Abstract
Single-molecule techniques have been used for only a subset of biological problems because of difficulties in studying proteins that require cofactors or post-translational modifications. Here, we present a new method integrating single-molecule fluorescence microscopy and immunopurification to study protein complexes. We used this method to investigate Lin28-mediated microRNA uridylation by TUT4 (terminal uridylyl transferase 4, polyU polymerase), which regulates let-7 microRNA biogenesis. Our real-time analysis of the uridylation by the TUT4 immunoprecipitates suggests that Lin28 functions as a processivity factor of TUT4. Our new technique, SIMPlex (single-molecule approach to immunoprecipitated protein complexes), provides a universal tool to analyse complex proteins at the single-molecule level.
Keywords: single-molecule fluorescence; single-molecule immunoprecipitation; TUT4 (ZCCHC11); Lin28 (Lin28a, Lin28b, Lin28); real-time hybridization
Introduction
Single-molecule techniques enable scientists to observe individual biological molecules with real-time resolution and nanometre accuracy (Walter et al, 2008), and their unprecedented power has helped to solve long-standing puzzles in molecular biology. However, in order for a technique to become a general tool for molecular biology, several technical challenges need to be overcome. Although single-molecule methods are readily applicable to purified recombinant protein systems, the majority of proteins, particularly those from higher eukaryotes, function in complex with other proteins that cannot be supplemented in bacterial or other expression systems. In addition, they often require post-translational modifications for functionality, which makes in vitro reconstitution of functional proteins even more challenging.
Single-molecule assays with cell extracts would therefore have advantages. These have been exploited for studies of the spliceosome, which is active only when the cell extracts are supplemented (Crawford et al, 2008; Abelson et al, 2010; Hoskins et al, 2011). In addition, a eukaryotic DNA replication system has been recently reconstituted at the single-molecule level with cell extracts from Xenopus eggs (Yardimci et al, 2011). However, direct investigation of proteins in cell extracts remains challenging. Proteins of interest are rare; therefore, the single-molecule events recorded might be dominated by nonspecific interactions and might also produce a low yield of reactions with low statistical confidence.
TUT4 (TUTase4, terminal uridylyl transferase 4 or ZCCHC11) was recently shown to be involved in the regulation of microRNA (miRNA; Norbury, 2010; Siomi & Siomi, 2010). MiRNA biogenesis is initiated when Drosha cleaves a primary miRNA transcript to release a hairpin RNA (pre-miRNA; Kim et al, 2009). The pre-miRNA is processed into a mature miRNA by Dicer. This maturation pathway of miRNA is suppressed when TUT4 attaches several uridines at the 3′ end of a particular set of pre-miRNAs, including pre-let-7 (Heo et al, 2008, 2009; Hagan et al, 2009). Our previous study showed that the Lin28 protein specifically recognizes the pre-miRNAs before TUT4 action (Heo et al, 2009). However, the way in which Lin28 coordinates the ternary interaction for the uridylation reaction remains unknown. Single-molecule observation will allow real-time detection of the interaction between TUT4, Lin28 and pre-miRNA, and thus might show the mechanism of TUT4 action. However, human TUT4 proteins (approximately 200 kDa) cannot be obtained from bacterial cells because of their poor expression (unpublished data), which prohibits analysis at the molecular level.
Here, we report an immunoprecipitation-based single-molecule assay that expands the scope of single-molecule approaches to new and complex protein systems. We applied this new technique to reveal the kinetic process of pre-let-7 uridylation by TUT4 complex and the role of its mediator, Lin28. We found that the Lin28 protein makes TUT4 action processive by strengthening the interaction between TUT4 and pre-let-7 by more than 200-fold.
Results and Discussion
Single-molecule immunoprecipitation
We sought to use the immunoprecipitation method in single-molecule microscopy using a surface-tethering scheme (Fig 1). A quartz surface was prepared with polymer coating to avoid nonspecific protein adsorption (Selvin & Ha, 2007). Biotinylated antibody was immobilized on the surface by biotin–streptavidin conjugation. TUT4 proteins, ectopically expressed in human embryonic kidney cells (HEK293T), would be captured by the immobilized antibody when injected into the microfluidic chamber (supplementary Fig S1 online). TUT4 was tagged in tandem with Flag epitope and mCherry protein for immunoprecipitation and/or fluorescence imaging (supplementary Fig S2A-C online). The two tags did not interfere with pre-let-7 elongation. Out of four different TUT4 constructs, TUT4–Flag–mCherry was chosen and used throughout this work.
