Abstract
TGF-β is a potent inducer of epithelial-to-mesenchymal transition (EMT), a process involved in tumour invasion. TIF1γ participates in TGF-β signalling. To understand the role of TIF1γ in TGF-β signalling and its requirement for EMT, we analysed the TGF-β1 response of human mammary epithelial cell lines. A strong EMT increase was observed in TIF1γ-silenced cells after TGF-β1 treatment, whereas Smad4 inactivation completely blocked this process. Accordingly, the functions of several TIF1γ target genes can be linked to EMT, as shown by microarray analysis. As a negative regulator of Smad4, TIF1γ could be crucial for the regulation of TGF-β signalling. Furthermore, TIF1γ binds to and represses the plasminogen activator inhibitor 1 promoter, demonstrating a direct role of TIF1γ in TGF-β-dependent gene expression. This study shows the molecular relationship between TIF1γ and Smad4 in TGF-β signalling and EMT.
Keywords: TIF1γ, EMT, TGF-β, Smad, breast
Introduction
The transforming growth factor-β (TGF-β) pathway is initiated by ligand-induced heterotetrameric complexes of type I and II serine/threonine kinase receptors and Smad (mothers against decapentaplegic homologue 4) transcriptional regulators (Massague et al, 2005). Alternatively, non-Smad pathways are involved in TGF-β signalling through mitogen-activated protein kinase, Rho-like or PI3K/AKT pathways (Moustakas & Heldin, 2005). Transcription intermediary factor 1γ (TIF1γ)—which was recently shown to be involved in TGF-β signalling—is characterized by several domains; RING finger, B boxes, coiled coil, PHD/TTC and bromodomain (Venturini et al, 1999). TIF1γ is involved in hematopoiesis, transcription elongation (Ransom et al, 2004; He et al, 2006; Bai et al, 2010) and iNKT-cell development, a subclass of natural killer T-cells (Doisne et al, 2009). In Xenopus embryos, TIF1γ (also known as ectodermin) is essential for ectoderm specification by restricting the mesoderm-inducing activity of TGF-β (Dupont et al, 2005). The negative control of Smad activity by TIF1γ controls early mouse development by tuning the responses of extra-embryonic and embryonic cells to Nodal (Morsut et al, 2010). It has been proposed that TIF1γ functions as a RING-finger ubiquitin ligase for Smad4. Smad4 monoubiquitination by TIF1γ promotes its nuclear export and inhibits the formation of Smad nuclear complexes (Dupont et al, 2009). TIF1γ has also been described as a selective binder of phosphorylated Smad2/3, competing with Smad4 to mediate an alternative, Smad4-independent TGF-β pathway (He et al, 2006).
The role of TGF-β in malignant transformation is ambiguous. At the early stages of tumorigenesis, it is an important tumour suppressor inducing apoptosis and cell-cycle arrest. At later stages, it stimulates tumour progression. Indeed, TGF-β promotes angiogenesis and modifications of the extracellular matrix facilitating invasion and metastasis (Thiery et al, 2009). In addition, cancer cells lose their ability to be growth-arrested by TGF-β, but retain their ability to undergo epithelial-to-mesenchymal transition (EMT; Massague, 2008; Heldin et al, 2009). Although the role of EMT in cancer has long been debated (Garber, 2008), recent advances indicate that EMT contributes to cancer-cell plasticity and to the invasive potential of transformed epithelial cells (Kalluri & Weinberg, 2009). It has been shown to endow normal and transformed mammary epithelial cells with stem-cell-like properties, including the ability to self-renew (Mani et al, 2008; Morel et al, 2008). Recent studies suggest that in cancer cells, EMT might help to prevent oncogene-induced senescence, thereby facilitating dissemination (Ansieau et al, 2008).
TGF-β is known to be a potent inducer of EMT. To better understand the role of TIF1γ in TGF-β signalling and its requirement for EMT, we analysed TGF-β1 response in human mammary epithelial cell lines. We show that TIF1γ inactivation facilitates the contribution of Smad4 to TGF-β1-induced EMT, but does not affect the anti-proliferative function of TGF-β1.
