Abstract
We report new functions of the cell-adhesion molecule E-cadherin in murine pluripotent cells. E-cadherin is highly expressed in mouse embryonic stem cells, and interference with E-cadherin causes differentiation. During cellular reprogramming of mouse fibroblasts by OCT4, SOX2, KLF4 and c-MYC, fully reprogrammed cells were exclusively observed in the E-cadherin-positive cell population and could not be obtained in the absence of E-cadherin. Moreover, reprogrammed cells could be established by viral E-cadherin in the absence of exogenous OCT4. Thus, reprogramming requires spatial cues that cross-talk with essential transcription factors. The cell-adhesion molecule E-cadherin has important functions in pluripotency and reprogramming.
Keywords: pluripotency, somatic cell reprogramming, E-cadherin, OCT4
Introduction
The regulation of pluripotency in mouse embryonic stem cells (mESCs) is a fascinating biological question with important implications for both basic science and the therapeutic potential of such cells. The same is true for induced pluripotent stem cells (iPSCs) of somatic origin. Both pluripotency and reprogramming are influenced by several cellular processes such as regulation of transcription, signal transduction, regulation of microRNAs and epigenetic changes (reviewed in Jaenisch & Young, 2008). The concept of somatic cell reprogramming began with somatic cell nuclear transfer (Wilmut et al, 1997) and was further developed by the finding that mouse embryonic fibroblasts (MEFs) can be converted into iPSCs by retroviral expression of four transcription factors: OCT4, SOX2, KLF4 and c-MYC (Takahashi & Yamanaka, 2006).
The cadherins are membrane-spanning proteins of adherens junctions that have crucial roles in cell–cell contact formation. A switch from epithelial components, such as E-cadherin (Cadherin 1, Ecad) expression, to mesenchymal components can occur, termed epithelial–mesenchymal transition, which promotes the cell migration in the development of many organs and in the metastasis of tumours (Heuberger & Birchmeier, 2010). Opposite mechanisms—mesenchymal–epithelial transitions (METs)—are important in strengthening tissue integrity following migratory processes in embryonic development. The function of E-cadherin in cell–cell adhesion is also connected to various signalling pathways that relay information regarding cell interactions to the cytoplasm and the nucleus (Stepniak et al, 2009). In early mammalian development, E-cadherin-based cell–cell contacts are required for the integrity of the embryonic blastocyst, and deletion of the E-cadherin gene results in failure of cell compaction at this stage (Larue et al, 1994). It has also been shown that E-cadherin controls processes of early differentiation (Larue et al, 1996) and has an important role in regulating pluripotency of different stem-cell compartments (Chou et al, 2008; Soncin et al, 2009).
In this study, we show that E-cadherin is essential for the maintenance of pluripotency of mESCs, and that disturbance or loss of E-cadherin induces epithelial–mesenchymal transitions. During reprogramming of MEFs to iPSCs, E-cadherin production is associated with the reprogramming process and, remarkably, exogenous expression of E-cadherin can replace the requirement for OCT4 during reprogramming.
Results And Discussion
E-cadherin is induced during reprogramming
We observe that undifferentiated mESCs show a high level of E-cadherin and OCT4 messenger RNA (mRNA) and protein expression, that is lost when cells differentiate (supplementary Fig S1A,B online). Moreover, by using short-hairpin RNA against E-cadherin, we showed that high expression of E-cadherin in pluripotent mESCs is crucial for maintenance of the undifferentiated state (supplementary Fig S1C–E online). Furthermore, mESCs that were treated either with a neutralizing antibody, DECMA-1, or short-interfering RNA against Ecad, or maintained in the absence of leukaemia inhibitory factor (LIF) for 7 days (−LIF, 7d), started to differentiate while changing their morphology from compact colonies to scattered cells (supplementary Fig S1F online). Differentiating cells underwent a cadherin switch to N-cadherin (Ncad) expression, whereas the expression of E-cadherin and OCT4 were lost. Our results show that E-cadherin is required to maintain the undifferentiated state of mESCs.
