Abstract
A 16-channel receive-only, closely-fitted array coil is described and tested in vivo for bilateral breast imaging at 3T. The primary purpose of this coil is to provide high SNR and parallel imaging acceleration in two directions for breast MRI. Circular coil elements (7.5 cm-diameter) were placed on a closed “cup-shaped" platform, and nearest neighbor coils were decoupled through geometric overlap. Comparisons were made between the 16-channel custom coil and a commercially-available 8-channel coil. SENSE parallel imaging noise amplification (g-factor) was evaluated in phantom scans. In healthy volunteers we compared SNR, parallel imaging in one and two directions, ARC g-factor and high spatial resolution imaging. When compared with a commercially-available 8-channel coil, the 16-channel custom coil shows 3.6× higher mean SNR in the breast and higher quality accelerated images. In patients, the 16-channel custom coil has facilitated high quality, high resolution images with bidirectional acceleration of R = 6.3.
Keywords: breast cancer, microscopy, parallel imaging, phased array, coil
Introduction
Screening dynamic contrast enhanced (DCE) MRI is recommended by the American Cancer Society for all women with a high risk for breast cancer [1]. Currently, DCE MRI of the breast gives information about lesion morphology, tissue perfusion and enhancement kinetics [2]. However, the lack of specificity as well as the difficulty in diagnosing ductal carcinoma in situ (DCIS) have limited the routine use of breast MRI to screening high risk populations and staging some women with newly diagnosed breast cancer [2]. Non-mass-like enhancement is particularly difficult to manage given the significant overlap between the appearances of benign breast enhancement and DCIS on conventional DCE MRI images.
These issues could potentially be addressed by the acquisition of high spatial and temporal resolution images[3–5]. Post-contrast images need to be acquired rapidly, and traditionally the tradeoff for rapid acquisition has been low spatial resolution[6, 7]. A recent study with a small surface coil has shown that high spatial and temporal resolution breast DCE MRI can improve sensitivity and specificity of DCIS diagnosis by visualizing smaller scale features such as ductal and periductal enhancement[5]. However, a small surface coil is not suitable for screening or bilateral staging examinations where volumetric coverage of both breasts is needed. Conversely, many commercially available breast coils offer volumetric coverage of the breasts, but the large coil elements severely limit the signal-to-noise ratio (SNR) and thus the ability to increase spatial and temporal resolution with high acceleration factors.
An array of small surface coils provides high SNR and volumetric coverage, and in addition, high temporal resolution can be achieved by the application of parallel imaging[8–11]. Arrays of many small elements (32 elements and above) have been applied to cardiac imaging and brain imaging for attaining highly-accelerated images[12–17], but there is little published data about similar arrays for breast imaging. The overall acceleration is limited for many commercially-available breast coils as they do not offer adequate superior/inferior (S/I) acceleration and compromise SNR in order to allow biopsy, which is rarely performed during diagnostic examinations. Recently, a prototype 16-channel open breast array (Sentinelle Medical, Toronto, Canada) has been shown to provide acceleration in both the left/right (L/R) and S/I direction (total R = 4) in phantom studies[18]. The array consists of an open design with some elements located in the adjustable side plates. In one volunteer at 1.5T, this coil has been shown to increase SNR by a factor of 2.2 when compared to the 8-channel High Density Breast Array Coil (GE Healthcare)[18].
In this paper, we describe a 16-channel receive-only breast coil for 3T that consists of an array of small surface coils placed on a closed mold, fitted for a medium-sized (cup B) woman. Although this design may not offer adequate flexibility for clinical imaging, the purpose of this work is to show the potential improvements that could be achieved. For clinical application, two or three different sized coil arrays would be needed to account for the variety in patient population. We detail the technical challenges related to building the coil including geometry design, coil element decoupling, and noise correlation. We compared SENSE g-factor maps from phantom studies acquired using both our 16-channel custom array and a commercially-available 8-channel standard array. Using both coils, we scanned volunteers and compared SNR, spatial resolution, ARC g-factor maps and parallel imaging. In addition, we also show high quality, high resolution scans of a patient with R = 6.3 (4× L/R and 2× S/I) using the 16-channel custom array.
MATERIALS AND METHODS
Breast Coils
Two breast coil arrays were compared in this study: a commercially available 8-channel High Density Breast Array Coil (GE Healthcare) and a prototype 16-channel closely-fitted custom array, hereafter referred to as 8-channel standard array and 16-channel custom array.
The 8-channel standard coil utilizes an open design that accommodates biopsy and also provides good homogeneity, but lower SNR, and poor S/I acceleration. For the 8-channel standard coil, preamplifiers are located less than 2 cm from each coil. Four of the coil elements have a surface area of about 325 cm each, and the other four elements are unreachable for measurements. Coil decoupling is accomplished through geometric overlap and low impedance preamplifiers. Coil elements are located on the surfaces of two “U-shaped" platforms with about 1000 ml volume each, as well as near the chest wall.
The 16-channel custom array has a closed design that is optimized for higher SNR and parallel imaging in two directions. To ensure that the coil elements are as close to the breast as possible, we took an impression of a medium-sized woman’s chest using a thermoplastic sheet, as shown in Fig. 1a. We designed the current breasts cups for a “cup B" size (410 ml volume each), but the housing can accommodate most breast sizes. If we define the filling factor as the volume of the sample divided by the volume of the coil, the filling factor will depend on the breast size of the subject. For a subject with a “cup B" size, the filling factor is close to 100% for the 16-channel custom prototype. The two main components of the housing are the structural support and the breast cups (Fig. 1d). We fabricated both components using G10 (FR4) - a versatile, thermosetting laminate that has high strength and excellent electrical properties.
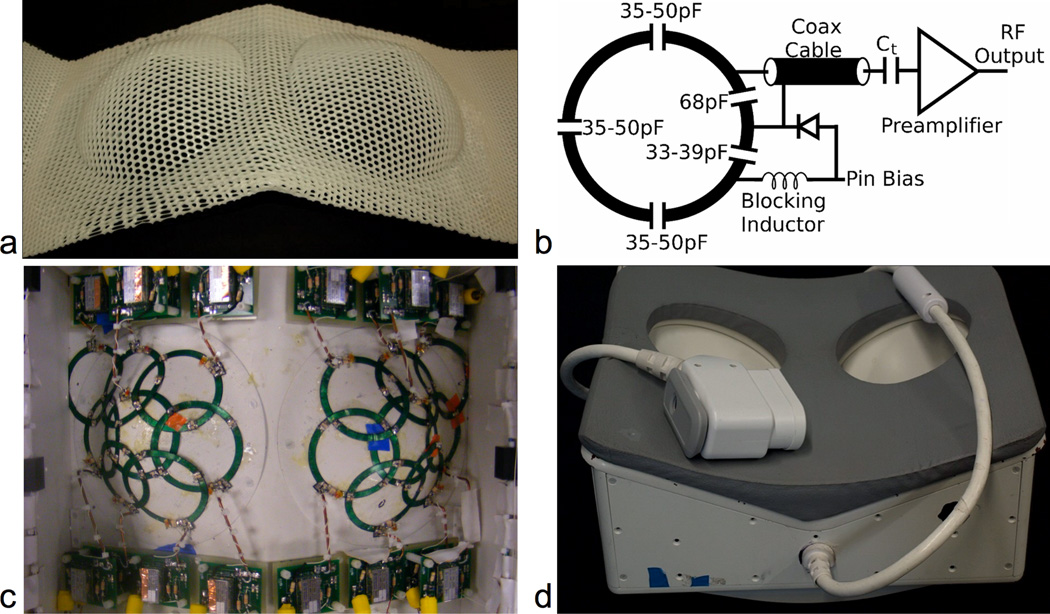
Figure 1.
