Abstract
Abstract The patterns of heat-induced synthesis (37°C to 45°C) of heat shock proteins (Hsps) in different tissues of grasshoppers and cockroaches from natural populations and in laboratory-reared gram-pest (Heliothis armigera) were examined by 35S-methionine labeling and sodium dodecyl sulfate–polyacrylamide gel electrophoresis fluorography. Whereas 45°C was lethal in most cases, optimal induction of Hsp synthesis was seen between 37°C and 42°C. The ongoing protein synthesis was not much affected at these temperatures, except in the tissues of adult H armigera exposed to 42°C. The profiles of the Hsps induced in the tissues of the insects, however, were different. From the relative abundance of the synthesis of 70-kDa (Hsp70) and 64-kDa (Hsp64) polypeptides, three categories of heat shock response were identified: (1) induction of abundant Hsp70 but little Hsp64 (malpighian tubules, male accessory glands, and ovaries of adult grasshoppers), (2) abundant Hsp64 but little Hsp70 (testes of adult grasshoppers, testes and malpighian tubules of adult cockroaches, and testes, malpighian tubules, and fat bodies of H armigera larvae), and (3) induction of both Hsp70 and Hsp64 in more or less equal abundance (ovaries of adult cockroaches, salivary glands of H armigera larvae, and malpighian tubules, male accessory glands, testes, and ovaries of adult H armigera). Cockroaches collected from storerooms showed detectable synthesis of Hsp64 and/or Hsp70 only after heat shock, but those collected from drains showed detectable synthesis of both Hsp70 and Hsp64 in different tissues without heat stress. Western blotting showed that the 64-kDa polypeptide in these insects is a member of the Hsp60 family. Grasshopper testes, which synthesized negligible Hsp70 but abundant Hsp64 after heat shock, developed thermotolerance. Thus, heat shock response is modulated by developmental and environmental factors in different tissues of insects.
INTRODUCTION
Cells of all organisms synthesize a small number of heat shock proteins (Hsps) in response to thermal and certain other stresses. Hsps function as molecular chaperones and protect cellular components from the injurious effects of thermal and other stresses. Hsps are grouped into five major families depending on their molecular sizes in kilodaltons (kDa): Hsp100, Hsp90, Hsp70, Hsp60, and the low-molecular-weight Hsp families (Schlesinger et al 1982; Nover 1984; Feder and Hofmann 1999). To our knowledge, the same set of Hsps is generally induced in different cell types as a response to heat shock in most of the model systems studied (Tissieres et al 1974; Lewis et al 1975; Dean and Atkinson 1983; Nath and Lakhotia 1989; Joplin and Denlinger 1990; Tiwari et al 1995). The malpighian tubules (MTs) of Drosophila melanogaster larvae, however, synthesize a completely different set of Hsps after a typical heat shock (Lakhotia and Singh 1989, 1996; Singh and Lakhotia 1995). None of the common Hsps are induced immediately after heat shock in this tissue (Lakhotia and Singh 1989). Instead, an entirely different set of MT-specific Hsps are seen, with a 64-kDa polypeptide being the most abundantly synthesized in the thermally stressed, larval MTs (Lakhotia and Singh 1989, 1996; Singh and Lakhotia 1995). This 64-kDa Hsp induced by heat shock in the larval MTs is a member of the Hsp60 family (Lakhotia and Singh 1996). Krebs and Feder (1997), using immunostaining, showed that Hsp70 appears in the MTs of D melanogaster only during recovery from heat shock. The biological significance of such a tissue-specific heat shock response, however, remains unknown.
The heat shock response is basically an adaptive cellular response that is linked to the ecologic conditions in which a given organism lives (Bijlsma and Loeschecke 1997). Because most studies on thermal stress–induced synthesis of Hsps have remained confined to a few model systems maintained under relatively constant environmental conditions (Feder and Hofmann 1999), this aspect has received lesser attention. To obtain information on the Hsp patterns in natural populations and to understand the significance of tissue-specific differences in Hsp induction, we examined Hsp synthesis in a variety of tissues from three insect species (grasshopper, cockroach, and Heliothis armigera). Our results revealed interesting developmental and environmental variations in the heat shock response of different tissues in these species, particularly regarding the induction of Hsp70 and Hsp64.
