We have all experienced the pain of an injection at some point in our lives. This level of discomfort and stress is the threshold that the new European Directive on “the protection of animals used for scientific purposes” sets for its applicability: if a procedure is more painful than the insertion of a needle, then the legislation will apply. Published last October in the Official Journal of the European Union (EC, 2010), the new Directive is the culmination of about 10 years of work by the European Commission. Nevertheless, reaction to it will probably be mixed. There are those for whom it will represent yet another bureaucratic burden in the practice of experimentation on animals, whereas others will perceive it as merely an affirmation of the right of scientists to cause animals to suffer in the name of progress, with limited oversight or control.
…if a procedure is more painful than the insertion of a needle, then the legislation will apply
In our opinion, the Directive (known as Directive 2010/63) represents a step forward in improving the quality of life of laboratory animals, while preserving the quality and reproducibility of experimental data. Furthermore, we believe that the Directive is a dynamic tool that provides the scientific community with the leeway to improve the living conditions of experimental animals. It therefore in no way represents the end of a scientific, ethical and legislative process, but instead functions as a starting point. Discussions about the details of how, when, where and why research using animals might be conducted are crucial and will continue to be so in the future. Our contribution should be seen in this light, and not simply as a critique of the work of the Commission.
There are both altered and new elements of legislation throughout the Directive, all of which are worthy of discussion. These range from the requirement to establish institutional animal-welfare bodies, to enriching the environment in which the animals are kept; from the inclusion of invertebrates in the species covered by the Directive (following the example of United Kingdom), to clearer, compulsory reference to the 3Rs Principle—replacement, refinement and reduction—that was first proposed by Russell & Burch (1959).
Our aim here, however, is to discuss just one fundamental aspect of the Directive that we feel is important: the level of suffering that an animal has to experience for the research to be governed by the Directive. Indeed, if we have understood what the legislators intend, researchers who impose a level of suffering below that caused by the introduction of a needle are not to be troubled by the requirements of the Directive. To wit, Article 1, Paragraph 5 of the Directive describes cases in which the new rules do not apply, including “practices not likely to cause pain, suffering, distress or lasting harm equivalent to, or higher than, that caused by the introduction of a needle in accordance with good veterinary practice”. This is confirmed by Article 3, Paragraph 1, which is dedicated to the term ‘procedure’, which is described as “any use, invasive or non-invasive, of an animal for experimental or other scientific purposes, with known or unknown outcome, or educational purposes, which may cause the animal a level of pain, suffering, distress or lasting harm equivalent to, or higher than that caused by the introduction of a needle in accordance with good veterinary practice”.
This definition of the scope of the Directive raises a serious question: who decides how to classify how painful a particular procedure is? If the researcher is to make the judgement, then he or she can effectively render their research invisible to the law by recording a low level of suffering. If it is not the researcher—and we think it is not intended to be—then a national committee, through the work of the newly-minted local animal-welfare bodies called for in the Directive, will need to set a clear scale of pain and suffering that should be standardized throughout the EU. It therefore becomes crucial to understand the meaning of the puncture of a needle as a threshold value.
We appreciate the need for legislators to establish threshold levels to regulate the feasibility and morality of scientific procedures involving the use of animals. Volumes have been written comparing the ways in which animals experience pain or distress with the ways in which humans suffer the same. Both intra- and inter-species differences are involved when trying to understand whether certain procedures cause higher, equal or lower discomfort for animals than the introduction of a needle. It might therefore be useful, for example, to establish guidelines or a scale of suffering for particular strains of rodents, which could help to determine a consistent and rational basis for deciding whether certain procedures with these animals fall above or below the ‘needle threshold’.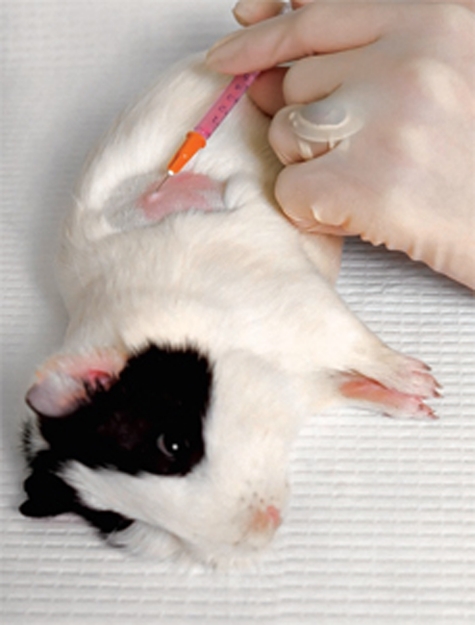
Pain is, of course, subjective. Even among humans we know that certain individuals are more or less sensitive to pain. Across species, then, it is clear that the same noxious stimuli can be perceived differently, and that even if the pain is equivalent, the level of trauma or stress that such discomfort leads to can be different. To set a threshold in relation to pain is therefore a challenging prospect, especially when the threshold is considered to be the same for all species.
