,17).
Two classes of divalent metal ion binding sites have also been suggested for the group I intron ribozyme derived from Tetrahymena, L-21 ScaI (32,38,39). Here, only Mg2+ and Mn2+ can fulfil the catalytic role, i.e. bind to class I sites, whereas the presumed structural class II sites can be occupied by Mg2+ and Mn2+ as well as by Ca2+ and Sr2+ (38,40). Taken together, with respect to divalent metal ion binding, the two large ribozyme systems RNase P RNA and group I intron RNA show clear similarities (41) but also differences such as the difference in catalytic performance in the presence of Ca2+ alone.
Acknowledgments
ACKNOWLEDGEMENTS
We acknowledge Drs D.Hughes and A.Virtanen for critical reading of the manuscript and the technical assistance of Ms S.Rosén. This work was supported by grants from the Swedish Natural Research Council and the Foundation for Strategic Research to L.A.K.
References
- 1.Gesteland R.F., Cech,T.R. and Atkins,J.F. (1999) The RNA World, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, NY.
- 2.Guerrier-Takada C., Gardiner,K., Marsh,T., Pace,N. and Altman,S. (1983) The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell, 35, 849–857. [DOI] [PubMed] [Google Scholar]
- 3.Kazakov S. and Altman,S. (1991) Site-specific cleavage by metal ion cofactors and inhibitors of M1 RNA, the catalytic subunit of RNase P from Escherichia coli. Proc. Natl Acad. Sci. USA, 88, 9193–9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith D., Burgin,A.B., Haas,E. and Pace,N.R. (1992) Influence of metal ions on the ribonuclease P reaction. J. Biol. Chem., 267, 2429–2436. [PubMed] [Google Scholar]
- 5.Smith D. and Pace,N.R. (1993) Multiple magnesium ions in the ribonuclease P reaction mechanism. Biochemistry, 32, 5273–5281. [DOI] [PubMed] [Google Scholar]
- 6.Kufel J. and Kirsebom,L.A. (1994) Cleavage site selection by M1 RNA, the catalytic subunit of Escherichia coli RNase P, is influenced by pH. J. Mol. Biol., 244, 511–521. [DOI] [PubMed] [Google Scholar]
- 7.Kufel J. and Kirsebom,L.A. (1996) Different cleavage sites are aligned differently in the active site of M1 RNA, the catalytic subunit of Escherichia coli RNase P. Proc. Natl Acad. Sci. USA, 93, 6085–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kufel J. and Kirsebom,L.A. (1996) Residues in Escherichia coli RNase P RNA important for cleavage site selection and divalent metal ion binding. J. Mol. Biol., 263, 685–698. [DOI] [PubMed] [Google Scholar]
- 9.Warnecke J.M., Fürste,J.P., Hardt,W.-D., Erdmann,V.E. and Hartmann,R.K. (1996) Ribonuclease P (RNase P) RNA is converted to a Cd2+-ribozyme by a single Rp-phosphorothioate modification in the precursor tRNA at the RNase P cleavage site. Proc. Natl Acad. Sci. USA, 93, 8924–8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y., Li,X. and Gegenheimer,P. (1997) Ribonuclease P catalysis requires Mg2+ coordinated to the pro-Rp oxygen of the scissile bond. Biochemistry, 36, 2425–2438. [DOI] [PubMed] [Google Scholar]
- 11.Brännvall M. and Kirsebom,L.A. (1999) Manganese ions induce miscleavage in the Escherichia coli RNase P RNA-catalyzed reaction. J. Mol. Biol., 292, 53–63. [DOI] [PubMed] [Google Scholar]
- 12.Milligan J.F., Groebe,D.R., Whiterell,G.W. and Uhlenbeck,O.C. (1987) Oligoribonucleotide synthesis using T7 RNA polymerase and DNA templates. Nucleic Acids Res., 15, 8783–8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vioque A., Arnez,J. and Altman,S. (1988) Protein–RNA interactions in the RNase P holoenzyme from Escherichia coli. J. Mol. Biol., 202, 835–848. [DOI] [PubMed] [Google Scholar]
- 14.Ciesiolka J., Hardt,W.-D., Schlegl,J., Erdmann,V.A. and Hartmann,R.K. (1994) Lead-ion-induced cleavage of RNase P RNA. Eur. J. Biochem., 219, 49–56. [DOI] [PubMed] [Google Scholar]
- 15.Behlen L.S., Sampson,J.R., DiRenzo,A.B. and Uhlenbeck,O.C. (1990) Lead-catalyzed cleavage of yeast tRNAPhe mutants. Biochemistry, 29, 2515–2523. [DOI] [PubMed] [Google Scholar]
- 16.Tallsjö A., Svärd,S.G., Kufel,J. and Kirsebom,L.A. (1993) A novel tertiary interaction in M1 RNA, the catalytic subunit of Escherichia coli RNase P. Nucleic Acids Res., 21, 3927–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattsson J.G., Svärd,S.G. and Kirsebom,L.A. (1994) Characterization of the Borrelia burgdorferi RNase P RNA gene reveals a novel tertiary interaction. J. Mol. Biol., 241, 1–6. [DOI] [PubMed] [Google Scholar]
- 18.Zito K., Hüttenhofer,A. and Pace,N.R. (1993) Lead-catalyzed cleavage of ribonuclease P RNA as a probe for integrity of tertiary structure. Nucleic Acids Res., 21, 5916–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardt W.-D., Schlegl,J., Erdmann,V.A. and Hartmann,R.K. (1995) Kinetics and thermodynamics of the RNase P RNA cleavage reaction: analysis of tRNA 3′-end variants. J. Mol. Biol., 247, 161–172. [DOI] [PubMed] [Google Scholar]