MATERIALS AND METHODS
Insect collection, culture, and conditioning
Grasshopper (Spathosternum prasiniferum, order Orthoptera), cockroach (Periplaneta americana, order Blatteria), and gram-pest (H armigera, order Lepidoptera) were used for this study. Grasshoppers were collected from the wild, conditioned in the laboratory at 24°C, and fed grass for 3 or 4 days before use. Cockroaches were collected from the storeroom of an animal house or the manholes of sewage drains and were fed standard cornmeal in the laboratory for 3 or 4 days at 24°C. H armigera larvae were reared in the laboratory on standard gram flour-agar-yeast food at 24°C; H armigera adults were fed honey.
Heat shock and analysis of protein synthesis
The desired insect tissues (6 to 8 individuals) were dissected in Poels' salt solution (PSS; Lakhotia and Mukherjee 1980). After thorough but gentle washing with PSS, the tissue was distributed to microfuge tubes, each having 40 μL of fresh PSS, for different temperature treatments (24°C, 37°C, 40°C, 42°C, or 45°C). The tissue was distributed in the different tubes so that each tube contained the particular tissue from all the dissected individuals. After 40 minutes of incubation at 24°C (control) or heat shock temperature (37°C, 40°C, 42°C, or 45°C), the tissues were labeled with 35S-methionine (activity, 100 μCi/mL; specific activity, >800 Ci/mM; BRIT, Bombay, India) for 40 minutes at the respective temperature. The labeled tissues were dissolved in the sodium dodecyl sulfate (SDS) sample buffer (0.0625 M Tris [pH, 6.8], 2% SDS, 1% β-mercaptoethanol, 20% glycerol) by transferring the tubes to a boiling water bath for 10 minutes. The dissolved protein samples were then ice-cooled, vortexed, and centrifuged. The supernatant was stored overnight at −20°C. The protein samples were separated in 12% SDS-polyacrylamide gels and fluorographed as described elsewhere (Singh and Lakhotia 1988). Triplicates of each sample were examined.
Western blotting
Protein samples from unstressed tissues were electrophoresed in SDS-polyacrylamide gel and electroblotted on nitrocellulose membrane as described elsewhere (Lakhotia and Singh 1993). The blot was challenged with the SPA-805 primary antibody (1:1000 dilution; StressGen Biotechnologies, Victoria, British Columbia, Canada), which specifically recognizes the Hsp60 family of proteins. The primary antibody was detected by the horse radish peroxidase (HRP)-conjugated anti-rabbit antibody (1:2000 dilution; catalog number A1949, Sigma, St Louis, MO, USA), which was followed by colorimetric detection as described elsewhere (Lakhotia and Singh 1993).
Thermotolerance
Thermotolerance in grasshopper testes was measured as the tissue's ability to incorporate 35S-methionine in acid-insoluble fraction (proteins) after thermal stress. Twenty testicular lobes from 5 grasshoppers (4 lobes per individual) in 40 μL of PSS were incubated at different temperatures (24°C, 40°C, or 45°C) for 40 minutes and then labeled at the respective temperature with 35S-methionine (activity, 100 μCi/mL; specific activity, >800 Ci/mM) for 30 minutes. Another sample was prepared after exposing the testicular lobes to 40°C for 30 minutes and then to 45°C for 40 minutes before labeling with 35S-methionine as described. Tissues were also dissolved in lysis buffer as described for gel electrophoresis. Ten microliters of each sample was mixed with 10 μL of 10% bovine serum albumin, and 5 μL of each mixture was spotted on a GF/C filter paper (Whatman International Ltd, Maidstone, England) and dried. The filters were washed twice with 10% ice-cold trichloroacetic acid, once for 30 minutes and once for 10 minutes. The filters were immediately dehydrated in alcohol, dried, and the 35S-methionine measured in a scintillation counter. The experiment was repeated 8 to 10 times, and the mean value of counts per minute was used for assessing the rate of protein synthesis under the different conditions.