16-channel custom bilateral breast coil. (a) Thermoplastic mold from a woman with a cup B breast size. (b) Circuit schematic of a single coil. (c) For each side, eight coil elements cover the breast region (including the axilla) with overlap to minimize the mutual inductance. (d) The finalized coil including a hand-craft replica of the thermoplastic mold made using G10 – a sturdy, MR-compatible material.
Simple circular planar elements would not fit appropriately on the breast cups due to their spherical curvature. Therefore, we approximated elements as a slice through a cone tangential to the sphere at the coil location. We laid the elements on a flexible printed circuit board, and shaped them to the final size. Fourteen out of sixteen coil elements have the same physical size (7.5 cm-inner diameter and 88 cm area), while we slightly decrease the size of the final two elements to an inner diameter of 5.0 cm (39 cm area) to provide the necessary geometric overlap between neighboring coils. The coil element layout covers the entire breast including the axilla and provides parallel imaging in two directions, as shown in Fig. 1c.
The circuitry for one coil element is shown in Fig. 1b. We use a 68 pF input capacitor for all coil elements. For the detuning traps, we use smaller capacitors (33 pF or 39 pF) to maximize the blocking impedance, improving decoupling between elements. We mounted on-coil preamplifiers between 5 and 15 cm from the coil element and connected the coil element to the preamplifier feed board with a copper coaxial cable. Balanced/unbalanced traps at the output of each feed board as well as halfway along each cable aid channel isolation.
SNR and g-factor Measurements
Numerous methods for estimating SNR have been described, each having advantages and limitations[19]. We implemented the “pseudo multiple replica" method for SNR calculation, which consists of adding appropriately scaled and correlated noise to the acquired k-space before image reconstruction[20]. First, we acquired noise-only data (with no RF) and only one image of the phantom or subject. We estimated the coil sensitivity data by applying low pass filters on theoriginal coil data. From the noise-only data, we calculated the noise covariance matrix (Ψ), which describes the Gaussian noise and coupling between coil elements.
We acquired 650,000 total samples to form the noise covariance matrix. Next, we synthesized gaussian noise with the same noise statistics and added the noise to the k-space of the original image. We repeated this synthesis procedure 100 times, each time reconstructing a full image using a SENSE reconstruction with R = 1[10]. The SNR map is the mean of the real part of the SENSE R = 1 reconstruction divided by the standard deviation of the real part of the SENSE R = 1 reconstruction, across all 100 synthesized images. This SNR estimation method is robust and does not add significant time to the scan. The acquisition of 650,000 noise-only samples requires only a few seconds.
The geometry factor, g-factor, is an important parameter when performing parallel imaging [10]. It is highly dependent on the sensitivity profile of each coil element, and it reflects the array’s ability to unfold accelerated images. The SNR of an accelerated image is inversely proportional to the g-factor and the square root of the reduction factor, R.
In our phantom experiments, we applied SENSE 1D and 2D acceleration and calculated the g-factors using [10]. For volunteer experiments, we calculated the ARC (GE Healthcare, Waukesha, WI) g-factor using the pseudo multiple replica method [20]. ARC uses coil-by-coil data driven calibration, a technique introduced by GRAPPA and shown to be robust without accurate coil sensitivity maps[9]. Utilizing a standard undersampling pattern, ARC also contains enhancements that allow it to take advantage of a full 3D unaliasing kernel in clinically feasible reconstruction times[21, 22].
Imaging Studies
All scans were performed on a 3T GE MR750 scanner with a 3D SPGR sequence. We compared the results from our 16-channel custom coil with the 8-channel standard coil with the same scan parameters. We formed each composite image using RSS for the parallel imaging and high resolution studies, and we used SENSE reconstruction for the SNR study[10, 23]. We analyzed all data using MATLAB (Matlab 7.1, The Mathworks, Inc.). For all parallel imaging experiments, we report the total reduction factor, R, as the unaccelerated scan time divided by the accelerated scan time.
SENSE g-Factor Maps
We imaged two homogeneous gelatin phantoms for comparison of SENSE g-factors [10]. In the coronal plane, the phantom shape was a square with rounded edges and 16 cm sides. We used the following protocol: coronal, TR/TE/α=4.6 ms/ 2.1 ms/ 3 flip angle, ±62.5 kHz receive BW, 256×256×64 matrix size, 1.4×1.4×1.0 mm resolution, and S/I readout. In post-processing, we applied SENSE acceleration of 2× in S/I and either 1×, 2×, 3×, or 4× in the L/R direction; therefore, the total reduction factors were 2, 4, 6 and 8. Using the unaccelerated data, we approximated each element’s sensitivity profile by smoothing each coil image with a low pass Hamming window and dividing the result by the RSS image. Ideally, for g-factor experiments and optimal estimation of coil sensitivities, we would use a homogeneous phantom that covered the entire FOV. Due to geometric differences between both coils, we could not cover the entire FOV with the same phantom. Background thresholding ensured g-factor values were calculated using data where the signal was adequate.
Using both coils, we performed in vivo experiments on 9 healthy volunteers, including evaluation of SNR, parallel imaging and high spatial resolution imaging. In addition, we performed DCE scans on 2 patients with known abnormalities using only the 16-channel coil. All volunteers and patients gave informed consent for the protocol approved by our investigational review board. For all imaging experiments with volunteers and patients, we used ARC for parallel imaging and acquired 24 calibration lines at the center of k-space for each undersampled direction.
SNR and ARC g-Factor Maps
We made SNR measurements for 2 healthy volunteers. We performed low resolution scans, measured the noise correlation matrices, and calculated SNR maps. We measured the noise correlation matrices on the scanner by turning off the RF amplifiers while maintaining the same analog and digital gains as the low resolution scans. For one of the volunteers, we calculated the ARC g-factor by undersampling the full data and using the pseudo multiple replica method. We used nominal accelerations of 2× S/I acceleration in addition to 1×, 2×, 3× or 4× L/R acceleration, effectively R = 1.9, 3.4, 4.6, and 5.7. The scan parameters were: axial, TR/TE/α=4.8 ms/2.2 ms/ 3, ±62.5 kHz receive BW, 256×160×64 matrix size, 1.4×2.2×3.0 mm resolution, 0:43 scan time, and A/P readout. The SNR was reported as the average SNR in the breast for central axial, sagittal and coronal slices.
Parallel Imaging
We assessed parallel imaging performance in one direction by applying 4× acceleration in the L/R direction, effectively R = 3.9. The scan parameters were: spectral-spatial RF pulse for fat suppression, axial, TR/TE/α=4.8 ms/ 2.2 ms/ 13, ±142.9 kHz receive BW, 512×272×312 matrix size, 0.7×1.3×1.0 mm resolution, 1:13 scan time, and A/P readout.
We compared parallel imaging in two directions using 2× S/I acceleration in addition to 1×, 2× or 4× L/R acceleration. We also acquired unaccelerated data as a comparison. We reformatted all images to the coronal plane and performed intensity correction based on low-pass filtering for all images. The scan parameters were: spectral fat suppression, axial, TR/TE/α=4.6 ms/ 1.1 ms/ 15 flip angle, ±62.5 kHz receive BW, 256×256×256 matrix, 1.4×1.4×1.0 mm resolution, 5:22/ 2:50/ 1:26/ 0:44 scan times for R = 1/ 1.9/ 3.7/ 7.3, and A/P readout.
High Spatial Resolution
High spatial resolution scans compared visualization of small scale features in the breast. The scan parameters were: spectral fat suppression, axial, TR/TE/α=10.6 ms/ 2.2 ms/ 10, ±62.5 kHz receive BW, 1024×512×32 matrix size, 0.3×0.6×0.6 mm resolution, 2:43 scan time, and A/P readout.