- 20.Loria A. and Pan,T. (1996) Domain structure of the ribozyme from eubacterial ribonuclease P. RNA, 2, 551–563. [PMC free article] [PubMed] [Google Scholar]
- 21.Guerrier-Takada C., Haydock,K., Allen,L. and Altman,S. (1986) Metal ion requirements and other aspects of the reaction catalyzed by M1 RNA, the RNA subunit of ribonuclease P from Escherichia coli. Biochemistry, 25, 1509–1515. [DOI] [PubMed] [Google Scholar]
- 22.Zarrinkar P.P., Wang,J. and Williamson,J.R. (1996) Slow folding kinetics of RNase P RNA. RNA, 2, 564–573. [PMC free article] [PubMed] [Google Scholar]
- 23.Kufel J. and Kirsebom,L.A. (1998) The P15-loop of Escherichia coli RNase P RNA is an autonomous divalent metal ion binding domain. RNA, 4, 777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan T. and Sosnick,T.R. (1997) Intermediates and kinetic traps in the folding of a large ribozyme revealed by circular dichroism and UV absorbance spectroscopies and catalytic activity. Nat. Struct. Biol., 4, 931–938. [DOI] [PubMed] [Google Scholar]
- 25.Misra V.K. and Draper,D.E. (1998) On the role of magnesium ions in RNA stability. Biopolymers, 48, 113–135. [DOI] [PubMed] [Google Scholar]
- 26.Mikkelsen N.E., Brännvall,M., Virtanen,A. and Kirsebom,L.A. (1999) Inhibition of RNase P RNA cleavage by aminoglycosides. Proc. Natl Acad. Sci. USA, 96, 6155–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feig A. and Uhlenbeck,O.C. (1999) The role of metal ions in RNA biochemistry. In Gesteland,R.F., Cech,T. and Atkins,J.F. (eds), The RNA World, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, NY, pp. 287–319,.
- 28.Perreault J.-P. and Altman,S. (1992) Important 2′ hydroxyl groups in model substrates for M1 RNA, the catalytic RNA subunit of RNase P from Escherichia coli. J. Mol. Biol., 226, 399–409. [DOI] [PubMed] [Google Scholar]
- 29.Perreault J.-P. and Altman,S. (1993) Pathway of activation by magnesium ions of substrates for the catalytic RNA subunit of RNase P from Escherichia coli. J. Mol. Biol., 230, 750–756. [DOI] [PubMed] [Google Scholar]
- 30.Pan T., Gutell,R.R. and Uhlenbeck,O.C. (1991) Folding of circularly permuted transfer RNAs. Science, 254, 1361–1364. [DOI] [PubMed] [Google Scholar]
- 31.Streicher B., von Ahsen,U. and Schroeder,R. (1993) Lead cleavage sites in the core structure of group I intron-RNA. Nucleic Acids Res., 21, 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streicher B., Westhof,E. and Schroeder,R. (1996) The enviroment of two metal ions surrounding the splice site of a group I intron. EMBO J., 15, 2556–2564. [PMC free article] [PubMed] [Google Scholar]
- 33.Krzyzosiak W.J., Marciniec,T., Wiewiorowski,M., Romby,P., Ebel,J.P. and Giegé,R. (1988) Characterization of the lead(II)-induced cleavages in tRNAs in solution and effect of the Y-base removal in yeast tRNAPhe. Biochemistry, 27, 5771–5777. [DOI] [PubMed] [Google Scholar]
- 34.Ross P.D. and Scruggs,R.L. (1964) Electrophoresis of DNA. II. Specific interactions of univalent and divalent cations with DNA. Biopolymers, 2, 79–89. [Google Scholar]
- 35.Gardiner K.J., Marsh,T.L. and Pace,N.R. (1985) Ion dependence of the Bacillus subtilis RNase P reaction. J. Biol. Chem., 260, 5415–5419. [PubMed] [Google Scholar]
- 36.Bock C.W., Kaufman Katz,A., Markham,G.D. and Glusker,J.P. (1999) Manganese as a replacement for magnesium and zinc: functional comparison of the divalent metal ions. J. Am. Chem. Soc., 121, 7360–7372. [Google Scholar]
- 37.Jack A., Ladner,J.E., Rhodes,D., Brown,R.S. and Klug,A. (1977) A crystallographic study of metal-binding to yeast phenylalanine transfer RNA. J. Mol. Biol., 111, 315–328. [DOI] [PubMed] [Google Scholar]
- 38.Grosshans C.A. and Cech,T.R. (1989) Metal ion requirements for sequence-specific endoribonuclease activity of the Tetrahymena ribozyme. Biochemistry, 28, 6888–6894. [DOI] [PubMed] [Google Scholar]
- 39.Wang J.-F. and Cech,T.R. (1994) Metal ion dependence of active-site structure of the Tetrahymena ribozyme revealed by site-specific photo-cross-linking. J. Am. Chem. Soc., 116, 4178–4182. [Google Scholar]
- 40.Celander D.W. and Cech,T.R. (1991) Visuzalizing the higher order folding of a catalytic RNA molecule. Science, 251, 401–407. [DOI] [PubMed] [Google Scholar]
- 41.Svärd S.G., Kagardt,U. and Kirsebom,L.A. (1996) Phylogenetic comparative mutational analysis of the base-pairing between RNase P RNA and its substrate. RNA, 2, 463–472. [PMC free article] [PubMed] [Google Scholar]
- 42.Haas E.S., Banta,A.B., Harris,J.K., Pace,N.R. and Brown,J.W. (1996) Structure and evolution of ribonuclease P RNA in gram-positive bacteria. Nucleic Acids Res., 24, 4775–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cowan J.A. (1995) The Biological Chemistry of Magnesium. VCH Publishers, Inc., New York, NY.