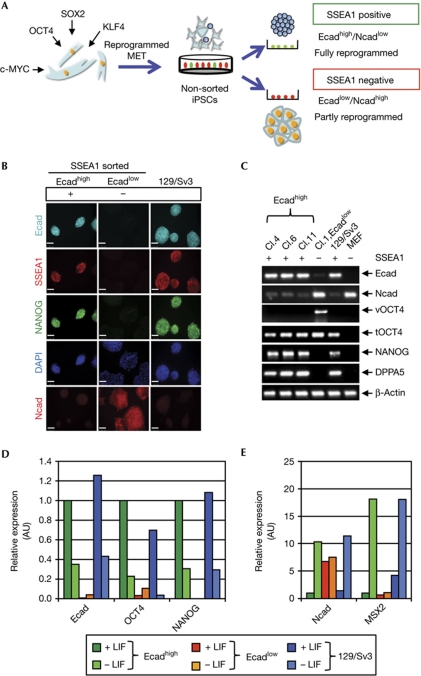
Reprogramming of MEFs through viral expression of OCT4, SOX2, KLF4 and c-MYC (OSKM) proceeds in a stepwise manner that gives rise to not only iPSCs, but also intermediate cell states. We used this procedure to generate iPSCs and sorted for cells that expressed the stem-cell marker stage-specific embryonic antigen 1 (SSEA1) by FACS analysis (Fig 1A). A total of 12.5% SSEA1-positive cells were obtained 8 days after retroviral transduction (supplementary Fig S2A online), and these cells maintained SSEA1 expression (supplementary Fig S2B online). Intriguingly, SSEA1-positive cell clones expressed E-cadherin but not N-cadherin, as detected by immunofluorescence analysis, whereas SSEA1-negative cell clones were low in E-cadherin but high in N-cadherin (Fig 1B). NANOG was only expressed in the SSEA1-positive cells, suggesting that these cells had undergone complete reprogramming. In line with the immunofluorescence analysis, reverse transcription–polymerase chain reaction (RT–PCR) analysis showed that SSEA1-positive cell clones expressed high levels of E-cadherin (Ecadhigh), as well as the pluripotency factors NANOG and DPPA5, and low levels of N-cadherin (Fig 1C). The Ecadlow cell clones showed residual expression of viral OCT4, SOX2, KLF4 and c-MYC and low levels of the endogenous genes, showing that retroviral expression was not silenced (Fig 1C, supplementary Fig S2C online).
Figure 1.
SSEA1-positive induced pluripotent stem cells express E-cadherin and pluripotency genes. (A) Mouse embryonic fibroblasts were retrovirally transduced with four factors, OCT4, SOX2, KLF4 and c-MYC, and sorted for SSEA1 expression after 8 days. (B) Characterization of established cell clones derived from SSEA1-positive (Ecadhigh) cells and SSEA1-negative (Ecadlow) cells, in comparison with 129/Sv3 mouse embryonic stem cells. Immunofluorescence for Ecad (cyan), SSEA1 (red) and NANOG (green) are shown. Nuclei were stained with DAPI (blue). Ncad (red) was analysed in independent clones. Magnification × 400; scale bars, 50 μm. (C) Agarose gel electrophoresis of reverse transcription–polymerase chain reaction products for marker expression of three stable independent Ecadhigh iPSC clones (clones 4, 6 and 11) in comparison with cells of an Ecadlow clone (clone 1, Ecadlow), 129/Sv3 and MEFs. SSEA1 expression was determined by flow cytometry and is indicated as (+/−). Expression of Ecad, Ncad, viral (v) and total (t) OCT4, NANOG and DPPA5 was analysed and related to β-actin. (D) Quantitative real-time RT–PCR analysis of spontaneously differentiated Ecadhigh (Ecadhigh) iPSCs, Ecadlow cells and 129/Sv3 cells grown for 4 days in the presence or absence of LIF. Messenger RNA levels for Ecad, OCT4 and NANOG, and (E) Ncad and MSX2. Expression levels are related to Ecadhigh iPSCs in LIF set to 1. Median values from biological duplicates are presented as bars, one representative experiment out of four independent experiments is shown. AU, arbitrary units; Cl., clone; DAPI, 4,6-diamidino-2-phenylindole; iPSC, induced pluripotent stem cell; MET, mesenchymal-to-epithelial transition; SSEA1, stage-specific embryonic antigen 1.
We tested the differentiation capacity of Ecadhigh and Ecadlow clones. E-cadherin, OCT4 and NANOG mRNA levels were similarly downregulated in Ecadhigh clones and 129/Sv3 mESCs on LIF withdrawal (Fig 1D), and the mRNAs for N-cadherin and MSX2 were induced (Fig 1E). Protein levels of cadherins and pluripotency factors and cell morphologies in the presence and absence of LIF were confirmed (supplementary Fig S2D,E online). Interestingly, Ecadlow cells that still contained high N-cadherin levels resulting from an inefficient MET process were unable to undergo spontaneous differentiation. We then examined the ability of our cells to undergo more stringent differentiations. When cells of both Ecadhigh and Ecadlow clones were used for embryoid-body formation, only Ecadhigh clones could form embryoid bodies (supplementary Fig S2E online). In further analyses, we used both cell clones for in vivo differentiation, such as teratoma formation (supplementary Fig S2F,G online) and chimera formation after blastocyst injection (supplementary Fig S2H online). We confirmed that Ecadhigh cells fulfilled all criteria of pluripotent cells, whereas Ecadlow cells were not able to form differentiated tumours or to integrate in blastocysts.
Deletion of E-cadherin in Ecadflox prevents reprogramming
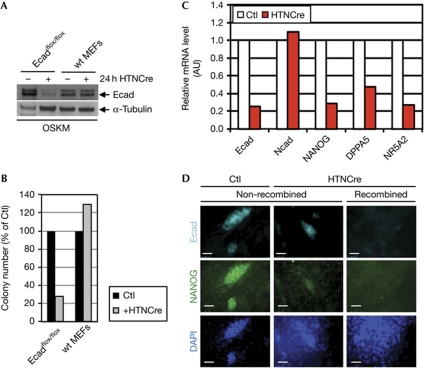
We isolated MEFs from mouse embryos that harboured two floxed E-cadherin alleles, Ecadflox/flox (Boussadia et al, 2002). Cre-mediated deletion of E-cadherin was achieved by the treatment of cultured cells with His-tat-NLS (HTN)-Cre, a membrane-penetrable Cre recombinase, which resulted in a reduction of E-cadherin-positive cell levels after viral infection with OSKM (Fig 2A). However, the ablation of E-cadherin by Cre-mediated recombination was not observed in all cells. We followed colonies by morphological inspection and observed an 80% reduction of colony numbers (Fig 2B). These MEFs were analysed for expression of pluripotency genes (Fig 2C). NANOG, DPPA5 and NR5A2 were expressed at reduced levels (25%, 45% and 25%, respectively) in HTNCre-treated MEFs. We conclude that reprogramming is impaired in the absence of E-cadherin, and that an MET with an induced expression of E-cadherin is required. We further analysed whether cell colonies that formed after Cre-mediated deletion of E-cadherin were E-cadherin negative or escaped the Cre-mediated deletion events. We found by immunofluorescence analysis of colonies in the Cre-treated pool that cell clones that converted to mESC-like morphology were still positive for E-cadherin, as well as for NANOG, showing that these clones are derived from cells that did not undergo deletion of E-cadherin (Fig 2D).
Figure 2.