For a patient, we present results for a highly-accelerated, high spatial resolution scan using the 16-channel custom coil. We administered Prohance (Gadoteridol) contrast agent at 0.1 mmol/kg over 10 seconds, and imaged two minutes after washout. The scan parameters were: spectral fat suppression, axial, TR/TE/α× =11.7 ms/6.5 ms/ 15, ±48.8 kHz receive BW, 800×720×156 matrix size, 220×264×93.6 mm FOV, 0.3×0.4×0.6 mm resolution, 3:15 scan time, and A/P readout. For this patient, we applied parallel imaging with R = 6.3 (4× L/R and 2× S/I).
RESULTS
g-Factor Maps
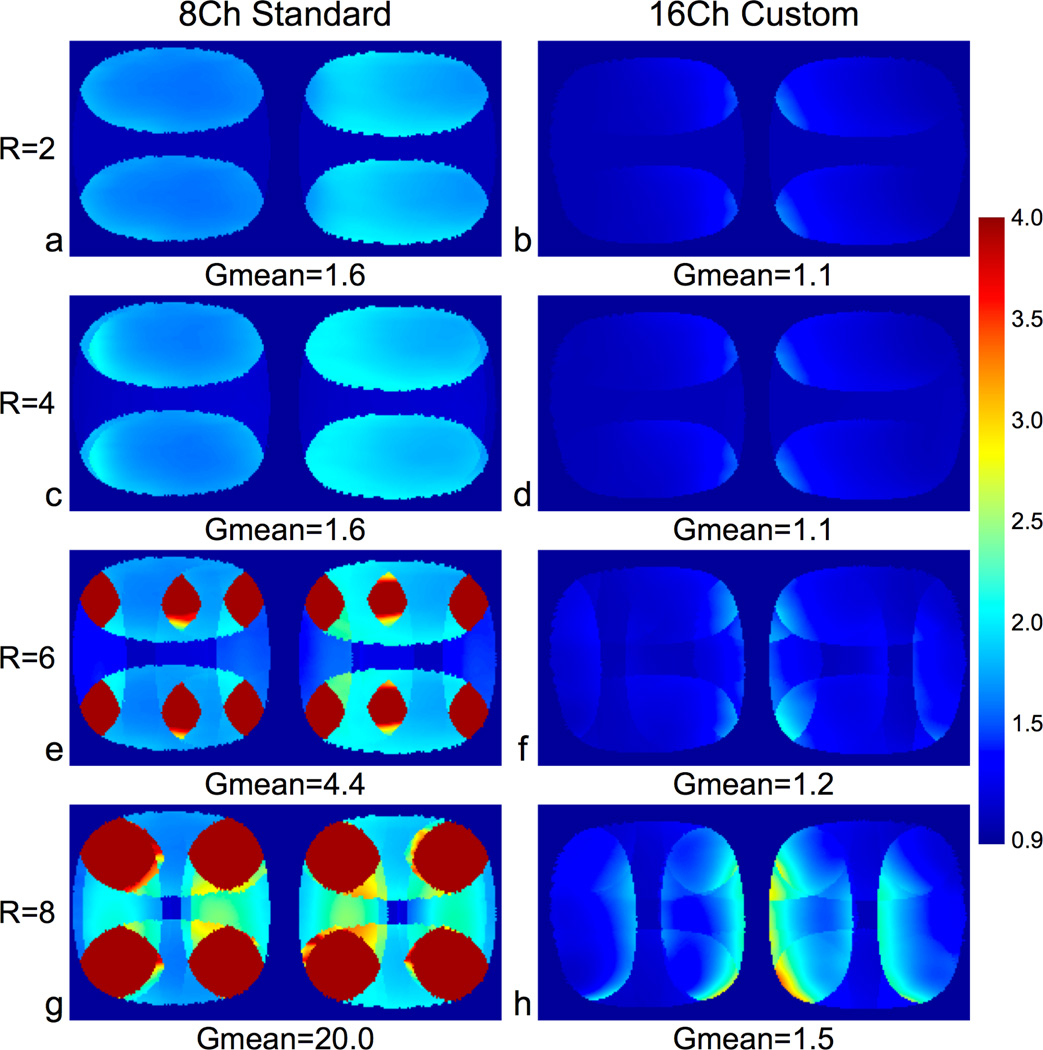
Fig. 2 shows the SENSE g-factor maps for a coronal slice of two gelatin phantoms. When compared to the 8-channel standard coil g-factor maps, the mean g-factor was lower for the 16-channel custom coil in all cases. The 8-channel standard coil performed poorly with high acceleration factors with mean g-factors of 4.4 and 20.0 for R = 6 and R = 8, respectively. The 16-channel custom coil had low mean g-factors in all cases, 1.2 and 1.5 for R = 6 and R = 8, respectively.
Figure 2.
G-Factor maps of a coronal slice of phantoms with L/R and S/I acceleration using the 8-channel standard coil (column 1) and the 16-channel custom coil (column 2). We acquired the full k-space data with a 3D SPGR sequence and applied SENSE acceleration in post-processing – 2× S/I and 1×, 2×, 3×, or 4× L/R. We applied background thresholding and smoothing prior to g-factor calculation. The 16-channel custom coil had much lower mean g-factors when compared to the 8-channel standard coil for all SENSE accelerated images.
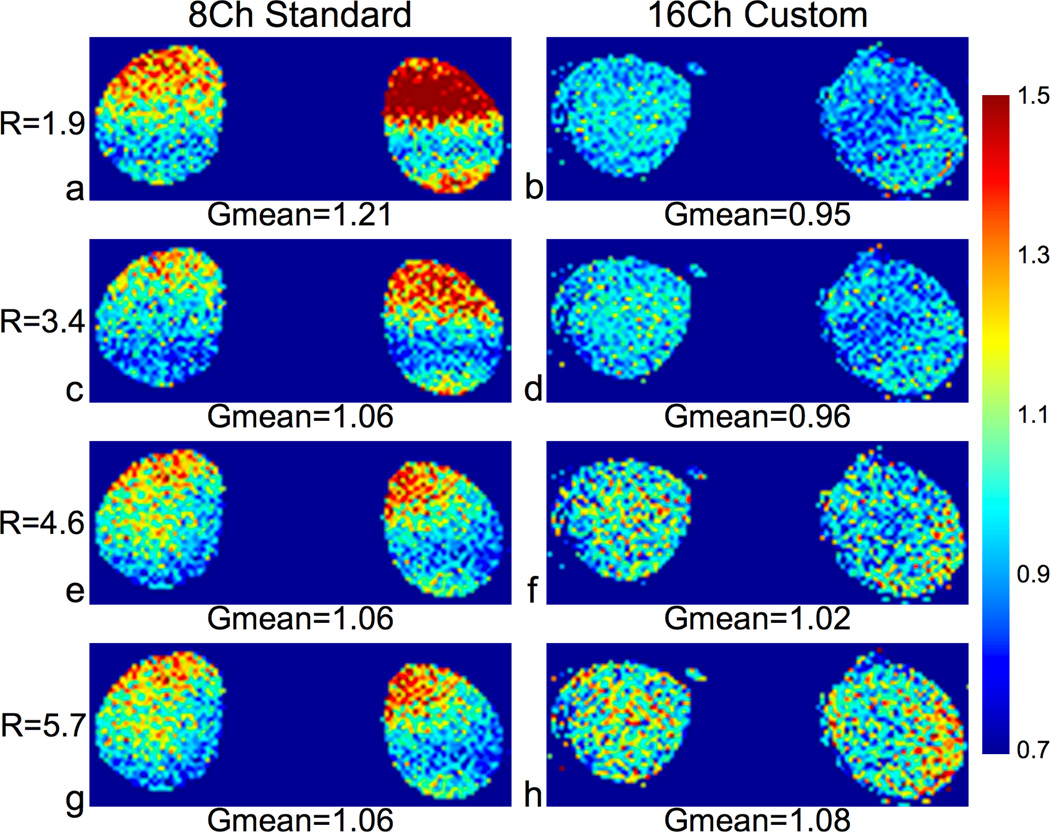
Fig. 3 shows the g-factor maps for a coronal slice of a healthy volunteer using ARC for parallel imaging. The mean g-factors were low for both coils for all acceleration factors, and the 16-channel custom coil had mean g-factors under 1 for R = 1.9 and R = 3.4.