RESULTS
As described, heat shock response was examined in three different insect species (grasshopper, cockroach, and gram-pest) at temperatures ranging from 37°C to 45°C. Heat shock at 45°C was lethal for most of the insect tissues, and in all three species, 42°C was optimal for induction of Hsp synthesis. The ongoing protein synthesis was not much affected at these temperatures except in the tissues of adult H armigera exposed to 42°C. The patterns of protein synthesis in control and heat shocked tissues of the three insect species are shown in Figures 1 through 5. The most striking feature in the different tissues of the three insect species related to the variable induction of 70-kDa and 64-kDa Hsps (Table 1). Depending on the relative induction of Hsp70 and Hsp64 (described later), we designated the heat shock response in these insect tissues as follows:
Hsp70 type, in which Hsp70 is most abundantly and Hsp64 is much less or little induced;
Hsp64 type, in which Hsp70 is minimally and Hsp64 is maximally induced; and
Mixed type, in which Hsp70 and Hsp64 are more or less equally induced.
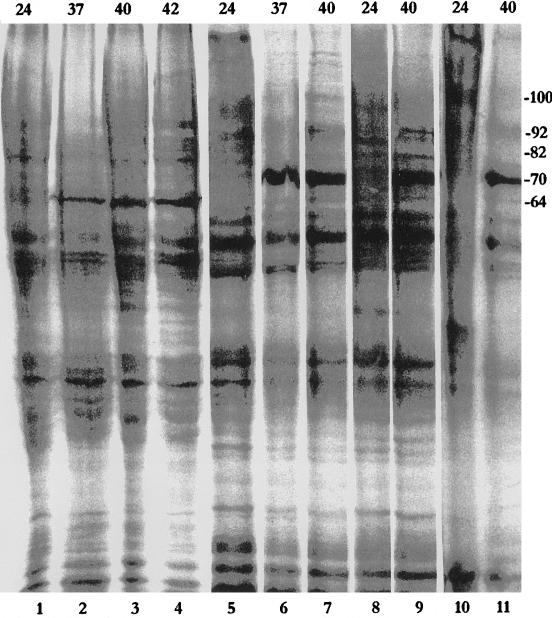
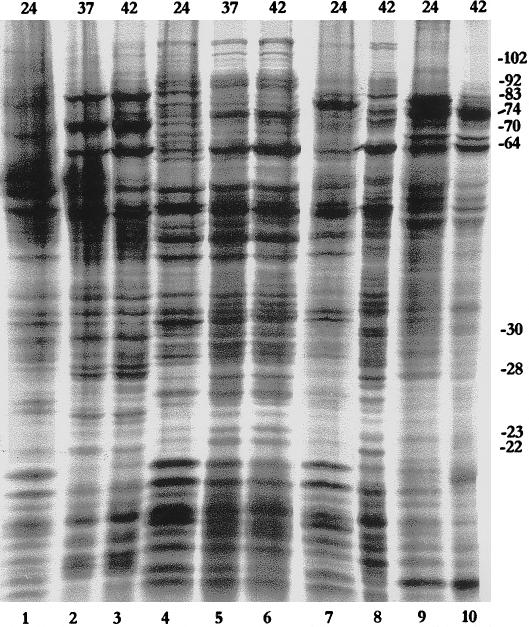
Fig 1.
Patterns of protein synthesis in different adult grasshopper tissues (testes, lanes 1–4; malpighian tubules, lanes 5–7; ovaries, lanes 8 and 9; and male accessory glands, lanes 10 and 11) at 24°C (lanes 1,5, 8, and 10), 37°C (lanes 2 and 6), 40°C (lanes 3, 7, 9, and 11), or 42°C (lane 4). Approximate sizes (kDa) are indicated on the right margin. Numbers on the top indicate the temperature in °C
Table 1.
Heat shock response types and their occurrence in different tissues at 42°C in grasshopper, cockroach, and gram-pest and at 37°C in D melanogaster
Induction of Hsp83 (82-kDa or 83-kDa polypeptide) varied in different insects, being less markedly induced in grasshoppers, moderately induced in cockroaches, and more pronouncedly induced in H armigera. In addition to the major Hsps such as Hsp83, Hsp70, and Hsp64, other polypeptides were also induced after heat shock. The high-molecular-weight, 100-kDa and 74-kDa polypeptides showed a specific pattern, because these polypeptides were generally induced only in tissues showing the Hsp64 type of heat shock response. A number of low-molecular-weight polypeptides (presumably belonging to the low-molecular-weight Hsp family) were commonly induced by heat shock in all three insect species.