Loss of E-cadherin expression shows its necessity during reprogramming. (A) Ecad protein expression in OCT4, SOX2, KLF4 and c-MYC (OSKM)-transduced non-Cre-treated (−) and His-tat-NLS (HTN)-Cre-treated (+) Ecadflox/flox MEFs and equally treated wild-type MEFs, respectively. (B) Determination of colony number by counting of mouse embryonic stem cell-like colonies in pools of non-Cre-treated, transduced Ecadflox/flox MEFs or wild-type MEFs (black) or Cre-treated Ecadflox/flox MEFs or wild-type MEFs (grey), respectively. Colony numbers were as follows: MEFs Ecadflox/flox without HTNCre (Ctl), 319 colonies (set to 100%); with HTNCre (+HTNCre), 88 colonies (28%); wild-type MEFs Ctl, 72 colonies (set to 100%); and +HTNCre, 93 colonies (129%). The difference in absolute colony numbers between Ecadvflox/flox and wild-type MEFs is due to the different viability of cells isolated from different animals at different times. The experiment was repeated four times and the values from one representative experiment are shown. (C) Relative mRNA expression level of Ecad, NANOG, DPPA5 and NR5A2 of a pool of OSKM-induced Cre-treated and non-treated Ecadflox/flox MEFs 10 days after infection. Median values from biological duplicates are presented as bars; one representative experiment is shown of four independent experiments. (D) Immunofluorescence analysis of cell colonies from OSKM-induced Ecadflox/flox MEFs for Ecad and NANOG either untreated (Ctl) or Cre-treated (HTNCre) showing no recombination (non-recombined first and second column) or recombination (recombined). DAPI was used for nuclear staining. Magnification × 400; scale bars, 50 μm. AU, arbitrary units; Ctl, control; DAPI, 4,6-diamidino-2-phenylindole; MEF, mouse embryonic fibroblast; mRNA, messenger RNA; wt, wild type.
E-cadherin can overcome the requirement of OCT4
We systematically examined whether the expression of exogenous E-cadherin influences the overall efficiency of reprogramming, and whether it might replace one of the OSKM factors during cellular reprogramming. Transduction of MEFs with the E-cadherin-expressing retrovirus, pMXs-Ecad, led to a high expression of E-cadherin (supplementary Fig S3A,B online). The expression of the viral constructs was confirmed by quantitative RT–PCR. The analysis of the reprogramming efficiency with different combinations of pluripotency factors and E-cadherin showed that E-cadherin did not induce a general increase in reprogramming efficiency, as measured by SSEA1 expression or alkaline phosphatase activity (supplementary Fig S3C,D online).
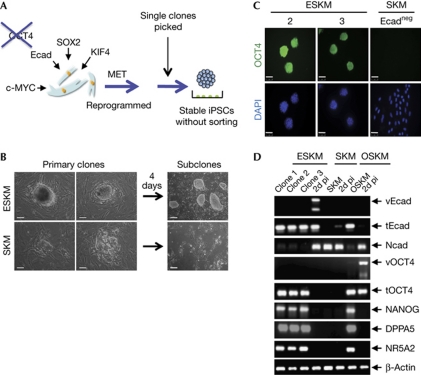
We also tested single cell clones that were produced by different factor combinations and scored them for the generation of those with iPSC-like morphology (Fig 3A). Intriguingly, the pMXs-Ecad retrovirus in combination with SOX2, KLF4 and c-MYC (ESKM)—that is, without OCT4—produced cells with the typical morphology of pluripotent iPSCs (Fig 3B), although at a lower frequency than with OSKM (supplementary Fig S4A online). By using Southern blot analysis, we confirmed that the ESKM clones showed no viral integration sites for OCT4 DNA sequences in comparison with an OSKM clone (supplementary Fig S3E online). When E-cadherin was omitted in the SKM combination, cell clones of iPSC-like morphology were rarely found (Fig 3B). Cell clones from ESKM and SKM combinations were cultivated for several passages and characterized. Remarkably, ESKM cell clones were positive for endogenous OCT4 (Fig 3C), NANOG and SSEA1 (supplementary Fig S4B online).
Figure 3.
E-cadherin can replace OCT4 in reprogramming. (A) Derivation of ESKM-induced pluripotent stem cell clones following viral transduction of MEFs in the presence of Ecad but the absence of OCT4. Transduced MEFs were seeded and embryonic-stem-cell-like colonies were observed. (B) Morphology of two independent primary clones photographed 12 days after viral transduction of MEFs with ESKM (upper panel) or SKM (lower panel). Primary clones were cultured for 4 days on feeder cells. Morphology of ESKM or SKM cells is shown in the right panels. Magnification × 400 (primary clones on feeder cells, two left panels); scale bars, 50 μm and × 100 (subclones, right panel); scale bars, 200 μm. (C) Immunofluorescence analysis of established ESKM (2, 3) and SKM cells for expression of OCT4. Nuclei were stained with DAPI. Magnification × 400; scale bars, 50 μm. (D) Reverse transcription–polymerase chain reaction analysis was performed with three independently generated ESKM clones (1, 2 and 3), ESKM-infected MEFs 2 days post-infection (2d pi), SKM subcloned cells (SKM), SKM-infected MEFs, OSKM-derived iPSCs and OSKM-infected MEFs. Expression levels of Ecad (v/t), Ncad, OCT4 (v/t), NANOG, DPPA5 and NR5A2 of cells were determined. DAPI, 4,6-diamidino-2-phenylindole; ESKM, pMXs-Ecad retrovirus in combination with SOX2, KLF4 and c-MYC; iPSC, induced pluripotent stem cell; MEF, mouse embryonic fibroblast; MET, mesenchymal-to-epithelial transition; OSKM, OCT4, SOX2, KLF4 and c-MYC.
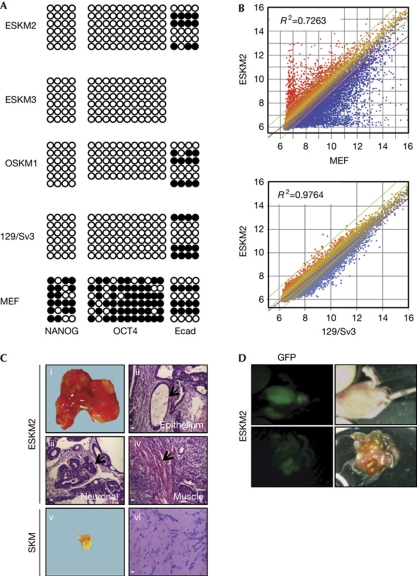
Independent ESKM clones (1,2,3) showed strong mRNA expression for E-cadherin and for the pluripotency genes OCT4, NANOG, DPPA5, and NR5A2, whereas expression of N-cadherin was not observed (Fig 3D). E-cadherin and OCT4 proteins were produced in ESKM clones, whereas N-cadherin protein was absent (supplementary Fig S4C online). ESKM-derived cell clones were able to form embryoid bodies (supplementary Fig S4D online) and showed expression of genes for endodermal (FoxA2), neuronal (Nestin) and mesodermal (α-SMA) lineages (supplementary Fig S4E online). The analysis of the methylation pattern of NANOG and OCT4 promoters showed a demethylated state in ESKM-derived and OSKM-derived iPSCs and mESCs, whereas in MEFs both promoters showed strong methylation marks (Fig 4A). We determined the methylation pattern of the E-cadherin promoter and observed no difference in the methylation statuses of iPSCs, mESCs and MEFs. Furthermore, we determined the global gene expression profiles of ESKM2 iPSCs, 129/Sv3 mESCs and MEFs (Fig 4B). Although the profile of ESKM iPSCs differed significantly from that of somatic MEFs (Pearson's coefficient: 0.7263), a significant similarity between ESKM iPSCs and mESCs (Pearson's coefficient: 0.9764) was observed.
Figure 4.
ESKM induced pluripotent stem cells fulfil all criteria of pluripotent stem cells. (A) Bisulphite sequencing of promoter regions of NANOG, OCT4 and Ecad, of ESKM cells (clone 2 and 3), OSKM cells (clone 1), mouse embryonic stem cells (mESCs; 129/Sv3) and mouse embryonic fibroblasts (MEFs). Shown are 5–6 reactions for each region. (B) Global gene expression profiling of ESKM-iPSCs (clone 2) in comparison with MEFs and mESCs. Similarity is shown by the R2 value. Three biological replicates of each cell line were included for analysis. (C) Whole morphology of tumours derived from ESKM-derived iPSCs (ESKM 2; 1) or SKM-derived cells (v). Histological analysis of tissue sections by haematoxylin and eosin staining of differentiated tumours (ESKM2) comprising epithelial (ii), neuronal (iii) and muscle-derived (iv) structures, or of a undifferentiated tumour (SKM; vi). Representative tumours are shown out of n=3 injections/cell clone, compare supplementary Table 3 for further details. Magnification of haematoxylin and eosin-stained sections × 25; scale bars, 500 μm. (D) Localization of green fluorescent protein (GFP)-positive areas in offspring following injection of stable GFP-expressing ESKM-iPSCs (clone 2) in blastocysts. ESKM, pMXs-Ecad retrovirus in combination with SOX2, KLF4 and c-MYC; OSKM, OCT4, SOX2, KLF4 and c-MYC.
In vivo differentiation showed that ESKM-reprogrammed iPSCs formed teratomas in non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice (Fig 4C, supplementary Fig S4F online). SKM clones showed only small cell lumps without significant growth. When stably green fluorescent protein (GFP)-transduced ESKM-iPSCs (ESKM2) were injected into blastocysts, these cells contributed to organ formation as indicated by GFP-positive areas, which were observed in different organs (Fig 4D). These data show that iPSCs can be derived in the presence of E-cadherin instead of OCT4 and show all the characteristics of pluripotent, reprogrammed cells.
Our analysis is focused on understanding the function of E-cadherin, both in ESCs during differentiation and in fibroblasts during iPSC reprogramming. First, we demonstrate that interference with E-cadherin in mESCs cause morphology changes and differentiation. It has been shown that E-cadherin is crucial to the conversion of Fgf2-, Activin-, BIO-derived (FAB, similar to EpiSC) mouse stem cells to pluripotent mESCs (Chou et al, 2008), and the loss of E-cadherin allows the maintenance of mESCs in suspension cultures (Mohamet et al, 2010), suggesting that, under these conditions, they might convert to intermediated cell-state-like mEpiSCs. These data indicate that E-cadherin has a crucial role in the maintenance of ESC pluripotency and the compact shape of the colonies. Second, in the reprogramming of MEFs by the four Yamanaka factors (OSKM), we found that iPSCs were exclusively E-cadherin-positive and could not be obtained from Ecadflox/flox cells after E-cadherin had been deleted by Cre. This latter finding is important as it is from genetic evidence and not on the basis of overexpression of factors or treatment with inhibitors. For instance, short-hairpin RNA viruses directed against E-cadherin could prevent iPSC reprogramming and chemical intervention leading to upregulation of E-cadherin-promoted somatic cell reprogramming (Chen et al, 2010). Recently, E-cadherin has also been found to be upregulated during reprogramming in global expression analyses (Samavarchi-Tehrani et al, 2010) and in a screen for cell adhesion molecules (Li et al, 2010). In our reprogramming system, overexpression of E-cadherin alone could not enhance the efficiency of iPSC production; such an effect was recently reported (Chen et al, 2010). Taken together, these findings add new aspects to the processes of nuclear reprogramming: as well as transcriptional networks (Jaenisch & Young, 2008), epigenetic changes (Hochedlinger & Plath, 2009) and changes in microRNA expression (Judson et al, 2009); apparently, spatial cues based on the function of E-cadherin are also of importance.
Third, our finding that the expression of E-cadherin is able to replace the exogenous transcription factor OCT4, adds to our understanding of reprogramming. It shows that iPSC reprogramming is not just a nuclear event, but that spatial cues also participate and that these cross-talk with essential transcription factors in a new manner. Specific cell–cell contacts of pluripotent cells seem to directly tie into the pluripotent transcription factor circuit, which influences the master transcription factor OCT4. Signalling events downstream from E-cadherin might provide a stimulus to upregulate endogenous OCT4. It is tempting to speculate that E-cadherin could control OCT4 through binding and sequestering β-catenin, that is, by modulation of canonical Wnt signalling. Interestingly, it has recently been shown that β-catenin enhances OCT4 activity through a T-cell factor (TCF)-independent mechanism (Kelly et al, 2011). Alternatively, the effects of E-cadherin on induced pluripotency might also require the involvement of Rho-family GTPases (Fukata & Kaibuchi, 2001). The elucidation of the molecular mechanisms that establish the cross-talk between the cell surface and nuclear machinery, especially the regulation of OCT4, awaits further analysis. With these three parts of our investigation, we have discovered new functions for the cell-adhesion molecule E-cadherin in pluripotency and reprogramming.
Methods
Cell culture. 129/Sv3-derived mESCs (from S. Noggle, New York, USA) and established stable iPSC lines generated were cultured on mitomycin-C-treated (2 mg/ml) MEFs or on gelatin-coated plates. MEFs were isolated according to standard procedures either from embryos of the mouse strain B6.129/Sv3-Cdh1tm2Kem/J (Ecadflox/flox) or from the outbred strain Cf1.
Generation of iPSCs. Generation of iPSCs was performed as described previously (Takahashi & Yamanaka, 2006). In brief, 5 × 106 Plat-E cells were seeded 1 day before transfection with pMXs-based retroviral vectors (Ecad or OCT4, SOX2, KLF4 and c-MYC; from Addgene) using calcium phosphate precipitation in the presence of 25 μM chloroquine. For expression of E-cadherin, the coding sequence of mouse E-cadherin was introduced into the pMXs vector. For the derivation of ESKM-iPSC clones, 2 × 105 MEFs/10 cm dish were seeded on Matrigel (BD) 4 days after viral infection in mESC medium. OSKM-induced cells were seeded to 5 × 104 cells/10 cm dish on mitomycin-C-treated MEFs.
Cell-based assays. Quantitative RT–PCR and western blot analysis were performed as described earlier (Diecke et al, 2008).
Cre-recombinase treatment: cells were treated with 3 μM purified, membrane-penetrable HTNCre for 24 h, as described previously (Peitz et al, 2002).
Bisulphite sequencing: genomic DNA was isolated from different cell lines. Bisulphite conversion of 2 μg DNA was done with the EpiTect Kit (Qiagen) and converted DNA was used for PCR using specific primers recognizing regions in the promoters of NANOG, OCT4 and E-cadherin. PCR products were cloned in the pGEM-Vector (Promega) and sequenced using SP6 primer.
Global gene expression analysis. Expression analysis was done in triplicates using the MouseWG-6 v2.0 Expression BeadChip Kits (Illumina), using standard protocols. The microarray analysis on the Illumina MouseWG-6 v2.0 expression beadchips (Fig 4B) has now been deposited at the National Center for Biotechnology Information (NCBI)/Gene Expression Omnibus (GEO). The data can be found at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE28594.
Animal-based assays. Teratoma formation and histological analysis: 1 × 106 cells were subcutaneously injected in NOD/SCID mice, as described previously (Takahashi & Yamanaka, 2006). Tumours were grown for 21 days and sections of surgically dissected tumours were stained with haematoxylin and eosin and biopsies were taken for RT–PCR analysis.
Blastocyst injection: stably GFP-expressing iPSCs transduced with the plasmid MP71-GFP were injected at the blastocyst stage. Vehicles were transferred in uteri of surrogate mothers. Pups were analysed for GFP-positive spots 2 days after birth.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank A. Kirschner, M. Mühlbauer and Anne Schäfer for technical assistance, and A. Klaus, H.-P. Rahn, B. von Eyss, K. Eckert, I. Fichtner, B. Jerchow and K. Becker for technical help. We express our appreciation to R. Hodge, U. Ziebold, I. Ibanez-Tallon and M. Gossen for critical reading of the manuscript. A Bundersministerium für Bildung und Forschung (BMBF) grant in the joint project START-MSC (standardisation for regenerative therapy–mesenchymal stem cells; 01GN0941) and a European Commission Marie Curie International Reintigration Grant (FP6-021407) supported this work.
Footnotes
The authors declare that they have no conflict of interest.
References
- Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R (2002) E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev 115: 53–62 [DOI] [PubMed] [Google Scholar]
- Chen T, Yuan D, Wei B, Jiang J, Kang J, Ling K, Gu Y, Li J, Xiao L, Pei G (2010) E-cadherin-mediated cell–cell contact is critical for induced pluripotent stem-cell generation. Stem Cells 28: 1315–1325 [DOI] [PubMed] [Google Scholar]
- Chou YF, Chen HH, Eijpe M, Yabuuchi A, Chenoweth JG, Tesar P, Lu J, McKay RD, Geijsen N (2008) The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell 135: 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diecke S, Quiroga-Negreira A, Redmer T, Besser D (2008) FGF2 signaling in mouse embryonic fibroblasts is crucial for self-renewal of embryonic stem cells. Cells Tissues Organs 188: 52–61 [DOI] [PubMed] [Google Scholar]
- Fukata M, Kaibuchi K (2001) Rho-family GTPases in cadherin-mediated cell–cell adhesion. Nat Rev Mol Cell Biol 2: 887–897 [DOI] [PubMed] [Google Scholar]
- Heuberger J, Birchmeier W (2010) Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol 2: a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K, Plath K (2009) Epigenetic reprogramming and induced pluripotency. Development 136: 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Young R (2008) Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132: 567–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson RL, Babiarz JE, Venere M, Blelloch R (2009) Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol 27: 459–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KF, Ng DY, Jayakumaran G, Wood GA, Koide H, Doble BW (2011) β-Catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell 8: 214–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R (1994) E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci USA 91: 8263–8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Antos C, Butz S, Huber O, Delmas V, Dominis M, Kemler R (1996) A role for cadherins in tissue formation. Development 122: 3185–3194 [DOI] [PubMed] [Google Scholar]
- Li R et al. (2010) A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell 7: 51–63 [DOI] [PubMed] [Google Scholar]
- Mohamet L, Lea ML, Ward CM (2010) Abrogation of E-cadherin-mediated cellular aggregation allows proliferation of pluripotent mouse embryonic stem cells in shake flask bioreactors. PLoS ONE 5: e12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitz M, Pfannkuche K, Rajewsky K, Edenhofer F (2002) Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. Proc Natl Acad Sci USA 99: 4489–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A, Woltjen K, Nagy A, Wrana JL (2010) Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 7: 64–77 [DOI] [PubMed] [Google Scholar]
- Soncin F et al. (2009) Abrogation of E-cadherin-mediated cell–cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells 27: 2069–2080 [DOI] [PubMed] [Google Scholar]
- Stepniak E, Radice GL, Vasioukhin V (2009) Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb Perspect Biol 1: a002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH (1997) Viable offspring derived from fetal and adult mammalian cells. Nature 385: 810–813 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.