Abstract
The aryl hydrocarbon receptor (AhR) is a ligand-inducible transcription factor that mediates biological responses to halogenated aromatic hydrocarbons. The unliganded AhR is a cytoplasmic, tetrameric complex consisting of the AhR ligand-binding subunit, a dimer of hsp90, and the hepatitis B virus X-associated protein 2 (XAP2). The role of XAP2 as a member of the AhR core complex is poorly understood. XAP2 shares significant homology with the immunophilins FKBP12 and FKBP52, including a highly conserved, C-terminal, tetratricopeptide repeat (TPR) domain. XAP2 forms a complex with hsp90 and the AhR but can also bind to both independently. This binding is mediated by the conserved TPR domain. Single-point mutations in this region are sufficient to disrupt the association of XAP2 with both the AhR and hsp90 in cells. Cotransfection of the AhR and XAP2 in COS-1 cells results in increased AhR levels compared with cells transfected with the AhR alone. In contrast, coexpression of the AhR with the TPR containing proteins FKBP52, protein phosphatase 5 (PP5), or XAP2 TPR-mutants deficient in binding to the AhR and hsp90 does not affect AhR levels and coexpression of the AhR with the TPR domain of PP5 results in AhR down-regulation. These results demonstrate that XAP2 is apparently unique among hsp90-binding proteins in its ability to enhance AhR levels. A yellow fluorescent protein (YFP)-XAP2-FLAG was constructed and biochemically characterized, and no loss of function was detected. YFP-XAP2-FLAG was transiently transfected into NIH 3T3 and was found to localize in both the nucleus and the cytoplasm when visualized by fluorescence microscopy. Treatment of Hepa-1 cells with the hsp90-binding benzoquinone ansamycin, geldanamycin, and the macrocyclic antifungal compound radicicol resulted in AhR but not XAP2 or FKBP52 turnover. Taken together, these results suggest that XAP2/hsp90 and FKBP52/hsp90 complexes are similar yet exhibit unique functional specificity.
INTRODUCTION
Heat shock protein 90 (hsp90) is highly abundant, accounting for approximately 1% to 2% of the total cytoplasmic protein pool. Hsp90 can act as a general molecular chaperone by facilitating the folding of proteins that are misfolded or denatured (reviewed in Csermely et al 1998). Most studies on hsp90 have focused on its role in maintaining soluble receptors in a conformation that is competent for ligand binding (reviewed in Pratt and Toft 1997; Buchner 1999). This chaperone activity has been shown to require other proteins, such as Hip, Hop, and heat shock protein 70 (reviewed in Bukau and Horwich 1998). Steroid hormone receptors (SHRs) that require hsp90 for formation into mature complexes include the progesterone (PR), estrogen (ER), and glucocorticoid receptors (GR) (reviewed in Pratt and Toft 1997). Protein kinases, such as Raf-1 and pp60v-src, have also been demonstrated to require hsp90 for proper function (reviewed in Buchner 1999). Mature SHR complexes consist of the SHR ligand-binding subunit, a dimer of hsp90, p23, and an immunophilin. The immunophilins associated with these complexes bind to the C-terminal end of hsp90 by their tetratricopeptide repeat (TPR) domains. These domains are believed to bind to the C-terminus of hsp90 by a MEEVD recognition site, which is part of a proposed TPR-binding motif (Carrello et al 1999). The immunophilins FK506-binding protein (FKBP) 52, FKBP51, and Cyclophilin 40 (CyP-40) have been identified in different SHR complexes (reviewed in Pratt and Toft 1997). FKBP52 has been observed to complex with the GR, mineralocorticoid receptor (MR), and progesterone receptor (PR) (Tai et al 1992; Milad et al 1995; Bruner et al 1997). FKBP51 interacts with unliganded GR and PR complexes and, to a lesser extent, with ER complexes (Barent et al 1998). CyP-40 has also been identified in GR and PR complexes (Johnson and Toft 1995; Milad et al 1995; Johnson et al 1996; Owens-Grillo et al 1996). The serine/threonine phosphatase PP5 has also been observed to complex with the GR and has certain properties similar to the FK506-binding immunophilins (Silverstein et al 1997).
Recently, a protein-sharing homology to the immunophilins FKBP12 and FKBP52, the hepatitis B virus X-associated protein 2 (XAP2), has been demonstrated to exist in complexes with hsp90 and the AhR (Carver and Bradfield 1997; Ma and Whitlock 1997; Meyer et al 1998). Human XAP2 was first identified by its ability to bind to the hepatitis B virus (HBV) X-protein in a yeast 2-hybrid screen and to repress the transactivation of the HBV X-protein (Kuzhandaivelu et al 1996). A murine homolog and another XAP2 human cDNA clone were later isolated in a yeast 2-hybrid screen with the aryl hydrocarbon receptor (AhR) and referred to as Ah-interacting protein and Ah receptor–activated 9, respectively (Carver and Bradfield 1997; Ma and Whitlock 1997). A simian cDNA clone of XAP2 was isolated as a member of the AhR-hsp90 core complex following the biochemical purification of XAP2 (Meyer et al 1998). This immunophilin-related protein contains a highly conserved TPR domain in its C-terminus, which is required to bind to hsp90 complexes both in vitro and in vivo (Meyer and Perdew 1999). The C-terminal TPR domains in FKBP52 and CyP-40 have also been demonstrated to be required for binding to hsp90 complexes, and their TPR domains compete for binding to hsp90 (Ratajczak et al 1993). Unlike FKBP52, however, XAP2 does not bind to an FK506 affinity column and is found in unliganded tetrameric AhR complexes (Carver and Bradfield 1997; Ma and Whitlock 1997; Carver et al 1998; Meyer et al 1998). XAP2 was recently demonstrated to bind to a C-terminal hsp90 domain, whereas the AhR was shown to bind to the middle of hsp90 (Meyer and Perdew 1999).
The role of XAP2 in protein complexes is unknown, although it has been demonstrated to enhance the transcriptional activation of endogenous and transiently transfected AhR and increases cytosolic AhR levels when overexpressed (Ma and Whitlock 1997; Meyer and Perdew 1999). To further characterize the function of XAP2 in AhR complexes, the ability of XAP2 to affect AhR stability was compared to the TPR-containing hsp90-binding proteins FKBP52, full-length PP5, and the TPR domain of PP5. These results would indicate that XAP2 is uniquely able to enhance AhR levels. In addition, the role of the conserved TPR in XAP2 was evaluated for its ability to facilitate the assembly of the AhR/hsp90 complex. To further compare the biochemical properties of XAP2 and FKBP52, the hsp90-binding antibiotic geldanamycin (GA) and the antifungal agent radicicol were assayed for their effect on XAP2 and FKBP52 stability. These studies demonstrate that XAP2 and FKBP52 protein levels are not altered by GA and thus reveal an additional similarity between these proteins.
MATERIALS AND METHODS
Bacterial strains and cell culture
Escherichia coli strain DH5α (Gibco BRL) was used for all plasmid preparations. Mammalian cells were grown in α-minimum essential medium (Sigma, St Louis, MO, USA) supplemented with 10% fetal bovine serum, 100 IU of penicillin/mL, and 0.1 mg streptomycin/mL at 37°C in 94% air, 6% CO2.