Figure 1.
SIMPlex workflow. (Top) TUT4–Flag–mCherry is immunopurified with Flag antibody beads and is eluted out with Flag peptide. (Middle) The eluate is tethered to RFP antibody on a single-molecule surface. (Bottom) Interaction of Lin28b-bound pre-let-7a-1 with the immobilized TUT4–Flag–mCherry is observed through single-molecule fluorescence total-internal-reflection microscopy. The evanescent wave is shown in green. Schematics are not to scale. RFP, red fluorescent protein; SIMPlex, single-molecule approach to immunoprecipitated protein complexes; TUT4, terminal uridylyl transferase 4.
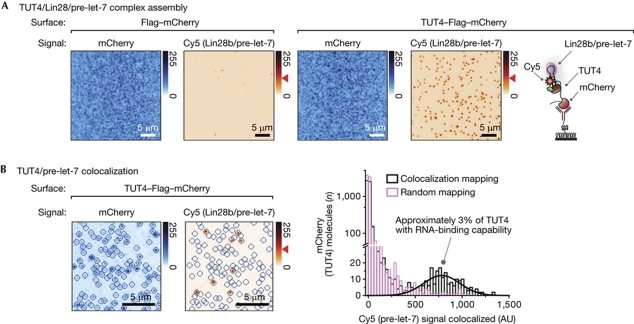
To immobilize TUT4 proteins that were directly extracted from human cells, yet had high purity, we used a tandem purification method (Rigaut et al, 1999; Gingras et al, 2007; Fig 1). We first enriched TUT4–Flag–mCherry proteins from crude cell extracts using Flag antibody beads. We then eluted the immunoprecipitates with a high concentration of free Flag peptide. The eluate was introduced onto a single-molecule surface coated with RFP (red fluorescent protein) antibody. The RFP antibody recognizes mCherry, an RFP family member. With this tandem purification, the surface showed a high amount of mCherry fluorescence signals (Fig 2A, left), and we observed only a few nonspecific RNA interactions on a control surface (supplementary Fig S3A, B online). These results indicated that the immunoprecipitates on the surface were pure enough to allow specific single-molecule observation.
Figure 2.
Specific interaction of single-molecule TUT4 immunoprecipitates with microRNA substrates. (A) The assembly of TUT4 and Lin28b-bound pre-let-7 complexes is shown in single-molecule images. mCherry signals are from Flag–mCherry (left) and TUT4–Flag–mCherry (right). Cy5 signals are from Cy5-labelled pre-let-7. (B) Colocalization of TUT4 and Lin28b-bound pre-let-7 (left). The concentration of TUT4 is 100 × lower than that in A. The blue circles indicate where mCherry signals are detected. The histogram (right) shows Cy5 intensity on the basis of the location of blue circles. Approximately 3% of mCherry spots are colocalized with Cy5 locations mapped (black bars; n=2). Random mapping leads to a negligible population of colocalized mCherry (pink bars). Blue (red) gradient bar: signal intensity upon 532 nm (640 nm) excitation. Red arrowhead: intensity of single Cy5. Scale bars, 5 μm. The intensity of mCherry varies between spots due to photobleaching during data acquisition. AU, arbitrary units; TUT4, terminal uridylyl transferase 4.
TUT4 immunoprecipitates with Lin28b-bound pre-let-7
The substrates of TUT4, pre-let-7 miRNAs, were labelled with Cy5 (supplementary Fig S2D,E online). They were pre-incubated with Lin28b before introduction to the microscopic chamber. There are two isoforms of full-length Lin28 proteins in humans: Lin28a and Lin28b. Throughout this study, we used one of the isoforms, Lin28b, as the paralogues were biochemically identical (Heo et al, 2008). The Lin28b-bound pre-miRNA docked on the surface in a TUT4-specific manner (Fig 2A, right). The binding affinity of Lin28b to pre-let-7 is high (dissociation constant, KD, 1.4±0.7 nM; supplementary Fig S2G online), suggesting that pre-let-7 was always bound to Lin28b during our single-molecule observation.
