Summary
The molecular mechanism behind alum adjuvanticity is probably the oldest secret of immunology. In this issue of Immunity, Kuroda et al. (Kuroda, 2011) and Kool et al. (Kool, 2011) identify NLRP3 protein-independent signaling to be crucial for the Th2 cell response induced by aluminum salts.
Edward Jenner’s vaccination against smallpox in 1789 is the first and still most dramatic record of a successful manipulation of the immune system. Early trials of vaccine development revealed that the efficiency of vaccines depends on the presence of so-called adjuvants (latin adiuvare: to help) in conjunction with the antigen. However, it took 200 years of research until Charles Janeway proposed the immunologic function of these little helpers - to stimulate the innate immune system. He suggested that adaptive immunity was not raised until the innate immune system provided clear evidence for the presence of pathogens - or for danger, as later extended by Polly Matzinger. The concept of PAMPs (pathogen-associated molecular patterns) and DAMPs (danger-associated molecular patterns) triggering innate PRRs (pattern recognition receptors) was born. The signals triggered by PAMPs and DAMPs strongly determines the type of adaptive immunity, ensuring an effective clearance of infection or appropriate inflammatory responses to sterile tissue damage. The most commonly used adjuvant in humans is alum. It induces so-called type 2 immune responses characterized by eosinophilia and production of IL-4, IgE, and IgG1. Although the discovery of alum’s adjuvanticity dates back to 1926, the underlying molecular mechanism is still a matter of debate.
The discovery that the NLRP3 inflammasome senses particulates including monosodium urate (MSU) crystals (Martinon et al., 2006), silica, asbestos as well as alum (Dostert et al., 2008; Hornung et al., 2008) suggested a plausible mechanism for alum’s effect as an adjuvant. Cellular uptake of particulates leads to reactive oxygen production and can inflict lysosomal damage. Both these effects were suggested to act upstream in the activation of NLRP3 (Dostert et al., 2008; Hornung et al., 2008). Aluminum salt mediated cytotoxicity can further induce cell death with subsequent release of uric acid (UA), which can also indirectly activate the NLRP3 inflammasome (Kool et al., 2008). Activated NLRP3 recruits the adapter molecule ASC, which binds to and activates procaspase-1. Active caspase-1 catalyzes the cleavage of proforms of the IL-1β cytokine family into biologically active cytokines (Martinon et al., 2002). The crucial role of the NLRP3 inflammasome for the secretion of IL-1β cytokines is undoubted. However, results are controversial when it comes to the question of whether NLRP3 plays a role in alum induced adjuvanticity and induction of type 2 immunity. Whereas some studies found NLRP3, ASC, and caspase-1 to be required for alum induced adjuvanticity (Eisenbarth et al., 2008), others failed to observe a role of NLRP3 (Franchi and Nunez, 2008).