Figure 3.
G-Factor maps of a coronal slice (reformatted from a 3D acquisition) of a healthy volunteer with L/R and S/I acceleration using the 8-channel standard coil (column 1) and the 16-channel custom coil (column 2). We acquired the full k-space data with a 3D SPGR sequence and applied ARC acceleration in post-processing – 2× S/I and 1×, 2×, 3×, or 4× L/R for effective accelerations of R = 1.9, 3.4, 4.6 and 5.7. We calculated ARC g-factors using the pseudo multiple replica method. Both coils had fairly low g-factors for all ARC accelerated images, but the custom coil had lower mean and peak values for R = 1.9, 3.4, and 4.6.
SNR
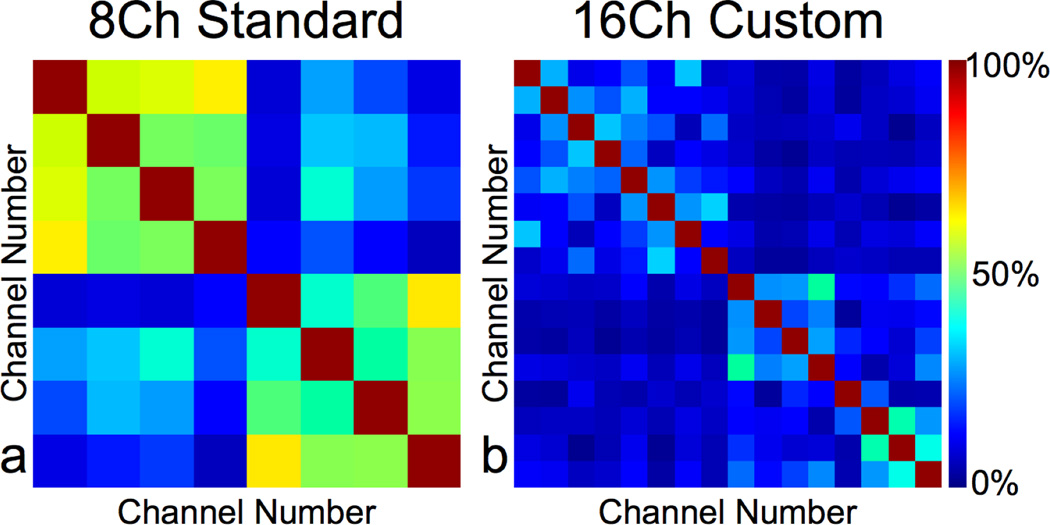
We used both the image data and the noise correlation matrix for calculating SNR maps for 2 volunteers. The noise correlation matrices for one of the volunteers are shown using the 8-channel standard coil (Fig. 4a) and the 16-channel custom coil (Fig. 4b). The mean cross correlation values were lower using the 16-channel custom coil (mean = 12%, maximum = 47%) versus the 8-channel standard coil (mean = 34%, maximum = 65%).
Figure 4.
Noise correlation matrices from a healthy volunteer using the 8-channel standard coil (a) and the 16-channel custom coil (b). The noise correlation was much lower using the 16-channel custom coil (mean=12%, maximum=47%) than the 8-channel standard coil (mean=34%, maximum=65%).
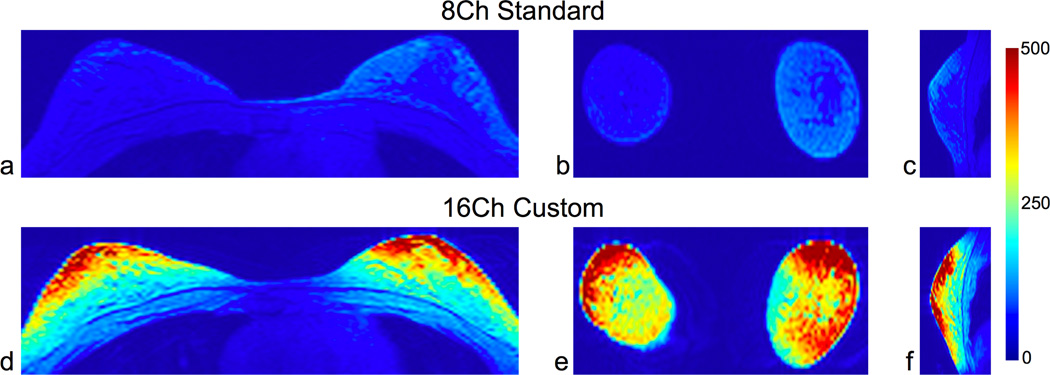
Fig. 5 shows the SNR maps using both coils for three orthogonal slices from the volunteer scan. For this volunteer, the mean SNR in the breast over all three slices was 4× higher using the 16-channel custom coil versus the 8-channel standard coil. Even though there is some falloff of the SNR at the chest wall using the 16-channel custom coil, this SNR is still higher than the corresponding SNR using the 8-channel standard coil. For both volunteers averaged, the mean SNR was 3.6× higher using the 16-channel custom coil versus the 8-channel standard coil (Table 1).
Figure 5.
SNR maps of healthy volunteer. Axial (a,d), coronal (b,e) and sagittal views (c,f) are shown. For this volunteer in all three views, the mean SNR in the breasts was 4× higher using the 16-channel custom coil than the 8-channel standard coil. For the 16-channel coil SNR maps, even though there is some falloff of the SNR at the chest wall, the SNR is still higher at the chest wall using the 16-channel custom coil versus the 8-channel standard coil. Table 1 summarizes the results from both volunteers. The scan parameters were: axial, 0:43 scan time, 256×160×64 matrix size, and 1.4×2.2×3.0 mm resolution.
Table 1.
Ratio of SNR Using 16-Channel Custom Coil versus 8-Channel Standard Coil for Bilateral Axial Breast Scans of Healthy Volunteers. Mean SNR measurements were made in central axial, sagittal and coronal slices.