For legislators applying norms of human behaviour to regulate other animal species, the use of thresholds facilitates the application of general rules to a complex reality in which a case-by-case approach cannot be applied. In the Directive, the idea of a threshold is functional—setting the level at which an intervention can be justified. The threshold is intended to minimize suffering, but makes the assumption that all animals suffer in the same way. The more-empirical ‘pain threshold’ is the actual point at which pain is first perceived during noxious stimulation—which has been shown to be essentially the same in humans and warm-blooded vertebrates (Vierck, 1976). Given this, the Directive's argument for assuming that the pain threshold is equivalent across species can be criticized, but is justified by the need for consistency and ease of application. Nevertheless, assumptions about the measurement of stimuli and similarity among species cannot be drawn with certainty (Committee on Pain and Distress in Laboratory Animals et al. 1992). In addition, measuring the impact of the introduction of a needle to different species is compounded by physical factors such as perfusion of the body region involved and skin thickness (De Marco & Pastoe, 2008).
…the Directive's argument for assuming that the pain threshold is equivalent across species can be criticized, but is justified by the need for consistency and ease of application
It is these and other variables—outlined in greater detail below—that trouble us. These are important factors, but we seek to discover whether—and by whom—these criteria can be used in a consistent and logical manner to decide whether a certain procedure applied to a particular organism is within the scope of the Directive. Therefore, we understand and approve of the need to standardize all of the procedures within the scope of the Directive, but we are sceptical about the prospect of an ‘experimental Far-West’, where nobody is sure of the law and whether or not it applies to what they are doing.
In this context, it is worth considering the idea of ‘replacement’, introduced by Russell and Burch as “any scientific method employing non-sentient material which may, in the history of experimentation, replace methods which use conscious living vertebrates” (Russell & Burch, 1959). This concept, though valid today, has been modified over time (Buchanan-Smith et al, 2005) to lead to a more-recent interpretation that implies the elimination of animals in a particular protocol—‘total replacement’—as well as the use of animals with less-complex nervous systems—‘partial replacement’. The latter concept can be found in the Directive under Article 13. The underlying assumption of partial replacement is that animals with less-complex nervous systems suffer less than animals with more-complex neurological organization. In biological terms, such an assumption is ambiguous. We do not yet have a clear understanding of what suffering means for any species other than our own. However, in normative terms, a definition of sensation and suffering has to be agreed and we think that in this case, this assumption is the best compromise between biological continuity and the need for operational thresholds.
Many factors probably have a role in the differences in pain sensitivity among members of the same species. A paradigmatic example of the prominent role that genetic factors may play is the impact of a polymorphism in the gene encoding catechol-O-methyltransferase (COMT). COMT is a ubiquitously expressed enzyme that degrades neurotransmitters such as dopamine and adrenaline. As such, COMT modulates pain sensitivity in mice, with higher COMT1 activity associated with behavioural differences in anxiety assays and decreased pain sensitivity (Diatchenko et al, 2006; Segall et al. 2010). Gender might also have an effect: recent data show that in addition to the well-established greater sensitivity of human women to pain (Greenspan et al, 2007), gender-based differences are also evident in rodents. In an operant test for pain sensitivity, female rats were found to be more sensitive to cold and less sensitive to heat, because of different sympathetic reactivity (Vierck et al, 2008). Age is another, often underestimated, factor: a decline in sensitivity concomitant with advanced age is well documented across species (Heft & Robinson, 2010). For laboratory rodents, housing conditions are also important. Social isolation can decrease pain sensitivity, perhaps due to changes in the number and activity of μ-opioid receptors (Bigi et al, 1993; Tuboly et al, 2009), and the fact that social interactions with peers modulate pain sensitivity (Norman et al, 2010). Interestingly, the effects of social isolation might also be different in different rat lines, suggesting that in certain experimental settings, social variables might override genetic predispositions (Raber & Devor, 2002).
The introduction of a needle represents only a moment of pain or distress, with no lasting harm. Nevertheless, to establish a needle threshold for individual species, all of the factors mentioned above that modulate pain sensitivity should be carefully considered when estimating equivalency between the introduction of a needle and the proposed procedure.