Construction and sources of vectors utilized
pcDNA3/βmAhR was used for expression of mAhR (Fukunaga and Hankinson 1996). The mammalian expression constructs pCMV6/PP5-Flag and pCMV6/PP5/TPR-Flag were provided by Michael Chinkers (Chen et al 1996). The vector pCI/FKBP52 was obtained from David Smith. Site-directed mutagenesis was performed on the conserved TPR domain of XAP2 with the QuikChange™ Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) using pCI/XAP2-FLAG as the template (Meyer and Perdew 1999). HPLC-purified oligonucleotides for site-directed mutagenesis of the XAP2 TPR were obtained from Operon Technologies, Inc. (Alameda, CA, USA). The nucleotide sequences of all XAP2-FLAG TPR mutants were confirmed by automated DNA sequencing. pCI/XAP2-FLAG (Meyer and Perdew 1999) was digested with Xho I and Sma I and the digested insert cloned into pEYFP-C1 (Clontech, Palo Alto, CA, USA) to generate pEYFP-XAP2-FLAG. The QuikChange™ Site-Directed Mutagenesis Kit was then used to insert 4 nucleotides 5′ to the XAP2 ATG start sequence, resulting in an in-frame fusion of EYFP and XAP2-FLAG.
Transient transfections of COS-1 cells
COS-1 cells were transfected at 70% confluency in 10 cm2 tissue culture plates by the lipofectAMINE procedure as specified by the manufacturer (Life Technologies, Gaithersburg, MD, USA). Transfected COS-1 cells were harvested 24–36 h after the beginning of the transfection with trypsin-EDTA and washed once in 1X PBS. The cells were lysed in MENG buffer (25 mM MOPS, 2 mM EDTA, 0.02% NaN3, 10% glycerol, pH 7.5), 1% NP-40, plus protease inhibitor cocktail (Sigma) for 15 min at 4°C. Cell lysates were centrifuged at 4°C at 10 000 × g for 15 min. The total amount of protein was quantitated using the bicinchoninic acid assay (Pierce, Rockford, IL, USA).
XAP2-FLAG immunoprecipitations
Cytosol isolated from transfected COS-1 cells was immunoprecipitated with the anti-FLAG M2 affinity gel (Sigma). XAP2-FLAG immunoprecipitations were performed in IP buffer (MENG buffer, 2 mg/mL ovalbumin, 2 mg/mL bovine serum albumin, 20 mM sodium molybdate) for 2 h at 4°C with rocking, in a total volume of 750 μL. Each immunoadsorption was washed 3 times in IP buffer, followed by 2 washes in MENG buffer + 20 mM sodium molybdate. The XAP2-FLAG complexes were eluted from the M2 affinity gel by incubating with FLAG peptide (Sigma) for 1 h at 4°C. Each eluted immunoprecipitation was centrifuged at 107 × g for 2 min at 4°C and the eluted fraction collected. Each eluted fraction was mixed with an equal volume of 2X tricine sample buffer (2X TSB), heated at 94°C for 5 min, and resolved by Tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis (TSDS-PAGE) (8% polyacrylamide) for 2 h at 30 V followed by 16 h at 60V. The gel was equilibrated in transfer buffer (20 mM Tris, 185 mM glycine, 20% [v/v] methanol), then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, USA) at 15 V for 3 h at 4°C in a Genie blotter (Idea Scientific Co., Minneapolis, MN, USA).
XAP2-AhR interaction analysis in a cell-free system
The anti-AhR antibody RPT9 was used to immunoprecipitate AhR/XAP2-FLAG complexes. RPT9 (5 μg) was bound to 35 μL of protein G sepharose (Sigma) in MENG buffer for 1 h. The resin was then washed in MENG buffer 3 times, followed by washing in IP buffer containing 0.75% w/v CHAPS and 20 mM sodium molybdate (IPCM buffer). The mAhR was in vitro translated using a Promega TNT coupled translation/transcription system, then added (50 μL) to the protein G-sepharose-mAb RPT9 in a total volume of 750 μL in IPCM buffer and incubated for 2 h at 4°C. The bound AhR was then washed in the same buffer 2 times, followed by washing 3 times in phosphate buffered saline (1X PBS) to remove hsp90 bound to the AhR from the translation mixture. The AhR-bound complexes were then washed in IPCM buffer 2 times prior to binding to XAP2-FLAG or XAP2-FLAG TPR mutant proteins. To generate XAP2-FLAG and XAP2-FLAG TPR mutant proteins, pCI/XAP2-FLAG and pCI/XAP2-FLAG TPR mutant constructs, respectively, were generated in an in vitro translation system. A 50 μL translation mixture was incubated with 50 μL of M2 affinity gel (Sigma) in MENG buffer at 4°C for 2 h with rocking. Immunoabsorbed complexes were washed 3 times in MENG buffer, then eluted with FLAG peptide for 1 h at 4°C. The M2 affinity gel and eluted proteins were centrifuged at 107 × g for 2 min at 4°C and the eluted supernatant fraction collected. An aliquot of the eluted fraction (80%) was incubated with the AhR immobilized on protein G sepharose for 2 h in IPCM buffer. AhR-XAP2-FLAG complexes were then washed in the same buffer 2 times, followed by washing in MENG buffer plus 20 mM sodium molybdate 2 times. An equal volume of 2X Tricine sample buffer was then added to the samples, heated at 94°C for 5 min, resolved by TSDS-PAGE (8% acrylamide), and immunoblotted to PVDF membrane as described previously.
Antibodies used in immunoabsorption and immunoblot analysis
RPT1, an anti-AhR mouse monoclonal antibody, was used to detect AhR in immunoblot analysis (Perdew et al 1995). Anti-hsp84/86 rabbit polyclonal antibodies were used to detect hsp90 by immunoblot analysis (Perdew et al 1993). M2 anti-FLAG mouse monoclonal antibody was used in immunoblot analysis to detect FLAG-tagged proteins (Sigma). The anti-FKBP52 mouse monoclonal antibody that was used to detect FKBP52 was provided by David Smith at the University of Nebraska (Nair et al 1997). Rabbit polyclonal anti-XAP2 antibodies were provided by E. Croze (Berlex Laboratories). Secondary antibody was added at a ratio of 1:1000 for 1 h at room temperature with rocking. Secondary antibodies were visualized with either HRP-conjugated GAM IgG, HRP-conjugated DAR IgG, 5 μCi [125I]-SAM IgG, or 5 μCi [125I]-DAR, depending on the assay. HRP-conjugated antibodies were visualized using the Vector VIP substrate Kit (Vector Laboratories, Inc., Burlingame, CA, USA). Blots were visualized by autoradiography or phosphorimager analysis after the use of radiolabeled secondary IgG.
Epifluorescence and laser scanning confocal microscopy analysis of YFP-XAP2
NIH 3T3 fibroblasts were grown on sterile coverslips in 6-well tissue culture dishes in α-MEM supplemented with 10% fetal bovine serum, 100 IU of penicillin/mL, and 0.1 mg streptomycin/mL. Cells were transfected using Lipofectamine (Gibco BRL) according to the manufacturer's instruction but with the following modifications. Transfection mixtures were supplemented with 10% fetal bovine serum after 1 h. After an additional 5 h, the medium was removed, cells were washed with PBS, and complete medium was added and incubated overnight. Before visualization, cells were rinsed twice with PBS, fixed for 30 min in 4% formaldehyde/PBS at room temperature, and rinsed twice with PBS, and the inverted coverslips were mounted on slides with Vectashield mounting medium (Vector). Epifluorescence micrographs were obtained on a SPOT SP100 cooled CCD camera using a Nikon Optiphot-2 upright microscope with EFD-3 episcopic-fluorescence attachment with a Nikon Pan Fluor 100X oil immersion objective. Confocal images were obtained on the same samples using a Zeiss Axiovert microscope with 63X oil immersion objective equipped with a BioRad MRC 1024 laser scanning confocal optical system using Lasersharp 3.1 image acquisition software.