It should be pointed out that many pre-let-7 (Cy5) were detected in the observation area of 50 × 50 μm2 only when the sample preparation of cell lysis and immunoprecipitation was optimized to minimize the possible denaturation of proteins (supplementary Fig S4A-D online). The ratio between Cy5 spots (pre-let-7) and the density of mCherry signals (TUT4) increased further when TUT4–Flag–mCherry was weakly expressed at a concentration comparable with that of endogenous TUT4, which would minimize the misfolding of ectopically expressed proteins as well as assuring optimal stoichiometry with its putative cofactors (supplementary Fig S4E,F online).
Single-molecule TUT4 immunoprecipitate complexes
When we directly showed the interaction between TUT4 proteins and Lin28b-bound pre-let-7 at the molecular level by immobilizing TUT4 proteins—diluted 100-fold on a surface—and colocalizing mCherry signals (TUT4; 532 nm excitation) with Cy5 signals (pre-let-7; 640 nm excitation; Fig 2B, left; supplementary Fig S5A online), we found that only approximately 3% of TUT4 molecules could accommodate pre-let-7, despite the excessive amount of RNA injected into a microfluidic chamber (Fig 2B, right). The colocalization result raises the intriguing question of why only a fraction of complexes interact with pre-miRNA substrates. We suspect that TUT4 might require cofactors, the association of which to TUT4 is sensitive to the cell lysis, immunoprecipitation and protein expression conditions, and that the cofactors are proteins with an RNA-binding motif that mediates the docking of Lin28/pre-miRNA to TUT4. Alternatively—although not mutually exclusively—TUT4 might reside in many types of complex with different compositions and functions, for example, acting on RNA substrates other than Lin28-bound pre-let-7. Despite our efforts, the experimental procedures might have induced the denaturation of TUT4 proteins. Artefacts from ectopic expression such as misfolding might not be significant, considering that a similarly low percentage was observed with a stable cell line of TUT4–Flag–mCherry in which misfolding was minimized (supplementary Fig S6 online). The low percentage did not impede our data analysis as the Cy5 signal, not the mCherry signal, was used in tracking the ternary interaction of TUT4/Lin28b/pre-let-7, and there were a statistically large number of active TUT4 molecules—several hundred on 50 × 50 μm2.
In singulo uridylation reaction
We carried out a uridylation reaction with the assembled ternary complex of TUT4/Lin28b/pre-let-7 by injecting UTP into the microfluidic chamber. Initially, we attempted to use Cy5-labelled UTP to follow the reaction in real time, but found that TUT4 was incapable of incorporating Cy5-labelled UTP (supplementary Fig S2F online). We therefore developed a detection method based on oligo-dA hybridization instead. When 10 nM Cy3-labelled dA15 was introduced to UTP-incubated TUT4/Lin28b/pre-let-7 complexes, localized Cy3 signals emerged, indicating that freely floating Cy3–dA15 were hybridized to elongated U tails (Fig 3). The signals were detected only when all the components of Lin28b, pre-let-7 and UTP were included (Fig 3A; supplementary Fig S5B online). The specific uridylation reaction was further confirmed by the colocalization of Cy3 signals (dA15; 532 nm excitation) to Cy5 signals (pre-let-7; 640 nm excitation; Fig 3B). Due to the photoinactive Cy5 population and the elongation of endogenous RNAs that were pulled down with TUT4 (Fig 3A; second, third and fourth panels), a fraction of Cy3 spots appeared where Cy5 signals were not detected (supplementary Fig S5C online).
Figure 3.
In vitro reaction of single-molecule TUT4 immunoprecipitate complexes. (A) Uridylation reaction is monitored with Cy3-labelled dA15 hybridization (left). The density of TUT4–Flag–mCherry (image not shown) was as saturated as in Fig 2A. The order of hybridization (1, 2 and 3 in the right panel) is indicated throughout in this figure. (B) Colocalization of pre-let-7 and dA15. The red circles indicate where Cy5 signals are detected. Right: histogram of Cy3 signals on the basis of the red circles. The 84±8% (n=6) of Cy5 spots are colocalized with Cy3 locations mapped. An arrow with two heads indicates single Cy3 intensity. Blue (red) gradient bar: intensity upon 532 nm (640 nm) excitation; blue (light blue; red) arrowhead: intensity of single Cy3 (mCherry; Cy5). Scale bar, 5 μm. (C) A contour plot of fluorescence time evolution, measured with 122 single-molecule TUT4 complexes (time resolution, 1 s). (D) A time trajectory of real-time dA15 hybridization (time resolution, 1 s). UTP was injected with 10 nM dA15 at t=0 (black arrow; marked by sudden increase of background signals). The elongated pre-let-7 dissociated at approximately 125 s (grey arrow). mCherry proteins were pre-photobleached before fluorescence recording. (E) Dwell time distribution of Δτ1 (left). Average dwell times of Δτ1, Δτ2 and Δτ3 (right) are from three independent experiments (778 and 697 molecules for dA10 and dA15, respectively). AU, arbitrary units; TUT4, terminal uridylyl transferase 4.