Accounting for different factors when considering pain thresholds in individuals of the same or different species is addressed in the Directive. Section II (assignment criteria) of Annex VIII (severity classification of procedures) suggests taking into consideration characteristics such as the species, genotype, age and gender of the animal, as well as the previous experience the animal has had of the procedure, before the category of invasiveness is decided.
As mentioned, our concern is whether the outlined differences can lead to a measured and sensible application of the Directive to all procedures for which it has merit. Decision criteria, by which one procedure is regulated and another is not, must be standardized and should be informed by a consensus of opinion. One possibility would be to establish a website on which researchers could anonymously describe procedures that they felt did not require regulation—those that are not more painful or harmful than a needle for a certain species. This would result in a database of cases that could constitute the basis for the evaluation of different procedures. Such an internationally derived catalogue of opinion could be extremely useful for animal-welfare bodies or other committees in deciding whether or not to apply the Directive.
a website on which researchers could anonymously describe procedures that they felt did not require regulation […] could constitute the basis for the evaluation of different procedures
The basis for this modus operandi is already present in the Directive. For example, Article 27, Paragraph 1 (on the tasks of the animal-welfare bodies) states that they should “establish and review internal operational processes as regards monitoring, reporting and follow-up in relation to the welfare of animals housed or used in the establishment”. Similarly, Article 27, Paragraph 2 states that “Member States shall ensure that the records of any advice given by the animal-welfare body and decisions taken regarding that advice are kept for at least 3 years.” Should we extend this particular point to be fully comprehensive and ensure the sharing of best practice? The records and advice of the animal-welfare bodies could remain available for a more-extensive period of time and could be organized into a user-friendly catalogue of decisions by the national committees (see Article 49 of the Directive). An online tool could then be used by the member states to better standardize procedures that are ambiguous in terms of the pain and suffering that they cause. This kind of arrangement could satisfy one of the main aims of the Directive: to better inform the exchange among European countries in terms of animal experiments.
This idea can also be found in the Directive in a more restricted form. Article 49 (national committees for the provision of animals used for scientific purposes), Paragraph 2 states that “the national committees […] shall exchange information on the operation of animal-welfare bodies and project evaluation and share best practice within the Union.”
We believe that member states should devise ways to interact and share information about the criteria they are using to decide which practices with which animals should fall within the scope of the Directive. If read optimistically, the Directive suggests this possibility, and we would like to see it realized. This is in line with our introductory comments that Directive 2010/63 should be seen as a starting point from which to improve experimental procedures with animals, not as a final set of rules to be lived by grudgingly. Some might say that we are pushing for the Directive to step beyond its intended boundaries, but we would encourage our critics to employ their imagination and expertise to use the Directive as a multi-purpose springboard from which to improve the ethical and scientific quality of research using animal subjects.
…we would encourage our critics to […] use the Directive as a multi-purpose springboard from which to improve the ethical and scientific quality of research using animal subjects
In this article we have tried to focus the attention of the reader on a particular problem: those procedures that might not fall within the scope of Directive 2010/63 because they impose a level of pain or suffering below the needle threshold with no lasting damage. We have suggested that institutional animal-welfare bodies—to which the Directive makes direct reference—could be the driving force behind the assessment of the inclusion or exclusion of particular procedures from the auspices of the Directive. Such assessment and standardization is required in order to adequately determine the threshold that invokes the Directive on a species or strain-specific basis, because pain and suffering are functions of the different sensitivities of individuals in different populations or species.
…pain and suffering are functions of [the] sensitivities of […] different populations or species
We offer one final, provocative thought about intra-species variability in the context of humans. It has been estimated that 10% of the general population have a phobia of needles, and that this percentage rises to 19% in children aged between 4 and 6 years (Rice, 1993). The paediatric and/or nursery management of needle injections or intravenous access has thus resulted in practical recommendations—selected positions, distraction procedures and parental training—to minimize anxiety and pain in needle-resistant or needle-phobic children (Cohen, 2008; Ives & Melrose, 2010). We raise this point only to reiterate that several factors must be taken into account in the ‘needle-equivalence’ issue, and that individual anticipatory responses to fearful stimuli are another piece of the jigsaw.
Open discussion of the points outlined in this article, as well as the creation of practical tools, are warranted for the engaging and difficult task facing animal-welfare bodies during the next few years, as they begin to apply the Directive. Practical tools should include an exhaustive review of the available literature, as well as of the first-hand experience and opinion of researchers. Formalized and user-friendly online catalogues of scientific evidence could help to identify and describe procedures that should or should not be regulated, which implies expanding the reach and effectiveness of the new European norms.