RESULTS AND DISCUSSION
Previous studies have demonstrated that the immunophilin-related protein XAP2 is associated with and is a member of the unliganded aryl hydrocarbon receptor core complex (Carver and Bradfield 1997; Ma and Whitlock 1997; Meyer et al 1998). Recently, XAP2 has been demonstrated to directly interact with the AhR in a cell-free system in the absence of hsp90 (Meyer and Perdew 1999). Additionally, XAP2 associates with hsp90 in COS-1 and Hepa-1 cells in the absence of the AhR (Ma and Whitlock 1997; Meyer and Perdew 1999). Overexpression of XAP2 in cells results in increased AhR-mediated transcriptional activity. However, the actual role of XAP2 in the unliganded complex is poorly understood. In this report, studies have focused on addressing whether XAP2 is unique among hsp90-binding proteins in its ability to enhance AhR stabilization.
XAP2 is expressed in a wide range of cell lines
After obtaining antibodies to XAP2, initial studies focused on examining whether various cell lines may express XAP2. Considering that rabbit reticulocyte lysate does not contain detectable levels of XAP2, we decided to see whether a cell line exists that has very low levels of XAP2 expression. Cytosol from 8 cell lines was examined on a protein blot for XAP2 levels; CV-1 cells exhibited the lowest level of expression (Fig 1). It is important to note that the level of expression did vary considerably in the cell lines tested, further underscoring the possibility that the pool size of XAP2 could mediate cell line–dependent differences in AhR regulation. COS-1 cells were chosen for further use in this report because of their ability to be easily transfected and their relatively low level of XAP2 expression.
Fig 1.

XAP2 is expressed in various established cell lines. Eight different cell lines were cultured, harvested, and homogenized. Total cytosolic extracts were isolated, and 75 μg of each lysate was resolved by SDS-PAGE, transferred to PVDF membrane, and analyzed by immunoblot analysis. XAP2 was detected with an XAP2 polyclonal antibody as primary antibody and [125I]-DAR as secondary antibody
XAP2 overexpression enhances the level of AhR in COS-1 cells, while FKBP52 or PP5 overexpression has essentially no effect on AhR levels
Previous studies have demonstrated that XAP2 is able to enhance AhR levels following its overexpression in COS-1 cells (Meyer and Perdew 1999). To further identify whether XAP2 specifically enhanced cytosolic AhR levels relative to other hsp90-binding proteins, immunophilin FKBP52 was selected for comparison to XAP2 because of its role in steroid receptor unliganded complexes. Increasing amounts of pCI/XAP2 or pCI/FKBP52 were cotransfected with pcDNA3/βmAhR in COS-1 cells. Following the transfection, cell lysates were isolated, resolved by SDS-PAGE, transferred to PVDF membrane, and analyzed by immunoblot analysis. The relative amounts of AhR in the 4 titration experiments described were quantitated by phosphorimaging analysis, and the results are depicted in Figure 2B. These results indicated that increasing amounts of XAP2 stabilized the AhR pool, whereas FKBP52 did not enhance cytosolic AhR levels (Fig 2A), suggesting that XAP2 specifically enhances the AhR pool when overexpressed.
Fig 2.
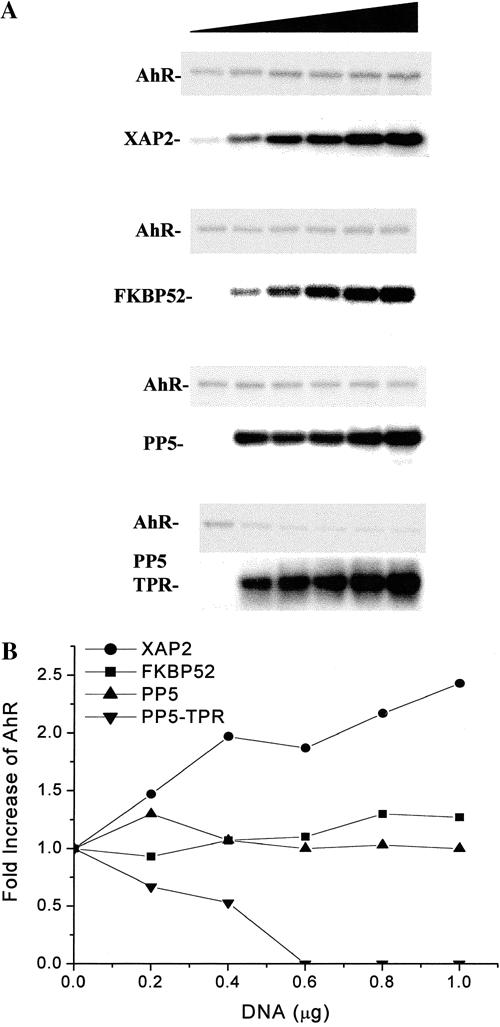
XAP2 specifically enhances the level of AhR in COS-1 cells when compared to other hsp90-binding TPR-containing proteins. COS-1 cells were transfected in 6 well dishes with 1 μg of pcDNA3/βmAhR and 0, 0.2, 0.4, 0.6, 0.8, or 1.0 μg of pCI/XAP2, pCI/FKBP52, pCMV6/PP5-FLAG, or pCMV6/PP5-TPR-FLAG (shaded triangle) and brought to a total of 2 μg vector/dish with pCI vector. (A) Total cell lysates were isolated, and 75 μg of each lysate were resolved by SDS-PAGE, transferred to PVDF membrane, and analyzed by immunoblot analysis. XAP2 and FKBP52 were detected with XAP2 polyclonal and FKBP52 monoclonal antibodies. PP5-TPR and PP5-TPR-FLAG were detected with anti-FLAG M2 antibody. AhR was detected with the anti-AhR monoclonal antibody RPT1. XAP2 was visualized with [125I]-DAR, and the AhR, FKBP52, PP5-FLAG, and PP5-TPR-FLAG were visualized with [125I]-SAM. (B) The graph depicts the fold change in AhR levels obtained in the presence of TPR-containing proteins after phosphorimaging of the blots. This experiment has been repeated 3 times with essentially the same results
Expression of another TPR-containing hsp90-binding protein, the serine/threonine phosphatase PP5, and its TPR domain alone were examined for their ability to enhance AhR levels. A titration of increasing amounts of pCMV6/PP5-FLAG or pCMV6/PP5TPR-Flag were cotransfected with pcDNA3/βmAhR in COS-1 cells. These results indicated that AhR levels remained unchanged with increasing amounts of PP5-FLAG (Fig 2A). Increasing amounts of PP5-FLAG-TPR, however, resulted in down-regulation of the AhR (Fig 2A). These results suggest that XAP2 specifically enhances AhR levels when compared to FKBP52 and full-length PP5. In contrast, expression of the PP5 TPR domain acted to down-regulate AhR levels (Fig 2B).