Direct observation of uridylation reaction
To observe the elongation process in real time, we added Cy3-labelled oligo-dA and UTP at t=0. The fluorescence signals from Cy3 emerged and increased in a stepwise manner (Fig 3C,D; supplementary Fig S7 online). The interval between the first and second hybridizations (Δτ1) suggests that the elongation rate is >0.22 nucleotides s−1 (Fig 3E, left; 15 nucleotides divided by 67.4 s). Similar rates were obtained with the following steps (Fig 3E, right). When a shorter oligo (dA10) was used, an equivalent kinetic rate (>0.23 nucleotides s−1; 10 nucleotides divided by 42.6 s) was obtained from a shorter interval distribution, indicating that the hybridization of the oligos occurred contiguously.
This real-time hybridization method for the step analysis might be used to investigate RNA synthesis processes by RNA polymerases (Herbert et al, 2008), telomerases (Stone et al, 2007; Alves et al, 2008; Wu et al, 2010) and non-template-dependent polymerases (Martin & Keller, 2007), which are otherwise difficult to observe in real time. DNA/RNA annealing was not a rate-limiting process in our assay, as rapid hybridization (time scale approximately 10 s) was observed when pre-let-7 was pre-elongated before the addition of Cy3–dA15 (supplementary Fig S8 online). Care should be taken, however, in using our hybridization approach. First, it could not cover the entire window of the elongation process, missing the first 20 s (Fig 3C), and the elongation rate might vary depending on the length of the U tail. A complementary bulk kinetics measurement suggests that the initial elongation over approximately 30 nucleotides might be faster than we estimated by single-molecule hybridization (supplementary Fig S9A online). Second, there was an observable artefact of oligo-dA hybridization, although it was negligible overall in measuring kinetics (supplementary Fig S9B online). Third, the hybridization of several oligos will occur contiguously only if the elongation is slower than the annealing process, as was the case with the TUT4-mediated uridylation. Finally, a photoinactive Cy3 population might lead to underestimation of the elongation rate.
Lin28 as a processivity factor of TUT4 action
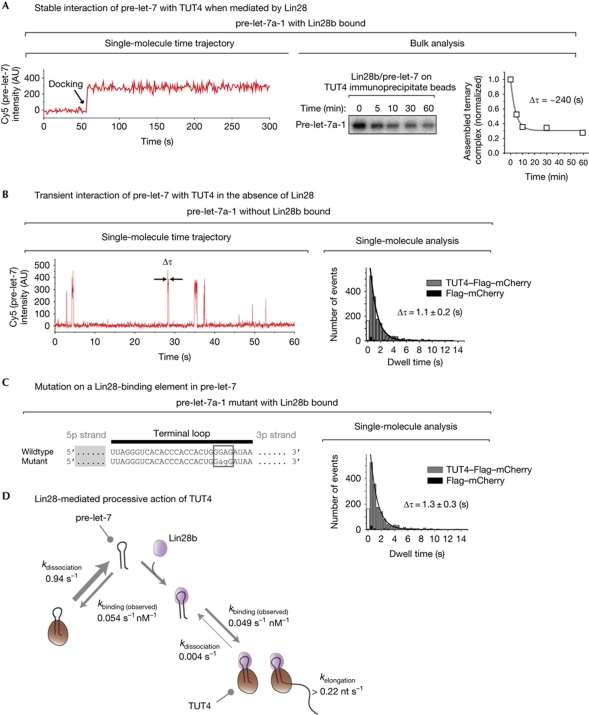
During the single-molecule measurements, we found that Lin28b/pre-let-7 often stayed bound to TUT4 for longer than our typical observation time of a few minutes (Fig 4A, left). To accurately estimate the dissociation kinetics, we measured the dissociation rate in bulk, as a single-molecule microfluidic chamber is only approximately 10 μl in volume, and thus reassociation might interfere with the dissociation kinetic measurement. Our bulk assay suggests that the ternary complex of TUT4/Lin28b/pre-let-7 is stable, with a characteristic time of approximately 240 s (Fig 4A, right). We speculate that the slow rate of TUT4 enzymatic activity (>0.2 nucleotides s−1) is compensated for by the Lin28-mediated stable interaction between TUT4 and pre-miRNA.