Animal experimentation is a multi-factorial enterprise that requires science, societal values, economics and ethics to find common ground
Animal experimentation is a multi-factorial enterprise that requires science, societal values, economics and ethics to find common ground. We think that if scientists embrace the message of Directive 2010/63 and seek ways in which the suffering of experimental animals can be minimized, then finding that common ground will prove easier in the long run and will be in the best interests of all parties, not least the animals.
Acknowledgments
We thank J.S. Vitale for help revising the English.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bigi S, De Acetis L, De Simone R, Aloe L, Alleva E (1993) Neonatal capsaicin exposure affects isolation-induced aggressive behavior and hypothalamic substance P levels of adult male mice (Mus musculus). Behav Neurosci 107: 363–369 [DOI] [PubMed] [Google Scholar]
- Buchanan-Smith HM, Rennie AE, Vitale A, Pollo S, Prescott MJ, Morton DB (2005) Harmonising the definition of refinement. Anim Welfare 14: 379–384 [Google Scholar]
- Cohen LL (2008) Behavioral approaches to anxiety and pain management for pediatric venous access. Pediatrics 122: S134–S139 [DOI] [PubMed] [Google Scholar]
- Committee on Pain and Distress in Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (1992) Recognition and Alleviation of Pain and Distress in Laboratory Animals. Washington, DC, USA: National Academies Press [Google Scholar]
- De Marco GJ, Pastoe PJ (2008) Anatomy, physiology, and the effects of pain. In Anesthesia and Analgesia in Laboratory Animals, RE Fish, PJ Danneman, M Brown, AZ Karas (eds), pp 3–25. Amsterdam, The Netherlands: Academic Press [Google Scholar]
- Diatchenko L, Nackley AG, Slade GD, Bhalang K, Belfer I, Max MB, Goldman D, Maixner W (2006) Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain 125: 216–224 [DOI] [PubMed] [Google Scholar]
- EC (2010) Directive 2010/63/EU of the European Parliament and the Council of 22 September on the protection of animals used for scientific purposes. Off J EU L 276: 33–79 (http://eurlex.europa.eu/LexUriServ) [Google Scholar]
- Greenspan JD et al. (2007) Studying sex and gender differences in pain and analgesia: a consensus report. Pain 132: S26–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heft MW, Robinson ME (2010) Age differences in orofacial sensory thresholds. J Dent Res 89: 1102–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives M, Melrose S (2010) Immunizing children who fear and resist needles: is it a problem for nurses? Nurs Forum 45: 29–39 [DOI] [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Morris JS, Zhang N, Cochran M, Courtney DeVries A (2010) Social interaction prevents the development of depressive-like behavior post nerve injury in mice: a potential role for oxytocin. Psychosom Med 72: 519–526 [DOI] [PubMed] [Google Scholar]
- Raber P, Devor M (2002) Social variables affect phenotype in the neuroma model of neuropathic pain. Pain 97: 139–150 [DOI] [PubMed] [Google Scholar]
- Rice LJ (1993) Needle phobia: an anesthesiologist's perspective. Pediatrics J 122: S9–S13 [DOI] [PubMed] [Google Scholar]
- Russell WMS, Burch RL (1959) The Principles of Humane Experimental Technique. London, UK: Methuen (reissued by UFAW; http://altweb.jhsph.edu/pubs/books/humane_exp/het-toc) [Google Scholar]
- Segall SK et al. (2010) Comt1 genotype and expression predicts anxiety and nociceptive sensitivity in inbred strains of mice. Genes Brain Behav 9: 933–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuboly G, Benedek G, Horvath G (2009) Selective disturbance of pain sensitivity after social isolation. Physiol Behav 96: 18–22 [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Acosta-Rua AJ, Rossi HL, Neubert JK (2008) Sex differences in thermal pain sensitivity and sympathetic reactivity for two strains of rat. Pain J 9: 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ Jr (1976) Extrapolations from the pain research literature to problems of adequate veterinary care. J Am Vet Med Assoc 168: 510–513 [PubMed] [Google Scholar]



 Laura Ricceri is in the Section of Neurotoxicology and Neuroendocrinology, Department of Cell Biology and Neuroscience, Istituto Superiore di Sanità, Rome, Italy.
Augusto Vitale is in the Section of Behavioural Neuroscience at the same institute.
E-mail:
Laura Ricceri is in the Section of Neurotoxicology and Neuroendocrinology, Department of Cell Biology and Neuroscience, Istituto Superiore di Sanità, Rome, Italy.
Augusto Vitale is in the Section of Behavioural Neuroscience at the same institute.
E-mail: