Abstract
The inflammasome pathway functions to regulate caspase-1 activation in response to a broad range of stimuli. Caspase-1 activation is required for the maturation of the pivotal pro-inflammatory cytokines of the pro-IL-1β family. In addition, caspase-1 activation leads to a certain type of cell death known as pyroptosis. Activation of the inflammasome has been shown to play a critical role in the recognition and containment of various microbial pathogens, including the intracellularly replicating Listeria monocytogenes; however, the inflammasome pathways activated during L. monocytogenes infection are only poorly defined. Here, we demonstrate that L. monocytogenes activates both the NLRP3 and the AIM2 inflammasome, with a predominant involvement of the AIM2 inflammasome. In addition, L. monocytogenes-triggered cell death was diminished in the absence of both AIM2 and NLRP3, and is concomitant with increased intracellular replication of L. monocytogenes. Altogether, these data establish a role for DNA sensing through the AIM2 inflammasome in the detection of intracellularly replicating bacteria.
Keywords: Listeria monocytogenes, Inflammasome, caspase-1, AIM2, NLRP3
Introduction
The innate immune system functions to sense microbial pathogens that are of danger to the host. In addition to its immediate effector functions, the innate immune system is also critically required to initiate adaptive immune responses. Several pattern recognition receptors (PRR) have been identified that are able to detect certain molecular structures that are unique to microbial pathogens but not to the host [1–3]. At the same time, PRR can also sense ligands, which are not necessarily specific for microbial pathogens, but appear for example in special compartments that are usually devoid of such ligands. Cell stress or tissue damage can also trigger PRR activation via the release of normally compartmentalized molecules or via chemically modified self-molecules. In contrast to pathogen-associated molecular patterns (PAMP) other endogenous inflammatory signals that appear after cellular damage or due to metabolic derangements, can also be summarized as danger-associated molecular patterns, or DAMP [4]. Activation of PRR leads to the transcription of pro-inflammatory genes that function to combat the microbial infection. Simultaneously, several signaling cascades are triggered that do not require de novo transcription and translation. Of these, the proteolytic activation of “inflammatory caspases” in particular caspase-1 plays a critical role in host defense [5].
Activation of caspase-1 leads to the proteolytic processing of pro-cytokines such as pro-IL-1β and pro-IL-18 [6]. In addition, active caspase-1 can also trigger a special type of cell death – so called pyroptosis [7]. Even though the critical role of caspase-1 in processing of inflammatory cytokines has been established for many years, it was only recently that upstream signaling components have been identified that lead to the activation of caspase-1 in a stimulus-specific manner. The term “inflammasome” has been coined for these distinct signaling platforms as the formation of high molecular weight complexes following their activation were observed [8]. To date, four independent inflammasomes have been characterized. NLRP1, NLRP3 and IPAF (also know as NLRC4) are all proteins of the nucleotide-binding domain leucine-rich repeat containing (NLR) family, whereas AIM2 belongs to the pyrin domain and HIN200 domain containing (PYHIN) protein family (reviewed in [9]). While the NLRP1, IPAF and AIM-2 inflammasome recognize specific molecules, the NLRP3 inflammasome can respond to a very broad spectrum of stimuli of diverse physio-chemical nature such as pore forming toxins, crystalline material or ATP [10–14]. In addition, a so-called priming signal is critically required for NLRP3 activation. We and others have recently shown that this priming signal (e.g. LPS) functions to upregulate NLRP3 expression, which is the limiting factor in the activity of the NLRP3 inflammasome [15, 16]. The clear nature of the signal that activates NLRP3 itself is currently unknown, but several hypotheses have been put forth to explain this phenomenon. For example, several lines of evidence suggest that lysosomal damage and concomitant release of lysosomal cathepsins lead to the activation of NLRP3 pathway [11, 12]. At the same time it has been demonstrated that the generation of reactive oxygen species is an upstream event of NLRP3 activation and a role for thioredoxin-interacting protein has been implicated in this process [10, 17]. Future studies will be required to formulate a unifying hypothesis of NLRP3 activation.