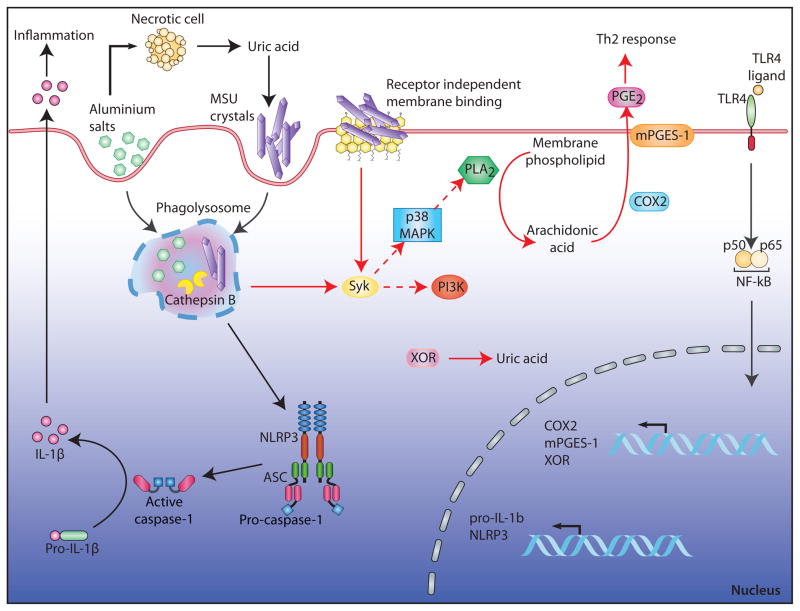
The studies from Kuroda et al. (2011) and Kool et al. (2011) aimed to clarify the molecular mechanisms by which immunogenic particulates initiate type 2 immune responses. Both studies argue for NLRP3 independent mechanisms. Kuroda et al. suggest a crucial involvement of prostaglandin PGE2. Kool et al. identify UA as an essential initiator and amplifier of alum and house dust mite allergen (HDM) induced type 2 immunity (Figure 1). Kuroda et al. report the initiation of two separate pathways upon stimulation with particulates. As shown in previous studies, silica and alum induced the secretion of IL-1β cytokines in an NLRP3 dependent manner. In addition, the particulates induced the production of the pro-inflammatory arachidonic acid metabolite prostaglandin PGE2. This second pathway, however, was independent of NLRP3, ASC, and caspase-1, but depended on cyclooxygenase-2 (COX2) and membrane associated PGE synthase-1 (mPGES-1), whereas neither COX2 nor mPGES-1 proved to be essential for IL-1β production. Furthermore, antigen-specific IgE amounts were reduced in mPGES-1-deficient mice after immunization using alum as an adjuvant. Conversely, immunization with NiO, which causes the secretion of PGE2 but not of IL-1β, substantially enhanced IgE amounts. These studies support a role of PGE2 rather than IL-1β in IgE production. Intriguingly, the mechanisms that lead to PGE2 production are very similar to those that operate upstream of NLRP3 although the former process is NLRP3 independent. A priming step by a proinflammatory stimulus is required (to induce the expression of COX2 and mPGES-1) and the induction of lysosomal damage by the particulate acts as a necessary second activation step. Based on studies with inhibitors, the authors further demonstrated that lysosomal damage leads to cPLA2 (cytosolic phospholipase A2) activation, probably via Syk and p38 MAP kinase, finally resulting in the release of arachidonic acid from membrane lipids and in the production of PGE2.
Figure 1. Mechanisms of particulate induced immunity - an updated model.
Upon phagocytosis, particulates like aluminium salts or monosodium urate crystals (MSU) cause lysosomal damage. This initiates at least two separate signaling pathways: First, the activation of the NLRP3 inflammasome due to the release of enzymes like cathepsin B into the cytoplasm. Autocatalytically activated caspase-1 mediates the proteolytic cleavage of pro-IL-1β in biologically active IL-1β, accounting for the inflammatory response to particulates. Second, the activation of Syk, which in turn activates cytosolic phospholipase A2 (cPLA2), probably via p38 MAP kinase resulting in the release of arachidonic acid from membrane lipids. Cyclooxygenase-2 (COX2) and membrane associated PGE synthase-1 (mPGES-1) convert arachidonic acid to prostaglandin E2 (PGE2).
The immunostimulatory effects of particulates do not only enable their use as adjuvants, but they also account for the role of particulates in inflammatory diseases. In allergic asthma for example particulates like HDM trigger a Th2 cell response characterized by eosinophilic airway inflammation, mucus hypersecretion, and airway obstruction. In gout, MSU crystals activate NLRP3 leading to inflammation (Martinon et al., 2006). Here, Kool et al. report a major role for MSU in allergic asthma. Sensitization of mice with OVA-alum or HDM induced an allergic Th2 cell response upon antigen challenge. This response was characterized by eosinophilia, increased Th2 cell cytokines and antibody concentrations, as well as migration and activation of inflammatory monocytes and dendritic cells (DCs). Notably, uricase treatment of mice just before sensitization reduced Th2 cell immunity suggesting that MSU played a critical role in alum and HDM induced asthma. Indeed, UA amounts increased upon treatment of mice with alum-OVA or house dust mite allergen. Consistently, asthmatic patients showed elevated UA concentrations after allergen challenge. Sensitization of mice with MSU-OVA revealed that MSU was not only necessary, but also sufficient for the induction of an allergic Th2 cell response. Most notably, and in contrast to the well-known inflammatory pathway initiated by MSU in gout (Martinon et al., 2006), the induction of Th2 cell immunity by MSU did not require NLRP3, ASC, or IL-1R.