| Subject | Coil | Axial | Coronal | Sagittal | Composite |
|---|---|---|---|---|---|
| 1 | 8-Ch Standard | 64 | 67 | 71 | 67 |
| 1 | 16-Ch Custom | 168 | 253 | 217 | 213 |
| 2 | 8-Ch Standard | 73 | 81 | 75 | 76 |
| 2 | 16-Ch Custom | 260 | 351 | 296 | 302 |
Parallel Imaging
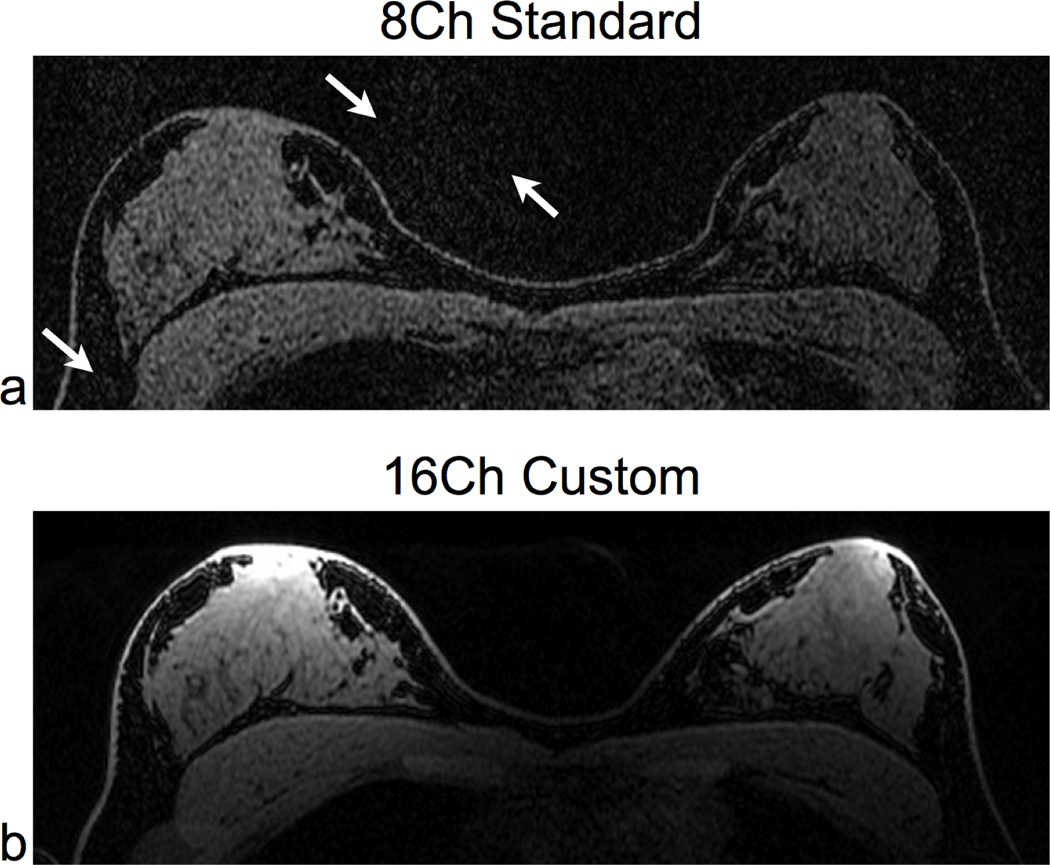
Fig. 6 compares image quality for 4× L/R acceleration using both the 16-channel custom coil and the 8-channel standard coil. In the 8-channel standard coil image, there is noticeable noise amplification in both the background and the breasts due to parallel imaging. There is significantly less noise in the corresponding 16-channel custom coil image.
Figure 6.
Accelerated (R = 3.9) 3D SPGR Scans of a normal volunteer using identical parameters for both coils: a 3D SPGR sequence, spectral-spatial fat suppression, and 4× ARC acceleration in the L/R direction (R = 3.9). We compared the 8-channel standard coil (a) and the 16-channel custom coil (b). There is a substantial improvement in signal quality in the 16-channel custom coil image, and there is little or no visible parallel imaging noise. The white arrows in (a) highlight the parallel imaging noise with the 8-channel standard coil. For the 16-channel custom coil image, the low sensitivity at the heart reduces cardiac motion artifact. We windowed both images to the same level and acquired both scans with identical parameters: axial, 1:13 scan time, 512×272×312 matrix size, and 0.7×1.3×1.0 mm resolution.
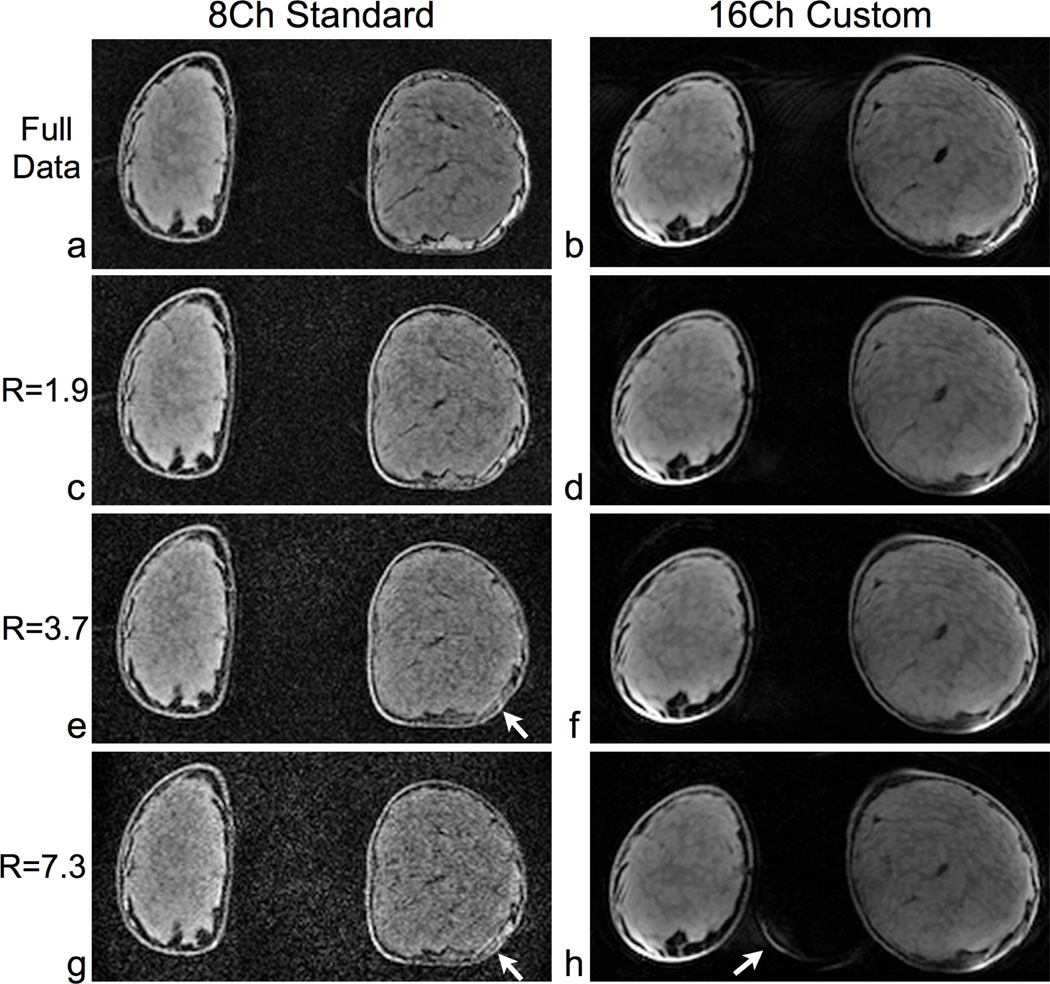
Fig. 7 compares parallel imaging performance in a coronal slice from another volunteer scan. For comparison with the accelerated images, we acquired and reconstructed the fully sampled data. Using the 8-channel standard coil, the image quality in the breast progressively degrades with increasing acceleration factors, and the noise obscures the detail in the breast. On the other hand, the image quality in the breast shows little change using the 16-channel custom coil for these acceleration factors. For the 16-channel custom coil image with R = 7.3, there is some aliasing of the edge of the breast into the background, but this aliasing does not affect the image quality in the breast.
Figure 7.
Coronal parallel imaging comparison in a normal volunteer, using identical scan parameters for both coils. We acquired full data (a,b) and accelerated data (c–h) with both the 8-channel standard coil (left column) and 16-channel custom coil (right column). For all accelerated images, we applied 2× acceleration in the S/I direction and additional acceleration in the L/R direction for a total acceleration of R = 1.9, 3.7 and 7.3. Using the 8-channel standard coil, significant noise (see arrows) obscures detail in the breast for R = 3.7 and R = 7.3. For the corresponding images with the 16-channel custom coil, there is excellent preservation of detail in the breast. Using the 16-channel coil, there is some aliasing of the edge of the breast into the background for R = 8 (see arrow), but this aliasing does not affect the image quality in the breast. Intensity correction was applied in post-processing to all images to account for B1 inhomogeneities in transmit and receive. Between the coils, the differences in shapes of the breasts are due to the passive support that the 16-channel custom coil provides. The scan parameters were: axial, 5:22/ 2:50/ 1:26/ 0:44 scan times for R = 1/ 1.9/ 3.7/ 7.3, 256×256×256 matrix size and 1.4×1.4×1.0 mm resolution.