Figure 4.
Lin28-mediated processive action of TUT4. (A) Left: a single-molecule time trace of a Lin28b-bound pre-let-7 (labelled with Cy5) docking to TUT4–Flag–mCherry at approximately 50 s (time resolution, 5 s). Right: the dissociation kinetics of Lin28b-bound pre-let-7 from TUT4–Flag–mCherry, measured in bulk. (B) Left: single-molecule events of pre-let-7 docking to TUT4–Flag–mCherry without Lin28b (time resolution, 30 ms). Right: a dwell time distribution that indicates the dissociation timescale (Δτ) of 1.1±0.2 s (n=5). The first column of the data was not included in the analysis to avoid an artefact from the limited time resolution. (C) When a Lin28-binding motif (GGAG) was mutated into GAGG, only brief docking events (Δτ, 1.3±0.3 s; n=5) were observed, even with Lin28 present. (D) A model of Lin28 as a processivity factor of TUT4. Whereas pre-miRNA dissociates rapidly from TUT4 when Lin28b is not bound (k=1/1.1 s=0.91 s−1), its dissociation rate decreases by more than 200-fold when Lin28b is bound (k=1/240 s=0.004 s−1). The binding rate with Lin28b (0.049 s−1 nM−1) was estimated from k(observed)=1/34.3 s/0.6 nM; the binding rate without Lin28b (0.054 s−1 nM−1) from k(observed)=1/30.8 s/0.6 nM. The binding kinetics were measured with 0.6 nM pre-let-7 (supplementary Fig S10B online). miRNA, microRNA; TUT4, terminal uridylyl transferase 4.
To investigate the role of Lin28 in the oligo-uridylation process, we carried out a single-molecule measurement without Lin28b as a control and observed that pre-let-7 frequently, but only briefly, docked onto TUT4 proteins (Fig 4B, Δτ=1.1±0.2 s). Only a negligible number of such events were observed with the negative control vector (Flag–mCherry; black, Fig 4B,C), indicating that the observed events were TUT4 specific. To further investigate the role of Lin28, we introduced a mutation on a Lin28-binding element in pre-let-7 (‘GGAG’ tetranucleotide in the RNA terminal loop mutated into ‘GAGG’). This RNA mutant, which can bind to Lin28b but fails to be oligo-uridylated (Heo et al, 2009), showed the same brief interactions, even with Lin28b present (Fig 4C, Δτ=1.3±0.3 s). This effect of the mutation suggests that stable TUT4 interaction requires well-coordinated organization of the Lin28/pre-miRNA binary complex. It will be interesting to obtain the high-resolution structural information of Lin28/pre-miRNA from crystallography or nuclear magnetic resonance analyses to learn how such coordination is achieved.
Our previous study indicated that TUT4 did not directly interact with either Lin28 or pre-miRNA when the other partner was missing; TUT4 interacted exclusively with the binary complex of Lin28/pre-miRNA (Heo et al, 2009). Our real-time observation with 30-ms time resolution (Fig 4B) suggests that TUT4 manages to make contact with pre-miRNA even without Lin28. The quantitative analysis suggests that Lin28 increases the stability of the interaction between pre-let-7 and TUT4 by two orders of magnitude, which must lead to effective oligo-uridylation (Fig 4D). We therefore propose that Lin28 is a processivity factor of the uridylation reaction of TUT4. To determine whether Lin28 might also enhance the association of pre-let-7 with TUT4, we compared the association kinetics with and without Lin28b (supplementary Fig S10 online). We found no difference between the two kinetic measurements, and therefore ruled out the possibility that Lin28 might increase the association rate (Fig 4D).