XAP2 represents a new member of the C-terminal hsp90-binding TPR-containing proteins. This protein appears to be specifically located in both AhR-hsp90 and hsp90 complexes. The ability of XAP2 to interact directly with the AhR supports the hypothesis that XAP2 in hsp90 complexes specifically recruits de novo synthesized AhR (Meyer and Perdew 1999). FKBP52 and PP5 have not been directly observed to interact with the AhR and have not been detected in AhR complexes along with hsp90 by immunoblot and silver stain analysis (Prokipcak et al 1989; Chen and Perdew 1994; Meyer et al 1998). Thus, FKBP52/hsp90 and PP5/hsp90 complexes may be unable to recruit the AhR or the resulting complexes are unstable. The mechanism by which the PP5-TPR domain down-regulates the AhR may be similar to the mechanism of PP5-TPR domain-mediated altered GR gene reporter activity (Chen et al 1996). It is not known, however, if the PP5 TPR is able to alter GR levels (Chen et al 1996). The overexpressed PP5-TPR domain may be able to efficiently compete with XAP2 for hsp90 C-terminal binding sites in the cell, resulting in predominantly PP5-TPR domain/hsp90 complexes. These complexes may not be recognized by de novo synthesized AhR, which may result in AhR turnover. In addition, overexpressed FKBP52 and PP5 may not compete with XAP2 as efficiently for hsp90, which results in the assembly of the AhR with an unaltered XAP2-hsp90 pool. Additional support for this model can be found by examination of the ability of XAP2, FKBP52, and PP5-TPR domain to complex in vitro with GST-C90, a C-terminal domain hsp90 fusion protein (Meyer and Perdew 1999). Results indicated that PP5-TPR binds with the highest efficiency to GST-C90. This result may explain the observation that transiently expressed FKBP52 or PP5 were unable to suppress AhR levels. Thus, transient expression of the PP5-TPR domain in cells would appear to be useful in assessing whether hsp90 is necessary for a given protein's activity.
Amino acid residues in a conserved tetratricopeptide repeat domain of XAP2 are required for interaction with hsp90 complexes in COS-1 cells
Previous studies have demonstrated that the conserved TPR domain and additional C-terminal amino acid residues of XAP2 are required for efficient hsp90 binding in COS-1 cells (Carver et al 1998; Meyer and Perdew 1999). To further characterize the role of this TPR domain, site-directed mutagenesis was performed on this highly conserved region. Each of the mutants was generated in the pCI vector and has a C-terminal FLAG epitope, which served 2 primary functions. The FLAG epitope was used to immunoadsorb XAP2-hsp90 complexes from COS-1 cells, and it was used as a recognition epitope to differentiate between endogenous XAP2 and XAP2-FLAG-TPR mutants. Five individual amino acid residues that were selected for mutation in the conserved TPR domain are depicted in Figure 3A. The G272D, G272E, and A284T mutations were selected on the basis of previous studies that demonstrated their functional significance in other TPR-containing proteins. The glycine at position 8 of the conserved TPR unit in the “A” domain is one of the most highly conserved residues in the TPR (Sikorski et al 1990; Lamb et al 1995). Additionally, mutation of this amino acid to either an aspartic or a glutamic acid residue results in a loss-of-function phenotype in both S. cerevisiae and human protein. The G-to-D mutation was observed in TPR 7 of the CDC23 gene in S. cerevisiae (Sikorski et al 1993). This mutant, designated 23-1, is unable to progress through the G2/M transition of the cell cycle (Sikorski et al 1993). The G-to-E mutation was observed to occur in the TPR region of the p67 phox protein, also referred to as NADPH oxidase (de Boer et al 1994). This mutation is associated with chronic granulomatous disease (CGD), which results in the failure of immune cells to generate superoxide in humans (de Boer et al 1994). The A-to-T mutation was observed in the ninth TPR repeat in the S. pombe gene cut9, which inhibits cell division at anaphase (Samejima and Yanagida 1994). The Y-to-A and F-to-A mutations were selected on the basis of the possibility that the change of a large side-chain amino acid to an amino acid with a methyl group would affect the function of the TPR. However, no such mutations in the TPR domains in other proteins have been observed or introduced for functional studies. These mutants were used in interaction studies with the AhR and hsp90.
Fig 3.
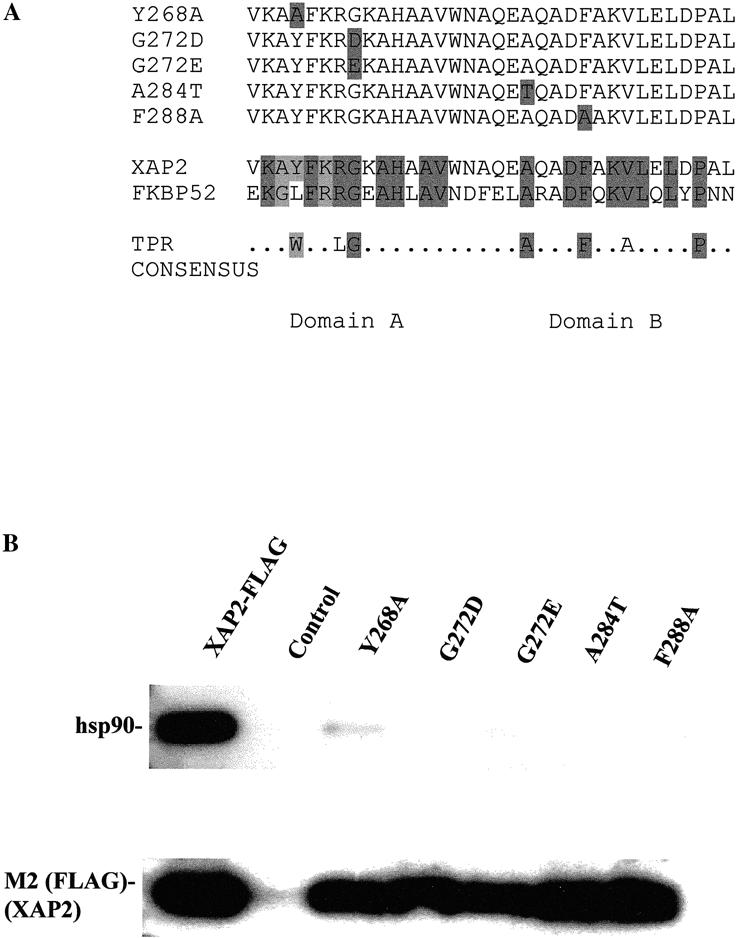
Schematic representation of XAP2 TPR mutants and their ability to bind to hsp90 in COS1 cells. (A) Top panel: Black boxes indicate amino acid substitutions in the respective mutant. Bottom panel: Black boxes indicate conserved amino acid residues between XAP2 and FKBP52. The TPR consensus was generated from CDC16, CDC23, CDC27, SSN6, and SK13 (Lamb et al 1995). (B) COS-1 cells were transiently transfected with pCI/XAP2-FLAG, pCI/XAP2-FLAG-TPR mutants, or pCI (control), and cell lysate was isolated, immunoabsorbed with the M2 anti-FLAG affinity resin, eluted with FLAG peptide, resolved by SDS-PAGE, and transferred to PVDF membrane, followed by immunoblot analysis. Hsp90 was visualized with polyclonal antibodies raised against hsp84/86 and [125I]-DAR, and XAP2-FLAG was visualized with anti-FLAG M2 antibody and [125I]-SAM
The ability of XAP2-TPR mutants to assemble into hsp90 complexes in cells was addressed. COS-1 cells were transiently transfected with pCI/XAP2-FLAG, pCI/XAP2-FLAG TPR mutants, or pCI vector alone. Total cell lysate was isolated and incubated with the anti-FLAG M2 affinity gel. Immunoadsorbed XAP2-associating protein complexes were washed in IP buffer, eluted with FLAG peptide, resolved by TSDS-PAGE, blotted to PVDF membrane, and followed by immunoblot analysis. These results indicated that, when compared to the nonmutated XAP2-FLAG, each mutant was impaired in its ability to associate in hsp90 complexes. The Y268A mutants had a low level of binding when compared to the other TPR mutants, in which no binding was detected (Fig 3B).