High Spatial Resolution
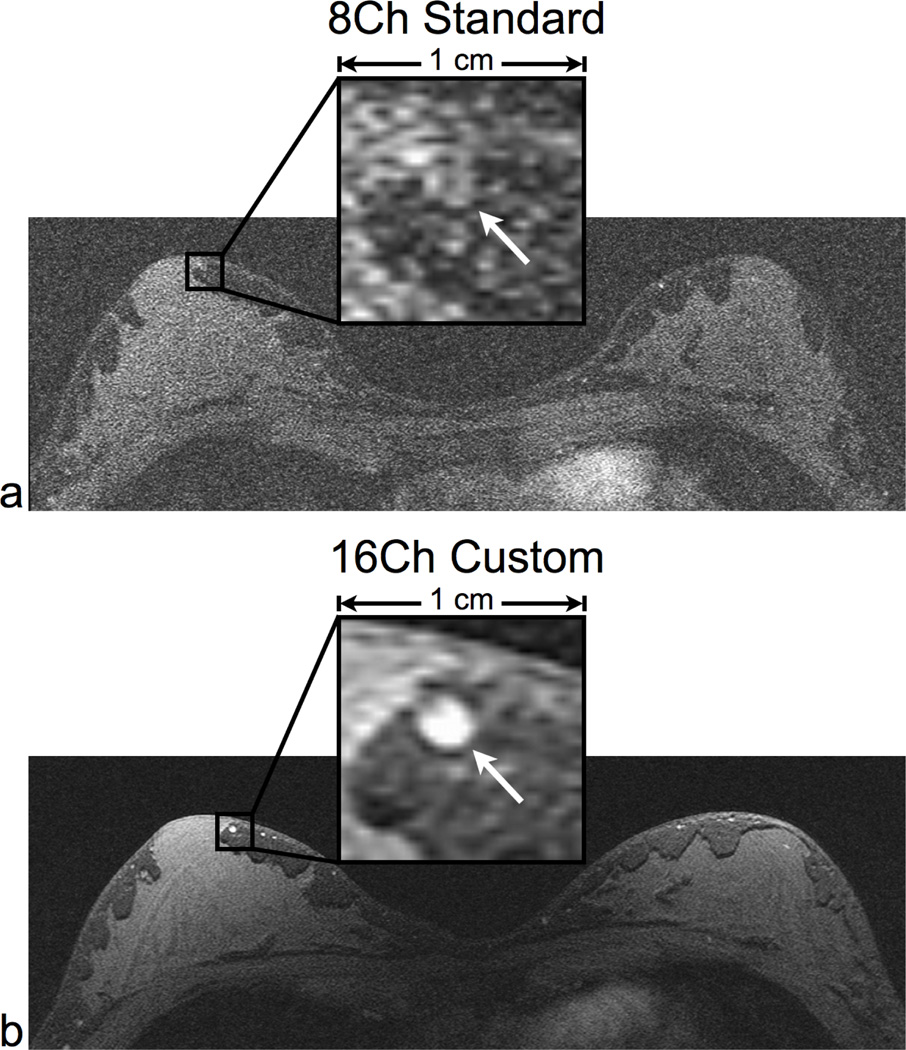
For high spatial resolution imaging, the ability to identify small-scale features is dependent on the image SNR. We display very high spatial resolution axial images (0.3×0.6×0.6 mm) of a volunteer scan for the 8-channel standard coil (Fig. 8a) and the 16-channel custom coil (Fig. 8b) as well as a zoomed-in box with 1 cm sides. The 16-channel custom coil image clearly resolves a 2 mm-diameter blood vessel. The low SNR in the 8-channel standard coil image limits the ability to identify this blood vessel.
Figure 8.
High spatial resolution axial scans in a normal volunteer using the same parameters for both coils: a 3D SPGR sequence with no parallel imaging. We compared the 8-channel standard coil (a) and the 16-channel custom coil (b). The 16-channel coil image clearly delineates fine morphological features such as a 2 mm wide blood vessel (see arrow). The low SNR in the 8-channel coil image limits the ability to identify these features. The scan parameters were: 2:43 scan time, 1024×512×32 matrix size, and 0.3×0.6×0.6 mm resolution.
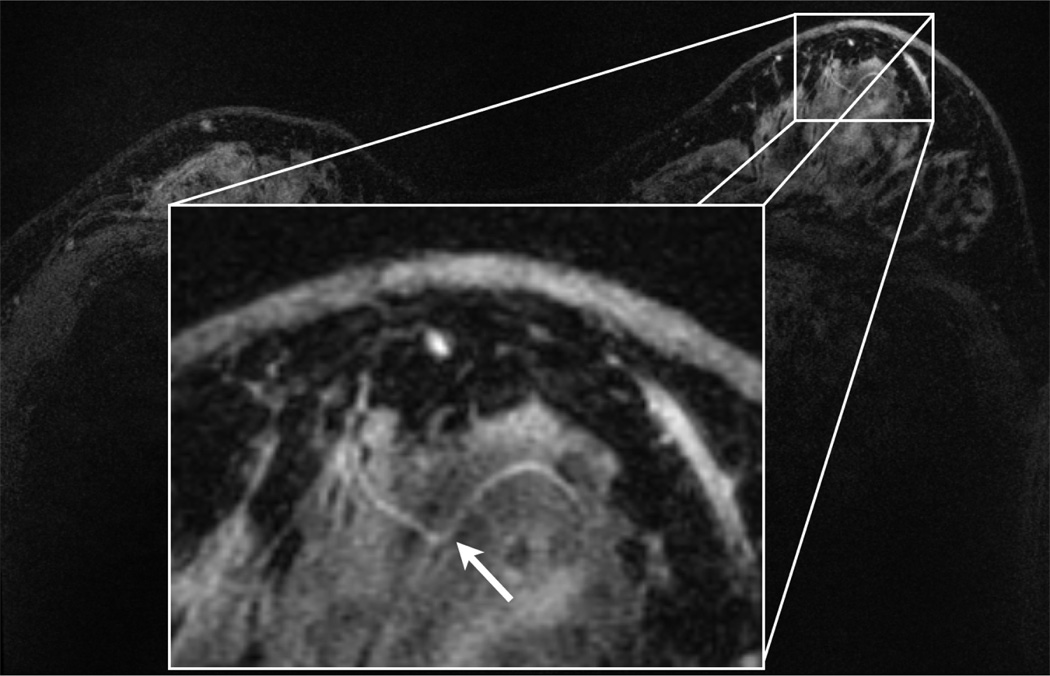
Fig. 9 shows a whole breast, “MR-microscopy” contrast-enhanced scan of a patient using the 16-channel custom coil. We can clearly visualize the fine morphological features of the ducts as well as a 0.4 mm-diameter blood vessel. The resulting voxel size, 61 nl, is comparable to the voxel size that has been reported for single “MR-Microscopy” surface coil breast imaging, but with bilateral whole-breast coverage.
Figure 9.
Axial DCE post-contrast scan using the 16-channel custom coil, showing ultrahigh spatial resolution scan using a 3D SPGR sequence. There is excellent visualization of small scale features including a 0.4 mm-diameter blood vessel (arrow). We applied ARC acceleration with R = 6.3 (4× L/R and 2× S/I). The scan parameters were: 3:15 scan time, 800×720×156 matrix size, 220×264×93.6 mm FOV, and 0.3×0.4×0.6 mm resolution.
DISCUSSION AND CONCLUSIONS
For breast MRI at 3T, we have shown the benefits of a 16-channel closely-fitted array of surface coils for bidirectional acceleration, increased SNR and a reduction in scan time. We developed this array for a “cup B” breast size, though similar coils can be constructed for different breast sizes. In studies of healthy volunteers, scans with the 16-channel custom coil led to a 3.6× increase in mean SNR and an improvement in parallel imaging quality when compared to corresponding scans with a commercially-available 8-channel standard coil. The 8-channel standard coil is biopsy-compatible and has larger coil elements for better homogeneity, but both of these features lead to lower SNR when compared with a closely-fitted, screening coil. Using the 16-channel custom coil, we also acquired high-quality, high spatial resolution images of both breasts for DCE breast scans in two patients.
A major goal of breast MRI, which this work seeks to advance, is the acquisition of very high-spatial resolution images that can be used to assess the morphology of areas of enhancement in the breast. However, these images must be acquired within approximately 5 minutes of scanning, to avoid decreased conspicuity of the target due to early washout of contrast and/or progressive gradual background enhancement that obscures the target margins. Our 16-channel custom coil provides the necessary SNR to enable dramatic reductions in voxel size required for high resolution images. The bidirectional parallel imaging capability of the coil is also critical as the absence of the high acceleration factors will prohibitively increase the scan time. For example, in the patient, we acquired ultrahigh resolution images (0.3×0.4×0.6 mm voxel size) in just over 3 minutes using R = 6.3. Using only 2× acceleration (which is typical for standard breast coils) the scan would have taken over 10 minutes, at which point, most of the contrast would have washed out.
An additional benefit of the 16-channel custom coil geometry is its minimal sensitivity to cardiac motion. We arranged the coils for acceleration in the S/I and L/R directions. This leaves frequency encoding in the A/P direction, which removes most of the cardiac motion ghost artifacts from the image. Additionally, the 16-channel custom coil further reduces cardiac motion artifacts as the heart is not in the sensitive volume of the coil.
The main SNR gain using the 16-channel custom coil is due to the reduction in coil element size when compared to the 8-channel standard coil. The smaller element size necessitates an increase in the number of coil elements to cover the FOV. We have built this coil for a “cup B" size, and for adapting this coil design for a “cup C/D" breast, there are two options: 1) maintain coil element size and increase the number of coil elements or 2) use 16 coil elements with a larger element size. The “cup C/D" women would better load the 8-channel standard coil and increase the coil’s SNR when compared to the “cup B" case. For option 1), there will be an increase in the local SNR at the edge of the breast and also improvements in parallel imaging quality when compared to the “cup B" case. For option 2), the parallel imaging quality will be comparable to the “cup B" case but the comparative SNR gain against the 8-channel standard coil will not be as high.
Ideally, a more uniform SNR profile is preferable; however, using the 16-channel custom coil, the SNR was higher at the anterior part of the breasts. This result occurs since in general, images from surface coil arrays have more coil shading when compared to volumetric coil images. For the images in Fig. 7, we used a low pass filter with a Hamming window for estimating the bias fields and corrected the images by dividing by the bias fields. In future experiments, we can investigate which various intensity correction and coil combination algorithms robustly lessen the intensity variations in our scans [10, 23–26].
The g-factor reflects a coil’s ability to perform parallel imaging acceleration. High g-factor values result in severe reduction in SNR. For the SENSE reconstructions, the 16-channel custom coil had low mean g-factors (1.5 and below) for all studies, while the 8-channel standard coil had an unacceptably high mean g-factor at high acceleration factors (20.0 for R = 8). The element layout of the 16-channel custom coil results in a better ability to separate folded, intermediate coil images. Upon examining individual coil images from the 8-channel standard coil, we found that there is not an appreciable difference in the S/I sensitivity profiles. Therefore, the 8-channel standard coil is not optimal for parallel imaging in the S/I direction for SENSE reconstructions. G-factor values less than 1 for GRAPPA-like reconstructions is a known phenomena, and it has been reported elsewhere in literature[20]. For the ARC in vivo studies, the 16-channel custom coil had mean g-factors less than 1 for R = 1.9 and R = 3.4. Interestingly, the 8-channel standard coil had lower mean g-factors at higher accelerations when compared with R = 1.9 (2× S/I). This is due to the 8-channel coil’s low g-factor in the L/R acceleration compensating for suboptimal performance in the S/I direction. In general, the ARC g-factors were low for all accelerations with both coils when compared with the SENSE g-factors.
The difference in SNR between the 16-channel custom and 8-channel standard coil is evident in human scans. Using the 16-channel custom coil, the image quality in the breasts is preserved for R = 7.3. The lower SNR using the 8-channel standard coil obscures image quality in the breasts for R = 3.7 and higher. For the 16-channel custom coil images with R = 7.3, there was some aliasing of the breasts into the background. This aliasing is due to the lack of coil sensitivity in the region between the breasts. Potential solutions include adding more coil elements between the breasts, utilizing a thresholding algorithm based upon a calibration scan, or increasing the number of acquisition lines for the ARC reconstruction. We utilize a standard simple grid for undersampling, and a CAIPIRINHA-type technique might be beneficial in reducing aliasing[27].
Geometric overlap of the custom coil elements and the low input resistance preamplifiers facilitated decoupling in the 16-channel custom coil. For this design, we avoid the additional cable noise associated with system preamplifiers by using on-coil preamplifiers. Using on-coil preamplifiers, cable noise is further minimized by placing the preamplifiers proximal to the coil. Even though the commercial 8-channel standard has a more optimal preamplifier location (less than 3 cm from the coil) than the 16-channel custom coil (between 5 and 15 cm), there is lower SNR with the 8-channel standard coil due to the larger coil elements. However, the major tradeoff with on-coil preamplifiers is the dependence of preamplifier tuning on the coil size. If we wanted to implement a system where the breast cups are interchangeable, system preamplifiers might lead to a more robust array by reducing tuning requirements.
In terms of SNR and g-factor, it is difficult to quantitatively compare the 16-channel custom coil to other published coil results due to differences in imaging parameters, loading and ROI selection. In addition, knowledge of the B1+ map is necessary since it affects the local SNR. The Sentinelle 16-element open prototype coil has shown SNR benefits compared to a commercially-available 8-channel coil and low g-factors for parallel imaging in two directions [18]. The geometry of the 16-channel Sentinelle prototype differs from that of our 16-channel custom-fitted array, and it would be interesting to directly compare SNR and parallel imaging in volunteers using the same protocol. As well, the geometry of the breast coil could also assist in reducing motion artifact. In the 16-channel Sentinelle coil geometry, reduced motion of the breast is achieved through moderate compression of the breast; however, this can also lead to patient discomfort. Conversely, improved comfort of the coil offers a better experience for the subject as well as reducing breast motion due to curtailed subject fidgeting.
While it is difficult to quantitatively assess the comfort level of each coil, our informal conversations with the subjects after the examination suggest that our 16-channel custom coil was more comfortable than the 8-channel commercially-available coil. Specifically, the subjects complained of pain in their sternum after scanning with the commercially-available coil. Perhaps the thinner, stiffer padding or the sternum support bar on the commercially-available coil causes this discomfort. The custom coil uses a thicker, more compliant padding around the sternum region and does not have a sternum support bar. In addition, some subjects remarked on the higher comfort of the custom coil due to the support that it provides for the breasts. In the commercially-available coil, the breasts hang freely in the coil, which can be awkward for the subject while the custom coil acts like a brassiere, providing passive support for the breasts while not actively compressing them. Again, this passive support helps to reduce respiratory motion artifacts since pendant non-compressed breasts have slight respiratory motion due to forces transmitted from the chest wall musculature. A side effect of the brassiere-style geometry is that the nipple may come in contact with the FR4 coil housing. That can lead to the false impression of nipple inversion, which can be a sign of breast disease. As such, radiologists using the 16-channel custom coil would need experience to distinguish between this effect and an actual case of nipple inversion.
In conclusion, we have developed and tested a 16-channel bilateral receive-only breast array for parallel imaging in two directions. Using this closely-fitted array, we have demonstrated highly-accelerated and high resolution imaging in volunteers and patients. Compared with an 8-channel standard array, images from the 16-channel custom coil show 3.6× higher SNR in the breast, as well as accelerated images with a reduced artifact level.
Acknowledgments
We would like to acknowledge GE Healthcare and Fifth Ave Design for supplying some electrical and mechanical components. We also thank Ronald Watkins and Mina Makram for their consultation during the coil fabrication and Misung Han and Kyung Sung for postprocessing techniques. As well, we thank the funding sources for this project.
Grant Sponsors:
This work was supported by P41RR009784, R01-EB009055, the Lucas Foundation, and General Electric Healthcare.
References
- 1.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, Morris E, Pisano E, Schnall MD, Sener S, Smith RA, Warner E, Yaffe M, Andrews KS, Russell CA. American cancer society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 2.Kuhl C. The current status of breast MR imaging: Part i. choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356–378. doi: 10.1148/radiol.2442051620. [DOI] [PubMed] [Google Scholar]
- 3.Orel SG, Schnall MD. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology. 2001;220:13–30. doi: 10.1148/radiology.220.1.r01jl3113. [DOI] [PubMed] [Google Scholar]
- 4.Konyer NB, Ramsay EA, Bronskill MJ, Plewes DB. Comparison of MR imaging breast coils. Radiology. 2002;222:830–834. doi: 10.1148/radiol.2223001310. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Kurihara Y, Kanemaki Y, Ogata H, Fukuda M, Nakajima Y, Maeda I. Diagnostic accuracy of high-resolution MRI using a microscopy coil for patients with presumed DCIS following mammography screening. Journal of Magnetic Resonance Imaging. 2007;25:96–103. doi: 10.1002/jmri.20809. [DOI] [PubMed] [Google Scholar]
- 6.Kuhl C, Schild HH. Dynamic image interpretation of MRI of the breast. Journal of Magnetic Resonance Imaging. 2000;12:965–974. doi: 10.1002/1522-2586(200012)12:6<965::aid-jmri23>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser WA, Zeitler E. MR imaging of the breast: Fast imaging with and without Gd-DTPA. Radiology. 1989;170:681–686. doi: 10.1148/radiology.170.3.2916021. [DOI] [PubMed] [Google Scholar]
- 8.Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magnetic Resonance in Medicine. 1997;38:591–603. doi: 10.1002/mrm.1910380414. [DOI] [PubMed] [Google Scholar]
- 9.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magnetic Resonance in Medicine. 2002;47:1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 10.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magnetic Resonance in Medicine. 1999;42:952–962. [PubMed] [Google Scholar]
- 11.Glockner JF, Hu HH, Stanley DW, Angelos L, King K. Parallel MR imaging: A user’s guide. Radiographics. 2005;25:1279–1297. doi: 10.1148/rg.255045202. [DOI] [PubMed] [Google Scholar]
- 12.Niendorf T, Sodickson DK. Parallel imaging in cardiovascular MRI: Methods and applications. NMR in Biomedicine. 2006;19:325–341. doi: 10.1002/nbm.1051. [DOI] [PubMed] [Google Scholar]
- 13.Schmitt M, Potthast A, Sosnovik DE, Polimeni JR, Wiggins GC, Triantafyllou C, Wald LL. A 128-channel receive-only cardiac coil for highly accelerated cardiac MRI at 3 tesla. Magnetic Resonance in Medicine. 2008;59:1431–1439. doi: 10.1002/mrm.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wichmann T, Lanz T, Griswold MA, Greiser A, Nittka M, Jakob PM. Highly accelerated real-time imaging of cardiac function using a 32 channel phased array at 3T. Proceedings of the 14th Annual Meeting of ISMRM; Seattle, WA, USA. 2006. p. 145. [Google Scholar]
- 15.Wintersperger BJ, Reeder SB, Nikolaou K, Dietrich O, Huber A, Greiser A, Lanz T, Reiser MF, Schoenberg SO. Cardiac CINE MR imaging with a 32-channel cardiac coil and parallel imaging: impact of acceleration factors on image quality and volumetric accuracy. Journal of Magnetic Resonance Imaging. 2006;23:222–227. doi: 10.1002/jmri.20484. [DOI] [PubMed] [Google Scholar]
- 16.Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 tesla receive-only phased-array head coil with soccer-ball element geometry. Magnetic Resonance in Medicine. 2006;56:216–223. doi: 10.1002/mrm.20925. [DOI] [PubMed] [Google Scholar]
- 17.Wiggins GC, Alagappan V, Potthast A, Schmitt M, Wiggins CJ, Fischer H, Jahns K, Benner T, Polimeni J, Wald LL. Design optimization and SNR performance of 3T 96 channel phased array head coils. Proceedings of the 15th Annual Meeting of ISMRM; Berlin, Germany. 2007. p. 243. [Google Scholar]
- 18.Marshall H, Devine PM, Shanmugaratnam N, Fobel R, Siegler P, Piron CA, Plewes DB. Evaluation of multicoil breast arrays for parallel imaging. Journal of Magnetic Resonance Imaging. 2010;31:328–338. doi: 10.1002/jmri.22023. [DOI] [PubMed] [Google Scholar]
- 19.Kellman P, McVeigh ER. Image reconstruction in SNR units: A general method for SNR measurement. Magnetic Resonance in Medicine. 2005;54:1439–1447. doi: 10.1002/mrm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robson PM, Grant AK, Madhuranthakam AJ, Lattanzi R, Sodickson DK, McKenzie CA. Comprehensive quantification of signal-to-noise ratio and g-factor for image-based and k-space-based parallel imaging reconstructions. Magnetic Resonance in Medicine. 2008;60:895–907. doi: 10.1002/mrm.21728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beatty PJ, Brau ACS, Chang S, Joshi SM, Michelich CR, Bayram E, Nelson TE, Herfkens RJ, Brittain JH. A method for autocalibrating 2-D accelerated volumetric parallel imaging with clinically practical reconstruction times; Proceedings of the 15th Annual Meeting of ISMRM; Berlin, Germany. 2007. p. 1749. [Google Scholar]
- 22.Brau ACS, Beatty PJ, Skare S, Bammer R. Comparison of reconstruction accuracy and efficiency among autocalibrating data-driven parallel imaging methods. Magnetic Resonance in Medicine. 2008;59:382–395. doi: 10.1002/mrm.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magnetic Resonance in Medicine. 1990;16:192–225. doi: 10.1002/mrm.1910160203. [DOI] [PubMed] [Google Scholar]
- 24.Brey WW, Narayana PA. Correction for intensity falloff in surface coil magnetic resonance imaging. Medical Physics. 1987;15:241–245. doi: 10.1118/1.596255. [DOI] [PubMed] [Google Scholar]
- 25.Cohen MS, Dubois RM, Zeineh MM. Rapid and effective correction of rf inhomogeneity for high field magnetic resonance imaging. Human Brain Mapping. 2000;10:204–211. doi: 10.1002/1097-0193(200008)10:4<204::AID-HBM60>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin FH, Chen YJ, Belliveau JW, Wald LL. A wavelet-based approximation of surface coil sensitivity profiles for correction of image intensity inhomogeneity and parallel imaging reconstruction. Human Brain Mapping. 2003;19:96–111. doi: 10.1002/hbm.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magnetic Resonance in Medicine. 2005;53:684–691. doi: 10.1002/mrm.20401. [DOI] [PubMed] [Google Scholar]