SIMPlex
We have developed a single-molecule assay that uses a classic pull-down method. Proteins are pulled down from their own cells with their natural cofactors bound, which is ideal for studying their endogenous function. In the light of this advantage, we named our technique SIMPlex (Single-molecule approach to IMmunoprecipitated Protein complexes). With SIMPlex we will be able to understand the multifunctional activity of protein complexes, as it allows the inspection of individual protein complexes. We hope that SIMPlex will be an innovative platform for identifying putative cofactors. SIMPlex also eliminates the need to use recombinant proteins for single-molecule observation and therefore permits the immediate investigation of large proteins that cannot be readily obtained from bacterial or insect expression systems, or proteins that require post-translational modification.
We have shown that tandem purification is sufficient for immunoprecipitation-based single-molecule observation. When we instead carried out immunoprecipitation with crude cell extracts directly on a single-molecule surface, the purity of immobilized immunoprecipitates was too low to make specific observations with RNA as substrates. When we first introduced HEK293T crude cell extracts that had Flag–mCherry proteins ectopically expressed onto a Flag antibody surface, only a few mCherry signals were recorded (supplementary Fig S3A online). This suggested that, of the antibody molecules, only a few captured mCherry molecules, whereas the majority captured other unwanted proteins. The low specificity of the immobilized immunoprecipitates was further confirmed when dye-labelled RNA was introduced onto the Flag–mCherry-treated surface; although Flag–mCherry did not contain an RNA-binding motif, there were nonspecific interactions between RNA molecules and the surface (supplementary Fig S3B online). We had to find an antibody with a higher specificity and/or increase the purity of the immunoprecipitates. When we used RFP antibody instead of Flag antibody, we observed a high amount of mCherry signals on the surface; however, nonspecific RNA interactions indicated that the immunoprecipitates were not pure enough (supplementary Fig S3A,B online). Only when the tandem purification was used with a combination of Flag and RFP antibodies did it lead to high coverage of mCherry signals and to a small number of nonspecific RNA interaction events.
The strategy of a two-tag system might consist of various combinations of other tags, such as HA, V5, MYC and fluorescent proteins of green fluorescent protein and other RFP families. For endogenous proteins from cell lines or tissues, two orthogonal antibodies that recognize different epitopes of a protein might be used. In addition, tandem purification might be executed with a combination of different approaches, such as size-exclusion purification (Post et al, 1998) and streptavidin conjugation with biotinylated proteins (Howarth & Ting, 2008). Our approach can also be applied to single-molecule studies with proteins translated in vitro in rabbit reticulocyte lysate (Honda et al, 2009; Wu et al, 2010). With a diverse number of variants, SIMPlex will be generally applicable to biological studies of protein–protein, protein–DNA and protein–RNA interactions.
Methods
HEK293T cells, grown for 1 day in a 10 cm dish, were transfected with 1 μg plasmids with the CaPO4 method and were grown for a further 1.5 days. Collected cells were suspended with buffer D (20 mM Tris (pH 8.0), 200 mM KCl and 0.2 mM EDTA), and lysis was carried out by passing the cells through a 30-gauge needle. For immunoprecipitation, the supernatant was incubated with Flag antibody-conjugated agarose beads. Immunoprecipitates were eluted with 100 μM 3 × Flag peptide. In vitro uridylation in bulk was performed with 3.2 mM MgCl2, 0.5 mM DTT, 100 μM UTP, 10 nM recombinant Lin28b and 0.1 nM pre-let-7 (γ-32P 5′-end labelled) in 0.5 × buffer D. Single-molecule fluorescence data were acquired with total-internal-reflection microscopy by exciting mCherry and Cy3 molecules with a 532 nm laser, and Cy5 with a 640 nm laser. Fluorescence signals, collected through a × 60 water immersion objective, were detected with an electron-multiplying charge-coupled device with 30–5,000 ms time resolution. On a single-molecule surface, which was coated with polyethyleneglycol, biotinylated RFP antibody was layered by conjugation with surface-immobilized streptavidin. Thereafter, immunoprecipitates were immobilized by antibody-epitope recognition. Dye-labelled pre-let-7 was injected with recombinant 2 nM Lin28b in 0.5 × buffer D and 3.2 mM MgCl2. Finally, uridylation reaction solution, composed of 100 μM UTP and 3.2 mM MgCl2 in 0.5 × buffer D, was injected with imaging buffer. The imaging buffer consists of 0.8% dextrose, 0.1 mg ml−1 glucose oxidase, 4 μg ml−1 Catalase and 1 mM Trolox. Cy3-labelled 10 nM oligo-dA was added together or afterwards to visualize the uridylation by hybridization.