Mutants G272D, G272E, and F288A are unable to interact with the AhR in the absence of hsp90 in a cell-free system
Previous studies have indicated that XAP2 can bind the AhR in the absence of hsp90 in a rabbit reticulocyte lysate (RL) system (Meyer and Perdew 1999). To determine whether the conserved TPR domain of XAP2 was required for this interaction, the same cell-free binding assay was performed. Plasmids pcDNA3/βmAhR and pCI/XAP2-FLAG were transcribed/translated independently in RL. The AhR was immunoabsorbed with the anti-AhR monoclonal antibody RPT9 coupled to Protein G sepharose, and XAP2-FLAG was immunoabsorbed with the anti-FLAG M2 affinity gel. The AhR immunoadsorption was washed sufficiently in PBS to remove hsp90, which complexes with the AhR following its translation (Meyer et al 1998). The XAP2-FLAG immunoadsorption was extensively washed and the presence of hsp90 examined using immunoblot analysis, and no hsp90 was detected (data not shown). It is worth noting that XAP2 alone does not complex with hsp90 directly in RL (Meyer et al 1998). XAP2-FLAG and XAP2-FLAG TPR mutants were then eluted from the M2 affinity gel with FLAG peptide for use in binding studies. Eluted XAP2-FLAG or various XAP2-FLAG TPR mutants were then incubated with immunopurified AhR immobilized to the RPT9/Protein G sepharose, and an equal amount was incubated with mouse IgG/Protein G sepharose in IP buffer for 1 h. All samples were washed 3 times in the same buffer, resolved by SDS-PAGE, and transferred to PVDF membrane. Immunoblot analysis, using the anti-AhR monoclonal antibody RPT1 and anti-FLAG M2 antibodies, indicated that XAP2-FLAG mutants Y268A and A284T bound with equal efficiency to the AhR, as did the nonmutated XAP2-FLAG (Fig 4A). XAP2-FLAG mutants G272D, G272E, and F288A, however, did not bind to the AhR (Fig 4A). These results indicated that amino acid residues G272 and F288 are important in mediating the direct AhR-XAP2 interaction in the absence of hsp90.
Fig 4.
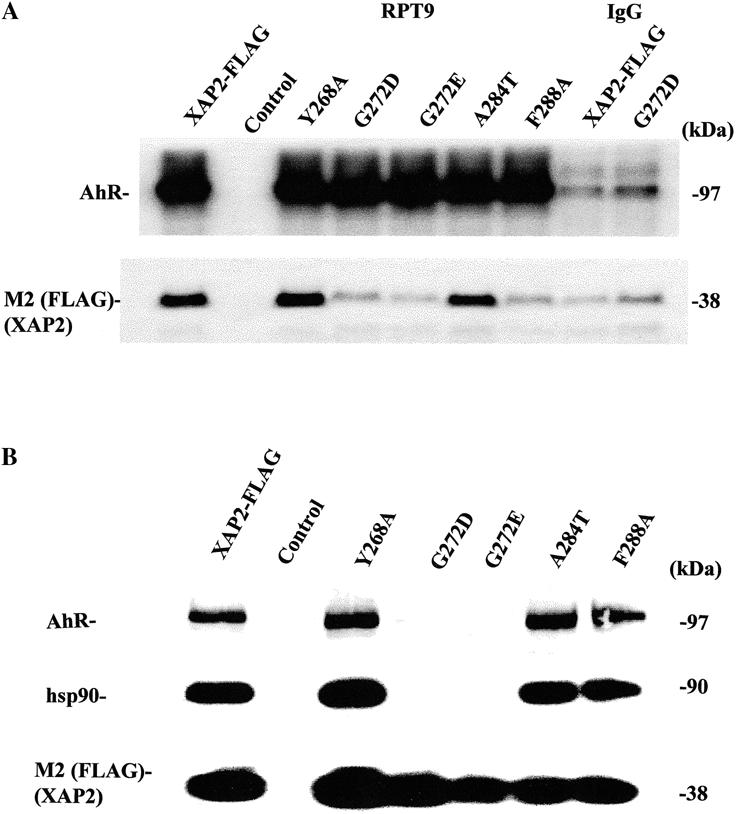
Mutants G272D, G272E, and F288A are unable to interact with the AhR in the absence of hsp90 in a cell-free system, and a conserved glycine in the XAP2 TPR complex is required for assembly of XAP2 in AhR-hsp90 complexes in COS-1 cells. (A) XAP2-FLAG, XAP2-FLAG-TPR mutants, or control (RL) were labeled with [35S] methionine in RL independently, immunoabsorbed with M2 resin, and eluted with FLAG peptide. mAhR was generated in RL (unlabeled) and immunoabsorbed with RPT9/protein G sepharose or with murine IgG/protein G sepharose (control, last 2 lanes). The AhR immunoabsorption was washed in PBS to remove hsp90. RPT9-sepharose-mAhR was mixed with eluted XAP2-FLAG or XAP2-FLAG TPR mutants, and complexes washed, resolved by SDS-PAGE, and transferred to PVDF membrane. Top panel: mAhR visualized with RPT1 and [125I]-SAM by autoradiography. Middle panel: XAP2-FLAG visualized by autoradiography. (B) COS-1 cells were transiently cotransfected with pCI/XAP2-FLAG, pCI/XAP2-FLAG-TPR mutants, or pCI (control) and pcDNA3/_mAhR, and cell lysate was isolated, immunoabsorbed with the M2 anti-FLAG affinity resin, eluted with FLAG peptide, resolved by SDS-PAGE, and transferred to PVDF membrane, followed by immunoblot analysis. AhR was visualized with RPT1 and [125I]-SAM, hsp90 was visualized with polyclonal antibodies raised against hsp84/86 and [125I]-DAR, and XAP2-FLAG was visualized with anti-FLAG M2 antibody and [125I]-SAM.><<002>><<002>><<002>>>
A conserved glycine residue in the XAP2 TPR is required for interaction of XAP2 in AhR/hsp90 complexes in COS-1 cells
COS-1 cells were cotransfected with pCI/XAP2 or pCI/XAP2-TPR mutants and pcDNA3/βmAhR to determine whether any of the observed amino acid changes in the conserved TPR domain affected the ability of XAP2 to bind to the AhR/hsp90 complex in cells. Following transfection, cell lysates were isolated, and complexes were immunoadsorbed with M2 anti-FLAG affinity resin, eluted with FLAG peptide, resolved by SDS-PAGE, and analyzed by immunoblot analysis. These results indicated that all pCI/XAP2 mutants, except G272D and G272E, bound to the AhR and hsp90 in cells with the same efficiency as the nonmutated XAP2-FLAG (Fig 4B). It is interesting to note that XAP2 mutant F288A was unable to bind to the AhR in vitro and did not bind to hsp90 alone yet was able to assemble into AhR/hsp90 complexes in COS-1 cells; the reason for this result is unknown. Taken together, these results suggest that the highly conserved glycine residue is important for mediating the ability of XAP2 to bind to either AhR or hsp90 alone or to AhR/hsp90 complexes. In addition, the highly conserved TPR domain also appears to be important for both AhR and hsp90 binding. These results also suggest that XAP2 TPR mutants that were unable to bind to hsp90 or to assemble in AhR/hsp90 complexes apparently have a similar half-life in COS-1 cells. This observation may indicate that XAP2 is stable, even in the absence of binding to hsp90.
XAP2 TPR mutants fail to enhance AhR levels in COS-1 cells
XAP2 TPR mutants G272D and G272E were compared with XAP2 for their ability to enhance AhR expression in COS-1 cells. Both of these mutant proteins were unable to alter the amount of AhR, except at high expression levels (Fig 5). These results would strengthen the assertion that direct binding of XAP2 is necessary for the increased level of AhR expression detected in this assay system. The modest increase in AhR levels at very high XAP2 mutant expression levels may reflect an ability of these mutants to weakly interact with the AhR. Nevertheless, these XAP2 mutants should serve as useful controls in studies examining the function of XAP2 in the AhR complex as well as in other cellular functions.