As previously reported, elevated UA amounts can reflect alum induced cell damage leading to the release of endogenous DAMPs (Kool et al., 2008). In the case of HDM, Kool et al. propose yet another mechanism, which is the enhanced production of UA due to upregulation of xanthine oxidoreductase (XOR). The XOR gene promoter region contains an NF-kB control element. Strikingly, in contrast to UA production upon alum treatment, UA production upon HDM treatment was TLR4 dependent suggesting that XOR upregulation might represent another important TLR priming effect.
Based on the finding that uricase treatment lost its profound effects if mice were sensitized by intratracheal instillation of OVA pulsed DCs, the authors concluded that MSU had to act upstream of DC activation and recruitment. The molecular pathway for activation involved Syk and PI3K∂. Notably, previous studies proposed that MSU was able to directly engage cholesterol rich cellular membranes of DCs in a receptor independent manner and thereby triggered the activation of Syk (Ng et al., 2008).
Together, the work of Kuroda et al. and Kool et al. provide another jigsaw piece in the understanding of particulate induced immunity. According to these authors, particulates activate not only the NLRP3 inflammasome to induce the secretion of IL-1β but also stimulate innate immunity in an NLRP3 independent manner. This latter pathway appears to be crucial for the initiation of Th2 cell immunity and could account for the controversy whether NLRP3 is required for alum’s adjuvant effect. Although representing two separate pathways, both pathways might go back to one common event induced by particulates, namely lysosomal damage.
While the herein discussed studies represent a substantial step forward towards solving the mystery of alum’s adjuvanticity, we are still struggling with depicting an exact molecular mechanism. It is likely, that multiple pathways are involved and triggering multiple pathways may actually be part of alum’s secret of success. The following mechanisms have been proposed so far to explain the utility of alum as an adjuvant. First, the so called “depot effect”, which refers to a slow release of antigen leading to prolonged stimulation of the immune system. Second, by converting soluble antigen into a particulate form its uptake could be enhanced. Third, NLRP3 stimulation induces inflammation and finally, the induction of type 2 immunity by NLRP3-independent signaling (Kool, 2011; Kuroda, 2011).
Our efforts to solve the oldest secret in immunology have taught us a great deal about our immune system and about what it takes to be a good adjuvant. These lessons have major implications for rational vaccine design. A good part of modern vaccines consist of purified antigens in combination with exogenous adjuvants. In order to achieve good efficiency, the formulation has to ensure efficient uptake of antigen in close conjunction with the adjuvant and the choice of the adjuvant will strongly influence the type of the adaptive immune response. Finally, we are starting to recognize the great potential of effectively targeting the innate immune system by combining multiple stimuli. A current approach to dually target TLR4 and TLR7 with a nanoparticle-based vaccine provides an excellent example how to gain effectiveness by making the secret a bit dirtier (Rhee et al., 2011).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011 doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda E. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity. 2011 doi: 10.1016/j.immuni.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, Li T, Lowell CA, Ling CC, Amrein MW, Shi Y. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee EG, Blattman JN, Kasturi SP, Kelley RP, Kaufman DR, Lynch DM, La Porte A, Simmons NL, Clark SL, Pulendran B, et al. Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J Virol. 2011;85:315–323. doi: 10.1128/JVI.01597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]