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We are grateful to J. Han, H. Son and A. Cho and all the members of N. Kim's and S. Hohng's laboratories. We thank N. Dekker for critical reading of the manuscript and B.-K. Kaang for providing materials. This work was supported by the Creative Research Initiatives Program (20090063603 and 20090081562) through the National Research Foundation of Korea and the BK21 Research Fellowships (K.H.Y., I.H., J.L. and C.J.) from the Ministry of Education, Science and Technology of Korea.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abelson J et al. (2010) Conformational dynamics of single pre-mRNA molecules during in vitro splicing. Nat Struct Mol Biol 17: 504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves D, Li H, Codrington R, Orte A, Ren X, Klenerman D, Balasubramanian S (2008) Single-molecule analysis of human telomerase monomer. Nat Chem Biol 4: 287–289 [DOI] [PubMed] [Google Scholar]
- Crawford DJ, Hoskins AA, Friedman LJ, Gelles J, Moore MJ (2008) Visualizing the splicing of single pre-mRNA molecules in whole cell extract. RNA 14: 170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Gstaiger M, Raught B, Aebersold R (2007) Analysis of protein complexes using mass spectrometry. Nat Rev Mol Cell Biol 8: 645–654 [DOI] [PubMed] [Google Scholar]
- Hagan JP, Piskounova E, Gregory RI (2009) Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol 16: 1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo I, Joo C, Cho J, Ha M, Han J, Kim VN (2008) Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell 32: 276–284 [DOI] [PubMed] [Google Scholar]
- Heo I, Joo C, Kim YK, Ha M, Yoon MJ, Cho J, Yeom KH, Han J, Kim VN (2009) TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138: 696–708 [DOI] [PubMed] [Google Scholar]
- Herbert KM, Greenleaf WJ, Block SM (2008) Single-molecule studies of RNA polymerase: motoring along. Annu Rev Biochem 77: 149–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Park J, Pugh RA, Ha T, Spies M (2009) Single-molecule analysis reveals differential effect of ssDNA-binding proteins on DNA translocation by XPD helicase. Mol Cell 35: 694–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins AA, Friedman LJ, Gallagher SS, Crawford DJ, Anderson EG, Wombacher R, Ramirez N, Cornish VW, Gelles J, Moore MJ (2011) Ordered and dynamic assembly of single spliceosomes. Science 331: 1289–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth M, Ting AY (2008) Imaging proteins in live mammalian cells with biotin ligase and monovalent streptavidin. Nat Protoc 3: 534–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10: 126–139 [DOI] [PubMed] [Google Scholar]
- Martin G, Keller W (2007) RNA-specific ribonucleotidyl transferases. RNA 13: 1834–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury CJ (2010) 3′ Uridylation and the regulation of RNA function in the cytoplasm. Biochem Soc Trans 38: 1150–1153 [DOI] [PubMed] [Google Scholar]
- Post PL, Bokoch GM, Mooseker MS (1998) Human myosin-IXb is a mechanochemically active motor and a GAP for rho. J Cell Sci 111: 941–950 [DOI] [PubMed] [Google Scholar]
- Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B (1999) A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol 17: 1030–1032 [DOI] [PubMed] [Google Scholar]
- Selvin PR, Ha T (2007) Single-Molecule Techniques: A Laboratory Manual, 1st edn. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Siomi H, Siomi MC (2010) Posttranscriptional regulation of microRNA biogenesis in animals. Mol Cell 38: 323–332 [DOI] [PubMed] [Google Scholar]
- Stone MD, Mihalusova M, O’Connor CM, Prathapam R, Collins K, Zhuang X (2007) Stepwise protein-mediated RNA folding directs assembly of telomerase ribonucleoprotein. Nature 446: 458–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter NG, Huang CY, Manzo AJ, Sobhy MA (2008) Do-it-yourself guide: how to use the modern single-molecule toolkit. Nat Methods 5: 475–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Stone MD, Zhuang X (2010) A single-molecule assay for telomerase structure-function analysis. Nucleic Acids Res 38: e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardimci H, Loveland AB, Habuchi S, van Oijen AM, Walter JC (2011) Uncoupling of sister replisomes during eukaryotic DNA replication. Mol Cell 40: 834–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.