Fig 5.
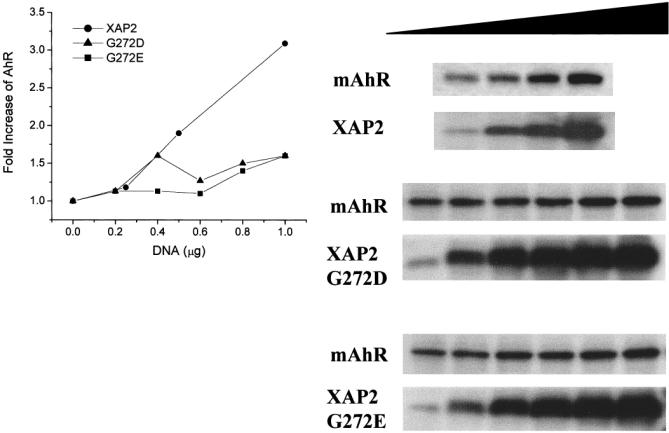
XAP2 mutants G272D and G272E are defective in enhancing the level of AhR in COS-1 cells. COS-1 cells were transfected in 6 well dishes with 1 μg of pcDNA3/βmAhR and 0, 0.2, 0.4, 0.6, 0.8, or 1.0 μg pCI/XAP2-G272D or pCI/XAP2-G272E or with 0, 0.25, 0.5, or 1.0 μg pCI/XAP2. The vector pCI was used to bring each transfection to a total of 2 μg plasmid/dish. Total cell lysates were isolated, and 75 μg of each lysate were resolved by SDS-PAGE, transferred to PVDF membrane, and analyzed by immunoblot analysis. XAP2 was detected with XAP2 polyclonal antibodies, and AhR was detected with the anti-AhR monoclonal antibody RPT1. XAP2 antibody was visualized with [125I]-DAR, and AhR antibody was visualized with [125I]-SAM. The graph depicts the fold change in AhR levels obtained in the presence of TPR-containing proteins after phosphorimaging of the blots. This experiment was performed twice with essentially the same results
XAP2 levels are resistant to geldanamycin and radicicol in Hepa-1 cells
XAP2 has been previously demonstrated to bind to the C-terminus of hsp90, much like FKBP52 and CyP-40 (Young et al 1998; Meyer and Perdew 1999). The chaperoned proteins, such as the AhR and the ER, have been shown to bind to the middle segment of hsp90 (Meyer and Perdew 1999). The antibiotic geldanamycin (GA) and the antifungal agent radicicol bind to the ATP/ADP-binding site in the N-terminus of hsp90 (Grenert et al 1997; Schulte et al 1998). Binding of GA to hsp90 results in the down-regulation of several different proteins, including the PR, AhR, and mutant p53, whereas other hsp90-binding proteins, such as p50cdc37, a component of Raf1/hsp90 complexes, are resistant to its effects (Smith et al 1995; Chen et al 1997; Whitesell et al 1998). Because of the observation that XAP2 is a new member of the TPR-containing C-terminal hsp90-binding proteins, it was unknown whether XAP2 levels would be affected by GA or radicicol. To determine whether XAP2 would be affected, Hepa-1 cells were utilized instead of COS-1 cells because they contain a relatively high endogenous level of AhR. Hepa-1 cells were treated with either 100 nM or 2 μM GA or radicicol for 6 h, and cytosol was isolated, resolved by TSDS-PAGE, and transferred to PVDF membrane, followed by immunoblot analysis with antibodies specific for the AhR, XAP2, or FKBP52. The results indicated that at 100 nM GA, AhR levels were depleted, as has been previously observed, whereas 100 nM radicicol did not affect the AhR to the same extent as did GA (Chen et al 1997) (Fig 6). Treatment with 2 μM GA or 2 μM radicicol resulted in total loss of the AhR. In contrast, XAP2 levels at all concentrations of GA and radicicol were unaffected as assessed by phosphorimaging analysis. FKBP52 levels were also unaffected at all concentrations (Fig 6). These results indicate that the chaperoned protein (eg, AhR) binds to the middle of hsp90 and is susceptible to GA or radicicol mediated turnover, whereas XAP2 and FKBP52, 2 TPR-containing C-terminal hsp90-binding proteins, are not altered. Thus, a general model is emerging that proteins involved as cochaperones with hsp90 (eg, FKBP52, XAP2, and p50cdc37) are not dependent on fully functional hsp90 complexes to maintain their steady-state levels.
Fig 6.
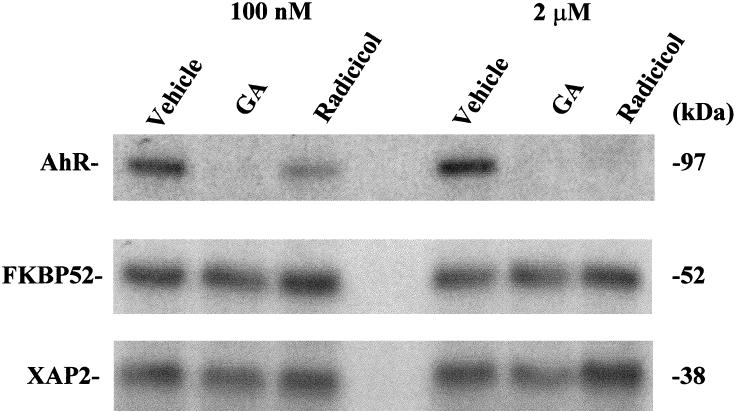
Cellular XAP2 is unaffected by geldanamycin or radicicol treatment. Hepa-1 cells were treated with GA or radicicol at 100 nM and 2 μM for 6 h. Following treatment, cytosol was isolated, and 100 μg were resolved by SDS-PAGE and transferred to PVDF membrane, followed by immunoblot analysis with antibodies specific for the AhR, XAP2, and FKBP52. AhR and FKBP52 were visualized with [125I]-SAM and XAP2 with [125I]-DAR and subsequent autoradiography
An EYFP-XAP2 fusion protein localizes in both the nucleus and cytoplasm
Using immunocytochemistry and cellular fractionation assays, previous studies have indicated that XAP2 is predominantly, if not entirely, cytoplasmic (Kuzhandaivelu et al 1996; Ma and Whitlock 1997). To more carefully examine the subcellular localization of XAP2, an enhanced yellow fluorescent protein (EYFP)-XAP2 fusion protein was generated. EYFP is a yellow-shifted variant of GFP, has a brighter emission spectra, is more resistant to photobleaching than GFP, and can be discriminated from other GFP variants with appropriate filters. The EYFP was fused to the N-terminal end of XAP2 because of previous observations that C-terminal sequences of XAP2 are required for its interaction with hsp90 and the AhR (Carver et al 1998; Meyer and Perdew 1999). To demonstrate the efficacy of the EYFP-XAP2 fusion protein, pEYFP-XAP2-FLAG or pEYFP alone was transiently transfected in COS-1 cells and the cytosol analyzed for the presence of XAP2 and EYFP by immunoblot analysis. These results indicated that a specific band at approximately 62 kDa was recognized by both the anti-XAP2 and the anti-GFP antibodies when compared to the control (Fig 7). To further characterize the EYFP-XAP2-FLAG fusion protein, its ability to complex with hsp90 alone or with the AhR and hsp90 in COS-1 cells was examined. Immunoblot analysis indicated that EYFP-XAP2- FLAG was indeed capable of binding to hsp90 complexes and to both the AhR and hsp90 in COS-1 cells (Fig 7). Fluorescence micrography and laser scanning confocal microscopy (LSCM) were used to study the intracellular location of EYFP-XAP2 protein in NIH 3T3 cells (Fig 8); this cell line was chosen because of its relatively flat morphology. LSCM would indicate that YFP-XAP2 distributes into both the cytoplasmic and the nuclear compartments. The distribution of the YFP-XAP2 G272D mutant appeared to be essentially the same, indicating that mutation of the conserved XAP2 TPR did not influence cellular distribution. This data is in contrast to the predominantly cytoplasmic localization reported by Kuzhanaivelu et al (1996). This apparent difference could be due to several factors, including the use of different cell lines, data from the previous report examined cells transfected with X protein, the antibody was unable to recognize XAP2 in the nucleus, and the localization of transiently expressed XAP2 vs endogenously expressed XAP2 could differ. Nevertheless, the data in this report clearly indicates that XAP2 can be localized throughout the cell. Finally, the development of YFP-XAP2 constructs should be useful in the further analysis of the role of XAP2 in interacting with the AhR and other possible targets of XAP2.