Grasshopper
Heat-induced protein synthesis was examined in the testes, ovaries, male accessory glands, and MTs of adult grasshoppers. Hsp synthesis in the testes was remarkable for the almost complete absence of Hsp70, whereas a 64-kDa polypeptide (Hsp64) was most abundantly induced at all temperatures studied (37°, 40°, and 42°C; Fig 1, lanes 1–4). Hsp70, however, was the most abundantly synthesized polypeptide in heat shocked MTs, ovaries, and male accessory glands in grasshoppers (Fig 1, lanes 5–11). Hsp64 was only marginally labeled in the ovaries (Fig 1, lanes 8 and 9) and accessory glands (Fig 1, lanes 10 and 11). In comparison, it was a little more strongly labeled in heat shocked MTs (Fig 1, lanes 5–7).
Cockroach
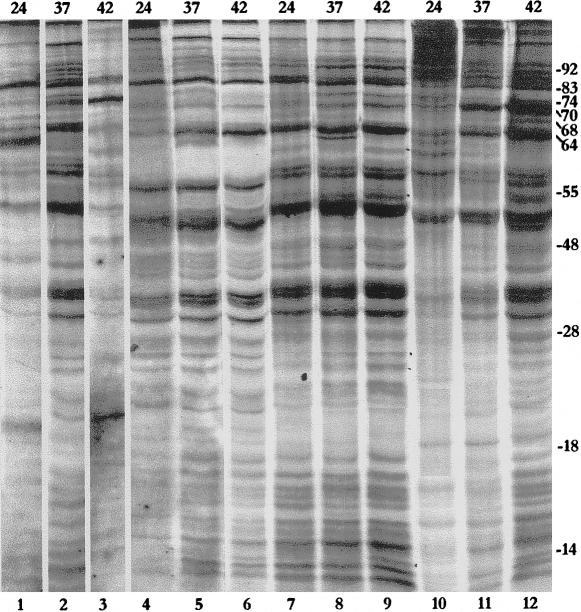
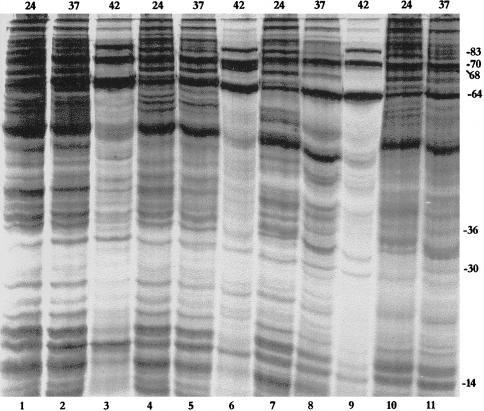
Heat shock–induced synthesis of proteins in the cockroach was examined in the testes, ovaries, salivary glands, and MTs of adults from two populations (storeroom and drains). Three Hsp patterns were observed in the tissues of the storeroom population. The salivary glands synthesized relatively more Hsp64 at 37°C, but this shifted in favor of Hsp70 at 42°C (Fig 2, lanes 1–3). The testes and MTs showed strong synthesis of Hsp64 but much less synthesis of Hsp70 (Fig 2, lanes 4–9). The ovaries synthesized both Hsp70 and Hsp64 in nearly equal abundance (Fig 2, lanes 10–12).
Fig 2.
Heat shock protein patterns in different tissues (salivary glands, lanes 1–3; malpighian tubules, lanes 4–6; testes, lanes 7–9; and ovaries, lanes 10–12) of adult cockroaches collected from storerooms at 24°C (lanes 1, 4, 7, and 10), 37°C (lanes 2, 5, 8, and 11), or 42°C (lanes 3, 6, 9, and 12). Approximate sizes (kDa) of the major induced Hsps are indicated on the right margin. Numbers on the top indicate temperature in °C
The Hsp patterns in the salivary glands and MTs of cockroaches collected from the sewer drains were similar to those in the ovaries of the storeroom population. The 70-kDa and 64-kDa polypeptides, however, were already significantly labeled in the control samples of this population (Fig 3, lanes 1 and 5). The heat shocked MT samples (Fig 3, lanes 6–8) showed synthesis of Hsp70 in abundance, which was not observed in the storeroom population (Fig 2, lanes 5 and 6). Labeling of Hsp64 was significantly higher in the MTs than in the salivary glands (Fig 3).
Fig 3.
Heat shock protein patterns in salivary glands (lanes 1–4) and malpighian tubules (lanes 5–8) at 24°C (lanes 1 and 5), 37°C (lanes 2–6), 40°C (lanes 3 and 7), or 42°C (lanes 4 and 8) of adult cockroaches collected from drains. Approximate sizes (kDa) are indicated on the right margin. Numbers on the top indicate the temperature in °C
H armigera
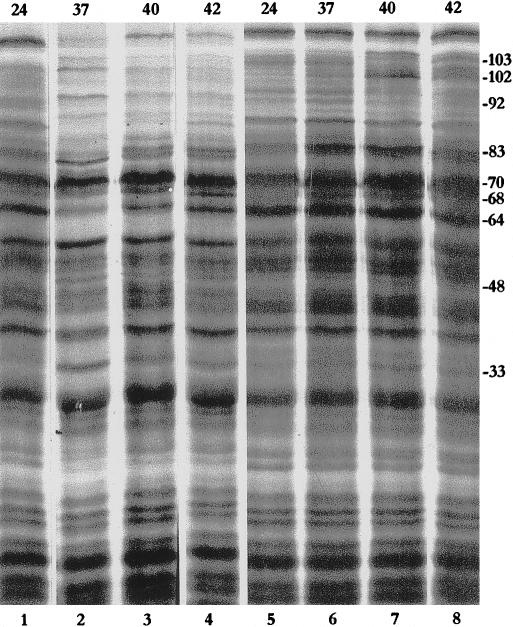
Heat shock protein synthesis was examined in the salivary glands, MTs, testes, and fat bodies of the laboratory-reared, fifth-instar larvae and adult H armigera (Figs 4 and 5). In the larval salivary glands, Hsp70 and Hsp64 were abundantly induced after heat shock, with Hsp70 being the most strongly labeled (Fig 4, lanes 1–3). Hsp70, however, was almost completely absent in larval MTs (Fig 4, lanes 4–6), testes (Fig 4, lanes 7 and 8), and fat bodies (Fig 4, lanes 9 and 10). In these three tissues, Hsp64 was the most strongly labeled polypeptide after heat shock at 37°C and 42°C. Induction of other high-molecular-weight Hsps also differed between the different larval tissues. Hsp83 was not much induced in the MTs and fat bodies but was fairly well labeled in the testes and salivary glands. An Hsp74 was strongly labeled after heat shock in larval MTs, testes, and fat bodies, but in the salivary glands, Hsp74 was detectably labeled only at 42°C (Fig 4, lane 3). Heat shocked larval testes showed good induction of an additional band at the 72-kDa position (Fig 4, lane 8).
Fig 4.
Protein synthesis in salivary glands (lanes 1–3), malpighian tubules (lanes 4–6), testes (lanes 7 and 8), and fat bodies (lanes 9 and 10) at 24°C (lanes 1, 4, 7, and 9), 37°C (lanes 2 and 5), or 42°C (lanes 3, 6, 8, and 10) of Heliothis armigera larvae. Approximate sizes (kDa) are indicated on the right margin. Numbers on the top indicate the temperature in °C.
Fig 5.
Heat shock protein pattern in malpighian tubules (lanes 1–3), male accessory glands (lanes 4–6), testes (lanes 7–9), and ovaries (lanes 10 and 11) of adult Heliothis armigera. Approximate sizes (kDa) are indicated on the right margin. Numbers on the top indicate the temperature in °C
Heat shock protein synthesis was also examined in the MTs, male accessory glands, testes, and ovaries of 4- to 5-day-old, adult H armigera (Fig 5). Unlike the previously noted variability in larval tissues, adult tissues showed more or less comparable induction of Hsp83, Hsp70, and Hsp64 after heat shock at 37°C and 42°C (Fig. 5).
Identification of the 64-kDa polypeptide as a member of the Hsp60 family
As shown in Figure 6, the Hsp60 antibody specifically cross-reacted with a 64-kDa polypeptide in the Western blots of the protein samples from unstressed MTs of cockroaches, from the testes and MTs of grasshoppers, and from the salivary glands of H armigera (lanes 1–4). Western blots challenged with 7.10 antibody (Kurtz et al 1986) that recognizes the Hsp70/Hsc70 class of proteins showed reactive bands around the 70-kDa region (not shown).
Fig 6.

Western blot for the presence of Hsp60 homologue at the 64-kDa position in unstressed malpighian tubules of the cockroach (lane 1), testes and malpighian tubules of the grasshopper (lanes 2 and 3), and salivary glands of Heliothis armigera (lane 4).
Acquisition of thermotolerance without Hsp70
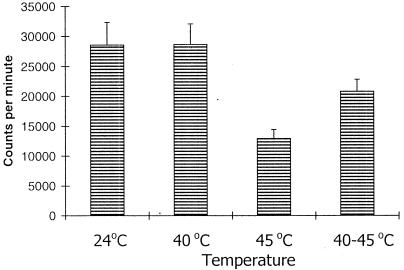
Because several tissues in the described analysis did not show any significant induction of Hsp70 by heat shock but synthesized Hsp64 instead, it was interesting to ascertain if these tissues developed thermotolerance. Testes of the grasshopper were taken as a representative example of tissues in which Hsp64, but not Hsp70, is abundantly induced by heat shock, and the rates of 35S-methionine incorporation in the newly synthesized proteins after the different heat shock regimens were compared. As shown in Figure 7, the rate of 35S-methionine incorporation was comparable at 24°C and at 40°C, whereas heat shock at 45°C caused a significant reduction in 35S-methionine incorporation. Interestingly, however, an exposure at 40°C before the more severe heat shock at 45°C considerably restored the rate of 35S-methionine incorporation in the testicular lobes (Fig 5). This suggests that these cells can acquire thermotolerance in the presence of Hsp 64 and other Hsps even though Hsp70 is absent.
Fig 7.
Rate of 35S-methionine incorporation in testes (counts/min) at different temperatures (24°C, 40°C, 45°C, and 40°C followed by 45°C)
DISCUSSION
Lakhotia and Singh (1989) first demonstrated that the patterns of heat shock–induced synthesis of Hsps in Drosophila sp. could be of three different types: (1) the common Hsp70 type seen in most tissues, (2) the Hsp64 type seen in larval MTs, and (3) the mixed type seen in adult MTs that showed induction of both sets of Hsps (see also Singh and Lakhotia 1995 and Lakhotia and Singh 1996). Results of the present study reveal that patterns of Hsp synthesis in different tissues of the three insect species examined can also be grouped into one of these three categories (Table 1).
The primary spermatocytes of D melanogaster do not show induction of Hsp70 transcripts, but other somatic and postmeiotic cells in testes transcribe hsp70 genes after heat shock (Bendena et al 1991; K.V. Prasanth and S.C. Lakhotia SC, unpublished observations). In our study, we noted that except in adult H armigera, synthesis of Hsp70 was not detectable in the testes of different insects. Compared with testes, however, ovaries always showed abundant expression of Hsp70. Therefore, Hsp70 synthesis in the testes appears to be regulated differentially. The induction of Hsp70 synthesis varied in the MTs and testes in relation to developmental stage or to species, but except in grasshoppers, Hsp64 synthesis was always strikingly high in the MTs. Our results show that the Hsp64 polypeptide of the three insect species belongs to the Hsp60 family of proteins. In several other insect species, polypeptides of the Hsp60 family are also synthesized at a high rate in the MTs, with or without heat shock (Nath and Lakhotia 1989; Tiwari et al 1995). Therefore, Hsp60 family proteins appear to have some special role in the MTs.
Krebs and Feder (1997) examined induction of Hsp70 in different tissues of Drosophila sp. larvae in situ by immunostaining. They reported that several tissues, such as parts of the gut, MTs, fat bodies, and so on, that do not show induction of Hsp70 immediately after the thermal stress do so after a recovery period of 4 hours. These authors did not examine the synthesis of other Hsps in these tissues, but our own results with the MTs of D melanogaster showed induction of Hsp64 synthesis immediately after heat shock. Other studies (K.V. Prasanth and S.C. Lakhotia, unpublished results) have shown that even though Hsp70 is not synthesized in the MTs and fat bodies of Drosophila sp. larvae either during or immediately after heat shock, the hsp70 transcripts are induced during heat shock in these tissues. Furthermore, when Hsp70 synthesis begins in the larval MTs and fat bodies during recovery, Hsp64 synthesis is inhibited (P. Srivastava and S.C. Lakhotia, unpublished results). The significance of and reasons for such specific variations in the induction of different Hsps in a temporally regulated manner both during and after the stress are not known. In our opinion, it appears that mounting one of the three types of heat shock responses (the Hsp70, Hsp64, or mixed type) is related to the specific cellular physiological conditions prevailing in different tissues in insects. This aspect needs further study.
It was interesting that the cockroaches collected from drains showed good expression of the 70-kDa and the 64-kDa polypeptides even without heat shock. This appears to result from the effect of the pollutants present in the drains. Constitutive expression of Hsp70 is used as a biomarker for the presence of these pollutants (Depledge et al 1995; de Pomerai 1996). Centipedes (Lithobios sp.) collected from an area near a smelter also had higher Hsp70 expression than did those collected from unpolluted areas (Pyza et al 1997).
Our results show that tissues that do not express Hsp70 can still develop thermotolerance, as seen in the improved rate of 35S-methionine incorporation in the grasshopper testes exposed to severe heat shock at 45°C after a 40°C pretreatment. Trypan blue exclusion test (data not presented) also showed that a milder heat shock before the more severe heat shock at 45°C resulted in a significant increase in the frequency (80%) of viable cells that exclude trypan blue compared with the frequency after a direct exposure to 45°C (35% viable cells). Therefore, the Hsp64 type of heat shock response also appears to confer thermotolerance.
Ruder et al (1989) found the pattern of Hsp synthesis in nerve cord and fat body cells of laboratory-reared cockroaches to be similar. In our study with a wild population of cockroaches, however, we noted subtle but significant differences in Hsp synthesis among different tissues. Additionally, whereas Ruder et al (1989) did not report the induction of Hsp64 in any tissue of the cockroach, we consistently found such induction (see Figs 2 and 3). Therefore, natural environmental variations appear to have a significant effect on the heat shock response of different tissues. Some earlier studies have also shown that variations in developmental conditions may influence the induction of different Hsps, even in laboratory-reared organisms such as Drosophila and Chironomus sp. (Singh and Lakhotia 1988; Nath and Lakhotia 1989). More studies on the heat shock response of different tissues in organisms within their natural habitats are necessary to understand the role and significance of developmental factors on Hsp synthesis in response to stress.
The results of this study show that tissue-specific differences in the induction of different Hsps, particularly regarding Hsp70 and Hsp64, that were reported earlier in Drosophila sp. (Lakhotia and Singh 1989) are fairly widespread among insects of diverse taxonomic groups. Nevertheless, the absence of a complete correlation between a given Hsp pattern and a given tissue in different insects remains intriguing.
The heat shock response has evolved in relation to the environmental conditions (internal as well as external) of cells (Bijlsma and Loeschecke 1997; Krebs et al 1998; Feder and Hofmann 1999). Therefore, it is not surprising that specific differences exist in the pattern of Hsp induction among various cell types in the same organism. To our knowledge, most studies regarding Hsp induction have generally been confined to model organisms grown under standard laboratory conditions and, therefore, have failed to model the real-life situations regarding thermal stress in natural populations. Further studies on the heat shock response of diverse organisms within their natural habitats are essential to understand the biological significance of the tissue-specific variations noted in this study.
Acknowledgments
This work was supported by a research grant for young scientists (SR/OY/Z-03/91) by the Department of Science and Technology, Government of India, New Delhi, to A.K.S.
REFERENCES
- Bendena WG, Ayme-Southgate A, Garbe JC, Pardue ML. Expression of heat shock locus hsr-omega in nonstressed cells during development in Drosophila melanogaster. Dev Biol. 1991;144:65–77. doi: 10.1016/0012-1606(91)90479-m. [DOI] [PubMed] [Google Scholar]
- Bijlsma R, Loeschecke V. eds. 1997 Environmental Stress, Adaptation and Evolution. Birkauser Verlag, Basel. [DOI] [PubMed] [Google Scholar]
- de Pomerai D. Heat shock proteins as biomarkers of pollution. Hum Exp Toxicol. 1996;15:279–285. doi: 10.1177/096032719601500401. [DOI] [PubMed] [Google Scholar]
- Dean RL, Atkinson BG 1983 The acquisition of thermal tolerance in larvae of Calpodes ethlius (Lepidoptera) and the in situ and in vitro synthesis of the heat shock proteins. Can J Biochem Cell. B61. 472–479. [DOI] [PubMed] [Google Scholar]
- Depledge MH, Aagaard A, Gyorkos P. Assessment of trace metal toxicity using molecular, physiological and behavioral biomarkers. Marine Pollut Bull. 1995;31:19–27. [Google Scholar]
- Feder ME, Hofmann GE. Heat shock proteins, molecular chaperones and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Joplin KH, Denlinger DL. Developmental and tissue specific control of the heat shock induced 70kDa related proteins in the flesh fly Sarcophaga crassipalpis. J Insect Physiol. 1990;36:239–249. [Google Scholar]
- Krebs RA, Feder ME. Tissue specific variation in Hsp70 expression and thermal damage in Drosophila melanogaster larvae. J Exp Biol. 1997;200:2007–2015. doi: 10.1242/jeb.200.14.2007. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Martin EF, Lee J. Heritability of expression of the 70kD heat shock protein in Drosophila melanogaster and its relevance to the evolution of thermotolerance. Evolution. 1998;52:841–847. doi: 10.1111/j.1558-5646.1998.tb03708.x. [DOI] [PubMed] [Google Scholar]
- Kurtz S, Rossi J, Petkol L, Lindquist S. An ancient developmental induction. Science. 1986;231:1154–1157. doi: 10.1126/science.3511530. [DOI] [PubMed] [Google Scholar]
- Lakhotia SC, Mukherjee T. Specific activation of puff 93D of Drosophila melanogaster by benzamide and the effect of benzamide treatment on heat induced puffing activity. Chromosoma. 1980;81:125–136. doi: 10.1007/BF00292427. [DOI] [PubMed] [Google Scholar]
- Lakhotia SC, Singh AK. A novel set of heat shock polypeptides in malpighian tubules of Drosophila melanogaster. J Genet. 1989;68:129–137. [Google Scholar]
- Lakhotia SC, Singh BN. A simple and inexpensive “Western blotting” apparatus. Ind J Exp Biol. 1993;31:301–302. [Google Scholar]
- Lakhotia SC, Singh BN. Synthesis of ubiquitously present new hsp60 family protein is enhanced by heat shock only in the malpighian tubules of Drosophila. Experientia. 1996;52:751–756. doi: 10.1007/BF01923984. [DOI] [PubMed] [Google Scholar]
- Lewis M, Helmsing PJ, Ashburner M. Parallel changes in puffing activity and patterns of protein synthesis in salivary glands of Drosophila. Proc Natl Acad Sci U S A. 1975;92:3604–3608. doi: 10.1073/pnas.72.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath BB, Lakhotia SC. Heat shock response in a tropical Chironomus: seasonal variation in response and the effect of developmental stage and tissue type on heat shock protein synthesis. Genome. 1989;32:676–686. [Google Scholar]
- Nover L 1984 Heat Shock Response of Eukaryotic Cells. Springer-Verlag, Berlin. [Google Scholar]
- Pyza E, Mak P, Kramarz P, Laskowaski R. Heat shock proteins (Hsp70) as biomarkers in ecotoxicological studies. Ecotoxicol Environ Safety. 1997;38:244–251. doi: 10.1006/eesa.1997.1595. [DOI] [PubMed] [Google Scholar]
- Ruder GK, Ovsenek N, Heikkila JJ, Downer RGH. Examination of heat shock gene expression in nerve cord isolated from heat stressed American cockroach, Periplaneta americana. Biochem Cell Biol. 1989;67:168–172. [Google Scholar]
- Schlesinger MJ, Ashburner M, and Tissieres A 1982 Heat Shock from Bacteria to Man. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Singh AK, Lakhotia SC. Effect of low temperature rearing on heat shock protein synthesis and heat sensitivity in D. melanogaster. Dev Genet. 1988;9:193–201. [Google Scholar]
- Singh BN, Lakhotia SC. The non-induction of hsp70 in heat shocked malpighian tubules of Drosophila larvae is not due to constitutive presence of hsp70 or hsc70. Curr Sci. 1995;69:178–182. [Google Scholar]
- Tissieres A, Mitchell HK, Tracy U. Protein synthesis in salivary glands of D. melanogaster. Relation to chromosome puffs. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Tiwari PK, Mohan DRK, Archana J. Developmental study of thermotolerance and the heat shock response in Lucilia cuprina (Weidemann) J Biosci. 1995;20:341–354. [Google Scholar]