Results
Identification of TIF1γ target genes
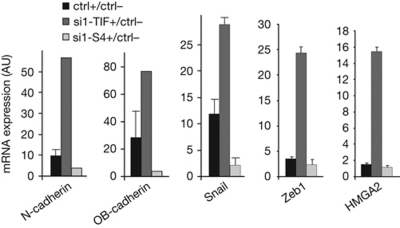
We characterized TIF1γ target genes during TGF-β1-induced EMT by transcriptomic analysis of HMEC-TR cells silenced for TIF1γ or Smad4 (supplementary Fig S1 online). The original data are available at the Gene Expression Omnibus database under accession number GSE28448. Microarray data were confirmed by quantitative reverse transcription (qRT)–PCR analysis of 14 TGF-β1 target genes (supplementary Fig S2 online). From the 707 TIF1γ-dependent genes (supplementary Table S2 online), we selected 96 genes encoding proteins involved in cytoskeleton dynamics, cell–cell and cell–matrix adhesion (supplementary Table S3 online), and potentially in EMT. Other functional groups are shown in supplementary Table S4 online. TGF-β1 target genes (fold change⩾2) regulated by TIF1γ and potentially involved in EMT are shown in Table 1. Of the 457 genes regulated by TIF1γ and Smad4, 37 showed opposite responses to Smad4 or TIF1γ downregulation (supplementary Table S5 online). These 37 genes could represent the signature of the repressive function of TIF1γ on Smad4. Of these, 12 encode proteins with functions linked to EMT, such as N-cadherin. The expression of TIF1γ-dependent genes was analysed in another mammary epithelial cell line (MCF10A) by qRT–PCR. The TGF-β1-induced upregulation of genes such as N-cadherin, OB-cadherin, SNAIL, ZEB1 and HMGA2, was abolished by Smad4 silencing, but enhanced by TIF1γ silencing (Fig 1). Together, these results show that TIF1γ target genes are not restricted to HMEC-TR cells and that TIF1γ could be involved in the regulation of TGF-β1-induced EMT.
Table 1. TGF-β1 target genes (fold change ⩾2 or ⩽0.5; P<0.05) regulated by TIF1γ and potentially involved in EMT.
| Gene symbol | Gene name | ctrl+/ctrl− | si-S4+/ctrl− | si-TIF+/ctrl− |
|---|---|---|---|---|
| ADAM12 * | A disintegrin and metalloproteinase domain 12 (meltrin-α) | 2.83 | 1.31 | 4.25 |
| AKAP9 | A kinase (PRKA) anchor protein (yotiao) 9 | 2.32 | 1.25 | 1.27 |
| CD58 | CD58 antigen (lymphocyte function-associated antigen 3) | 3.92 | 1.90 | 1.77 |
| CDH11 * | Cadherin 11, type 2, OB-cadherin (osteoblast) | 2.25 | 1.42 | 6.29 |
| CDH2 * | Cadherin 2, type 1, N-cadherin (neuronal) | 2.38 | 1.05 | 4.76 |
| CLDN14 | Claudin 14 | 3.38 | 1.45 | 1.39 |
| CLDN4 | Claudin 4 | 3.15 | 0.89 | 1.43 |
| CLU | Clusterin | 2.03 | 1.56 | 1.24 |
| COL1A1 | Collagen, type I, α1 | 6.54 | 0.19 | 0.41 |
| COL5A1 | Collagen, type V, α1 | 5.59 | 1.60 | 9.40 |
| COL5A2 | Collagen, type V, α2 | 2.29 | 0.64 | 1.10 |
| FANK1 | Fibronectin type 3 and ankyrin-repeat domains 1 | 2.27 | 0.66 | 0.82 |
| HNT | Neurotrimin | 2.65 | 0.52 | 0.68 |
| ITGB3 | Integrin β3 (platelet glycoprotein IIIa, antigen CD61) | 8.26 | 1.26 | 3.89 |
| JPH1 | Junctophilin 1 | 0.41 | 0.76 | 0.27 |
| KAL1 | Kallmann syndrome 1 sequence | 2.13 | 0.91 | 1.20 |
| KRT16 | Keratin 16 | 2.13 | 0.4 | 0.61 |
| KRTHA4 | Keratin, Hair, Acidic, 4 (intermediate filament) | 5.78 | 2.28 | 12.02 |
| MLPH | Melanophilin | 2.07 | 2.85 | 9.46 |
| MMP10 | Matrix metalloproteinase 10 (stromelysin 2) | 4.44 | 0.15 | 0.48 |
| PIK3CD | Phosphoinositide-3-kinase, Catalytic, delta polypeptide | 2.48 | 1.35 | 4.01 |
| PKP1 | Plakophilin 1 (ectodermal dysplasia/skin fragility syndrome) | 2.13 | 1.16 | 3.66 |
| PODXL | Podocalyxin-like | 2.73 | 0.43 | 1.65 |
| SERPINE1 * | Plasminogen activator inhibitor type 1 (PAI1) | 5.95 | 1.14 | 10.26 |
| TPM1 | Tropomyosin 1 (α) | 4.55 | 1.18 | 2.0 |
| TSPAN-2 | Tetraspan 2 | 3.64 | 0.85 | 2.33 |
| WASF3 | WAS protein family, member 3 | 0.50 | 2.01 | 1.54 |
| WNT5A | Wingless-type MMTV integration site family, member 5A | 2.44 | 1.44 | 3.90 |
| WNT5B * | Wingless-type MMTV integration site family, member 5B | 4.29 | 2.87 | 8.87 |
| WNT9A * | Wingless-type MMTV integration site family, member 9A | 2.23 | 0.44 | 7.17 |
| ctrl, control; EMT, epithelial-to-mesenchymal transition; S4, mad4; TGF-β1, transforming growth factor-β1; TIF1γ, transcription intermediary factor 1γ. | ||||
| Genes showing opposite responses to Smad4 or TIF1γ downregulation are presented in bold. | ||||
| *Target genes for which quantitative reverse transcription PCR was performed. | ||||
Figure 1.
MCF10A cells inactivated for TIF1γ or Smad4 were treated with TGF-β1 for 9 h. Expression of the indicated genes was determined by qRT–PCR. Values were normalized to the amount of HPRT mRNA and expressed relative to the value obtained in TGF-β1-untreated controls. Error bars represent s.d. The experiment shown is representative of at least two separate experiments conducted in triplicate. AU, arbitrary units; ctrl, control; HPRT, hypoxanthine-guanine phosphoribosyltransferase; mRNA, messenger RNA; siRNA, short-interfering RNA; S4, Smad4; TGF-β1, transforming growth factor-β1; TIF, transcription intermediary factor 1γ.
TIF1γ inactivation enhances TGF-β-induced EMT
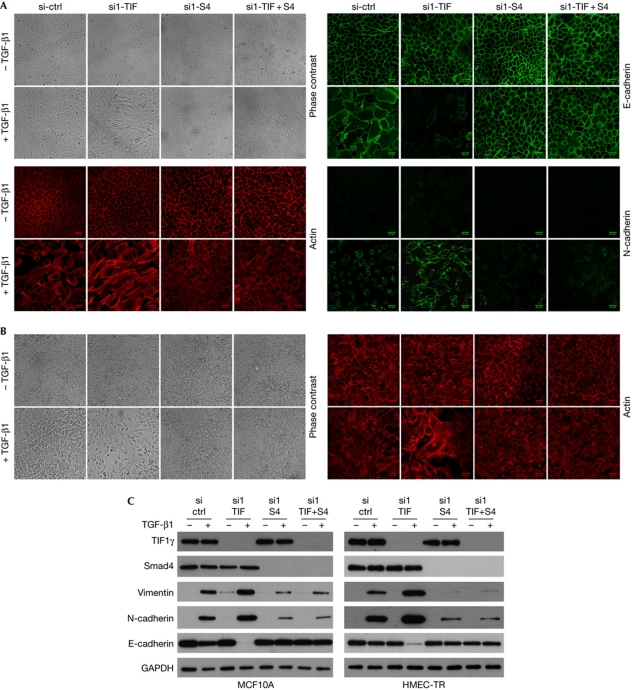
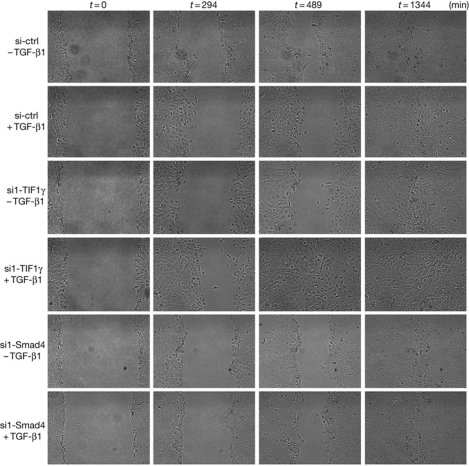
Results from transcriptomic analysis showed that several TIF1γ target genes are involved in EMT. To address the role of TIF1γ during EMT, we used the mammary epithelial cell lines MCF10A and HMEC-TR. First, we validated that TGF-β1 induces characteristic features of EMT in these cells (Fig 2). Compared with control cells treated with TGF-β1, TIF1γ-silenced cells had increased actin polymerization, reorganization of stress fibres into large linear and parallel bundles and fibroblast-like elongated morphology (Fig 2A,B). Immunofluorescence analysis and western blotting also showed increased expression of N-cadherin and vimentin and downregulation of E-cadherin (Fig 2). Similar results were obtained with a second short-interfering RNA (siRNA) targeting human TIF1γ messenger RNA (mRNA; supplementary Fig S3 online). By contrast, Smad4 silencing or double Smad4 and TIF1γ knockdown led to a complete block of TGF-β1-induced EMT (Fig 2). These results support the idea that Smad4 has a key role in TGF-β1-induced EMT (Valcourt et al, 2005; Deckers et al, 2006). To check whether TGF-β1-induced EMT is reversible, MCF10A cells that were stably inactivated for TIF1γ were exposed to TGF-β1 for 21 days. A full EMT phenotype was observed, with gradual reversion to epithelial cells resembling parental MCF10A cells after TGF-β1 removal and adjunction of TβRI kinase inhibitor (SB-431542; supplementary Fig S4 online). We next tested whether EMT in TIF1γ knockdown cells was accompanied by a modification of cell motility. Results of wound healing assays in MCF10A cells monitored by time-lapse video microscopy indicated that untreated cells on both sides of the wound moved as a cohesive unit (Fig 3). This organized migration was compromised in cells that had undergone EMT under TGF-β1 treatment. By contrast, TIF1γ-silenced cells quickly moved into the wound (supplementary Fig S5 online), resulting in faster closure. As expected, Smad4 silencing reduced cell motility. These results show that TIF1γ has an important regulatory role in TGF-β1-induced EMT.
Figure 2.
Inactivation of TIF1γ expression enhances TGF-β1-induced EMT. MCF10A (A) and HMEC-TR (B) cells were knocked down using siRNA and treated or not with TGF-β1 for 96 h. To block any signalling arising from autocrine production of TGF-β, experiments without TGF-β were performed using the TβRI kinase inhibitor SB-431542. Phase-contrast images ( × 10) and immunofluorescence confocal acquisitions showing actin subcellular localization detected by phalloidin or indirect immunofluorescence using E- or N-cadherin-targeting antibodies. Scale bars, 26 μm. (C) Endogenous TIF1γ, Smad4, vimentin, N-cadherin and E-cadherin levels were determined by immunoblotting. GAPDH was used as a loading control. ctrl, control; EMT, epithelial-to-mesenchymal transition; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; siRNA, short-interfering RNA; S4, Smad4; TGF-β1, transforming growth factor-β1; TIF, transcription intermediary factor 1γ.
Figure 3.
Inactivation of TIF1γ expression enhances TGF-β1-induced cell migration. After the wound, the healing process of MCF10A cells was observed over 24 h using time-lapse photography. Images were combined into a film. Illustrative stills from the time-lapse recording are representative of at least three independent experiments. ctrl, control; TGF-β1, transforming growth factor-β1; TIF1γ, transcription intermediary factor 1γ.
Inactivation of TIF1γ has no effect on cell proliferation
As Smad4 is a transmitter of growth-inhibitory effects of TGF-β1, we analysed the effect of TIF1γ inactivation on MCF10A and HMEC-TR proliferation. Growth inhibition was observed in TGF-β1-treated cells (Fig 4A). TIF1γ silencing had no effect on TGF-β1-induced growth arrest, whereas, as expected, Smad4 silencing suppressed this arrest. We next examined the extent to which TIF1γ downregulation altered cell-cycle progression in HMEC-TR cells. Four days after TGF-β1 treatment, DNA content was analysed by flow cytometry. Cell-cycle distribution was not modified by TIF1γ silencing, whereas, as expected, Smad4 silencing suppressed the effect of TGF-β1 (supplementary Fig S6 online). These results are consistent with qRT–PCR data for the known Smad4 target genes p21 (CDKN1A) and MYC. In contrast to Smad4 silencing, inactivation of TIF1γ did not affect the modulation of p21 and MYC expression induced by TGF-β1 in MCF10A and HMEC-TR cell lines (Fig 4B). Moreover, several Smad4 target genes involved in the regulation of cell proliferation did not seem to be TIF1γ target genes (supplementary Table S6 online). Taken together, these results indicate that TIF1γ is not involved in the regulation of the proliferation of mammary epithelial cells upon TGF-β1 treatment.
Figure 4.
Inactivation of TIF1γ expression has no effect on cell proliferation. (A) Proliferation of TIF1γ or Smad4-inactivated cells cultured with or without TGF-β1 for 96 h. (B) Cells were treated with TGF-β1 for 9 h. TGF-β-induced fold changes of p21 (CDKN1A) and MYC were analysed by qRT–PCR. All values were normalized to the amount of HPRT messenger RNA and expressed relative to the value obtained for TGF-β-untreated controls. The experiments shown are representative of three separate experiments performed in triplicate. Error bars indicate s.d. ctrl, control; NT, not transfected; qRT–PCR, quantitative reverse transcription PCR; S4, Smad4; TGF-β1, transforming growth factor-β1; TIF, transcription intermediary factor 1γ.
TIF1γ inhibits TGF-β1 transcriptional response
The TGF-β1-induced accumulation of plasminogen activator inhibitor 1 (PAI1) mRNA was abolished by Smad4 silencing, but strongly enhanced upon TIF1γ silencing (Fig 5A; supplementary Fig S2 online). We next tested whether TIF1γ could modulate the transcriptional activity of PAI1, which is known to respond to TGF-β1 in a Smad4-dependent manner. As observed for the (CAGA)9-Luc reporter (supplementary Fig S7A,B online), exogenous TIF1γ inhibited the TGF-β1-induced transactivation of PAI1-Luc, whereas TIF1γ inactivation enhanced signalling (Fig 5B). The Ub-ligase activity of TIF1γ is essential for inhibiting luciferase reporters that are sensitive to TGF-β (Dupont et al, 2009). Isolated domains of TIF1γ (TRIM, middle or PHD/Br) have no biological effect on luciferase reporter construct (Dupont et al, 2009; supplementary Fig S7C online), suggesting that the full length of the protein is required. These findings indicate that TIF1γ negatively affects Smad4 function, compromising TGF-β1 signalling.
Figure 5.
TIF1γ inhibits TGF-β1 signalling. (A) HMEC-TR cells inactivated for TIF1γ or Smad4 were treated with TGF-β1 for 9 h. TGF-β1-induced fold changes of PAI1 were analysed by qRT–PCR. All values were normalized to the amount of HPRT messenger RNA and expressed relative to the value obtained for TGF-β1-untreated controls. The experiment shown is representative of three separate experiments performed in triplicate; error bars represent s.d. (B) HMEC-TR cells were co-transfected with the pGL3(PAI1)-Luc vector together with the pRL-CMV (cytomegalovirus) internal control vector and expression vectors or short-interfering RNA, as indicated. Relative luciferase activity is given as the mean±s.d. of an experiment performed in triplicate, representative of three experiments. (C,D) ChIP assay was performed on HMEC-TR cells transfected (D) or not (C) with TIF1γ expression vector as indicated and treated with TGF-β1 for 2 h. PCR amplification of endogenous PAI1 promoter (733/484) was performed to detect protein-bound DNA. Rabbit preimmune serum was used as a negative control. Primers specific to PAI1 were used before (input) and primers specific for actin were used after immunoprecipitation as a control to monitor immunoprecipitation specificity. (E,F) ChIP assay of the kinetics of Smad4 and TIF1γ binding to DNA after TGF-β1 stimulation. HMEC-TR cells transfected with PAI1 promoter were subjected to PCR analysis (E). The relative binding of Smad4 and TIF1γ proteins to the human PAI1 promoter was also measured by qRT–PCR analysis of the precipitated DNA and input DNA (F). Results are expressed as percentages of the total DNA input. The data shown correspond to one of two independent experiments performed with comparable results. (G) ChIP assay of the kinetics of Smad4 binding to DNA after TGF-β1 stimulation. HMEC-TR cells transfected with PAI1 promoter and inactivated for TIF1γ were subjected to PCR analysis. AU, arbitrary units; ChIP, chromatin immunoprecipitation; ctrl, control; IgG, immunoglobulin G; IP, immunoprecipitation; PAI1, plasminogen activator inhibitor 1; qRT–PCR, quantitative reverse transcription PCR; S4, Smad4; TGF-β1, transforming growth factor-β1; TIF, transcription intermediary factor 1γ.
To test whether TIF1γ can bind to the PAI1 promoter region harboring the Smad-binding elements, we performed chromatin immunoprecipitation (ChIP) analyses. As expected, Smad4 was specifically immunoprecipitated with the endogenous PAI1 promoter after TGF-β treatment. Interestingly, TIF1γ was able to bind to the same region of the PAI1 promoter (Fig 5C). Consistent with results showing that TIF1γ inhibits the Smad3/4 complex (He et al, 2006; supplementary Fig S8A,B online), TIF1γ protein expression reduced the Smad4–DNA association and enhanced its association with DNA (Fig 5D). Next, we analysed the kinetics of Smad4 and TIF1γ binding to DNA after TGF-β stimulation. TIF1γ binding to the promoter was detected under basal condition. TGF-β1 caused TIF1γ release from the promoter and induced Smad4 recruitment. Two hours after TGF-β1 treatment, TIF1γ was recruited instead of Smad4 (Fig 5E,F). Interestingly, 90 min after TGF-β1 treatment, both Smad4 and TIF1γ bound to the PAI1 promoter, suggesting that an intermediary and transitory complex could exist between TIF1γ and Smad4. These results are in agreement with data showing that TIF1γ interacts with Smad4 and promotes its nuclear export (Dupont et al, 2009; supplementary Fig S8D online). TIF1γ silencing enhanced Smad4 binding to DNA with the same kinetics (Fig 5G). These results indicate that TIF1γ can function as a repressor of Smad4 and thus has a direct role in TGF-β1-dependent gene expression.
Discussion
This study demonstrates that TIF1γ participates in TGF-β signalling as a negative regulator of Smad4 during TGF-β-induced EMT in mammary epithelial cell lines. Inactivation of TIF1γ markedly intensifies the mesenchymal characteristics triggered by TGF-β. By contrast, Smad4 downregulation leads to complete inhibition of TGF-β-induced EMT. These results are supported by microarray data showing that several genes involved in EMT are antagonistically regulated by Smad4 and TIF1γ. Taken together, our results show that TIF1γ inactivation enhances the function of Smad4 during TGF-β-induced EMT, but does not affect the anti-proliferative function of TGF-β. In the light of the morphogen-like properties of TGF-β and the role of TIF1γ in the embryo and in trophoblast stem cells (Morsut et al, 2010), TGF-β-mediated induction of growth arrest can be considered a low-threshold response, requiring prolonged association of Smad with its target promoter. Other responses, such as EMT induction, might correspond to higher threshold responses.
As shown previously (He et al, 2006), we have found that TIF1γ expression decreases Smad3/4 interaction and favours Smad3/TIF1γ complex, whereas TIF1γ downregulation favours Smad3/4 complex required for Smad4 function (supplementary Fig S8A,B online). TIF1γ interacts with Smad4 (Dupont et al, 2009; supplementary Fig S8D online) and our demonstration that TIF1γ inhibits Smad4 function is consistent with the TGF-β-dependent ubiquitination of Smad4 by TIF1γ (Dupont et al, 2009). TIF1γ could destroy the association of Smad4 with Smad2/3, causing inhibition of Smad4 function. In TIF1γ-depleted cells, Smad4 is more available for association with Smad2/3, leading to enhanced TGF-β signalling. These results corroborate data from ChIP assays showing that TIF1γ can function as a repressor of Smad4. TIF1γ expression inhibits Smad4 binding to DNA and is involved in the interplay of Smad4 with the transcriptional machinery in TGF-β-mediated transcription. TIF1γ can interact with phosphorylated Smad2/3 (He et al, 2006; supplementary Fig S8A–C online), and thus we cannot exclude its role in an alternative, Smad4-independent TGF-β pathway.
EMT triggered by TGF-β is regulated by TIF1γ, which limits Smad4 function, suggesting that TIF1γ alterations could contribute to the nature of aggressive cancer cells by promoting EMT, probably facilitating cell invasion. Finally, these results corroborate data from mouse models, showing that loss of TIF1γ function cooperates with KrasG12D activation to induce pancreatic tumours (Vincent et al, 2009).
Methods
Cell culture and siRNA transfection. Human mammary epithelial cells infected with a retrovirus carrying hTERT and the oncogenic allele H-RasV12 (HMEC-TR) were provided by RA Weinberg (Elenbaas et al, 2001). The mammary epithelial cell line MCF10A was obtained from American Type Culture Collection. Recombinant TGF-β1 (Peprotech) was used at 10 ng/ml. A total of 1.5 × 105 cells were collected in OptiMEM medium (Invitrogen) and transfected with 5 nM siRNA and 0.5 μl/ml lipofectamine RNAiMax (Invitrogen). Cells were cultured for 48 or 96 h in complete fresh medium supplemented or not with TGF-β1. The siRNA sequences used are listed in supplementary Table S7 online.
Microarray analysis. Replicates of total RNA from HMEC-TR cells silenced for TIF1γ or Smad4 were obtained from two independent cell cultures. The microarrays were performed using GeneChip Human Genome U133 plus 2.0 Arrays (Affymetrix) as described previously (Hesling et al, 2007). The original data are available from the Gene Expression Omnibus (accession number GSE28448). Normalized data were statistically filtered with GeneSpring software (Agilent) using Pairwise comparison (supplementary information online).
qPCR. One microgram of total RNA was used for complementary DNA synthesis with the SuperScript II Reverse Transcriptase system (Invitrogen). mRNA levels were quantified using the SYBR Green StepOne Plus Real Time PCR system (Applied Biosystems). Primers are listed in supplementary Table S8 online.
Western blot analysis. Lysates of cells prepared in RIPA lysis buffer were separated by SDS–polyacrylamide gel electrophoresis, electroblotted onto polyvinylidene difluoride transfer membrane, stained with specific primary antibodies (Smad4, SantaCruz Biotechnology; TIF1γ, Euromedex; vimentin, Dako; E-cadherin and N-cadherin, BD Biosciences) and horseradish-peroxidase-linked secondary antibodies, and then detected with electrochemiluminescence plus reagent (Roche).
Proliferation assays. SiRNA-treated cells were seeded in triplicate, cultured for 4 h and then treated with TGF-β1. At each time point, cells were treated with Uptiblue (Interchim) and incubated for 4 h at 37°C. Fluorescence intensity was monitored at 530–560 nm excitation wavelength and 590 nm emission wavelength (CytoFluor, PerSeptive Biosystem).
Immunofluorescence. Cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS), permeabilized with 0.4% Triton X-100 in PBS for 5 min and subsequently stained with 0.25 mM tetramethyl rhodamine iso-thiocyanate-conjugated phalloidin (Sigma-Aldrich), E-cadherin or N-cadherin antibodies. Fluorescence was examined by confocal laser scanning microscopy (Carl Zeiss).
Wound healing. MCF10A cells were grown to confluence with or without TGF-β1. Medium was aspirated and a gel filter tip was drawn carefully through the monolayer to create a wound between cells. After washing with PBS, fresh medium was added, with or without TGF-β1. Plates were transferred to a heated stage on a Zeiss microscope. The field of view was centred on a section of the wound. Phase images were recorded every minute for up to 24 h and then processed into a film.
Luciferase assays. HMEC-TR cells transfected or not with siRNAs or expression vectors were co-transfected with a PAI1 promoter (p800-Luc) luciferase construct (supplementary information online). Luciferase activity was measured in equivalent amounts of each lysate using the dual luciferase kit (Promega).
ChIP. HMEC-TR cells transfected or not with PAI1 promoter (p800-Luc) were treated or not with TGF-β1 for the indicated time. Assays were carried out using the kit from Upstate Biotechnology. Cell lysates were subjected to anti-Smad4 (SantaCruz) or anti-TIF1γ (Bethyl) immunoprecipitation. Rabbit IgG (Abcam) was used as a negative control. Following reverse crosslinking, DNA was treated with proteinase K and purified. Smad4- or TIF1γ-precipitated genomic DNA was subjected to PCR. The 351 bp PAI1 promoter region harbouring the Smad-binding elements was amplified with primers 5′-AGCCAGACAAGGTTGTTG-3′ and 5′-GACCACCTCCAGGAAAG-3′. An unrelated genomic DNA sequence (actin) was amplified with primers 5′-AGCCATGTACGTTGCTATCCAG-3′ and 5′-CTTCTCCTTAATGTCACGCACG-3′. The relative binding of proteins of interest Smad4 and TIF1γ to the human PAI1 promoter was also measured by real-time quantitative PCR analysis of the precipitated DNA and input DNA. Results are presented as ‘percent input’ values.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank R.A. Weinberg for providing HMEC derivatives and C. Languilaire for excellent technical assistance. We also thank M.D. Reynaud for help in manuscript preparation, S. Croz from ProfileXpert for microarray study and CeCIL platform (Structure Fédérative de Recherche; SFR Santé Lyon-Est) for videomicroscopic study. This work was supported by grants from the Ligue Nationale contre le Cancer (Savoie, Saone & Loire), Institut National du Cancer (INCA), Association pour la Recherche sur le Cancer (ARC) and IFR62.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ansieau S et al. (2008) Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 14: 79–89 [DOI] [PubMed] [Google Scholar]
- Bai X et al. (2010) TIF1γ controls erythroid cell fate by regulating transcription elongation. Cell 142: 133–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers M, van DM, Buijs J, Que I, Lowik C, van der PG, Ten DP (2006) The tumor suppressor Smad4 is required for transforming growth factor β-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res 66: 2202–2209 [DOI] [PubMed] [Google Scholar]
- Doisne JM et al. (2009) iNKT cell development is orchestrated by different branches of TGF-β signaling. J Exp Med 206: 1365–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Zacchigna L, Cordenonsi M, Soligo S, Adorno M, Rugge M, Piccolo S (2005) Germ-layer specification and control of cell growth by Ectodermin, a Smad4 ubiquitin ligase. Cell 121: 87–99 [DOI] [PubMed] [Google Scholar]
- Dupont S et al. (2009) FAM/USP9x, a deubiquitinating enzyme essential for TGFβ signaling, controls Smad4 monoubiquitination. Cell 136: 123–135 [DOI] [PubMed] [Google Scholar]
- Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL, Popescu NC, Hahn WC, Weinberg RA (2001) Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev 15: 50–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K (2008) Epithelial-to-mesenchymal transition is important to metastasis, but questions remain. J Natl Cancer Inst 100: 232–233, 239 [DOI] [PubMed] [Google Scholar]
- He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MA, Massague J (2006) Hematopoiesis controlled by distinct TIF1γ and Smad4 branches of the TGFβ pathway. Cell 125: 929–941 [DOI] [PubMed] [Google Scholar]
- Heldin CH, Landstrom M, Moustakas A (2009) Mechanism of TGF-β signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol 21: 166–176 [DOI] [PubMed] [Google Scholar]
- Hesling C, Oliveira CC, Castilho BA, Zanchin NI (2007) The Shwachman-Bodian-Diamond syndrome associated protein interacts with HsNip7 and its down-regulation affects gene expression at the transcriptional and translational levels. Exp Cell Res 313: 4180–4195 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA et al. (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J (2008) TGFβ in cancer. Cell 134: 215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D (2005) Smad transcription factors. Genes Dev 19: 2783–2810 [DOI] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A (2008) Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 3: e2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsut L et al. (2010) Negative control of Smad activity by ectodermin/Tif1γ patterns the mammalian embryo. Development 137: 2571–2578 [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH (2005) Non-Smad TGFβ signals. J Cell Sci 118: 3573–3584 [DOI] [PubMed] [Google Scholar]
- Ransom DG et al. (2004) The zebrafish moonshine gene encodes transcriptional intermediary factor 1γ, an essential regulator of hematopoiesis. PLoS Biol 2: E237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139: 871–890 [DOI] [PubMed] [Google Scholar]
- Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A (2005) TGF-β and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell 16: 1987–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini L et al. (1999) TIF1γ, a novel member of the transcriptional intermediary factor 1 family. Oncogene 18: 1209–1217 [DOI] [PubMed] [Google Scholar]
- Vincent DF et al. (2009) Inactivation of TIF1γ cooperates with Kras to induce cystic tumors of the pancreas. PLoS Genet 5: e1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.