In the early phase of infection, inflammasome activation is critical for host defense against many pathogens, including L. monocytogenes. L. monocytogenes is a facultative intracellular pathogen and the causative agent of the food-borne disease, listeriosis. While causing only mild symptoms of gastroenteritis in healthy individuals, infection with L. monocytogenes can cause life-threatening illness in immunocompromised individuals, including newborns and the elderly [18]. Upon invasion, L. monocytogenes is internalized by both non-phagocytic cells and professional phagocytes such as macrophages. Following uptake into phagocytic vacuoles, L. monocytogenes escapes from phagosomal compartments, employing the pore-forming cytolysin listeriolysin O (LLO). It replicates robustly within the cytosol and spreads directly from cell to cell avoiding extracellular immune recognition.
The activation of innate immune PRR in response to infection with L. monocytogenes is still not completely understood. L. monocytogenes is sensed by several PRR that lead to the de novo transcription and translation of pro-inflammatory cytokines. PRR that have been identified to play a role in this sensing process are TLR2, NOD1 and NOD2, while yet unknown pathways seem to be involved in addition [19–26]. At the same time, cytosolic localization of L. monocytogenes leads to the activation of the inflammasome and caspase-1 has shown to be required for effective clearance of L. monocytogenes during murine infection [27]. Initial reports indicated that L. monocytogenes induces caspase-1 activation through the NLRP3 inflammasome [13] and similar findings were reported recently [28]. However, subsequent studies have not confirmed solely NLRP3-dependent activation of caspase-1 induced by L. monocytogenes [29]. These discrepancies may be explained at least in part by variations in the expression of bacterial factors that contribute to caspase-1 activation. For example, it has been shown that L. monocytogenes can induce caspase-1 activation through both, the NLRP3 and IPAF inflammasomes as well as another yet-to-be-identified ASC-dependent inflammasome [26]. IPAF activation is dependent on the cytosolic release of flagellin but flagellin-expression in L. monocytogenes is temperature-regulated with expression in some strains being turned off at 37°C [30].
In this study we comprehensively explore the role of known inflammasome pathways in the recognition of L. monocytogenes and hereby identify a critical role for both AIM2 and NLRP3 in L. monocytogenes-triggered inflammasome activation.
Results and Discussion
Activation of caspase-1 by Listeria monocytogenes is independent of IPAF, but requires ASC
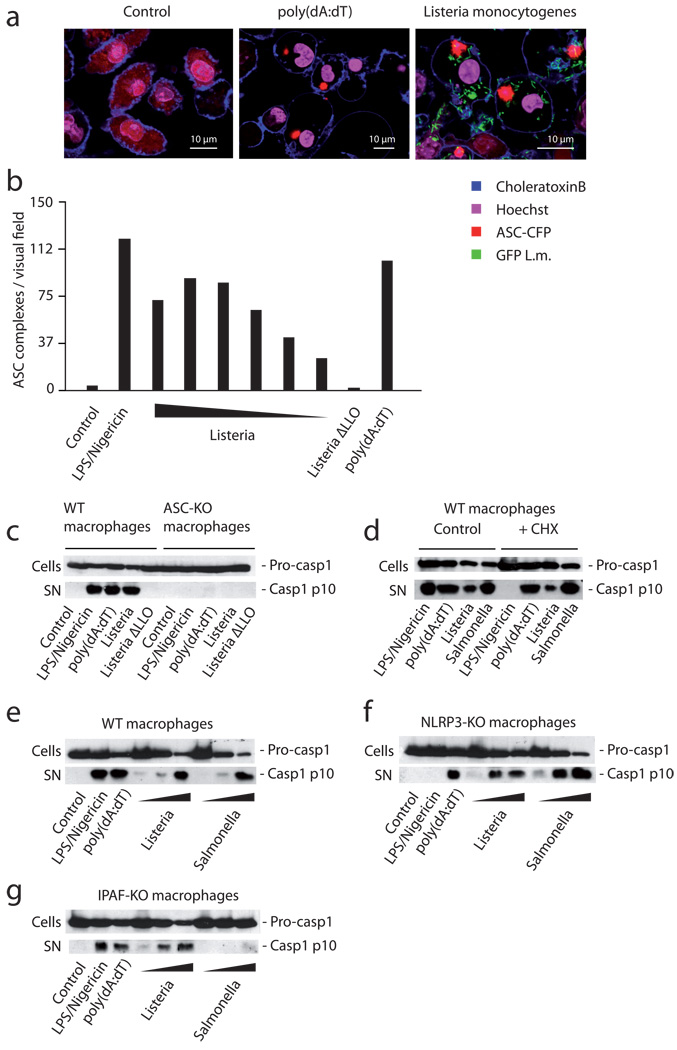
We first studied the requirement of the signaling molecule ASC, using ASC-CFP overexpressing macrophages that allow the direct visualization and quantification of ASC pyroptosome formation and thus are able to directly report ASC-dependent inflammasome activation. In these cells ASC forms large oligomers after its activation, which leads to visible changes in the cytoplasmic fluorescence pattern of CFP-ASC [15]. Cytoplasmic presence of Listeria was readily followed by ASC pyroptosome formation (Fig. 1a) in a dose-dependent fashion (Fig. 1b), whereas non-invasive Listeria devoid of LLO did not lead to ASC activation. Listeria-mediated ASC pyroptosome formation was in the same range as the NLRP3 stimulus LPS/ATP or the AIM2-trigger poly(dA:dT) (Fig. 1b). Listeria infection not only resulted in ASC activation, yet ASC was also critically required for Listeria-mediated caspase-1 activation (Fig. 1c). As expected, an absolute ASC requirement was also seen for NLRP3 or AIM2 activation (Fig. 1c). Altogether these results are in line with the majority of studies demonstrating that Listeria trigger inflammasome activation in an ASC-dependent fashion with a requirement for LLO. To study the contribution of individual upstream NLR, we next addressed the role of de novo protein synthesis for the inflammasome responsiveness towards Listeria. NLRP3 activation critically requires a priming signal that leads to the induction of NLRP3 expression thus licensing the NLRP3 inflammasome, whereas the AIM2 inflammasome is responsive towards dsDNA independent of de novo transcription or translation [15, 16]. Cycloheximide-treated macrophages were readily responsive to intracellular DNA and replicating Listeria or Salmonella, while stimulation with the NLRP3 trigger LPS/ATP was unable to prompt caspase-1 activation in these cells (Fig. 1d). These data already excluded a non-redundant role for NLRP3 as a sensor for Listeria. Indeed, NLRP3-deficient macrophages showed no loss in caspase-1 activity in response to Listeria (Fig. 1e). In addition, IPAF and NLRP1 also showed a redundant role, if any, in the recognition of Listeria-mediated inflammasome activation. IPAF-deficient macrophages still displayed strong caspase-1 activation in response to Listeria (Fig. 1e–g). Moreover, a non-redundant involvement of NLRP1 was excluded due to the fact that macrophages derived from C57BL/6 mice, which are devoid of functional NLRP1 [31], also showed a normal caspase-1 response (data not shown). While these data did not exclude the possibility of a NLRP3, IPAF or NLRP1-involvement in the response to Listeria, they demonstrated that additional mechanisms were critically required in the inflammasome response to Listeria.
Figure 1. Listeria triggers caspase-1 activation through ASC, yet is unimpaired in cells solely deficient in NLRP3 or IPAF.
(A) Wild type macrophages stably expressing ASC-CFP were treated with L. monocytogenes expressing GFP, transfected with poly(dA:dT) or left untreated. 6 hours after stimulation, the formation of ASC complexes was quantified using confocal microscopy. (B) ASC-CFP expressing macrophages were treated with ATP/LPS, decreasing amounts of L. monocytogenes (MOI 20 – 0.625 in steps of 50%), a mutant L. monocytogenes strain lacking LLO (Listeria ΔLLO, MOI 20) or transfected with poly(dA:dT) and formation of ASC complexes was quantified using epifluorescence microscopy. (C) Wild type macrophages or ASC-deficient macrophages were treated with the stimuli as above (L. monocytogenes MOI of 10). 6 hours after stimulation pro-caspase-1 (pro-casp1) expression was assessed in cell lysates (cells), while caspase-1 activation (casp1 p10) was assessed in the supernatant (SN) using western blot. (D) Wild type macrophages were treated with 20 ng /ml cycloheximide or left untreated. After 30 min cells were stimulated as indicated (L. monocytogenes and S. enterica MOI of 10) and caspase-1 expression and activation was assessed as above. (E) Wild type, (F) NLRP3- or (G) IPAF-deficient macrophages were stimulated as indicated (L. monocytogenes / S. enterica MOI of 50, 10 or 1) and 6 hours after stimulation caspase-1 expression and activation was assessed. One representative experiment out of three (A, B, D) or four (C, E, F, G) independent experiments is depicted.
NLRP3 and AIM2 are both required for L. monocytogenes-mediated inflammasome activation
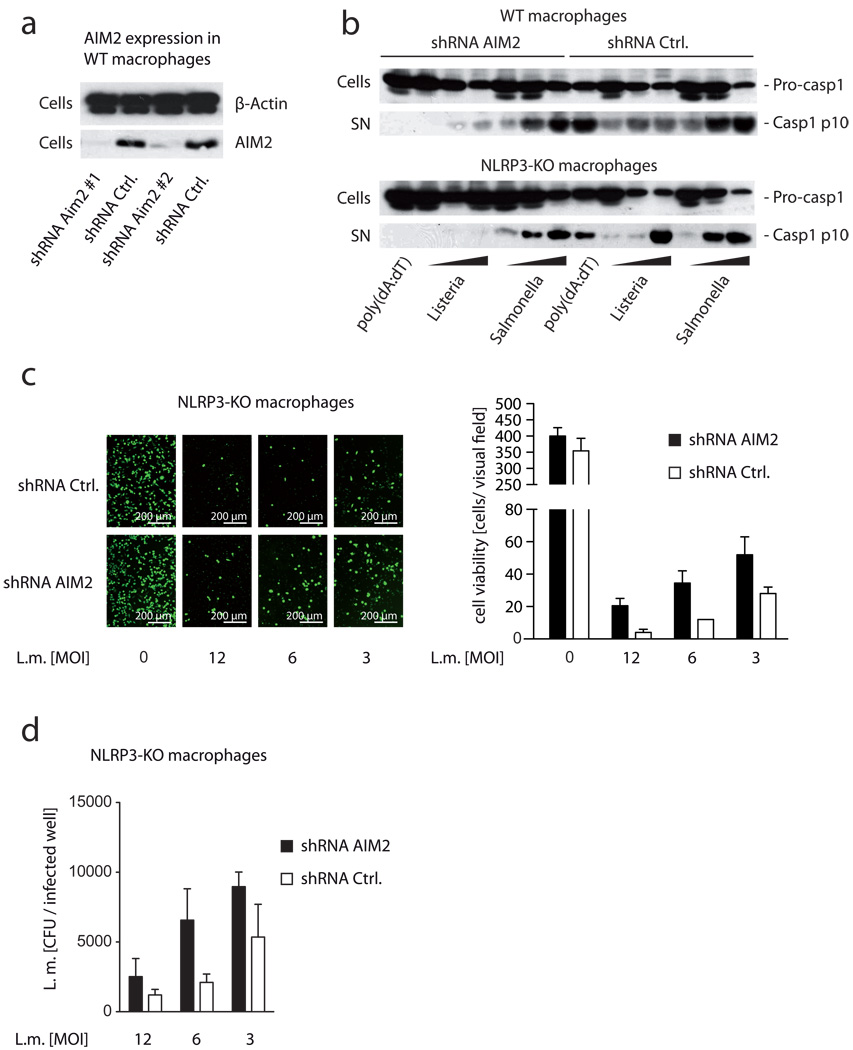
Recognition of Listeria by intracellular PRR has been ascribed to the detection of DNA by a so far unknown DNA sensing machinery [32]. However, up to now no direct data exist proving the involvement of pathogen-derived DNA in the detection of Listeria infection. Yet there is indirect evidence that Listeria release DNA into the cytosol of the host cell given the fact that Listeria DNA can serve as a template for RNA Pol II-mediated transcription by the host [33]. Thus, we hypothesized that the recently identified DNA sensor AIM2 might be the missing inflammasome PRR in the recognition of replicating Listeria. To test this hypothesis, we knocked down AIM2 expression in macrophages via shRNA. Employing this approach, we were able to strongly decrease AIM2 expression at both the mRNA (data not shown) and the protein level (Fig. 2a). Wild type macrophages silenced for AIM2 expression showed a considerable decrease in Listeria-mediated caspase-1 activation, while Salmonella-triggered inflammasome activation was unaffected (Fig. 2b). Since LLO has been shown to activate NLRP3 during Listeria infection, we next knocked down AIM2 expression in NLRP3-deficient macrophages. Indeed, when we targeted AIM2 expression in NLRP3-deficient macrophages, we observed an almost complete loss of caspase-1 activity in response to replicating Listeria (Fig. 2b). Salmonella, however, were not compromised in the absence of NLRP3 and AIM2 with regards to inflammasome activation.
Figure 2. Knockdown of AIM2 in NLRP3-deficient macrophages leads to a complete impairment of Listeria-mediated inflammasome activation.
(A) Wild type macrophages were transduced with two individual shRNA targeting AIM2 or two control shRNA. 7 days after transduction, β-Actin and AIM2 expression was analyzed by western blot. (B) Wild type macrophages or NLRP3-deficient macrophages were transduced using shRNA targeting AIM2 or a control shRNA and 7 days after selection cells were stimulated as indicated (L. monocytogenes / S. enterica MOI of 50, 10 or 1). 6 hours after stimulation caspase-1 expression and activation was assessed by western blot. (C) NLRP3-deficient macrophages transduced as in (B) were stimulated with L. monocytogenes as indicated and 24h after transfection viable cells were labeled using calcein AM and subsequently visualized using fluorescent microscopy. One representative visual field (20 × objective) for the conditions tested is depicted in the left panel. In addition, cells in two independent visual fields (20 × objective) were counted using ImageJ. Data are depicted as mean + SD. (D) Lysates of macrophages as in (C) were prepared and colony forming units of L. monocytogenes were quantified. Data from two independent replicates are depicted as mean + SD. One representative experiment out of three independent experiments is depicted (A–D).
Inflammasome activation has also been linked to a certain type of cell death known as pyroptosis. The type of cell death involved in L. monocytogenes-infected macrophages was initially described as necrosis and distinct from apoptosis [34]. However, the mechanisms of cell death in response to L. monocytogenes are not fully understood. Both caspase-1-dependent and –independent cell death has been reported after L. monocytogenes infection of macrophages [35–37]. In fact, macrophages lacking caspase-1 have been shown to survive Listeria infection for a prolonged time frame therefore giving rise to higher bacteria replication. Indeed mice lacking caspase-1 show higher levels of Listeria replication, which is accompanied with a dramatic increase in mortality. Assessing cell viability following Listeria-infection, we observed that knocking down AIM2 in NLRP3-deficient macrophages led to increased cell viability 24 hours after infection (Fig. 2c). This indicated that inflammasome activation was also associated with induction of cell death. Yet, while Listeria-mediated cell death was decreased in the absence of NLRP3 and AIM2, only a partial rescue in cell viability was achieved in the absence of these two receptors. Similar results were obtained when ASC-deficient macrophages were challenged with Listeria. These data are in line with previous findings that Listeria-mediated cell death is for the most part independent of caspase-1 [36]. Moreover, the enhanced survival in the absence of AIM2 and NLRP3 was mirrored by higher intracellular Listeria burden 24 hours after infection. Assessing bacterial numbers 24 hours after Listeria challenge showed that the absence of both NLRP3 and AIM2 enhanced Listeria replication by two to three-fold at the MOI tested (Fig. 2d). Altogether these results indicated that activation of the AIM2 and NLRP3 inflammasome pathway plays an essential role in the activation of caspase-1 in response to Listeria and also an important role in the containment of Listeria replication.
Concluding Remarks
Our studies clearly argue for a critical role of AIM2 in the recognition of replicating L. monocytogenes. Given the fact that AIM2 is a DNA sensing inflammasome receptor, these data are the first mechanistic indication that L. monocytogenes is indeed sensed in the cytosol by its DNA. While previous studies have shown that Listeria-derived DNA can trigger innate immune responses, a direct link to a DNA receptor has been missing so far. As many sources of DNA can trigger innate immune responses, these reports were only able to allude to a possible role of PRR-dependent DNA recognition. A contribution of NLRP3 to Listeria-mediated inflammasome activation, most likely due to LLO-triggered lysosomal disruption, might to some extent explain the conflicting reports in the literature and at the same time provide evidence for functional redundancy of inflammasome activation by bacteria. Presumably a major role for NLRP3-dependent sensing of L. monocytogenes can be observed under conditions with predominant lysosomal damage, yet limited cytosolic replication. On the other hand it is conceivable that AIM2 recognition predominates under condition with high cytosolic replication. Altogether, based on our data we propose a two-step model for Listeria-mediated inflammasome activation. Listeria that are taken up by phagocytic cells can escape the phagolysosome by LLO. Disruption of the phagolysosome leads to release of lysosomal content into the cytosol and translocation of Listeria into the cytoplasm. NLRP3 senses the disruption of the lysosomal membrane whereas Listeria-derived DNA is sensed by AIM2. The net effect of these events is activation of caspase-1, which is critically required to regulate the proteolytic activation of pro-IL-1β and pro-IL-18 and also involved the induction of pyroptotic cell death.
Materials and Methods
Cells and cell culture
NLRP3-, ASC- and IPAF-deficient mice were kindly provided by Millennium Pharmaceuticals. C57BL/6 mice were from Jackson Laboratories. Macrophages from these mice were transformed as previously described [11]. All experiments were performed with transformed macrophages at a density of 1×106 cells per ml in DMEM medium supplemented with L-glutamine, sodium pyruvate and 10% (v/v) FBS (Hyclone). All experiments for immunoblot analysis were performed in serum free medium. C57BL/6 wild type macrophages overexpressing ASC-CFP were generated as previously described [11]. Transduction with lentivirus-encoded shRNA was performed as previously described [38]. ShRNA targeting AIM2 were either shRNA TRCN0000096105 (#1) or TRCN0000096108 (#2). The control shRNA were either TRCN0000103646 (#1) or TRCN0000103648 (#2) (directed against murine IFIH1) and were confirmed not to have any effect on NLRP3 or AIM2 expression.
Reagents
Poly(dA:dT), nigericin and cycloheximide (CHX) were from Sigma-Aldrich. Ultra pure LPS from E.coli were from Invivogen. Poly(dA:dT) was transfected using Lipofectamine 2000 (Invitrogen). If indicated 5 mM nigericin or 5 mM ATP was added 1 h before supernatants were collected.
Bacterial infections
Listeria monocytogenes strains (EGD wt and EGD Δhly = LLO deficient strain) and Salmonella enterica were grown overnight at 37°C in brain heart infusion (BHI) broth. One volume of the overnight culture was added to 20 volumes of fresh BHI medium and bacteria were grown to the logarithmic growth phase (OD600 = 0.6 – 0.8). Bacteria were subsequently washed in PBS and diluted in DMEM medium for infection. Macrophages at a density of 1×106/ml were infected with bacteria at the indicated multiplicities of infection (MOI). 1 h post-infection 20 µg/ml gentamicin was added to remove extracellular bacteria. Macrophages were further cultured at 37°C in humidified 10% CO2. MOI was controlled by plating serial dilutions on BHI agar plates and counting colonies after growth at 37°C for 24 h.
For intracellular replication experiments, 5×104 macrophages were seeded per well in 96-well plates and infected at the indicated MOI. 24 h after infection macrophages were lysed in water for 10 min. Serial dilutions of cellular lysate were plated on BHI agar to enumerate CFU after growth at 37°C for 24 h.
Immunoblot analysis
Caspase-1 cleavage was detected by immunoblot in supernatants (SN) that were prepared as previously described [12], whereas whole cell lysates (cells) were analyzed for pro-caspase-1. Blots were incubated with rabbit polyclonal antibody to mouse caspase-1 subunit p10 (sc-514 Santa Cruz Biotechnology). β-Actin was detected using sc-47778 (Santa Cruz Biotechnology) and AIM2 was detected using a rabbit polyclonal antibody raised against full length murine AIM2.
Microscopy
Confocal microscopy was performed on a Leica SP2 AOBS confocal laser-scanning microscope. Cell membranes were stained with choleratoxin subunit B. Hoechst dye was used for nuclear stains. ASC speckles per visual field were calculated with ImageJ software.
Acknowledgements
This work was supported by grants to V.H. from the German Research Foundation (SFB704) and the European Research Council (ERC-2009-StG 243046) and by grants by the NIH (1R01HL093262 to E.L. and 1R01AI083713-01 to E.L. and K.A.F).
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 3.Wilkins C, Gale M., Jr. Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 5.Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 7.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 9.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 14.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 15.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 21.O'Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc Natl Acad Sci U S A. 2002;99:13861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Opitz B, Puschel A, Beermann W, Hocke AC, Forster S, Schmeck B, van Laak V, Chakraborty T, Suttorp N, Hippenstiel S. Listeria monocytogenes activated p38 MAPK and induced IL-8 secretion in a nucleotide-binding oligomerization domain 1-dependent manner in endothelial cells. J Immunol. 2006;176:484–490. doi: 10.4049/jimmunol.176.1.484. [DOI] [PubMed] [Google Scholar]
- 23.Park JH, Kim YG, Shaw M, Kanneganti TD, Fujimoto Y, Fukase K, Inohara N, Nunez G. Nod1/RICK and TLR signaling regulate chemokine and antimicrobial innate immune responses in mesothelial cells. J Immunol. 2007;179:514–521. doi: 10.4049/jimmunol.179.1.514. [DOI] [PubMed] [Google Scholar]
- 24.Stockinger S, Reutterer B, Schaljo B, Schellack C, Brunner S, Materna T, Yamamoto M, Akira S, Taniguchi T, Murray PJ, Muller M, Decker T. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J Immunol. 2004;173:7416–7425. doi: 10.4049/jimmunol.173.12.7416. [DOI] [PubMed] [Google Scholar]
- 25.Torres D, Barrier M, Bihl F, Quesniaux VJ, Maillet I, Akira S, Ryffel B, Erard F. Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect Immun. 2004;72:2131–2139. doi: 10.1128/IAI.72.4.2131-2139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008;180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuji NM, Tsutsui H, Seki E, Kuida K, Okamura H, Nakanishi K, Flavell RA. Roles of caspase-1 in Listeria infection in mice. Int Immunol. 2004;16:335–343. doi: 10.1093/intimm/dxh041. [DOI] [PubMed] [Google Scholar]
- 28.Meixenberger K, Pache F, Eitel J, Schmeck B, Hippenstiel S, Slevogt H, N'Guessan P, Witzenrath M, Netea MG, Chakraborty T, Suttorp N, Opitz B. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1beta, depending on listeriolysin O and NLRP3. J Immunol. 184:922–930. doi: 10.4049/jimmunol.0901346. [DOI] [PubMed] [Google Scholar]
- 29.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 30.Way SS, Thompson LJ, Lopes JE, Hajjar AM, Kollmann TR, Freitag NE, Wilson CB. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell Microbiol. 2004;6:235–242. doi: 10.1046/j.1462-5822.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 31.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 32.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Grillot-Courvalin C, Goussard S, Courvalin P. Wild-type intracellular bacteria deliver DNA into mammalian cells. Cell Microbiol. 2002;4:177–186. doi: 10.1046/j.1462-5822.2002.00184.x. [DOI] [PubMed] [Google Scholar]
- 34.Barsig J, Kaufmann SH. The mechanism of cell death in Listeria monocytogenes-infected murine macrophages is distinct from apoptosis. Infect Immun. 1997;65:4075–4081. doi: 10.1128/iai.65.10.4075-4081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cervantes J, Nagata T, Uchijima M, Shibata K, Koide Y. Intracytosolic Listeria monocytogenes induces cell death through caspase-1 activation in murine macrophages. Cell Microbiol. 2008;10:41–52. doi: 10.1111/j.1462-5822.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 36.Zwaferink H, Stockinger S, Hazemi P. Lemmens-Gruber, R. and Decker, T., IFN-beta increases listeriolysin O-induced membrane permeabilization and death of macrophages. J Immunol. 2008;180:4116–4123. doi: 10.4049/jimmunol.180.6.4116. [DOI] [PubMed] [Google Scholar]
- 37.Zwaferink H, Stockinger S, Reipert S, Decker T. Stimulation of inducible nitric oxide synthase expression by beta interferon increases necrotic death of macrophages upon Listeria monocytogenes infection. Infect Immun. 2008;76:1649–1656. doi: 10.1128/IAI.01251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]