Fig 7.
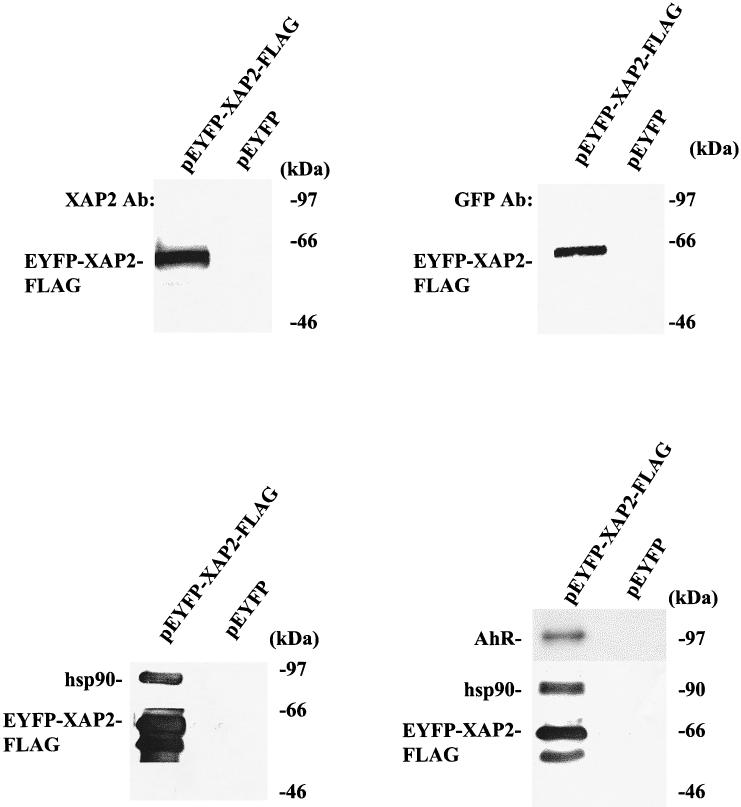
EYFP-XAP2 binds to hsp90 and to AhR/hsp90 complexes in COS-1 cells. Upper panels: pEYFP-XAP2-FLAG or pEYFP alone was transiently transfected in COS-1 cells, cytosol was isolated, and 150 μg of protein were resolved by SDS-PAGE and transferred to PVDF, followed by immunoblot analysis with XAP2 polyclonal antibodies or anti-GFP monoclonal antibodies. Antibodies were visualized with DAR-P and GAM-P, respectively. Lower panels: pEYFP-XAP2-FLAG or pEYFP were transiently transfected in COS-1 cells (left panel); pEYFP-XAP2-FLAG or pEYFP were transiently cotransfected with pcDNA3/βmAhR in COS-1 cells (right panel). In both experiments, cytosol was isolated and immunoabsorbed with the M2 affinity resin, and complexes were eluted with FLAG peptide and resolved by SDS-PAGE, transferred to PVDF membrane, and analyzed by immunoblot analysis. pEYFP-XAP2-FLAG was visualized with the M2 antibody, AhR with the RPT1 antibody, and hsp90 with rabbit polyclonal antibodies against hsp84 and hsp86. M2 antibody was visualized with GAM-P, hsp90 with DAR-P, and AhR with GAM-P by ECL
Fig 8.
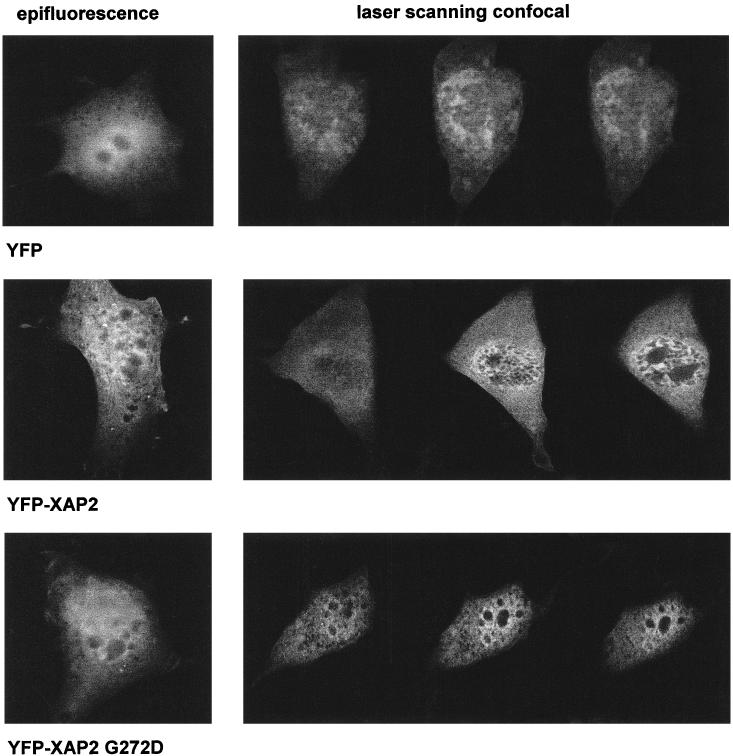
YFP-XAP2/Flag and YFP-XAP2/Flag G272D are localized in both the cytoplasmic and nuclear compartments. NIH 3T3 cells were transiently transfected with pEYFP (top panels), pEYFP-XAP2/Flag (middle panels), or pEYFP-XAP2 G272D (bottom panels). After 24 h, the transfected cells were visualized by either epifluorecence (left panels) or scanning confocal microscopy (right panels). The confocal images represent a horizontal midsectional view of the cell. Each image is representative of the population of transfected cells
Acknowledgments
This work was supported by NIEHS grant ES04869. Oliver Hankinson, Ed Seto, Micheal Chinkers, and David Smith are acknowledged for providing pcDNA3/βmAhR, XAP2 cDNA, pCMV6/PP5-Flag and pCMV6/PP5/TPR-Flag, and pCI/FKBP52, respectively. Radicicol was kindly provided by Shiro Akinaga.
REFERENCES
- Barent RL, Nair SC, Carr DC, Ruan Y, Rimerman RA, Fulton J, Zhang Y, Smith DF. Analysis of FKBP51/FKBP52 chimeras and mutants for hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol. 1998;12:342–354. doi: 10.1210/mend.12.3.0075. [DOI] [PubMed] [Google Scholar]
- Bruner KL, Derfoul A, Robertson NM, Guerriero G, Fernandes-Alnemri T, Alnemri ES, Litwack G. The unliganded mineralocorticoid receptor is associated with heat shock proteins 70 and 90 and the immunophilin FKBP-52. Recept Signal Transduct. 1997;7:85–98. [PubMed] [Google Scholar]
- Buchner J. Hsp90 & Co.—a holding for folding. TIBS. 1999;24:136–141. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Carrello A, Ingley E, Minchin RF, Tsai S, Ratajczak T. The common tetratricopeptide repeat acceptor site for steroid receptor-associated immunophilins and Hop is located in the dimerization domain of Hsp90. J Biol Chem. 1999;274:2682–2689. doi: 10.1074/jbc.274.5.2682. [DOI] [PubMed] [Google Scholar]
- Carver LA, Bradfield CA. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog. in vivo. J Biol Chem. 1997;272:11452–11456. doi: 10.1074/jbc.272.17.11452. [DOI] [PubMed] [Google Scholar]
- Carver LA, LaPres JJ, Jain S, Dunham EE, Bradfield CA. Characterization of the Ah receptor-associated Protein, ARA9. J Biol Chem. 1998;273:33580–33587. doi: 10.1074/jbc.273.50.33580. [DOI] [PubMed] [Google Scholar]
- Chen H-S, Perdew GH. Subunit composition of the heteromeric cytosolic aryl hydrocarbon receptor complex. J Biol Chem. 1994;269:27554–27558. [PubMed] [Google Scholar]
- Chen HS, Singh SS, Perdew GH. The Ah receptor is a sensitive target of geldanamycin-induced protein turnover. Arch Biochem Biophys. 1997;348:190–198. doi: 10.1006/abbi.1997.0398. [DOI] [PubMed] [Google Scholar]
- Chen MS, Silverstein A, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of phosphoprotein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem. 1996;271:32315–32320. doi: 10.1074/jbc.271.50.32315. [DOI] [PubMed] [Google Scholar]
- Csermely P, Schnaider T, Soti C, Proháska Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications—a comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- de Boer M, Hilarius-Stokman PM, Hossle JP, Verhoeven AJ, Graf N, Kenney RT, Seger R, Roos D. Autosomal recessive chronic granulomatous disease with absence of the 67-kD cytosolic NADPH oxidase component: identification of mutation and detection of carriers. Blood. 1994;83:531–536. [PubMed] [Google Scholar]
- Fukunaga BN, Hankinson O. Identification of a novel domain in the aryl hydrocarbon receptor required for DNA binding. J Biol Chem. 1996;271:3743–3749. doi: 10.1074/jbc.271.7.3743. [DOI] [PubMed] [Google Scholar]
- Grenert JP, Sullivan WP, Fadden P, et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- Johnson J, Corbisier R, Stensgard B, Toft D. The involvement of p23, hsp90, and immunophilins in the assembly of progesterone receptor complexes. J Steroid Biochem Mol Biol. 1996;56(0):31–37. doi: 10.1016/0960-0760(95)00221-9. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Toft DO. Binding of p23 and hsp90 during assembly with the progesterone receptor. Mol Endocrinol. 1995;9:670–678. doi: 10.1210/mend.9.6.8592513. [DOI] [PubMed] [Google Scholar]
- Kuzhandaivelu N, Cong YS, Inouye C, Yang WM, Seto E. XAP2, a novel hepatitis B virus X-associated protein that inhibits X transactivation. Nucleic Acids Res. 1996;24:4741–4750. doi: 10.1093/nar/24.23.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JR, Tugendreich S, Hieter P. Tetratricopeptide repeat interactions: to TPR or not to TPR. TIBS. 1995;20:257–259. doi: 10.1016/s0968-0004(00)89037-4. [DOI] [PubMed] [Google Scholar]
- Ma Q, Whitlock JP Jr. A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1997;272:8878–8884. [PubMed] [Google Scholar]
- Meyer BK, Perdew GH. Characterization of the AhR-hsp90-XAP2 core complex and the role of the immunophilin-related protein XAP2 in AhR stabilization. Biochemistry. 1999;38:8907–8917. doi: 10.1021/bi982223w. [DOI] [PubMed] [Google Scholar]
- Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol. 1998;18:978–988. doi: 10.1128/mcb.18.2.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M, Sullivan W, Diehl E, Altmann W, Nordeen S, Edwards DP, Toft DO. Interaction of the progesterone receptor with binding proteins for FK506 and cyclosporin A. Mol Endocrinol. 1995;9:838–847. doi: 10.1210/mend.9.7.7476967. [DOI] [PubMed] [Google Scholar]
- Nair SC, Rimerman RA, Toran EJ, Chen S, Prapapanich V, Butts RN, Smith DF. Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with hsp90 and progesterone receptor. Mol Cell Biol. 1997;17:594–603. doi: 10.1128/mcb.17.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens-Grillo JK, Czar MJ, Hutchison KA, Hoffmann K, Perdew GH, Pratt WB. A model of protein targeting mediated by immunophilins and other proteins that bind to hsp90 via tetratricopeptide repeat domains. J Biol Chem. 1996;271:13468–13475. doi: 10.1074/jbc.271.23.13468. [DOI] [PubMed] [Google Scholar]
- Perdew GH, Abbott B, Stanker LH. Production and characterization of monoclonal antibodies directed against the Ah receptor. Hybridoma. 1995;14:279–283. doi: 10.1089/hyb.1995.14.279. [DOI] [PubMed] [Google Scholar]
- Perdew GH, Hord N, Hollenback CE, Welsh MJ. Localization and characterization of the 86- and 84-kDa heat shock proteins in Hepa 1c1c7 cells. Exp Cell Res. 1993;209:350–356. doi: 10.1006/excr.1993.1320. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin co-chaperones. Endocrinol Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Prokipcak RD, Faber LE, Okey AB. Characterization of the Ah receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin: use of chemical crosslinking and a monoclonal antibody directed against a 59-kDa protein associated with steroid receptors. Arch Biochem Biophys. 1989;274:648–658. doi: 10.1016/0003-9861(89)90480-3. [DOI] [PubMed] [Google Scholar]
- Ratajczak T, Carrello A, Mark PJ, Warner BJ, Simpson RJ, Moritz RL, House AK. The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59) J Biol Chem. 1993;268:13187–13192. [PubMed] [Google Scholar]
- Samejima I, Yanagida M. Bypassing anaphase by fission yeast cut9 mutation: requirement of cut9+ to initiate anaphase. J Cell Biol. 1994;127:1655–1670. doi: 10.1083/jcb.127.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, Neckers LM. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3:100–108. doi: 10.1379/1466-1268(1998)003<0100:arbttn>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Boguski MS, Goebl M, Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990;60:307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Michaud WA, Hieter P. p62cdc23 of Saccharomyces cerevisiae: a nuclear tetratricopeptide repeat protein with two mutable domains. Mol Cell Biol. 1993;13:1212–1221. doi: 10.1128/mcb.13.2.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein AM, Galigniana MD, Chen MS, Owens-Grillo JK, Chinkers M, Pratt WB. Protein phosphatase 5 is a major component of glucocorticoid receptor·hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem. 1997;272:16224–16230. doi: 10.1074/jbc.272.26.16224. [DOI] [PubMed] [Google Scholar]
- Smith DF, Whitesell L, Nair SC, Chen S, Prapapanich V, Rimerman RA. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai PK, Albers MW, Chang H, Faber LE, Schreiber SL. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992;256:1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Sutphin PD, Pulcini EJ, Martinez JD, Cook PH. The physical association of multiple molecular chaperone proteins with mutant p53 is altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol. 1998;18:1517–1524. doi: 10.1128/mcb.18.3.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Obermann WM, Hartl FU. Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of hsp90. J Biol Chem. 1998;273:18007–18010. doi: 10.1074/jbc.273.29.18007. [DOI] [PubMed] [Google Scholar]


